Abstract
Background:
Although a clear negative influence of chronic exposure to stressful experiences has been repeatedly demonstrated, the outcome of acute stress on key brain regions has only just started to be elucidated. Although it has been proposed that acute stress may produce enhancement of brain plasticity and that antidepressants may prevent such changes, we still lack ultrastructural evidence that acute stress-induced changes in neurotransmitter physiology are coupled with structural synaptic modifications.
Methods:
Rats were pretreated chronically (14 days) with desipramine (10mg/kg) and then subjected to acute foot-shock stress. By means of serial section electron microscopy, the structural remodeling of medial prefrontal cortex glutamate synapses was assessed soon after acute stressor cessation and stress hormone levels were measured.
Results:
Foot-shock stress induced a remarkable increase in the number of docked vesicles and small excitatory synapses, partially and strongly prevented by desipramine pretreatment, respectively. Acute stress-induced corticosterone elevation was not affected by drug treatment.
Conclusions:
Since desipramine pretreatment prevented the stress-induced structural plasticity but not the hormone level increase, we hypothesize that the preventing action of desipramine is located on pathways downstream of this process and/or other pathways. Moreover, because enhancement of glutamate system remodeling may contribute to overexcitation dysfunctions, this aspect could represent a crucial component in the pathophysiology of stress-related disorders.
Keywords: stress, glutamate, synapse, desipramine
Introduction
Emotional arousing and stressful experiences represent constitutive parts of our daily life; tight and efficient regulation of the systems in charge of mediating the stress response is therefore required to ensure adaptation (Sterling and Eyer, 1988). However, when dysregulation of the stress response network or too much stress output occur, an overload of the system and its physical and psychological manifestations such as depressed mood and inflammation may be promoted (McEwen and Stellar, 1993). Among the key targets for stress hormones is the prefrontal cortex (PFC) (Meaney and Aitken, 1985; McEwen, 2007), a limbic region mediating highly evolved executive functions, including working memory and cognitive flexibility (McEwen and Morrison, 2013). Depending on the severity and duration of the stimulus, stressful episodes have been reported to exert differential effects on the PFC (Popoli et al., 2012). Preclinical studies in animal models of stress have shown that chronic exposure to repeated stressors produces a number of physiological, cognitive, and structural deficits within medial PFC (mPFC); these changes cover suppression of glutamate receptor expression and function, reduced glial metabolism, and decreased expression of synaptic proteins as well as impaired decision-making and working memory (Dias-Ferreira et al., 2009; Banasr et al., 2010; Li et al., 2011; Yuen et al., 2012). Chronic stress has also been shown to induce profound structural remodeling of mPFC layer II-III pyramidal neurons, including dendritic shortening, spine loss, and neuronal atrophy (Cook and Wellman, 2004; Radley et al., 2004). In contrast, short-term activation of the stress response systems, as produced by acute stressors, has been reported to cause plasticity-enhancing effects, including potentiation of glutamate transmission in conjunction with enhanced working memory and increase of excitatory aminoacid levels and of glutamate release in parallel with increased release probability in layer III pyramidal neurons of mPFC (Moghaddam, 1993; Yuen et al., 2009; Musazzi et al., 2010). Less, however, is known about the effects of acute stress on mPFC structural remodeling. It is well established that excitatory synapses play a major role in mediating synaptic transmission, plasticity, and memory; on the other hand, it has recently been demonstrated that stress and glucocorticoids, the major stress hormones, can actively and dynamically regulate these synapses (Krugers et al., 2012; Timmermans et al., 2013). Thus, in the present study we aimed at investigating the immediate effects of acute stress on presynaptic and synaptic remodeling of layer II-III pyramidal neurons within the mPFC. It has recently been demonstrated that a single foot-shock (FS) stress exposure may produce hippocampal synapse loss 24 hours after stress (Hajszan et al., 2009). Moreover, we have recently shown that acute FS stress may strongly and rapidly increase the number of glutamate vesicles available for release within mPFC layer II-III (Treccani et al., 2014); however, whether the previously observed rearrangement of synaptic vesicles towards the presynaptic membrane may be coupled with changes in the number of mPFC synapses has never been explicitly demonstrated soon after acute stress. In this study, we also aimed to determine whether pretreatment with the traditional antidepressant desipramine (DMI) may affect any such change. It has recently been demonstrated that pretreatment with the norepinephrine reuptake inhibitor DMI dampens acute FS stress-induced increase of depolarization-evoked glutamate release from synaptic terminals of frontal and PFC cortex, and enhancement of the release probability in layer III pyramidal neurons within mPFC (Musazzi et al., 2010). However, it has never been explicitly demonstrated whether the preventive action of DMI on FS stress-induced enhancement of glutamate transmission may be coupled with any structural changes of mPFC after acute FS stress.
Materials and methods
Animals and Drug Treatment
Male Sprague-Dawley rats (175–200g, Charles River, Calco, Italy) were housed 2 per cage in a light-controlled room (under a 12-h-light/-dark cycle; lights on at 7:00 am) at room temperature (22ºC) with food and water ad libitum. After 5 days of housing, one-half of the animals were subjected to chronic treatment (14 days) with DMI (10mg/kg) delivered in drinking water. Drug solutions were changed every 2 days according to the animals’ weight and water intake. All experimental procedures involving animals were performed in accordance with the European Community Council Directive 86/609/EEC and approved by Italian legislation on animal experimentation (Decreto Ministeriale 116/1992).
Stress Paradigms and Corticosterone Levels
Twenty-four hours after the last drug or vehicle administration, animals were subjected to 2 different acute stressors. For the FS stress, rats were placed in a plastic chamber for 40 minutes, and 20 minutes of total actual shock (0.8 mA) was delivered with a random inter-shock length between 2 and 8 seconds (Vollmayr and Henn, 2001). Control animals were kept in the stress apparatus for the same interval of time (40 minutes) without delivering the shocks. For the acute restraint (RS) stress, rats were immobilized in air-accessible cylinders for 1 hour (Deolindo et al., 2013). Stress exposure was conducted between 8:00 am and 12:00 am in a room different from that used for housing.
Trunk blood corticosterone (CORT) levels were estimated using the IDS ELISA kit (Immunodiagnostic System, Herlev, Denmark). Inter- and intra-assay coefficient of variation was <10% (supplementary Material).
