Abstract
Background:
Desensitization and blockade of 5-HT2C receptors (5-HT2CR) have long been thought to be central in the therapeutic action of antidepressant drugs. However, besides behavioral pharmacology studies, there is little in vivo data documenting antidepressant-induced 5-HT2CR desensitization in specific brain areas.
Methods:
Mice lacking the 5-HT reuptake carrier (5-HTT-/-) were used to model the consequences of chronic 5-HT reuptake inhibition with antidepressant drugs. The effect of this mutation on 5-HT2CR was evaluated at the behavioral (social interaction, novelty-suppressed feeding, and 5-HT2CR–induced hypolocomotion tests), the neurochemical, and the cellular (RT-qPCR, mRNA editing, and c-fos–induced expression) levels.
Results:
Although 5-HTT-/- mice had an anxiogenic profile in the novelty-suppressed feeding test, they displayed less 5-HT2CR–mediated anxiety in response to the agonist m-chlorophenylpiperazine in the social interaction test. In addition, 5-HT2CR–mediated inhibition of a stress-induced increase in 5-HT turnover, measured in various brain areas, was markedly reduced in 5-HTT-/- mutants. These indices of tolerance to 5-HT2CR stimulation were associated neither with altered levels of 5-HT2CR protein and mRNA nor with changes in pre-mRNA editing in the frontal cortex. However, basal c-fos mRNA production in cells expressing 5-HT2CR was higher in 5-HTT-/- mutants, suggesting an altered basal activity of these cells following sustained 5-HT reuptake carrier inactivation. Furthermore, the increased c-fos mRNA expression in 5-HT2CR–like immune-positive cortical cells observed in wild-type mice treated acutely with the 5-HT2CR agonist RO-60,0175 was absent in 5-HTT-/- mutants.
Conclusions:
Such blunted responsiveness of the 5-HT2CR system, observed at the cell signaling level, probably contributes to the moderation of the anxiety phenotype in 5-HTT-/- mice.
Keywords: antidepressant drugs, c-fos mRNA, editing, frontal cortex, serotonin2C
Introduction
One prominent feature of many antidepressant drugs (ADs) is their antagonistic action at 5-HT2C receptors (5-HT2CR). Other ADs, including selective serotonin reuptake inhibitors, increase extracellular 5-HT concentration, and their chronic administration markedly decreases behavioral responses to 5-HT2CR agonists (Yamauchi et al., 2004; Mongeau et al., 2010), suggesting 5-HT2CR desensitization. In this context, mutant rodents lacking the 5-HT reuptake carrier (5-HTT) are an interesting tool, which displays high extracellular 5-HT levels (Fabre et al., 2000; Shen et al., 2004; Homberg et al., 2007) and decreased behavioral responses to the anxiogenic 5-HT2CR agonist m-chlorophenylpiperazine (mCPP; Mongeau et al., 2008; Moya et al., 2011). However, behavioral studies are insufficient to infer that authentic 5-HT2CR desensitization underlays the reduced 5-HT2CR–mediated responses, because causal alterations might occur at neurotransmitters and neuronal circuits downstream of 5-HT2CR.
5-HT2CR are positively coupled to phospholipase C, mobilizing intracellular Ca2+ and subsequently triggering cell signaling processes, such as the transcription of immediate early genes (IEGs). Many in vitro studies indicate rapid (minutes/hours) decrease/inactivation of this signaling function in response to elevated concentrations of 5-HT (Akiyoshi et al., 1996; Seitz et al., 2012). In contrast, AD treatments require several days to weeks to induce tolerance of behavioral responses to 5-HT2CR agonists (for a review, see Martin et al., 2014). Furthermore, whereas in vitro desensitization can reverse rapidly, in vivo tolerance remains for hours/days after AD treatment (Mongeau et al., 2010). The extrapolation of in vitro desensitization data to in vivo regulatory tolerance processes is therefore not straightforward. Furthermore, differential desensitization might occur in various brain areas depending on 5-HT2CR density and other factors (pre-mRNA editing, constitutive activity, etc.). We thus used various approaches to assess mechanisms possibly contributing to the apparent 5-HT2CR desensitization inferred from behavioral investigations in 5-HTT-/- mice. In particular, 5-HT2CR–mediated negative feedback on 5-HT release, a mechanism mainly occurring under high serotonergic tone conditions and stress (Mongeau et al., 2010), allowed us to set up an in vivo biochemical assay to assess the functional state of 5-HT2CR in specific brain areas of 5-HTT-/- mice.
Particular focus was put on the frontal cortex (FC), an area of interest regarding anxiety and depression (Mann et al., 2000; Prater et al., 2013). We investigated whether the decrease in 5-HT2CR responses could be explained by changes in expression of the 5-HT2CR protein and/or mRNA or pre-mRNA editing (Werry et al., 2008). Interestingly, 5-HT2A receptor desensitization had already been shown in the cortex of 5-HTT-/- mice by measuring regional brain phospholipase A2 response to a preferential 5-HT2AR agonist (DOI; Qu et al., 2005). Unfortunately, a similar assay to measure coupling with the Gαq/11 protein cannot be used in the case of 5-HT2CR because of relatively low receptor density in all brain regions, except the choroid plexus (Mannoury La Cour et al., 2009). Therefore, we set up an in vivo 5-HT2CR signaling assay that consisted of quantifying 5-HT2CR–induced c-fos mRNA in 5-HT2CR–immune-positive cells. This assay was performed in stress-free handling conditions and was adapted for stereological quantification of c-fos mRNA at the cell level. Changes in anxiety-related behaviors were tentatively interpreted in view of alterations in 5-HT2CR function found at both the brain and cellular levels.
Materials and Methods
Animals
Male mice, issued from the C57Bl/6J strain (F10), expressing or not expressing the 5-HT transporter (5-HTT+/+ and 5-HTT-/- mice), were generated by the group of KP Lesch (Bengel et al., 1998) and were bred in our Paris laboratory.
Experiments performed in 8–16-week-old mice were carried out under standard laboratory conditions (12h light-dark cycle, lights on at 07:00h, temperature 21±1°C) with free access to food and water. For at least 30 minutes before each behavioral study, animals were left quiet in a room nearby the experimental room. All the procedures utilizing animals were conducted in full agreement with institutional guidelines (council directive no. 87–848, October 19, 1987, MAF, protocol agreement 0096.02 and authorization # 75–977 to LL).
Pharmacological Treatments
Although the preferential 5-HT2CR agonists used here— (S)-6-Chloro-5-fluoro-1H-indole-2-propanamine (RO-60,0175) and mCPP—also have some agonist activity at other 5-HT receptor types (5-HT2B and 5-HT1B receptors, respectively), their effects in the various tests used in our studies were previously shown to be prevented by the selective 5-HT2CR antagonist, SB-242,084 (Maj and Moryl, 1992; Kennett et al., 2000; Hayashi et al., 2004; Mongeau et al., 2010), indicating their mediation essentially through 5-HT2CR activation. Even though mCPP is less selective than RO-60,0175, it was used in the social interaction test, because at the low systemic dose used in our studies (0.3mg/kg i.p.), mCPP triggers 5-HT2CR–mediated anxiety without causing hypolocomotion, which could possibly interfere with the anxiety-related response in this test (Mongeau et al., 2010). Sigma-Aldrich and Tocris manufactured the mCPP and RO-60,0175 (as hydrochloride salts), respectively. Both drugs were dissolved in 0.9% NaCl with a brief sonication just prior to i.p. administration in mice.
Behavioral Studies
Novelty-Suppressed Feeding Test
Mice were isolated and food-deprived for 30h before the experiment. They were then placed in a brightly lit (450 lux) open arena (40 x 40cm) without bedding. Food pellets were placed at the center and latency to feed (with a cut-off time of 300 s) was measured, using video recording, from the time the mouse was placed in the arena. Latency to feed was also measured in the home cage.
Social Interaction Test
Following two consecutive days of habituation to the social interaction arena (40 x 40cm; Mongeau et al., 2010), mice of the same genotype and unfamiliar to each other were paired and injected with mCPP (0.3mg/kg i.p.), then kept in individual cages for 30 minutes before placing them in the dimly lit arena (5 lux). Fresh bedding was renewed after each test.
Animal behaviors were recorded with a camera, and analyzed by an observer unaware of the treatment groups. Active social interaction events (sniffing, fighting, chasing, grooming, etc.) were scored using ODlog (Macropod Software). In our test conditions, social interactions were rarely aggressive. Total social interactions scored reflected all types of active social interactions, but not passive contacts.
Locomotor Activity Test
Activity was measured using a computer-based photo-beam apparatus (Actisystem II, Panlab). Actimeter boxes (area: 30 x 15cm; height: 18cm; with grid floor) detected mouse movements by means of infrared light beams. Mice were placed in the boxes 30 minutes after i.p. administration of saline or the 5-HT2CR agonist RO-60,0175 (3mg/kg) and horizontal activity was monitored for 15 minutes (Mongeau et al., 2010).