Confocal Microscopy of Synapses
Four male Sprague-Dawley rats (175–200g) were treated for 14 days with either vehicle or DMI, as described above. Following a 24-hour wash-out, rats were deeply anesthetized with pentobarbital sodium (Unikem A/S, Copenhagen, Denmark; intra-peritoneal, 75mg/kg) and transcardially perfused with 100mL heparinized (10mg/mL) saline (NaCl 0.9%, pH=7.4) for 5 minutes followed by 2% fresh paraformaldehyde in 0.1M phosphate buffered saline (pH=7.4, 4°C). Brains were paraffin-embedded and processed for immunofluorescence staining against Piccolo and glutamate vesicular transport 1 protein (VGLUT1); for quantitative analysis, sections were examined in a 2-photon Zeiss Confocal LSM 510 META microscope (supplementary Material).
Total Number of Synapses and Synaptic Vesicles Using Electron Microscopy
Directly after the end of acute stress, animals were anesthetized and transcardially perfused with heparinized (10mg/mL) saline (NaCl 0.9%, pH=7.4) for 5 minutes followed by fixative containing 2.5% glutaraldehyde and 2% fresh paraformaldehyde in 0.1M cacodylate buffer (pH 7.4). Series of 100-µm-thick sections were cut coronally on a vibratome 3000 (Vibratome, St. Louis, MO); based on a systematic, uniform random sampling principle and a sampling fraction of one-sixth, 2 series were chosen. Given their exquisite sensitivity to chronic and acute stressors (Radley et al., 2004; Musazzi et al., 2010), mPFC layer II-III containing the apical dendrites of the vast majority of cortical pyramidal neurons were unambiguously identified and processed for electron microscopy (supplementary Material) (Nava et al., 2014).
Synaptic vesicle quantification was performed on a series of 45-nm-thick sections sampled from 2 regional areas per animal. Briefly, neurotransmitter-containing vesicles were classified into docked vesicles, when overlapping with the presynaptic membrane; all nondocked vesicles were defined as reserve pools. Synaptic vesicles were quantified in perforated (PSs) (Figure 1a) and nonperforated (NPSs) (Figure 1b) synapses. Quantification was performed as previously described (supplementary Material) (Nava et al., 2014).
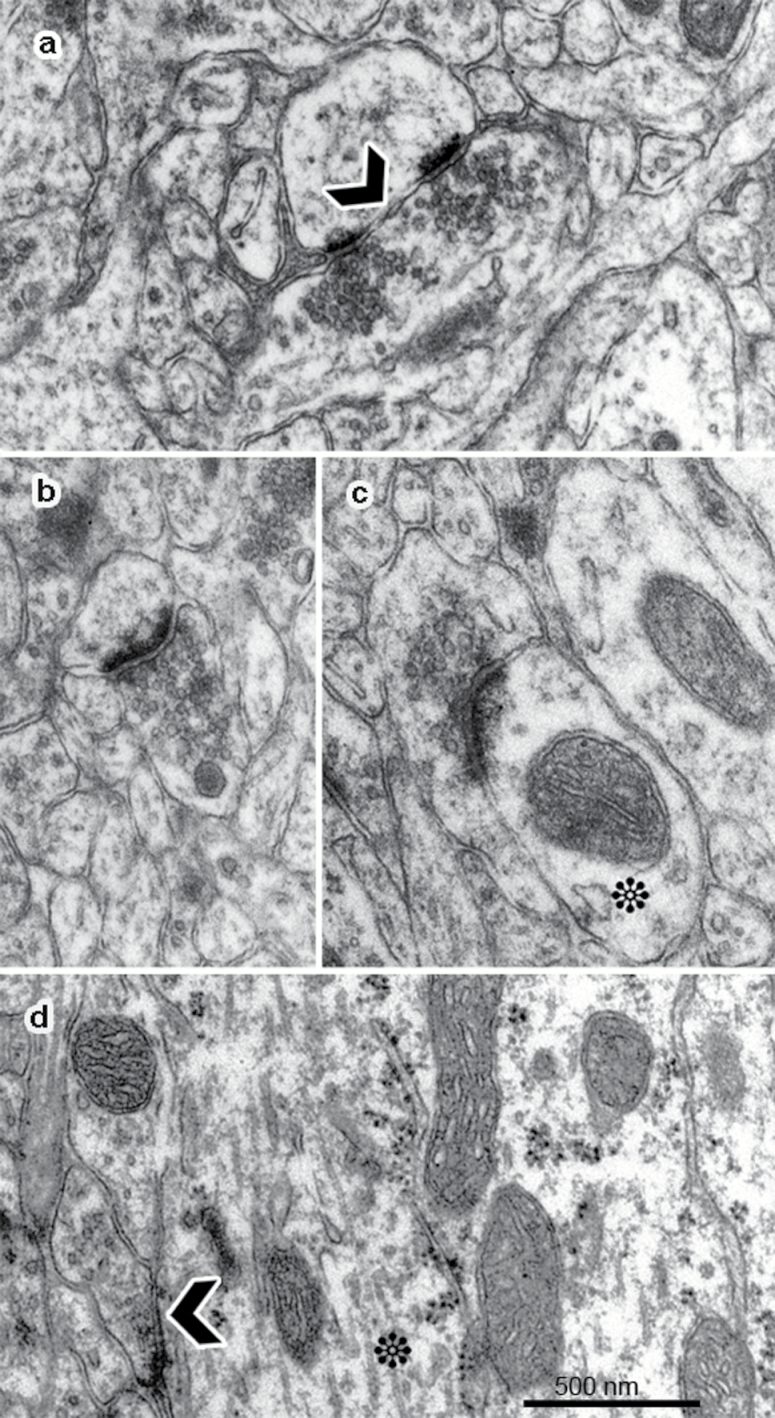
Figure 1.
Electron micrographs identifying subpopulations of synapses analyzed. Axo-spinous synapses were classified into perforated (a) and nonperforated (b) based on the presence of a discontinuity (black arrow in a) in the postsynaptic density. Axo-dendritic synapses (c-d) were identified by the presence of a mitochondrion on the postsynaptic compartment (asterisk in c and d). Scale bar=500nm.
For synapse number estimation, four epon blocks were sliced into series of 65-μm-thick serial sections with a MC MT-7 ultramicrotome (Tucson, AZ), and for each block two regions were sampled and imaged in a FEI Morgagni transmission electron microscope at final magnification of 18000x. Synapses were classified into axo-spinous, including PSs (Figure 1a) and NPSs (Figure 1b) synapses, based on the presence of a perforation on their postsynaptic density (black arrow in 1a), and axo-dendritic, namely axo-shafts (AXSs) (Figure 1c-d), synapsing directly onto the dendritic shaft (asterisk in Figure 1c-d) (Geinisman et al., 2001). For quantification, a modified physical disector was adopted (Sterio, 1984) (supplementary material). All animals were assigned code numbers and decoded only after completion of the quantification.
mPFC Volume Estimation
mPFC total volume was obtained by applying the Cavalieri estimator in combination with the 2D-nucleator (6 half-lines; newCAST software) on thionin-stained sections (supplementary Material) (Gundersen and Jensen, 1987).
Preparation of Figures
Electron micrographs, as shown in the figures, were adjusted for contrast and brightness using Adobe Photoshop (San Jose, CA). Three-dimensional reconstructions were performed by applying of the RECONSTRUCTT softare (Fiala, 2005).
Statistical Analysis
All results were expressed as mean and relative coefficient of variation and calculated as the standard deviation of the estimates, divided by the mean. N represented the number of animals per group, and n represented the number of sections per animal analyzed for immunofluorescence study.
Coefficients of error (CEs) for synapse and vesicle number estimation were estimated as previously described ( Nava et al., 2014 ) ( supplementary Tables S1 and S2 ). Data were analyzed using 2-way (stressxtreatment) and 1-way (stress subtypes) analysis of variance. When analysis of variance revealed significant group differences, the Bonferroni posthoc test was employed. For the immunofluorescence study, an unpaired t test was used. Statistical analysis and graphical representation was carried out using Systat 12.0 and GraphPad Prism 5.0. The degrees of significance were considered *P<.05, ** P<.01, and ***P<.001.
Results
Glutamatergic Synaptic Terminal Distribution within mPFC
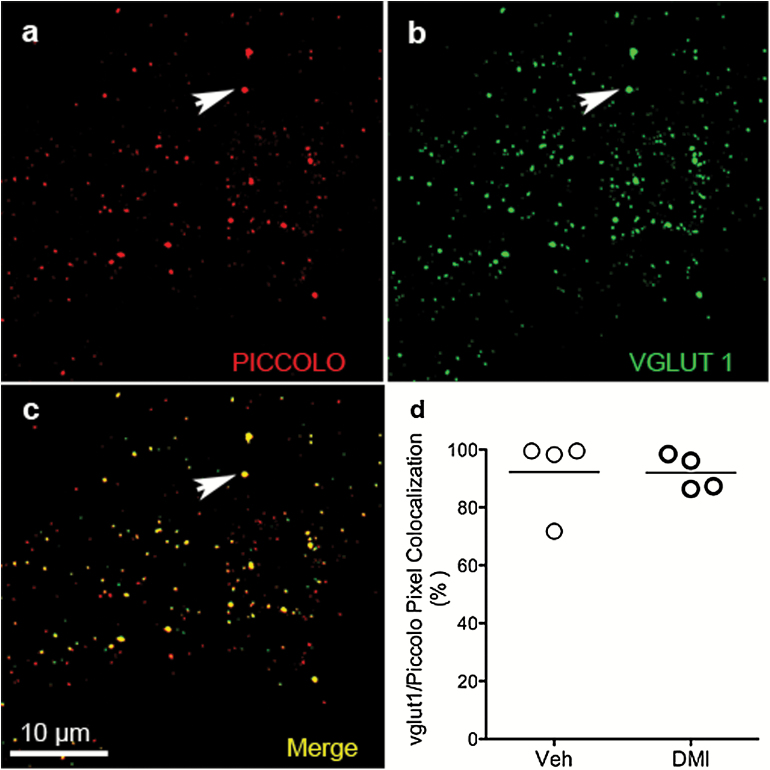
It is believed that excitatory synapses represent the majority of synapses within neocortex (Sanacora et al., 2012). However, to verify this, we assessed the relative distribution of mPFC excitatory terminals in rats subjected to chronic treatment with vehicle or DMI. A presynaptic active zone was visualized with antibody to Piccolo protein (Figure 2a) (Dondzillo et al., 2010); glutamate-specific synaptic terminals were identified using antibody to VGLUT1 (Figure 2b), regarded as expressed primarily in glutamatergic neurons and also in glial cells within cortical areas (Sanacora et al., 2012). In line with this, transporter VGLUT1 and Piccolo showed a high level of colocalization (Figure 2c) in both vehicle- and DMI-treated groups, with an average of 92.3% and 92.1%. Unpaired t test revealed no significant difference in the degree of colocalization of the 2 proteins between the 2 groups (Figure 2d). Taken together, these results confirm that the large majority of mPFC synaptic contacts are excitatory.
Figure 2.
Relative distribution of excitatory synaptic terminals within medial prefrontal cortex (mPFC). Dual staining for active-zone marker Piccolo (white arrow) (a) and transporter glutamate vesicular transport 1 protein (VGLUT1) (white arrow) (b) shows high degree of colocalization (white arrow) (c). Scale bar=10 μm. (d) Summary of quantitative data of colocalized punctate on dual-stained mPFC sections from rats treated with either vehicle (Veh) or chronic desipramine (DMI), obtained using VIS Image Analysis Software (Visiopharm, Hørsholm, Denmark). P value, ns. (N=4; n=5).
Acute Stress-Induced Increase of Docked Vesicles Is Partially Prevented by Chronic Pretreatment with DMI
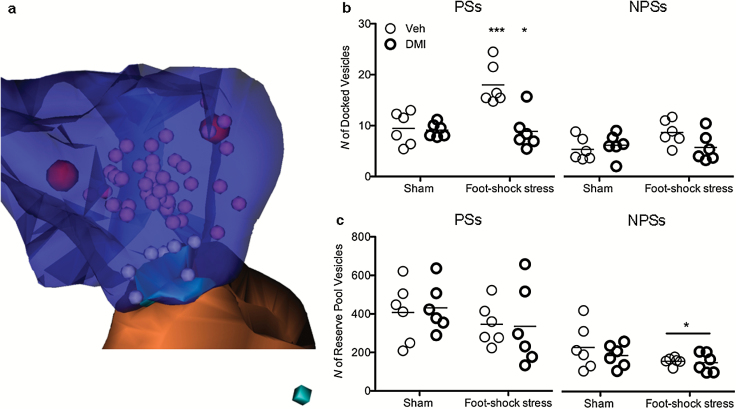
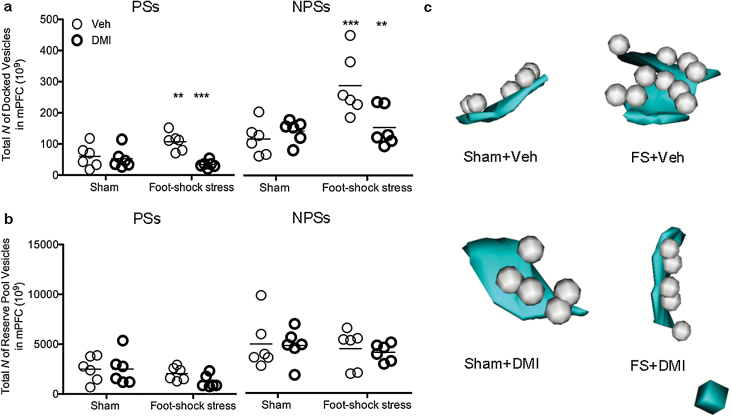
At the synaptic level, presynaptic terminals (blue region in Figure 3a) are enriched with neurotransmitter-containing vesicles of varying release competence (Dobrunz, 2002), so that vesicles docked to the active zone (cyan in Figure 3a) are thought as ready to be released (white spheres in Figure 3a) (Schikorski and Stevens, 2001); instead, nondocked vesicles (purple spheres in Figure 3a) comprise the reserve pool.