Measurement of 5-HT Turnover
Tissue levels of 5-HT and its metabolite 5-hydoxyindoleacetic acid (5-HIAA) were determined in the amygdala, FC, hippocampus, nucleus accumbens (Acb), and ventral tegmental/substantia nigra (VTA/SN) using HPLC-ED detection, as previously described (Mongeau et al., 2010; see Supplementary Material).
For some animals, saline or RO-60,0175 (3mg/kg) was administered i.p. 30 minutes before restraint stress, performed by placing mice inside a 50mL syringe, perforated at the tip to allow them access to air. Mice were sacrificed immediately after restraint stress (45min), and HPLC-ED determinations of 5-HT and 5-HIAA proceeded as described (Mongeau et al., 2010).
Histological Assays
Characterization of the 5-HT2CR Antibody
The polyclonal 5-HT2CR antibody Ab32172 (Abcam) was characterized using Chinese hamster ovary (CHO-K1) cells, which do not natively express 5-HT2AR or 5-HT2CR mRNA (Berg et al., 2001; Anastasio et al., 2010). CHO-K1 cells were transiently transfected with the rat 5-HT2CR encoding sequence, which shares 96% homology with the mouse 5-HT2CR at the level of the domain (amino acids 400–460) recognized by the antibody (Abcam). Plasmids encoding the rat 5-HT2CR (RR207006, OriGene), 5-HT2AR (generously provided by Dr Bryan L. Roth; Bhatnagar et al., 2001), or empty vector were transfected overnight into CHO-K1 cells using lipofectamine 2000 (Invitrogen), following the manufacturer’s protocol. The following day, transfected cells were seeded on coverslips and grown overnight (37°C, 5% CO2). They were then fixed in 3% paraformaldehyde for 30min and washed in Tris buffer (50mM Tris-HCl, 500mM NaCl, 0.6% Tween-20, pH 7.5) before blockade with 3% normal donkey serum in Tris buffer for 1h at room temperature. Incubation with 5-HT2CR antibody Ab32172 (1:400) or 5-HT2AR antibody Ab16028 (1:400; Abcam) was then performed overnight at 4°C, prior to exposure to Alexa Fluor 555 donkey anti-rabbit IgG (1 hour at room temperature; 1:1000; Life Technologies). Then, coverslips were mounted with Vectashield fluorescent medium containing DAPI (Vector Laboratories). All images of CHO-K1 cells were taken with an Olympus BX51 fluorescent microscope (objective 40x) and Hamamatsu digital camera without additional zoom, using identical exposure settings across all slides.
Intravenous Injections and Tissue Preparation
Intravenous catheter surgery was carried out as previously described (Trigo et al., 2007). Mice were anesthetized with a ketamine⁄xylazine mixture (5:1; 1 mL⁄100g body weight, i.p.) and then implanted with an indwelling i.v. silastic catheter. A 5cm length of silastic tubing (0.3mm inner diameter, 0.6mm outer diameter; Silastic®, Dow Corning) was fitted to a 22-gauge steel cannula (Semat) that was bent at a right angle and then embedded in a cement disk (Dentalon Plus) with an underlying nylon mesh. The catheter tubing was inserted 1.3cm into the right jugular vein and anchored with suture. The remaining tubing ran subcutaneously to the cannula, which exited at the midscapular region. All incisions were sutured and coated with an antibiotic ointment. Following surgery, mice were individually housed and allowed to recover for 4 days prior to drug administration. The day of the experiment, mice were connected to the injection apparatus and left quiet and alone for at least 3 hours. Mice were then remotely injected i.v. with either the vehicle (sterile 0.9% NaCl) or RO-60,0175 (0.3mg/kg; 12.5 µl; Di Matteo et al., 2000). The catheter was flushed with sterile saline 1 minute before drug administration to prevent clogging.
Thirty minutes later, the animals were decapitated and their brains were dissected on ice, cut in 3mm coronal slabs containing the FC, and fixed in a 4% paraformaldehyde solution (Sigma-Aldrich). After this fixation step, the brain slabs were put in a solution of sucrose (30%) overnight at 4°C before being stored at -20°C in a cryoprotectant solution (adapted from Watson et al., 1986; sucrose 30% w/v, polyvinylpyrrolidone 1% w/v, ethylene glycol 30% v/v, 0.2% ProtectRNA™ [Sigma-Aldrich] in phosphate buffered saline [PBS] treated with 0.05% v/v of diethyl-pyrocarbonate [DEPC-PBS; Sigma-Aldrich] before autoclaving). On the experiment day, the slabs were washed in cold-DEPC-PBS, cut at 40 µm using a vibratome, and kept in well-meshes (Costar).
5-HT2CR Immunohistochemistry Coupled to c-fos mRNA In Situ Hybridization
ProtectRNA™ 0.2% and DEPC-treated components were used throughout the immunohistochemistry procedure to prevent mRNA degradation before the in situ hybridization assay, performed 3 days later.
The 5-HT2CR immunohistochemistry assay (with the Ab32172®) followed by the c-fos mRNA in situ hybridization assay, were performed as previously described (Mongeau et al., 2003; Martin et al., 2013; see Supplementary Material).
Stereological Analysis
Preliminary tests confirmed that all antibodies adequately penetrated inside the 40 µm–thick sections. Stereology analysis was performed using a semiautomatic stereology computer-based system (Mercator; ExploraNova). Counting outlines of the FC (including infralimbic and prelimbic cortices) were defined at low magnification (4x) according to the mouse brain atlas (Paxinos and Franklin, 2001). Doubled, stained c-fos mRNA and 5-HT2CR–like immunoreactive (IR) cells were counted at high magnification (63x) using the Optical Fractionator (50 x 50 x 20 µm3 counting bricks, randomly sampled) operated by the Mercator software using an automated stage. We defined a counting exclusion zone of 5 µm at the top and bottom of the sections to avoid biases.
The sampling error, which takes into account the variability both within sections and between sections, was always ≤5% (Schaeffer test). Volumes (in mm3) and density estimation (number of cells/mm3) were measured.
5-HT2CR mRNA Quantification by RT-qPCR
Mice were decapitated and their brains were immediately dissected on ice. The FC was isolated and kept in liquid nitrogen, then stored at -80°C. RNA extraction and quantification were done as described in the Supplementary Material.
Measurements of Pre-mRNA Editing
The FC was isolated and stored as for RT-qPCR determinations. Purified mRNA samples were extracted and treated with DNAse (DNAse kit, Invitrogen). Total RNA was linearized before reverse transcription using a reverse transcriptase kit (Thermoscript Real-Time Polymerase Chain Reaction system, Invitrogen). Nested-polymerase chain reaction (PCR) single-strand conformational polymorphism analyses, capillary electrophoresis, and the identification/relative quantification of each isoform were then performed as previously described (Poyau et al., 2007; see Supplementary Material).
Statistics
Data for most behavioral tests, the quantitative RT-PCR, and the 5-HT2CR–like IR-cells density were all analyzed by the student’s t-test, comparing 5-HTT-/- mutants and 5-HTT+/+ mice. The effect of meta-chlorophenylpiperazine (mCPP) in the social interaction test was analyzed using a two-way analysis of variance (ANOVA) followed by the Bonferroni’s post hoc test.
The effect of RO-60,0175 on the stress-induced increase of 5-HT turnover was analyzed with a three-way ANOVA using the brain area, drug treatment, and genotype as factors, and then two-way ANOVAs to assess the effect of the drug treatment in each brain area independently, and the genotype effects as a function of brain areas in either the RO-60,0175 or saline conditions. Pairwise comparisons were done using the Bonferroni’s post hoc test. Stereological histological data was analyzed by two-way ANOVA to assess the respective effects of treatment and genotype.