Figure 3.
Foot shock stress-induced presynaptic ultrastructural plasticity is partially prevented by chronic desipramine (DMI). (a) A reconstructed synapse showing a typical spine contact. Presynaptic terminal (blue) is enriched with neurotransmitter-containing vesicles, divided into docked vesicles (white spheres) overlapping with the presynaptic membrane at the level of the active zone (cyan) and into reserve pool vesicles (purple spheres). Red spheres represent large, dense core vesicles, which were not included in the quantification. Cube side = 50nm. (b) Acute FS-stressed rats showed significantly higher number of vesicles docked to the presynaptic membrane in synapses with perforation of postsynaptic density (PSs) (P<.001); treatment with chronic desipramine (DMI) prevented the stress-induced increase of the number of docked vesicles (left panel) (P<.05). Conversely, the number of docked vesicles in small nonperforated synapses (NPSs) was shown to be stable (right panel). (c) The total number of reserve pool vesicles was stable among experimental groups in PSs (left panel). The number of reserve pool vesicles in NPSs was slightly decreased after acute FS stress (right panel) (P<.05).
Acute FS stress has been reported to induce a strong and rapid increase of glutamate release from frontal and PFC synaptic terminals. This effect was paralleled by increased release probability and changes of excitatory postsynaptic current (EPSC) kinetics in layer III pyramidal neurons, two electrophysiological measurements of pre- and postsynaptic activity, respectively (Musazzi et al., 2010). Moreover, we have recently shown that an FS stress-induced increase of glutamate release occurs in parallel with an increased number of glutamate vesicles available for release within mPFC (Treccani et al., 2014). Chronic treatment with DMI dampened the stress-induced increase of glutamate release and release probability, but not the kinetics of EPSC, suggesting a mechanism of action limited at the presynaptic level (Musazzi et al., 2010). Thus, to evaluate the effect of chronic DMI on stress-induced glutamate vesicle redistribution within the presynaptic compartment, we quantified the number of docked and reserve pool vesicles in stressed animals pretreated with chronic DMI. At the end of acute FS stress exposure, the average number of docked vesicles was increased almost 2-fold in PSs (+90.3%; N=6; P<.001), and this increase was partially prevented by chronic DMI (−6.9%; N=6; P<.05) (Figure 3b, left panel). No effect of either stress or DMI was observed in NPSs (N=6; P, ns) (Figure 3b, right panel). In contrast, the average number of reserve pool vesicles significantly decreased after stress in NPSs (−16.0%; N=6; P<.05) (Figure 3c, right panel) with no effect on PSs (N=6; P, ns) (Figure 3c, left panel).
These results confirm that acute FS stress induces a dramatic and selective increase in the number of docked vesicles available for release in PSs, as previously reported (Treccani et al., 2014). Moreover, the results show that, in PSs, the stress-induced major redistribution of synaptic vesicles towards the presynaptic membrane is attenuated by DMI pretreatment. Overall, these results suggest that, by reducing the stress-induced increase of docked vesicles, chronic DMI acts at the presynaptic level by preventing the stress-induced potentiation of a morphological correlate of excitatory synaptic strength.
Acute Stress Induces a Rapid Increase of mPFC Excitatory Synapse Number, Which Is Prevented by Chronic DMI
An altered number of docked vesicles has been observed during long-term potentiation in conjunction with fast synaptic remodeling, as shown by an increased number of synapses in hippocampal Cornu Ammonis 1 as soon as 30 minutes after the stimulus induction (Bourne and Harris, 2011; Bourne et al., 2013). Moreover, high vulnerability of synapses to acute stressful events has previously been reported in studies showing hippocampal synaptic marker increase and synapse loss, respectively, shortly and 24 hours after stress termination (Hajszan et al., 2009; Sebastian et al., 2013).
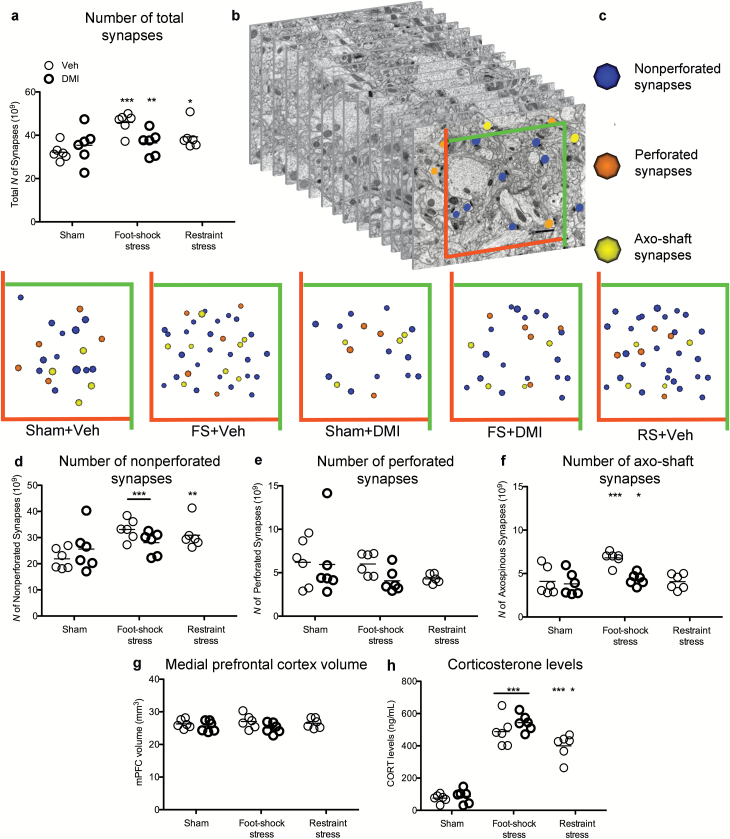
Hence, to investigate the effects of acute FS stress and DMI treatment on mPFC excitatory terminal remodeling, we quantitated the number of mPFC asymmetric synapses regarded as excitatory by means of physical disector and electron microscopy. Interestingly, the total number of excitatory synapses was found to be rapidly and dramatically increased by 42.6% (N=6; P<.001) after 40 minutes of FS stress compared with the sham group, whereas chronic administration of DMI partially prevented the stress-induced increase (+13.3% compared with control group; N=6; P<.01) (Figure 4a).