Results
Behavioral Features in 5-HTT-/- Versus 5-HTT+/+ Mice
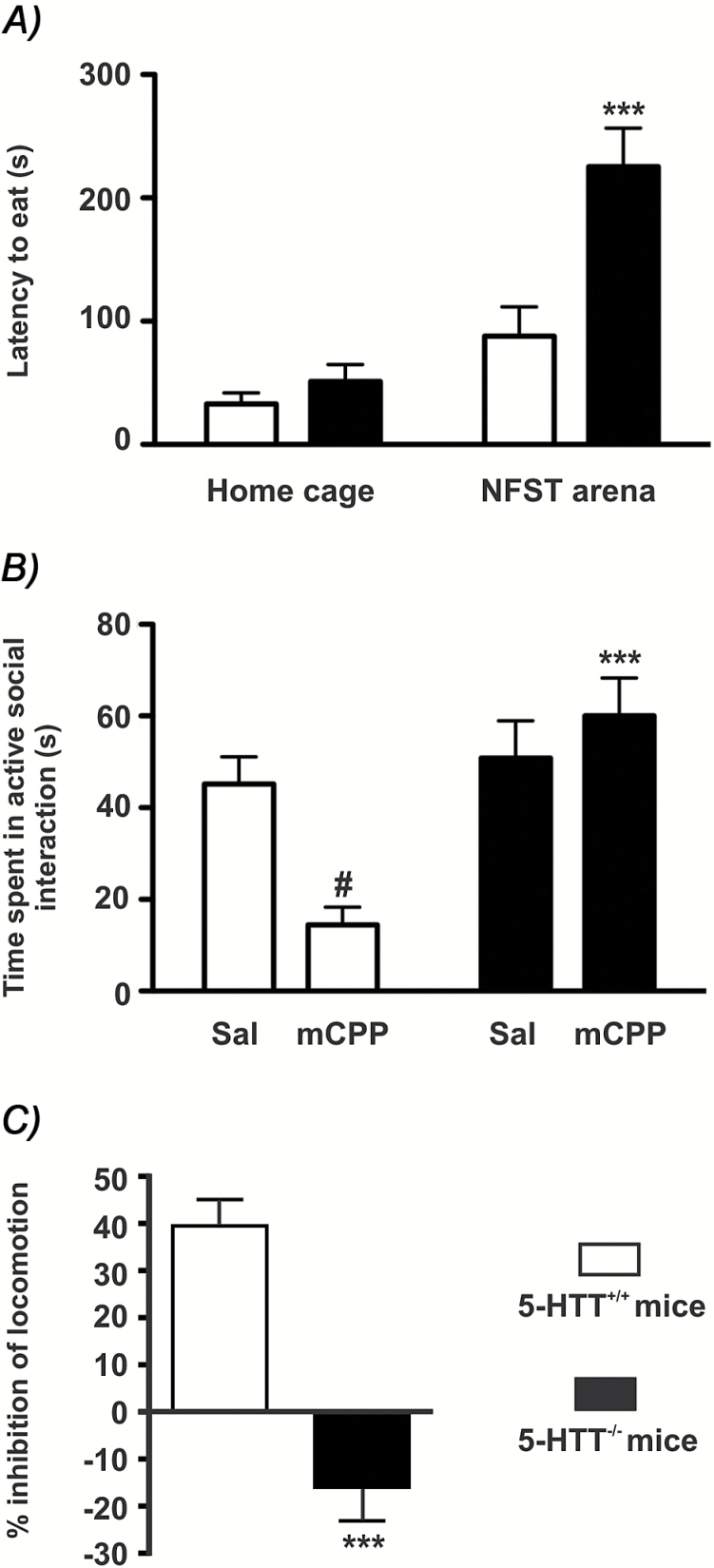
No differences were observed between 5-HTT-/- and 5-HTT+/+ mice in latency to feed in their home cage (Figure 1) or in spontaneous locomotor activity assessed in an actimeter (in counts per 15 minutes; 5-HTT+/+: 266±23; 5-HTT-/-: 238±53; means ± standard error of the mean [SEM]; n = 6; p > 0.05 student’s t-test). Mice were then tested in the novelty-suppressed feeding test (a standard model of anxiety commonly used to assess the anxiolytic effect of ADs; Bodnoff et al., 1988). The weight loss during the food-deprivation period that preceded the novelty-suppressed feeding test was not significantly different between the two genotypes (5-HTT+/+ = -20±1.6 %; 5-HTT-/- = -17.3±1.7 %; n = 4; p > 0.05, student’s t-test). However, a longer latency to initiate feeding was noted for 5-HTT-/- mice in the unfamiliar environment (Figure 1), indicating an anxiety-like profile compared to 5-HTT+/+ mice, as previously observed (Lira et al., 2003; Ansorge et al., 2004). However, in the social interaction test, 5-HTT-/- mice did not appear more anxious (Figure 1B), instead displaying a trend for more social interactions. Using the same test, we confirmed that a low dose of mCPP (0.3mg/kg, i.p) markedly reduced the time spent by 5-HTT+/+ mice in active social interactions (-68±8%), an effect previously shown to be mediated by 5-HT2CR activation (Mazzola-Pomietto et al., 1996; Mongeau et al., 2010). In contrast, mCPP was ineffective in 5-HTT-/- mice (Figure 1B).
Figure 1.
Anxiety-like profile and 5-HT2C receptor-mediated hypolocomotion in mice lacking the 5-HT reuptake carrier (5-HTT-/-).
(A) The latencies to feed were compared in food-deprived 5-HTT-/- versus 5-HT reuptake carrying (5-HTT+/+) mice, placed in the novelty-suppressed feeding test (NSFT) arena or left in their home cage. Values are mean ± standard error of the mean (SEM; n = 7–8). Two-way ANOVA indicated a significant effect of environment [F(1,27) = 27, p < 0.0001] and of genotype [F(1,27) = 12.6, p = 0.001], and a significant environment x genotype interaction [F(1,27) = 7.4, p = 0.01]. *p < 0.001. Bonferroni’s post hoc test indicated a significant effect of genotype in the NSFT arena. (B) Time spent in active social interaction in saline- or m-chlorophenylpiperazine (mCPP)-treated mice. Values are mean ± SEM (n = 6–7). Two-way ANOVA indicated a significant effect of genotype [F(1,26) = 13.6, p = 0.001] and no overall effect of mCPP [F(1,26)= 2.4], but a significant genotype x mCPP interaction [F(1,26) = 8.3, p < 0.01]. Bonferroni’s post hoc test indicated a significant effect of genotype in the mCPP groups (*p < 0.001) and a significant effect of mCPP vs. saline-only treatment in the 5-HTT+/+ group (#p < 0.05). (C) The hypolocomotion induced by RO-60,0175 in 5-HTT+/+ mice (in counts/15min; saline: 266±23; RO: 159±15; means ± SEM, - 40.2±6 %, n = 6, p < 0.01) was not observed in 5-HTT-/- mice (saline: 238±53; RO: 277±17; +16±7 %; n = 6, n.s.). *p ≤ 0.001; student’s t-test comparing the percentage inhibition of locomotion produced by RO-60,0175.
Although mCPP is the drug of choice to induce anxiety via 5-HT2CR, with minimal locomotor effects when used at low doses (<1mg/kg i.p.; Mongeau et al., 2010), the more selective agonist, RO-60,0175, was previously demonstrated to be more appropriate for assessment of 5-HT2CR–mediated hypolocomotion (Martin et al., 1998). Accordingly, we noted that RO-60,0175 (3mg/kg i.p.) induced a 40±6 % (n = 6) reduction in locomotor activity in 5-HTT+/+ mice. In contrast, no hypolocomotor effect of RO-60,0175 (3mg/kg i.p.) was observed in 5-HTT-/- mice (Figure 1C).
Brain 5-HT Turnover in 5-HTT-/- Versus 5-HTT+/+ Mice
Significant differences in basal 5-HT turnover in 5-HTT-/- versus 5-HTT+/+ mice were observed in three out of the five brain areas examined (Table 1). Indeed, 5-HIAA/5-HT ratios in the Acb (+35±3%), hippocampus (+78±7%), and particularly the VTA/SN (+112±6%) were significantly higher in mutants than in wild-type mice, and marginally increased in the FC. In contrast, this ratio did not differ between strains in the amygdala (Table 1), although both 5-HIAA and 5-HT tissue levels were similarly decreased in the latter area, as in all brain areas examined in mutants (see Supplementary Table 1), in line with previous reports (Fabre et al., 2000; Kim et al., 2005).
Table 1.
The effect of genetic invalidation of 5-HTT on basal 5-HT turnover in various brain areas.
| 5-HTT+/+ | 5-HTT-/- | p-value | |
|---|---|---|---|
| Accumbens | 0.46±0.02 | 0.62±0.01 | p < 0.001 |
| Amygdala | 0.50±0.04 | 0.56±0.02 | n.s. |
| Frontal cortex | 0.52±0.03 | 0.63±0.04 | p = 0.08 |
| Hippocampus | 0.60±0.04 | 1.07±0.04 | p < 0.001 |
| VTA/SN | 0.76±0.04 | 1.61±0.05 | p < 0.001 |
Values are mean 5-HIAA/5-HT ratio ± standard error of the mean (n = 6–8). p-values were determined with the student’s t-test, comparing mice lacking (5-HTT-/-) and posessing (5-HTT+/+) the 5-HT reuptake carrier in each area. VTA/SN: ventral tegmental/substantia nigra.
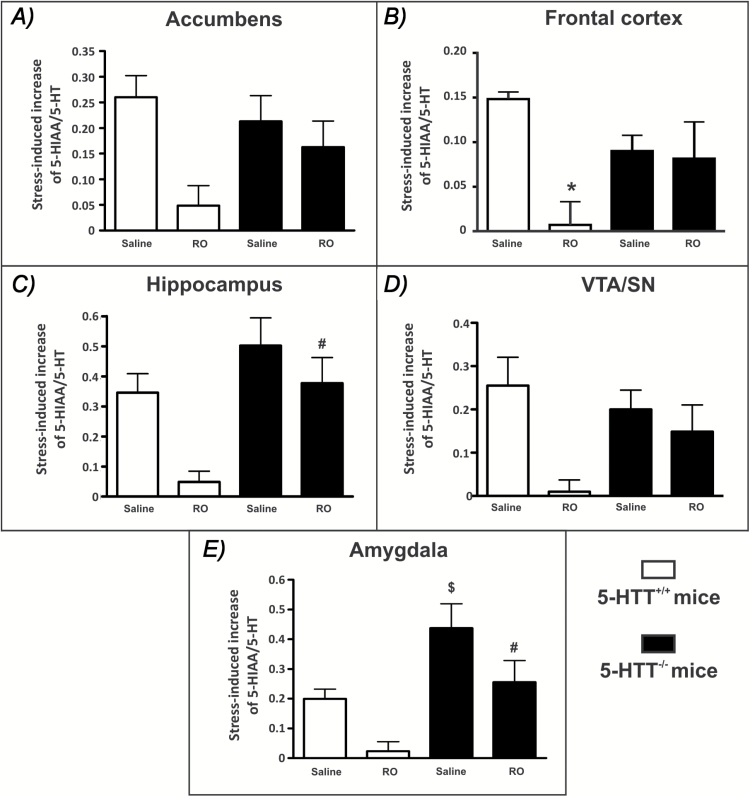
Determination of the RO-60,0175 inhibitory effect on the stress-induced increase of 5-HT turnover also indicated differences between 5-HTT-/- and 5-HTT+/+ mice (Figure 2). Stress produced a similar increase in the 5-HIAA/5-HT ratio in most brain areas of both genotypes, except the amygdala, where the stress-induced increase in 5-HT turnover appeared twice as high in mutants as in wild-type mice (Figure 2E). Furthermore, in contrast to what was observed in WT mice (Mongeau et al., 2010), RO-60,0175 did not induce major inhibitory effects on the stress-induced increase of 5-HIAA/5-HT ratio in most brain areas of 5-HTT-/- mice, except in the amygdala of 5-HTT-/- mice, where the effect was nevertheless smaller than that observed in 5-HTT+/+ mice (5-HTT-/- = -41±16%; 5-HTT+/+ = -88±16%; n = 10; mean ± SEM, p = 0.05 in student’s t-test; Figure 2E). Three-way ANOVA with repeated measures indicated a significant effect of the drug RO-60,0175 [F(1,122) = 37, p < 0.0001], of genotype [F(1,122) = 21, p < 0.0001], and a significant drug x genotype interaction [F(1,122) = 7, p < 0.01]. There were also significant differences in the stress-induced increase of 5-HT turnover between brain areas [F(4,122) = 10, p < 0.001] and an interaction between brain areas and genotype [F(4,122) = 5, p < 0.01]. A two-way ANOVA using drug and genotype as factors indicated a significant effect of RO-60,0175 in all the brain areas tested (p < 0.05– 0.01): the Acb [F(1,24) = 8.3], amygdala [F(1,31)= 9.4], FC [F(1,22)= 7.6], hippocampus [F(1,23)=8.8], and VTA/SN [F(1,21) = 10.5]. There was also a significant effect of genotype in the amygdala [F(1,31) = 16.2, p < 0.001] and in the hippocampus [F(1,23) = 11.6, p < 0.01]. The drug x genotype interaction reached significance only in the FC [F(1,22) = 5.7; p < 0.05] and the Bonferroni’s post hoc analysis revealed a significant effect of RO-60,0175 compared to saline treatment in 5-HTT+/+ mice (p < 0.001), but not in 5-HTT-/- mice (Figure 2B).
Figure 2.
Effects of acute administration of RO-60,0175 (RO) on the stress-induced increase of 5-HT turnover in various brain areas of mice lacking (5-HTT-/-) and possessing (5-HTT+/+) the 5-HT reuptake carrier. ∆ 5-HIAA/5-HT values (5-HIAA/5-HT ratios in stressed mice minus the mean 5-HIAA/5-HT ratios found in naive mice) are mean ± standard error of the mean of n = 6–10 mice. In mutant mice, RO-60,0175 did not induce major inhibitory effects on the stress-induced increase of 5-HIAA/5-HT ratio in (a) the nucleus accumbens, (b) the frontal cortex, (c) the hippocampus, (d) the ventral tegmental/substantia nigra (VTA/SN), except in (e) the amygdala. Data were analyzed using the three-way analysis of variance (ANOVA) following by two-way ANOVAs (see text for details). *p < 0.001 compared to saline-treated 5-HTT+/+ mice using Bonferroni’s post hoc test after a two-way ANOVA (using drug and genotype as factors). $p < 0.05 compared to saline-treated 5-HTT+/+ mice and #p < 0.01 compared to RO-60,0175–treated 5-HTT+/+ mice using Bonferroni’s post hoc test after two-way ANOVA (using brain areas and genotype as factors).
Two-way ANOVAs were also performed using brain areas and genotype as factors in each treatment condition. In the saline condition, there was a significant difference in the stress-induced increase in 5-HT turnover between areas [F(4,55) = 7.43; p < 0.0001] and a significant area x genotype interaction [F(4,55) = 2.78; p < 0.05], and the Bonferroni’s post hoc test indicated that wild-type and mutant mice differed only in the amygdala (p < 0.05). In the RO-60,0175 condition, there was also a significant difference in the stress-induced increase in 5-HT turnover between areas [F(4,66) = 3.1; p < 0.05] and an effect of genotype [F(1,66) = 29.6; p < 0.001], and the Bonferroni’s post hoc test indicated that mice differed in the hippocampus (p < 0.001) and in the amygdala (p < 0.01).
Molecular and Cellular Investigations of 5-HT2CR Sensitivity in the Frontal Cortex of 5-HTT-/- Versus 5-HTT+/+ Mice
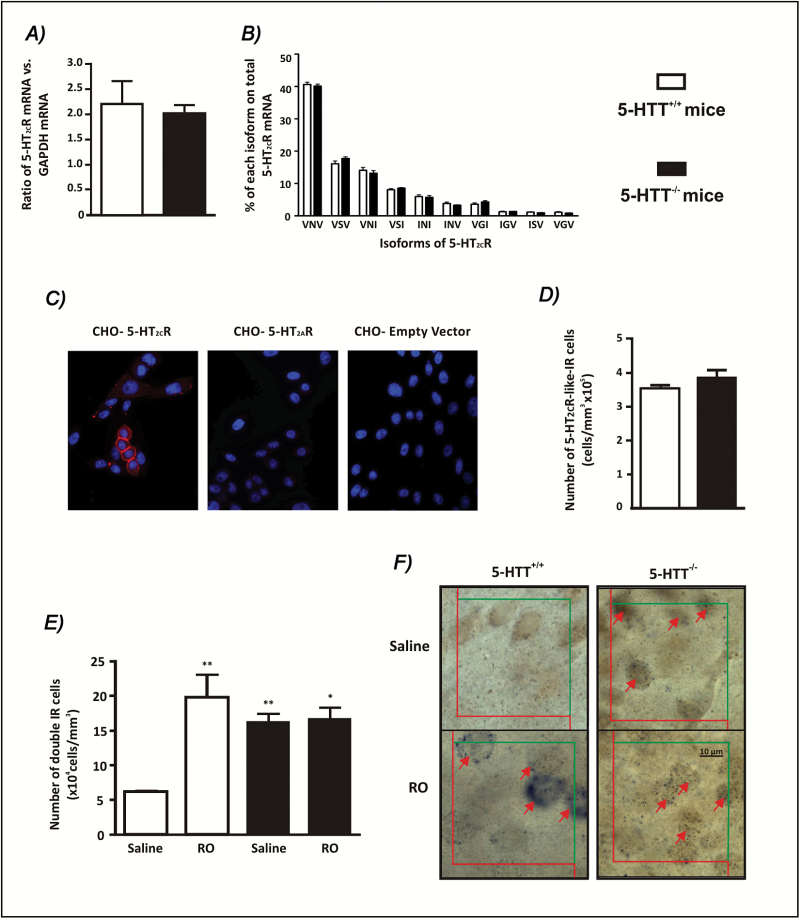
To unveil the molecular/cellular processes underlying the 5-HTT-/--associated decrease in the 5-HT2CR function, as evidenced in the assays reported above, investigations were carried out at the mRNA and protein levels. RT-qPCR determinations first showed unaltered tissue levels of total 5-HT2CR encoding mRNA in the 5-HTT-/- mutants, compared to the 5-HTT+/+ mice (Figure 3A). Measurements of each editing isoform of 5-HT2CR pre-mRNA using nested-PCR followed by capillary electrophoresis (Poyau et al., 2007) showed that the predominant isoforms were those encoding the VNV, VSV, and VNI variants in both genotypes (Figure 3B). However, no differences in the respective percentages of these or other variants were found in the FC of 5-HTT-/- compared to 5-HTT+/+ mice (Figure 3B).
Figure 3.