Figure 4.
Total number of medial prefrontal cortex (mPFC) excitatory synapses is rapidly and selectively increased after 40 minutes of acute foot shock (FS) stress and after 1 hour of restraint (RS) stress, whereas treatment with chronic desipramine (DMI) partially prevents FS stress-induced synaptogenesis. (a) Total number of mPFC synapses was found to be rapidly increased at the end of 40 minutes of FS stress exposure (P<.001); however, FS stress-induced synaptogenesis was partially prevented by treatment with chronic DMI (P<.01). RS stress also increased the total number of synapses compared with sham vehicle-treated (Veh) group (P<.05). (b) For synapse quantification, a modified physical disector was applied (Tang et al., 2001); a grid was superimposed over 15 serial sections; section 1 was look-up section, sections from 2 to 10 were used as the reference section of the disectors, whereas the last 5 sections were used as look-up sections to ensure that all counted postsynaptic densities were included in the section series. Synaptic profiles touching the inclusion (green) lines were included in the analysis, whereas those touching the exclusion (red) lines were excluded (scale bar=1 μm). Synapses were classified between axo-spinous, synapsing onto spines, including nonperforated (blue dots) and perforated (orange dots). Contacts synapsing directly onto the dendritic shaft were classified as axo-shaft (AXSs) (yellow dots). (c) Application of disector technique allowed unbiased estimates of the number of neocortical synapses, reconstructed here as spheres, within neuropil volume. Blue spheres represent nonperforated synapses (NPSs), orange spheres perforated synapses (PSs), and yellow spheres AXSs. Representative estimates of the number of synapses are reported as the number of 3D spheres for each experimental group: Sham+Veh (n=21); FS+Veh (n=37); Sham+DMI (n=18); FS+DMI (n=26); RS+Veh (n=35). (d) Acute FS stress led to a strong significant increase in small NPSs (P<.001) regardless of DMI treatment. The number of NPSs was also increased in RS animals compared with the sham group (P<.01). (e) The total number of great PSs was shown to be stable among experimental groups. (f) AXSs were found strongly increased after acute FS stress (P<.001), whereas treatment with chronic DMI prevented the stress-induced increase (P<.05). (g) mPFC volume estimated by the Cavalieri estimator did not show any significant difference among experimental groups. (h) Corticosterone concentration was found significantly increased after both FS and RS stress, regardless of previous DMI treatment (P<.001). Compared with RS-stressed animals, rats subjected to FS stress showed higher levels of CORT (P<.05).
To further verify whether the observed synaptogenic effect was specific for FS stress or common to other acute stress models, we included rats previously subjected to 1 hour of RS stress in our analysis. When comparing the total number of asymmetric synapses across different stress subtypes, acute RS stress also increased asymmetric synapses by 22% compared with the sham group (N=6; P<.05) (Figure 4a).
We have recently shown that the majority of mPFC asymmetric spine synapses, namely PSs and NPSs, are endowed with heterogeneous structural features that may reflect differences in synaptic efficacy. For example, NPSs have shown smaller bouton volume and lower numbers of neurotransmitter-containing vesicles compared with PSs (Nava et al., 2014). Moreover, asymmetric synapses may undergo differential activity-dependent remodeling, depending on the subtype. For example, long-term potentiation, a synaptic model of memory, has been shown to selectively increase the number of PSs only (Geinisman et al., 1991, 1993).
Therefore, to clarify whether the synaptogenic effect of acute stress is selective for a specific synaptic population, we further classified asymmetric synapses between axospinous, including NPSs and PSs, and axo-dendritic contacts, namely AXSs (Figure 4c), in our serial section electron microscopy analysis (Figure 4b). Small NPSs were increased after FS stress compared with the sham group (+39.6%; N=6; P<.001) regardless of treatment with DMI. Comparison across stressor subtypes showed that RS stress also significantly increased the number of NPSs (+41.2%; N=6; P<.01) (Figure 4d). Interestingly, big PSs were stable across groups (N=6; P, ns) (Figure 4e). Moreover, only acute FS stress, but not RS stress, induced a strong increase in AXSs (+51.2%; N=6; P<.001), and this effect was partially prevented by treatment with chronic DMI (+6.01%; N=6; P<.05) (Figure 4f). When estimating overall mPFC volume, no significant effect of stress-induced new synapse formation was observed (Figure 4g).
To determine whether the increase of mPFC asymmetric synapses in acutely stressed animals is correlated with the elevated levels of adrenal corticosteroid hormones, we performed enzyme-linked immunosorbent assay to measure CORT levels. As shown in Figure 4h, compared with unstressed animals, rats exposed to acute stressors had significantly higher blood concentration of CORT (FS stress: +532%, P<.001; RS stress: +390%, P<.001) regardless of previous treatment with DMI (Musazzi et al., 2010). When comparing among stressor subtypes, CORT levels were found to be significantly higher after FS compared with RS stress animals (+29.1%, P<.05) (Figure 4h).
Thus, an acute severe stressor rapidly induces strong sprouting of asymmetric synaptic contacts, mainly by increasing the number of small and less efficacious NPSs; on the other hand, the number of glutamate synapses is only modestly increased after exposure to a less severe stressor. Moreover, DMI pretreatment, ineffective in the absence of stress, partially prevents the stress-induced increase. These results suggest that an acute stressor can rapidly boost the mPFC glutamate system by enhancing the number of glutamate release sites and apposed postsynaptic specializations. On the other hand, the preventive effect of DMI on stress-induced synapse sprouting, but not on CORT increase, suggests that the site of action of DMI in the previously observed modulation of stress-induced glutamate release (Musazzi et al., 2010) is downstream of CORT release, as previously found (Conti et al., 2004; Musazzi et al., 2010), and may directly involve the blockade of new pre- and postsynaptic element formation.