Cellular investigations of the apparent 5-HT2CR desensitization in the frontal cortex of mice lacking the 5-HT reuptake carrier (5-HTT-/-). The 5-HT2CR mRNA expression (A) and the quantitative distribution of 5-HT2CR mRNA isoforms expressed at more than 1% of total mRNA (B) are compared between 5-HTT-/- and 5-HTT+/+ (posessing the 5-HT reuptake carrier) mice. Values are mean ± standard error of the mean (SEM) of (A) total 5-HT2CR mRNA normalized to GaPDH mRNA (p > 0.05, student’s t-test, n = 9–10) and (B) respective percentages of various 5-HT2CR mRNA isoforms over total 5-HT2CR mRNA (n = 5–6; only values above 1% are represented). The two-way analysis of variance (ANOVA) indicated a difference between isoforms [F(9,90) = 1156, p < 0.0001], but no effect of genotype [F(1,90) = 0.1] and no interaction [F(9,90) = 1]. (C) To validate the Ab32172 antibody before its use in mouse cortical tissue, Chinese hamster ovary (CHO-K1) cells, which do not express 5-HT2AR or 5-HT2CR mRNA, were transiently transfected with either the 5-HT2CR (CHO-5-HT2CR) or 5-HT2AR (CHO-5-HT2AR) encoding vector or the empty vector (CHO-Empty Vector) and stained with Ab32172, followed by Alexa Fluor 555 secondary antibody (red) and counterstained with DAPI (blue). Significant immunofluorescence was observed in cells transfected with the 5-HT2CR, but not the 5-HT2AR or the empty vector. 5-HT2AR expression in 5-HT2AR-transfected CHO cells was confirmed by immunofluorescence using the 5-HT2AR antibody Ab16028 (data not shown). (D) The overall density of 5-HT2CR–like immunoreactive cells was determined in 5-HTT-/- mutants compared to 5-HTT+/+ mice. Values are mean ± SEM of the total number of 5-HT2CR–like immunoreactive cells/mm3. There was no difference in the volume of the region investigated between mutants and wild-type mice (5-HTT+/+ = 0.97±0.03mm3; 5-HTT-/- = 0.87±0.09mm3; means ± SEM, n = 6,). (E) Analysis of RO-60,0175–induced c-fos mRNA expression in 5-HT2CR–like immunoreactive cells, in 5-HTT+/+ and 5-HTT-/- mice. Two-way ANOVA indicated a significant effect of RO-60,0175 [F(1,8) = 13, p < 0.01] and no effect of genotype [F(1,8) = 3], but a significant RO-60,0175 x genotype interaction [F(1,8) = 11, p = 0.01]. The one-way ANOVA, followed by the Newman Keuls’ test, indicated further differences [F(3,8) = 9; p < 0.01]; *p < 0.05 and **p < 0.01 compared to saline-treated 5-HTT+/+ mice (n = 3). (F) Representative micrographs of double-stained cells (red arrows). 5-HT2CR–like immunopositive cells have brown deposits while digoxigenin-immunopositive cells have dark dots indicating the presence of c-fos mRNA (scale bar = 10 µm).
We then performed immunofluorescence labeling with a specific 5-HT2CR antibody. Prior experiments using CHO cells showed that this antibody (Ab32172) labeled cells transfected with the 5-HT2CR–encoding plasmid but not those transfected with the 5-HT2AR–encoding plasmid or the empty vector (Figure 3C). As shown in Figure 3D, quantitative estimates of immunohistochemical labeling by this anti-5-HT2CR antibody did not reveal differences in the density of 5-HT2CR–like positive cells in the FC between genotypes.
In contrast, combined 5-HT2CR and c-fos mRNA labeling (Figure 3E) showed that, in saline-treated mice, the density of double IR cells in the FC was significantly higher in 5-HTT-/- mutants than in 5-HTT+/+ mice (Figure 3E). Furthermore, acute treatment with RO-60,0175 (0.3mg/kg i.v.) markedly increased the density of 5-HT2CR–positive c-fos mRNA-double IR cells in 5-HTT+/+ mice (+224±55%, p < 0.01), but had no effect on these cells in 5-HTT-/- mice (Figure 3E and F). However, in RO-60,0175-treated mice, the percentage of 5-HT2CR–like IR cells not expressing c-fos mRNA did not significantly differ between genotypes (5-HTT+/+: 44±9%; 5-HTT-/-: 56±4%) suggesting that the differential effect of the 5-HT2CR agonist in mutants versus wild-type mice could not be accounted for by a saturating capacity of 5-HT2CR–like IR cells, to be activated by RO-60,0175 in the mutants.
Discussion
5-HTT-/- mice are a useful tool to model chronic 5-HT reuptake inhibition with ADs (Holmes et al., 2002). Nevertheless, the mutation is obviously not identical to an AD treatment, as many events taking place during development in 5-HTT-/- mice do not occur during adulthood AD treatments. In fact, the developmental loss of 5-HTT was argued to alter mouse behaviors in models of depression and anxiety, but in opposite ways to those expected from AD treatments (Ansorge et al., 2004). In humans, the 5-HTT polymorphism, generating a short variant allele of the 5-HTT promoter, reduces 5-HT reuptake capacity in homozygote individuals and predisposes them to affective disorders (Lesch et al., 1996; Gillespie et al., 2005). In mice, an early deficit of 5-HT reuptake during development triggers an anxious phenotype in adulthood (Ansorge et al., 2004; Olivier et al., 2011).
Most authors point to the anxiety phenotype of 5-HTT-/- mice in standard tests (Holmes et al., 2003; Carroll et al., 2007), but these mice can be less—or at least not more—anxious than 5-HTT+/+ mice in other validated behavioral tests (Lira et al., 2003; Zhao et al., 2006; Olivier et al., 2008). We clearly showed here (Figure 1), that 5-HTT-/- mutants had a longer latency to feed in the novelty-suppressed feeding assay, as previously reported (Lira et al., 2003; Ansorge et al., 2004; Olivier et al., 2008). Anxiety-related changes most likely explain this behavior, because latency to feed was unaffected under usual, non-anxiogenic, environmental conditions. Furthermore, previous studies showed that changes in 5-HTT expression do not, on their own, influence feeding behavior (Pringle et al., 2008). However, 5-HTT-/- mutants were less responsive than 5-HTT+/+ mice to the anxiogenic compound mCPP in the social interaction test, another test shown to detect the effects of serotonergic agents on anxiety (Bristow et al., 2000; Bagdy et al., 2001; File and Seth, 2003). These results suggest that genetic 5-HTT invalidation alters circuits regulating conflict (inhibition of feeding in an anxiogenic context) but not those regulating social fear, in line with the well-established mediation of distinct aspects of fear and anxiety by different brain areas (Gray and McNaughton, 2000). Interestingly, our results are also in accordance with those of Moya and colleagues (2011) who used a variant of the social interaction test that is often used to model the reduced social interaction in autism (Nadler et al., 2004). Finally, our behavioral investigations confirmed that the hypocolomotion normally induced by RO-60,0175 in wild-type mice was not observed in 5-HTT-/- mice (Mongeau et al., 2008; Moya et al., 2011), further arguing for tolerance to 5-HT2CR agonists’ effects.
In view of the neurodevelopmental changes caused by early 5-HT reuptake deficits, which lead to amygdala hyperactivity and fear (Wellman et al., 2007), it is retrospectively surprising that anxiety in 5-HTT-/- mice is not unequivocal, as one might expect. Their behavioral phenotype may be moderated by an apparent desensitization of 5-HT2CR, notably in the cerebral cortex, as convergent data clearly demonstrated that a decrease in 5-HT2R function mediates anxiolysis (Weisstaub et al., 2006; Heisler et al., 2007; Mongeau et al., 2010). The tolerance to 5-HT2CR agonist stimulation might not only be at the core of ADs’ anxiolytic effect, but could also be a physiological compensatory phenomenon limiting anxiety symptoms in predisposed individuals. It was thus of utmost interest to determine how this putative desensitization occurs.
We previously showed that 5-HT2CR activation prevents the enhancement of brain 5-HT turnover and extracellular concentration triggered by restraint stress (Mongeau et al., 2010). This indirect 5-HT2CR–mediated effect, which involves gamma-aminobutyric acid (GABA) ergic interneurons exerting a negative feedback on 5-HT release, probably plays a pivotal role in moderating the high serotonergic tone occurring during stress. However, an excessively low 5-HT tone at other receptors can limit stress-coping ability and ultimately lead to anxiety (Keck et al., 2005). We used this GABA-mediated, 5-HT2CR, negative-feedback control of 5-HT turnover as an in vivo functional assay to assess the sensitivity of 5-HT2CR. A major decrement in the inhibitory effect of RO-60,0175 was observed in the FC of 5-HTT-/- compared to 5-HTT+/+ mice. This change was very similar to that reported after chronic 5-HT reuptake inhibition with paroxetine in wild-type mice and may account for the behavioral phenotype observed here in the mutants (Whitton and Curzon, 1990; Mongeau et al., 2010). Deletion or chronic blockade of the 5-HTT very likely caused this tolerance via the sustained increase in extracellular 5-HT concentration reached in the vicinity of 5-HT2CR. Previous microdialysis studies have shown that extracellular 5-HT is increased in the hippocampus, striatum, and VTA/SN of 5-HTT-/- versus 5-HTT+/+ mice (Fabre et al., 2000; Shen et al., 2004; Homberg et al., 2007), which translated here into an increased 5-HT turnover (Table 1).
In the amygdala, acute administration of RO-60,0175 still produced a decrease in stress-enhanced 5-HT turnover in 5-HTT-/- mutants (Figure 2), probably because there were no genotype differences in basal 5-HT turnover in that area (Table 1). Interestingly, however, an increased 5-HT turnover did become apparent in the amygdala of stressed 5-HTT-/- mutants (Figure 2E). A possible explanation of these peculiar responses in the amygdala might be related to the higher expression of the organic cation transporter (OCT) 3 in this area compared to other brain areas (Gasser et al., 2009). Indeed, the non-specific monoamine OCTs are overexpressed in several brain areas of 5-HTT-/- mice (Chen et al., 2001; Schmitt et al., 2003; Baganz et al., 2008), and this might compensate for the 5-HTT invalidation, as previously suggested by Daws (2009). In addition, OCT reuptake blockade by corticosterone released during stress (Wu et al., 1998; Arndt et al., 2001; Gasser et al., 2006) might account, at least in part, for the enhanced, stress-induced increase of 5-HT turnover in the amygdala of 5-HTT-/- mice.
Such specific alterations in the amygdala might be related to the increased anxiety-like phenotype of 5-HTT-/- mice in some models, especially because an increased 5-HT2CR binding was observed in this area of the mutants (Li et al., 2003). But if more amygdala 5-HT2CR stimulation means more anxiety, this could not account for the reduced anxiogenic effect of mCPP in the social interaction test. However, because 5-HT2CR mRNA editing would lead to reduced 5-HT2CR functions in vivo, as inferred from clear-cut in vitro data on downstream 5-HT2CR–signaling pathways (McGrew et al., 2004), an increased 5-HT2CR mRNA editing in the amygdala of 5-HTT-/- mice might contribute to the reduced anxiogenic effect of mCPP (Moya et al., 2011). In fact, in contrast to the expectations created from in vitro data, mutant mice expressing only the fully-edited isoform of 5-HT2CR show an increase rather than a decrease in their responses to 5-HT2CR agonists (Martin et al., 2013). Accordingly, the reduced anxiogenic effect of 5-HT2CR stimulation in 5-HTT-/- mice is most likely accounted for by reduced sensitivity of 5-HT2CR in areas outside the amygdala, where there is no compensatory increase in 5-HT2CR density.
Among these brain areas, the FC appeared to us of special interest because it is interconnected with the amygdala; it is also involved in social anxiety regulation (Prater et al., 2013), and a local decrease in 5-HTT binding has been found to correlate with depression (Mann et al., 2000). In contrast to the amygdala, the FC in 5-HTT-/- mutants displays increases in extracellular 5-HT (Shen et al., 2004; Homberg et al., 2007) and a clear-cut reduction in 5-HT2CR–mediated inhibition of stress-induced 5-HT turnover (Figure 2). In many brain areas, in particular the FC, the resulting increased tone at other 5-HT receptors probably has important consequences relevant to the anxiolytic/antidepressant effects, as well as some of the negative psychotropic side effects of sustained 5-HTT inhibition. Chronic 5-HTT blockage with ADs was previously shown to enhance 5-HT1AR–mediated inhibition of neural circuitry in the FC, in conjunction with attenuated effects related to 5-HT2R activation (Bobula et al., 2003). An optimized balance between the excitatory and inhibitory effects of 5-HT through various receptors (including 5-HT2CR and 5-HT1AR) is central to behavioral inhibition, impulsivity, and other controls over emotions operated by the FC (Puig and Gulledge, 2011). It was thus crucial to determine whether changes in 5-HT2CR density, transcription, editing, or some alteration in downstream cell signaling account for the tolerance to 5-HT2CR stimulation observed in the FC.
5-HT2CR distribution has been well characterized by immunohistochemistry in rodent FC, where these receptors are mainly expressed by parvalbumin-positive GABA interneurons (Liu et al., 2007). The turnover (5-HIAA/5-HT ratio) assay does not provide information as to whether the decreased inhibitory effect of 5-HT2CR activation can be explained by changes at the 5-HT2CR themselves or by other changes, notably affecting GABAergic transmission. However, immunohistochemical data indicated no changes in the density of cells expressing 5-HT2CR protein in the FC of 5-HTT-/- mice, nor did quantitative RT-PCR show any decrease in 5-HT2CR mRNAs (as previously shown with chronic selective serotonin reuptake inhibitors; Spurlock et al., 1994). Our data agree with those of Li et al. (2003), who reported no changes in cortical 5-HT2CR binding sites in 5-HTT-/- mutants versus 5-HTT+/+ mice.
Because 5-HT2CR density was not altered, we next explored whether the decrease in 5-HT2CR functions might alternatively be explained by alterations in 5-HT2CR pre-mRNA editing in 5-HTT-/- mutants. Indeed, Englander et al. (2005) reported significant editing changes in the cerebral cortex of BALB/c mice after chronic 5-HT reuptake inhibition. However, such changes were not found in C57Bl/6 mice (Englander et al., 2005). Also, our assessment of up to 32 mRNAs of the 5-HT2CR using capillary electrophoresis (Poyau et al., 2007) did not reveal any editing change in the FC of 5-HTT-/- mice of this same genetic background (Figure 3).
These negative findings spurred us into setting up an in vivo cellular assay to assess the ability of 5-HT2CR activation to induce the expression of the IEG c-fos mRNA. This assay was validated using 5-HT2CR–like immunostaining coupled with c-fos mRNA in situ hybridization, rather than with a classical pharmacological approach. This choice was made because 5-HT2CR antagonists produce on their own distinct patterns of Fos, or c-fos mRNA, expression (Herdegen and Leah, 1998; De Deurwaerdère et al., 2010). To avoid strong c-fos mRNA induction generated by animal handling during injections, mice were injected remotely using an i.v.-implanted catheter. Contrary to the Fos protein, the expression of c-fos mRNA is transient, with maximal expression being reached within 30 minutes after stimulus (Rahmsdorf et al., 1987; Herdegen and Leah, 1998). In the present study, basal c-fos mRNA expression was not likely to reflect animal handling because catheterizations were performed 4 days before the experiment. Furthermore, tubing connection was made 3 hours before i.v. injections.
In these conditions, we found that acute administration of RO-60,0175 increased c-fos mRNA expression in 5-HT2CR–positive cells in 5-HTT+/+ mice, but not in 5-HTT-/- mutants. The latter had, however, an increased baseline expression of c-fos mRNA (in agreement with Hoyle et al., 2011), which is very probably related to the increased Fos levels reported after chronic ADs (Morinobu et al., 1995). In 5-HTT-/- mutants, 5-HT2CR–mediated responses might be decreased because cells expressing 5-HT2CR have blunted intracellular signaling capacities, including those required to trigger c-fos mRNA production. Knowing that c-fos mRNA induction is linked to a rise in intracellular Ca2+ concentration (Herdegen and Leah, 1998), cells regulated by 5-HT2CR might have a diminished capacity to raise intracellular Ca2+ levels above baseline in 5-HTT-/- mice.
Major alterations in GABAergic neurons expressing 5-HT2CR have been evidenced in 5-HTT-/- mice, including decreased GABA synthesis capacity, reduced GABAA receptor numbers, and lower GABA-stimulated Cl- uptake compared to wild-type mice (Fox et al., 2007; Guidotti et al., 2012). In addition, two markers of GABAergic interneurons, the intracellular Ca2+ buffering proteins parvalbumin and calbindin, are decreased in 5-HTT-/- mice (Guidotti et al., 2012), as well as in mice chronically treated with fluoxetine (Ohira et al., 2013), and low parvalbumin concentration is associated with increased Fos expression (Keilhoff et al., 2004). Interestingly, Zhong and Yan (2011) reported that chronic blockade of 5-HTT desensitizes the excitatory effect of 5-HT on GABAergic interneurons while increasing their basal excitability. In view of these data, we propose a hypothetic mechanism of functional 5-HT2CR desensitization, as detailed in Figure 4. Our data suggest that this desensitization does not involve changes at the receptor itself, but implicates downstream signaling pathways. Furthermore, the basal increase in c-fos mRNA is interesting, as this IEG has been involved in synaptic plasticity (Tischmeyer and Grimm, 1999). It is thus possible that consequent neural circuit changes in the FC, impacting the physiology of GABAergic interneurons (Ohira et al., 2013), also explain the altered behavioral responses of 5-HTT-/- mice.