Acute FS Stress Induces a Generalized Increase of Glutamate Vesicles Available for Release within mPFC, Which Is Prevented by DMI Pretreatment
The observed effects of FS stress and DMI pretreatment on synaptogenesis and presynaptic ultrastructural plasticity showed a high degree of selectivity towards different synaptic subtypes. Thus, acute stress increased the number of docked vesicles in PSs only and increased the number of NPSs and AXSs. Therefore, we examined how the stress-induced increase in number of NPSs could affect the total number of docked vesicles and, possibly, excitatory synaptic strength within mPFC by combining the data obtained on docked vesicles and synapse number estimation.
The total number of docked vesicles, calculated by multiplying the total number of synapses within mPFC with the average number of vesicles, was significantly increased after FS stress in both PSs (+78.1%; N=6; P<.01) and NPSs (+145.4%; N=6; P<.001) compared with the control group, with a more remarkable effect on the latter subtype. Treatment with DMI, ineffective in the absence of stress, fully and partially normalized the stress-induced increase in PSs (−11.9%; N=6; P<.001) and NPSs (+31.4%; N=6; P<.01), respectively (Figure 5a, left and right panels). No effect of either stress or treatment was observed on reserve-pool vesicles in PSs and NPSs (Figure 5b, left and right panels). These results suggest that, following the stress-induced increase of synapses, acute FS stress produces a strong and generalized increase in the number of vesicles available for release in both synapse subtypes, which is strongly normalized by DMI pretreatment (Figure 5c).
Figure 5.
Acute foot shock (FS) stress dramatically increases morphological correlates of synaptic strength by increasing the number of vesicles available for release in medial prefrontal cortex (mPFC) perforated (PSs) and nonperforated (NPSs) synaptic population. (a) The total number of docked vesicles in PSs within mPFC was significantly increased by almost 2-fold after acute FS stress (P<.01); treatment with chronic desipramine (DMI) completely prevented the stress-induced increase in the number of docked vesicles within mPFC PSs (left panel) (P<.001). (b) Similarly, in mPFC NPSs, FS stress significantly increased the total number of docked vesicles (P<.001), whereas treatment with DMI partially prevented the stress-induced effects (right panel) (P<.01). In PSs, the total number of mPFC reserve pool vesicles was not affected by stress or DMI treatment in either PSs or NPSs. (c) Three-dimensional representation of synaptic vesicles (white spheres) docked to the active zone (cyan) for each experimental group. Cube side=50nm.
Discussion
The results from the present study suggest that a single brief exposure to stress potentiates excitatory neurotransmission by inducing presynaptic structural plasticity, as shown by an increased number of docked vesicles and large sprouting of excitatory synapses. Moreover, these changes were partially blocked by DMI pretreatment. Our ultrastructural findings were localized in layers II-III of mPFC, which contains predominantly somata of pyramidal neurons, and is known to be particularly sensitive to chronic stress insults (McEwen and Morrison, 2013). With regards to the effects of acute stress on mPFC structural plasticity, the first result of the present study showed that acute FS stress strongly increases the number of docked vesicles in PSs only, confirming our previous observation on the selective effect of acute FS stress (Treccani et al., 2014). These results suggest that following acute FS stress, more vesicles from the reserve pool become competent to fuse with the presynaptic membrane and empty their content by being released from the cytoskeletal matrix (Schikorski and Stevens, 2001; Sudhof, 2004). In line with this hypothesis, acute FS stress has shown to increase presynaptic release probability (Musazzi et al., 2010). We also observed for the first time that a single severe stressor can rapidly induce a strong increase in the number of asymmetric synapses, whereas synapse increase is only modest after a less severe stressor. This finding deserves particular attention considering that the general synapse increase was differentially provided for the 2 stressors by the counting of AXSs and NPSs. Interestingly, AXSs were found increased only after FS (+51.2%) but not RS stress. Most likely due to their relatively higher strength through their strategic location directly on dendritic shafts rather than on spines (Figure 1c-d), we postulate that potentiation of AXSs only after FS stress may be linked to significantly higher levels of CORT found after FS compared with RS stress, as well as to a different degree of complexity among the 2 stressors (Maras et al., 2014). Small NPSs were increased after both FS (+39.6%) and RS stress (+41.2%), with greater stability being observed among PSs. Hippocampal PSs mediate greater synaptic efficacy compared with small NPSs by expressing a larger number of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors (AMPARs) (Ganeshina et al., 2004b). Because NPSs express fewer AMPARs and are regarded as less efficacious (Ganeshina et al., 2004a, 2004b), we hypothesize that the observed stress-induced increase of NPSs is consistent with an increase in the so-called silent synapses, containing mainly N-methyl-d-aspartate receptors (NMDARs) and few or no AMPARs (Isaac et al., 1995). Despite being functionally silent at resting potentials, these synapses represent a new substrate for the formation, by insertion of AMPARs, of new functional synapses more likely to be potentiated (Suvrathan et al., 2014). In line with this hypothesis, a number of recent studies strongly suggest postsynaptic changes in glutamate receptors, as shown by an increased level of surface NMDARs and AMPARs in PFC slices examined 1 to 4 hours poststress and altered EPSC kinetics likely occurring at the postsynaptic level (Yuen et al., 2009; Musazzi et al., 2010).
According to a well-accepted model of synaptic remodeling following long-term potentiation, an increased number of synapses is provided by splitting small NPSs into large PSs (Geinisman, 2000). However, in the present study, the stress-induced increase in small NPSs and AXSs was not accompanied by a corresponding decrease in PSs. Therefore, we postulate that additional AXSs and NPSs observed after acute stress are formed mostly ex novo rather than being converted from another synapse subtype. Although a number of in vitro studies have shown that activity-dependent synaptogenesis can occur within 2 hours from the application of the stimulus (Bourne et al., 2013), only few studies investigated the formation of new synaptic contacts ex vivo, reporting 24 hours as the shortest interval required for the formation of large efficacious boutons (Medvedev et al., 2012). Interestingly, our findings provide the first ex vivo evidence that activity-dependent synaptogenesis of small and less efficacious NPSs can occur locally ex novo as early as 40 minutes after the application of a severe stressful stimulus. This shows remarkable dynamics of synapse structure observed in a short time-frame following application of the stressor. Given that the effects of acute stress on synapse number were observed immediately after the end of the stress protocol, it is conceivable that these changes may be accounted for by changes in local protein translation in dendrites. One of the crucial mediators of activity-dependent synaptic plasticity is the neurotrophin brain derived neurotrophic factor involved in the translation of a subset of dendritic mRNAs at synapses, including cytoskeletal proteins involved in synaptic rearrangement (Leal et al., 2014). This new knowledge can be of great importance in clarifying the dynamics leading to the long-term behavioral and structural impairments that exposure to CORT may produce (Gourley et al., 2013).