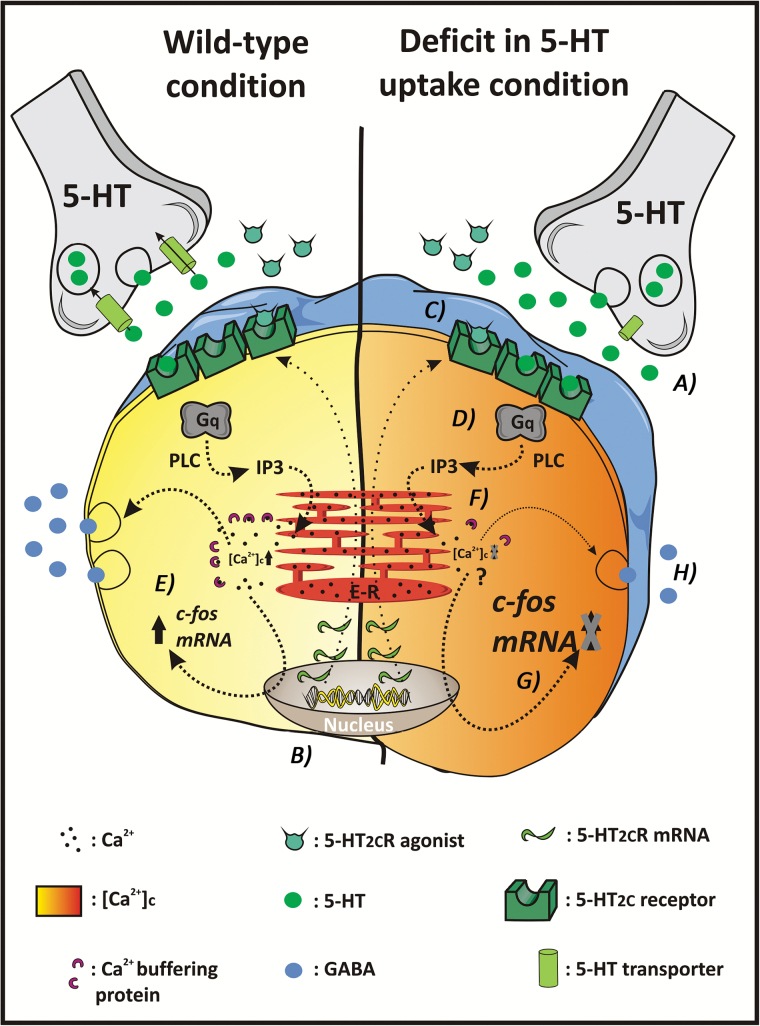
Figure 4.
Hypothetical mechanisms underlying the decreased 5-HT2C receptor-mediated responses in areas such as the frontal cortex following sustained 5-HT uptake inhibition.
(A) A deficit in 5-HT reuptake leads to an increased extracellular concentration of 5-HT. Despite this increased synaptic 5-HT, there are no changes in either 5-HT2CR mRNA or pre-mRNA editing (B) or 5-HT2CR density (C). (D) 5-HT and 5-HT2CR agonists exert an excitatory effect on GABAergic cells via Gq protein activation of PLC and IP3-induced mobilization of Ca2+ from endoplasmic reticulum. (E) c-fos mRNA expression is increased by cell depolarization and elevation in cytosolic [Ca2+]c, normally triggered by 5-HT2CR activation (left). (F) Antidepressant drugs and 5-HTT inactivation appear to decrease Ca2+ buffering proteins, which would lead to increased basal levels of both [Ca2+]c and c-fos expression (Guidotti et al., 2012; Keilhoff et al., 2004; Ohira et al., 2013; right). (G) The enhanced basal [Ca2+]c and c-Fos protein would prevent further increases in these intracellular messengers following 5-HT2CR activation (right). (H) These alterations in intracellular signaling could somehow be linked to the altered gamma-aminobutyric acid (GABA) ergic transmission observed after 5-HTT inactivation and antidepressant treatments (Fox et al., 2007; Guidotti et al., 2012; Gray et al., 1987; Suzdak and Gianutsos, 1986). Overall, long-term 5-HTT inhibition would lead to both an increased basal excitability of these cells and their blunted response to 5-HT2CR stimulation (Zong and Yan, 2011).
In conclusion, the primary change explaining the decrease in the responsiveness of 5-HT2CR in 5-HTT-/- mice might be the high extracellular 5-HT concentration reached after reuptake inactivation, but no secondary changes in 5-HT2CR protein, mRNA, or editing were observed in the FC. Although not investigated here, developmentally-triggered changes in 5-HT2CR density in the amygdala might explain the mainly anxiogenic profile of 5-HTT-/- mice (Li et al., 2003; Moya et al., 2011) as this structure remained responsive to 5-HT2CR stimulation in 5-HTT-/- mice (Figure 2). However, the blunted capacity of neurons expressing 5-HT2CR in other areas to trigger 5-HT2CR–mediated intracellular signaling would strongly limit the expression of anxiety in 5-HTT-/- mice. Such an adaptive functional desensitization of 5-HT2CR opens new perspectives not only in the context of AD treatments, but also in relation to 5-HTT polymorphisms predisposing to anxiety disorders.
Statement of Interest
All authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
This work was supported by grants from INSERM, UPMC, the European Community (FP7, “Devanx” consortium, F2-2007–201714), and ANR (Sercedit 2008–2010, contract ANR-06-Neuro-045-01). Dr CBP Martin was recipient of a fellowship from the French Ministère de la Recherche during performance of this work. Dr Trigo was supported by Fundacion Alicia Koplowitz. Dr Fink (DA07287, DA034488) and Dr Cunningham (DA024157, DA020087) were supported by the National Institute on Drug Abuse grants. We are especially grateful to Professor KP Lesch (University of Wuerzburg, Germany) for providing 5-HTT mutant mice to set up breeding colonies, to Laurent Vincent and Dr Jean-François Pujol (Biocortech, Paris) for capillary electrophoresis analysis of pre-mRNA editing and Dr Theresa Smith (University of Texas Medical Branch) for her guidance and assistance with CHO cell analyses.
References
- Akiyoshi J, Isogawa K, Yamada K, Nagayama H, Fujii I. (1996). Effects of antidepressants on intracellular Ca2+ mobilization in CHO cells transfected with the human 5-HT2C receptors. Biol Psychiatry 39:1000–1008. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. (2010). Serotonin 5-HT(2C) receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem 113:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306:879–881. [DOI] [PubMed] [Google Scholar]
- Arndt P, Volk C, Gorboulev V, Budiman T, Popp C, Ulzheimer-Teuber I, Akhoundova A, Koppatz S, Bamberg E, Nagel G, Koepsell H. (2001). Interaction of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. Am J Physiol Renal Physiol 281:F454–F468. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. (2008). Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci USA 105:18976–18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. (2001). Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychop 4:399–408. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mössner R, Westphal H, Lesch KP. (1998). Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53:649–655. [DOI] [PubMed] [Google Scholar]
- Berg KA, Stout BD, Maayani S, Clarke WP. (2001). Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J Pharm Exp Ther 299:593–602. [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. (2001). The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem 276(11):8269–8277. [DOI] [PubMed] [Google Scholar]
- Bobula B, Tokarski K, Zahorodna A, Hess G. (2003). Adaptive changes in the reactivity of 5-HT1A and 5-HT2 receptors induced in rat frontal cortex by repeated imipramine and citalopram. Naunyn Schmiedebergs Arch Pharmacol 367:444–450. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. (1988). The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology 95:298–302. [DOI] [PubMed] [Google Scholar]
- Bristow LJ, O’Connor D, Watts R, Duxon MS, Hutson PH. (2000). Evidence for accelerated desensitisation of 5-HT(2C) receptors following combined treatment with fluoxetine and the 5-HT(1A) receptor antagonist, WAY 100,635, in the rat. Neuropharmacology 39:1222–1236. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. (2007). Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behav Genet 37:214–222. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. (2001). Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci 21:6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. (2009). Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther 121:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, Le Moine C, Chesselet M-F. (2010). Selective blockade of serotonin 2C receptor enhances Fos expression specifically in the striatum and the subthalamic nucleus within the basal ganglia. Neurosci Lett 469:251–255. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. (2000). Biochemical and electrophysiological evidence that RO 60–0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res 865:85–90. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. (2005). How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci 25:648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. (2000). Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci 12:2299–2310. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. (2003). A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53. [DOI] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch K-P, Holmes A, Murphy DL. (2007). A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology 195:147–166. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. (2006). Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci 26:8758–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. (2009). Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol 512:529–555. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. (2005). The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med 35:101–111. [DOI] [PubMed] [Google Scholar]
- Gray JA, Goodwin GM, Heal DJ, Green AR. (1987). Hypothermia induced by baclofen, a possible index of GABAB receptor function in mice, is enhanced by antidepressant drugs and ECS. Br J Pharmacol 92:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. (2000). The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system, 2nd ed. New York: Oxford Science Publications; [Google Scholar]
- Guidotti G, Calabrese F, Auletta F, Olivier J, Racagni G, Homberg J, Riva MA. (2012). Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: modulation by antidepressant treatment. Neuropsychopharmacology 37:746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Suzuki M, Sasamata M, Miyata K. (2004). Thermogenic effect of YM348, a novel 5-HT2C-receptor agonist, in rats. J Pharm Pharmacol 56:1551–1556. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. (2007). Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav 6:491–496. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. (1998). Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev 28:370–490. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. (2002). Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27:914–923. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. (2003). Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav 2:365–380. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JDA, Smits BMG, Mul JD, Mudde J, Verheul M, Nieuwenhuizen OFM, Cools AR, Ronken E, Cremers T, Schoffelmeer ANM, Ellenbroek BA, Cuppen E. (2007). Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience 146:1662–1676. [DOI] [PubMed] [Google Scholar]
- Hoyle D, Juhasz G, Aso E, Chase D, del Rio J, Fabre V, Hamon M, Lanfumey L, Lesch K-P, Maldonado R, Serra M-A, Sharp T, Tordera R, Toro C, Deakin JFW. (2011). Shared changes in gene expression in frontal cortex of four genetically modified mouse models of depression. Eur Neuropsychopharmacol 21:3–10. [DOI] [PubMed] [Google Scholar]
- Keck ME, Sartori SB, Welt T, Müller MB, Ohl F, Holsboer F, Landgraf R, Singewald N. (2005). Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: effects of chronic paroxetine treatment. J Neurochem 92:1170–1179. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein H-G. (2004). Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience 126:591–598. [DOI] [PubMed] [Google Scholar]
- Kennett G, Lightowler S, Trail B, Bright F, Bromidge S. (2000). Effects of RO 60 0175, a 5-HT(2C) receptor agonist, in three animal models of anxiety. Eur J Pharmacol 387:197–204. [DOI] [PubMed] [Google Scholar]
- Kim D-K, Tolliver TJ, Huang S-J, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch K-P, Murphy DL. (2005). Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 49:798–810. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van De Kar LD, Garcia F, Murphy DL. (2003). Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem 84:1256–1265. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. (2003). Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry 54:960–971. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. (2007). Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience 146:1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Moryl E. (1992). Effects of sertraline and citalopram given repeatedly on the responsiveness of 5-HT receptor subpopulations. J Neural Transm Gen Sect 88:143–156. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. (2000). A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 57:729–738. [DOI] [PubMed] [Google Scholar]
- Mannoury La Cour C, Chaput C, Touzard M, Millan MJ. (2009). An immunocapture/scintillation proximity analysis of G alpha q/11 activation by native serotonin (5-HT)2A receptors in rat cortex: blockade by clozapine and mirtazapine. Synapse 63:95–105. [DOI] [PubMed] [Google Scholar]
- Martin CBP, Ramond F, Farrington DT, Aguiar AS, Chevarin C, Berthiau A-S, Caussanel S, Lanfumey L, Herrick-Davis K, Hamon M, Madjar J-J, Mongeau R. (2013). RNA splicing and editing modulation of 5-HT(2C) receptor function: relevance to anxiety and aggression in VGV mice. Mol Psychiatry 18:656–665. [DOI] [PubMed] [Google Scholar]
- Martin CBP, Hamon M, Lanfumey L, Mongeau R. (2014). Controversies on the role of 5-HT2C receptors in the mechanisms of action of antidepressant drugs. Neurosci Biobehav Rev 42:208–223. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bös M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Köhler C, Delft AM. (1998). 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharm Exp Ther 286:913–924. [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Aulakh CS, Wozniak KM, Murphy DL. (1996). Evidence that m-chlorophenylpiperazine-induced hyperthermia in rats is mediated by stimulation of 5-HT2C receptors. Psychopharmacology 123:333–339. [DOI] [PubMed] [Google Scholar]
- McGrew L, Price RD, Hackler E, Chang MSS, Sanders-Bush E. (2004). RNA editing of the human serotonin 5-HT2C receptor disrupts transactivation of the small G-protein RhoA. Mol Pharmacol 65:252–256. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Miller GA, Chiang E, Anderson DJ. (2003). Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J Neurosci 23:3855–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R, Martin CBP, Chevarin C, Hamon M, Lanfumey L. (2008). Reduced anxiogenic effect of m-chlorophenyl-piperazine in 5-HTT and 5-HT1B knock-out mice. Behav Pharmacol 19:661. [Google Scholar]
- Mongeau R, Martin CBP, Chevarin C, Maldonado R, Hamon M, Robledo P, Lanfumey L. (2010). 5-HT2C receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J Neurochem 115:438–449. [DOI] [PubMed] [Google Scholar]
- Morinobu S, Nibuya M, Duman RS. (1995). Chronic antidepressant treatment down-regulates the induction of c-fos mRNA in response to acute stress in rat frontal cortex. Neuropsychopharmacology 12:221–228. [DOI] [PubMed] [Google Scholar]
- Moya PR, Fox MA, Jensen CL, Laporte JL, French HT, Wendland JR, Murphy DL. (2011). Altered 5-HT2C receptor agonist-induced responses and 5-HT2C receptor RNA editing in the amygdala of serotonin transporter knockout mice. BMC Pharmacol 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. (2004). Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 3:303–314. [DOI] [PubMed] [Google Scholar]
- Ohira K, Takeuchi R, Iwanaga T, Miyakawa T. (2013). Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Mol Brain 6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier J, Van Der Hart M, Van Swelm R, Dederen P, Homberg J, Cremers T, Deen P, Cuppen E, Cools A, Ellenbroek B. (2008). A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience 152:573–584. [DOI] [PubMed] [Google Scholar]
- Olivier JDA, Vallès A, van Heesch F, Afrasiab-Middelman A, Roelofs JJPM, Jonkers M, Peeters EJ, Korte-Bouws GAH, Dederen JP, Kiliaan AJ, Martens GJ, Schubert D, Homberg JR. (2011). Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology 217:419–432. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2001). The mouse brain in stereotaxic coordinates (Deluxe Edition), Second Edition. Academic Press. [Google Scholar]
- Poyau A, Vincent L, Berthommé H, Paul C, Nicolas B, Pujol J-F, Madjar J-J. (2007). Identification and relative quantification of adenosine to inosine editing in serotonin 2c receptor mRNA by CE. Electrophoresis 28:2843–2852. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. (2013). Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 30:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle A, Jennings KA, Line S, Bannerman DM, Higgs S, Sharp T. (2008). Mice overexpressing the 5-hydroxytryptamine transporter show no alterations in feeding behaviour and increased non-feeding responses to fenfluramine. Psychopharmacology 200:291–300 [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. (2011). Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 44:449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. (2005). 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology 180:12–20. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf HJ, Schönthal A, Angel P, Litfin M, Rüther U, Herrlich P. (1987). Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res 15:1643–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Mössner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. (2003). Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res 71:701–709. [DOI] [PubMed] [Google Scholar]
- Seitz PK, Bremer NM, McGinnis AG, Cunningham KA, Watson CS. (2012). Quantitative changes in intracellular calcium and extracellular-regulated kinase activation measured in parallel in CHO cells stably expressing serotonin (5-HT) 5-HT2A or 5-HT2C receptors. BMC Neurosci 13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch K-P, Murphy DL, Hall FS, Uhl GR, Sora I. (2004). Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29:1790–1799. [DOI] [PubMed] [Google Scholar]
- Spurlock G, Buckland P, O’Donovan M, McGuffin P. (1994). Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5-HT transporter. Neuropharmacology 33:433–440. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Gianutsos G. (1986). Effect of chronic imipramine or baclofen on GABA-B binding and cyclic AMP production in cerebral cortex. Eur J Pharmacol 131:129–133. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. (1999). Activation of immediate early genes and memory formation. Cell Mol Life Sci 55:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch K-P, Robledo P, Maldonado R. (2007). 3,4-methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol Psychiatry 62:669–679. [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE. (1986). Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung J-P, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313:536–540. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch K-P, Murphy DL, Holmes A. (2007). Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Loiacono R, Sexton PM, Christopoulos A. (2008). RNA editing of the serotonin 5-HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther 119:7–23. [DOI] [PubMed] [Google Scholar]
- Whitton P, Curzon G. (1990). Anxiogenic-like effect of infusing 1-(3-chlorophenyl) piperazine (mCPP) into the hippocampus. Psychopharmacology 100:138–140. [DOI] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. (1998). Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem 273:32776–32786. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Tatebayashi T, Nagase K, Kojima M, Imanishi T. (2004). Chronic treatment with fluvoxamine desensitizes 5-HT2C receptor-mediated hypolocomotion in rats. Pharmacol Biochem Behav 78:683–689. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein R, Jaing C, Murphy D, Lanthorn T, Holmes A. (2006). Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience 140:321–334. [DOI] [PubMed] [Google Scholar]
- Zhong P, Yan Z. (2011). Differential regulation of the excitability of prefrontal cortical fast-spiking interneurons and pyramidal neurons by serotonin and fluoxetine. PLOS ONE 6:e16970. [DOI] [PMC free article] [PubMed] [Google Scholar]