A number of previous studies have shown that tricyclic antidepressants, including DMI, can reduce glutamate release and transmission under both basal conditions in hippocampus (Bonanno et al., 2005; Tokarski et al., 2008) and in the presence of an acute stressful challenge in prefrontal and frontal cortex (Musazzi et al., 2010). In the last study, electrophysiological recordings showed that DMI exerts its effect mainly by acting at the presynaptic level, with little or no effect at the postsynaptic side (Musazzi et al., 2010). In the present study, we examined whether DMI may also dampen the enhanced structural plasticity induced by acute FS stress; this was done by investigating the size of synaptic vesicle pools, as presynaptic markers, and the number of glutamate synapses as an overall measure of synaptic changes. Here we showed that DMI treatment prevents the effects of acute FS stress on structural plasticity. Although subchronic treatment (5–7 days) with DMI has been shown to reverse the detrimental effects of inescapable FS on hippocampal spine synapse number (Hajszan et al., 2009), the present results suggest that DMI may also have a preventive action on the effects of stress on structural plasticity, as shown by normalized vesicle docking and small synapse sprouting.
On the other hand, the dampening action of DMI on the stress-induced structural potentiation was not accompanied by a reduction of CORT levels. Thus, we suggest that the potential mechanism underlying the blockade of stress effects by chronic DMI must be located on pathways downstream of CORT release or on alternative pathways. Several mechanisms may account for the DMI-induced prevention of the stress-induced elevation of structural plasticity. As previously discussed (Musazzi et al., 2010), by increasing noradrenaline availability, DMI can induce downregulation of chronically activated autoreceptors inhibiting noradrenaline release and leading in turn to disinhibition of noradrenaline exocytosis (Raiteri et al., 1986; Starke et al., 1989). In parallel, activation of α2-adrenergic heteroreceptors located on glutamatergic neurons does not seem to lead to their downregulation, thereby inhibiting glutamate release (Raiteri et al., 1983; Kamisaki et al., 1992; Pittaluga et al., 2007). In particular, a major role in these processes may be played by synapsin I, which is known to control the trafficking of synaptic vesicles between the reserve and the readily releasable pool, depending on phosphorylation state (Greengard et al., 1993; Ceccaldi et al., 1995). Because we have recently shown that acute FS stress increases levels of Phospho-Ser9 synapsin I in synaptic membranes (Treccani et al., 2014), it will be interesting to analyze the effects of DMI pretreatment on the acute stress-induced increase of Phospho-Ser9 synapsin I. Overall, this effect of DMI on stress-induced vesicle accumulation may account for the previously described blockade of acute stress-induced enhancement of glutamate release and transmission by antidepressants (Musazzi et al., 2010).
Moreover, the effect of DMI on stress-induced structural plasticity was found double-sided. Besides limiting the accumulation of presynaptic-located docked vesicles, DMI was found to act at the postsynaptic level by limiting the number of highly electron-dense PSD, regarded as containing postsynaptic receptors (Gray, 1959). Antidepressants have long been known to regulate glutamate receptors by limiting NMDAR function through decreased protein expression level of its subunits (Pittaluga et al., 2007) and, conversely, potentiating AMPAR-mediated transmission (Barbon et al., 2006). Therefore, it will be interesting to assess the effect of DMI pretreatment on the postsynaptic surface level of glutamate receptors.
By limiting the number of glutamate vesicles available for release and the number of excitatory synapses as potential glutamate release sites, DMI may help prevent FS stress-induced boosting of the mPFC glutamate system and subsequent hyper-glutamate–induced noxious effects (Sanacora et al., 2008; Popoli et al., 2012).
In conclusion, by increasing vesicle docking and forming new small synapses, acute stress promotes an immediate form of ultrastructural plasticity of mPFC pyramidal neurons. This heterogeneous population of neurons tends to segregate inputs with extracortical afferents (from the mediodorsal nucleus of the thalamus and hippocampal CA3) clustering on distal dendrites (layer I) (Swanson and Cowan, 1977; Groenewegen, 1988) and synapses of local cortical circuits clustering on proximal apical and basal dendrites (Scheibel and Scheibel, 1970). The acute FS stress-induced enhancement of structural plasticity as observed here was circumscribed to mPFC layer II-III containing primarily the proximal portions of the apical and basilar arbor: in turn, this may suggest a shift in emphasis from subcortical to intracortical information, with important implications for the functioning of mPFC and cognitive behaviors mediated by it.
Although further research is needed to assess how long the stress-induced synaptogenesis and presynaptic potentiation can be sustained and which pathways mediate the preventive effects of DMI, these results support our initial hypothesis that acute stress-induced presynaptic plasticity occurs in conjunction with increased synaptic remodeling, suggesting in turn an overall FS stress-induced enhancement of mPFC structural plasticity. In addition to the previously observed preventive effect on stress-induced increase of glutamate release, pretreatment with classic antidepressant DMI can strongly counteract FS stress-induced mPFC structural plasticity. This preventing action of DMI may be directly linked with its therapeutic effect in the treatment of mood and anxiety disorders.
While our observations on stress-induced structural plasticity confirms the high vulnerability of mPFC to even a single stress exposure, our data on the preventive effects of DMI suggest synapse remodeling as a neuronanatomical marker predicting the effectiveness of antidepressant compounds on preventing long-term noxious consequences.
Statement of Interest
N.N. is supported by Aarhus University Mobility Fellowship. The Centre for Stochastic Geometry and Advanced Bioimaging is supported by the Villum Foundation.
Acknowledgments
We thank Herdis Krunderup, Lone Lysgaard, and Anette Larsen for skillful technical assistance. Laura Musazzi is gratefully acknowledged for advice and support during the study.
References
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. (2010). Glial Pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, Tiraboschi E, Musazzi L, Giambelli R, Gennarelli M, Racagni G, Barlati S. (2006). Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry 59:713–720. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, Raiteri M, Racagni G, Popoli M. (2005). Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci 25:3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. (2011). Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus 21:354–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Chirillo MA, Harris KM. (2013). Presynaptic ultrastructural plasticity along CA3-->CA1 axons during LTP in mature hippocampus. J Comp Neurol 521:3898–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi PE, Grohovaz F, Benfenati F, Chieregatti E, Greengard P, Valtorta F. (1995). Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J Cell Biol 128:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. (2010). Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus 20:1376–1384. [DOI] [PubMed] [Google Scholar]
- Conti AC, Kuo YC, Valentino RJ, Blendy JA. (2004). Inducible camp early repressor regulates corticosterone suppression after tricyclic antidepressant treatment. J Neurosci 24:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60:236–248. [DOI] [PubMed] [Google Scholar]
- Deolindo MV, Reis DG, Crestani CC, Tavares RF, Resstel LB, Correa FM. (2013). NMDA receptors in the lateral hypothalamus have an inhibitory influence on the tachycardiac response to acute restraint stress in rats. Eur J Neurosci 38:2374–2381. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325:621–625. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE. (2002). Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int J Dev Neurosci 20:225–236. [DOI] [PubMed] [Google Scholar]
- Dondzillo A, Satzler K, Horstmann H, Altrock WD, Gundelfinger ED, Kuner T. (2010). Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins bassoon and piccolo in the rat calyx of held before and after the onset of hearing. J Comp Neurol 518:1008–1029. [DOI] [PubMed] [Google Scholar]
- Fiala JC. (2005). Reconstruct: a free editor for serial section microscopy. J Microsc 218:52–61. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. (2004a) Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol 468:86–95. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. (2004b) Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience 125:615–623. [DOI] [PubMed] [Google Scholar]
- Geinisman Y. (2000). Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cerebral Cortex 10:952–962. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F. (1991). Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res 566:77–88. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE, Rossi M, Parshall RF. (1993). Structural synaptic correlate of long-term potentiation: formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus 3:435–445. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van Der Zee EA. (2001). Associative learning elicits the formation of multiple-synapse boutons. J Neurosci 21:5568–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. (2013). Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J Neurosci 33:3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. (1959). Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature 183:1592–1593. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. (1993). Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259:780–785. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. (1988). Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24:379–431. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. (1987). The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. (2009). Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry 65:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. (1995). Evidence for silent synapses: implications for the expression of LTP. Neuron 15:427–434. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Hamahashi T, Hamada T, Maeda K, Itoh T. (1992). Presynaptic inhibition by clonidine of neurotransmitter amino acid release in various brain regions. Eur J Pharmacol 217:57–63. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Karst H, Joels M. (2012). Interactions between noradrenaline and corticosteroids in the brain: from electrical activity to cognitive performance. Front Cell Neurosci 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB. (2014). BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76 Pt C:639–656. [DOI] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ. (2014). Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry 19:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- Mcewen BS, Morrison JH. (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS, Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Arch Intern Med 153:2093–2101. [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. (1985). [3H]Dexamethasone binding in rat frontal cortex. Brain Res 328:176–180. [DOI] [PubMed] [Google Scholar]
- Medvedev NI, Dallerac G, Popov VI, Rodriguez Arellano JJ, Davies HA, Kraev IV, Doyere V, Stewart MG. (2012). Multiple spine boutons are formed after long-lasting LTP in the awake rat. Brain Struct Funct 219:407–414. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. (1993). Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem 60:1650–1657. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M. (2010). Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. Plos One 5:E8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava N, Chen F, Wegener G, Popoli M, Nyengaard JR. (2014). A new efficient method for synaptic vesicle quantification reveals differences between medial prefrontal cortex perforated and nonperforated synapses. J Comp Neurol 522:284–297. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Raiteri L, Longordo F, Luccini E, Barbiero VS, Racagni G, Popoli M, Raiteri M. (2007). Antidepressant treatments and function of glutamate ionotropic receptors mediating amine release in hippocampus. Neuropharmacology 53:27–36. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, Mcewen BS, Sanacora G. (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, Mccall T, Hof PR, Mcewen BS, Morrison JH. (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125:1–6. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Marchi M, Maura G. (1983). Chronic drug treatments induce changes in the sensitivity of presynaptic autoreceptors but not of presynaptic heteroreceptors. Eur J Pharmacol 91:141–143. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Bonanno G, Maura G. (1986). Changes of sensititvity of presynaptic Α2-adrenoreceptors induced by chronic clonidine in rat brain. J Hyperthens 4:S122–S124. [Google Scholar]
- Ribic A, Zhang M, Schlumbohm C, Matz-Rensing K, Uchanska-Ziegler B, Flugge G, Zhang W, Walter L, Fuchs E. (2010). Neuronal MHC class I molecules are involved in excitatory synaptic transmission at the hippocampal mossy fiber synapses of marmoset monkeys. Cell Mol Neurobiol 30:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. (2008). Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7:426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. (1970). Of pattern and place in dendrites. Int Rev Neurobiol 13:1–26. [Google Scholar]
- Schikorski T, Stevens CF. (2001). Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci 4:391–395. [DOI] [PubMed] [Google Scholar]
- Sebastian V, Estil JB, Chen D, Schrott LM, Serrano PA. (2013). Acute Physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus. Plos One 8:E79077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K, Gothert M, Kilbinger H. (1989). Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69:864–989. [DOI] [PubMed] [Google Scholar]
- Sterio DC. (1984). The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. (1988). Allostasis: a new paradigm to explain arousal pathology. In: Handbook of life stress, cognition, and health. (Fisher S, Reason JT, eds). Chicester, NY: Wiley. [Google Scholar]
- Sudhof TC. (2004). The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S. (2014). Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci 369:20130151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. (1977). An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172:49–84. [DOI] [PubMed] [Google Scholar]
- Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. (2006). Expression and function of SNAP-25 as a universal SNARE component in gabaergic neurons. J Neurosci 26:7826–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ. (2001). Total regional and global number of synapses in the human brain neocortex. Synapse 41:258–273. [DOI] [PubMed] [Google Scholar]
- Timmermans W, Xiong H, Hoogenraad CC, Krugers HJ. (2013). Stress and excitatory synapses: from health to disease. Neuroscience 248:626–636. [DOI] [PubMed] [Google Scholar]
- Tokarski K, Bobula B, Wabno J, Hess G. (2008). Repeated administration of imipramine attenuates glutamatergic transmission in rat frontal cortex. Neuroscience 153:789–795. [DOI] [PubMed] [Google Scholar]
- Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, Lamanna J, Malgaroli A, Drago F, Racagni G, Nyengaard JR, Wegener G, Bonanno G, Popoli M. (2014). Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry 19:433–443. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA. (2001). Learned helplessness in the rat: improvements in validity and reliability. Brain Res Brain Res Protoc 8:1–7. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, Mcewen BS, Yan Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A 106:14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73:962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]