Abstract
Background:
Zinc may act as a neurotransmitter in the central nervous system by activation of the GPR39 metabotropic receptors.
Methods:
In the present study, we investigated whether GPR39 knockout would cause depressive-like and/or anxiety-like behavior, as measured by the forced swim test, tail suspension test, and light/dark test. We also investigated whether lack of GPR39 would change levels of cAMP response element-binding protein (CREB),brain-derived neurotrophic factor (BDNF) and tropomyosin related kinase B (TrkB) protein in the hippocampus and frontal cortex of GPR39 knockout mice subjected to the forced swim test, as measured by Western-blot analysis.
Results:
In this study, GPR39 knockout mice showed an increased immobility time in both the forced swim test and tail suspension test, indicating depressive-like behavior and displayed anxiety-like phenotype. GPR39 knockout mice had lower CREB and BDNF levels in the hippocampus, but not in the frontal cortex, which indicates region specificity for the impaired CREB/BDNF pathway (which is important in antidepressant response) in the absence of GPR39. There were no changes in TrkB protein in either structure. In the present study, we also investigated activity in the hypothalamus-pituitary-adrenal axis under both zinc- and GPR39-deficient conditions. Zinc-deficient mice had higher serum corticosterone levels and lower glucocorticoid receptor levels in the hippocampus and frontal cortex.
Conclusions:
There were no changes in the GPR39 knockout mice in comparison with the wild-type control mice, which does not support a role of GPR39 in hypothalamus-pituitary-adrenal axis regulation. The results of this study indicate the involvement of the GPR39 Zn2+-sensing receptor in the pathophysiology of depression with component of anxiety.
Keywords: GPR39, zinc receptor, depression, HPA axis, CREB
Introduction
Depression is a leading psychiatric illness, with high morbidity and mortality (Ustun, 2004). The lack of appropriate, rapidly acting antidepressants is probably due to the direct pathomechanism of depression being unknown, and this leads to the high suicide statistics. Approximately 50% of those diagnosed with major depressive disorder do not respond to antidepressants when using them for the first time (Fava et al., 2008). Long-term antidepressant treatment generates many side effects, and more than 30% of depressed patients do not experience any mood improvement at all (Fava and Davidson, 1996). Until now, only one drug, ketamine, has shown rapid action even in treatment-resistant patients (Mathew et al., 2012; Lara et al., 2013; Haile et al., 2014). One drug, ketamine, has shown rapid and sustained action even in treatment-resistant patients (Mathew et al., 2012; Lara et al., 2013; Haile et al., 2014). This indicates promise for modulators of the glutamatergic system, which may lead to the establishment of homeostasis between glutamate and GABA in the central nervous system (CNS) (Skolnick, 2002; Skolnick et al., 2009; Malkesman et al., 2012; Pilc et al., 2013; Pochwat et al., 2014). In addition, some trace elements, such as magnesium and zinc, are involved in glutamatergic attenuation through their binding sites at the N-methyl-d-aspartate (NMDA) receptor (Swardfager et al., 2013b). Preclinical findings indicate that zinc deficiency has been shown to produce depressive-like behavior (Singewald et al., 2004; Tassabehji et al., 2008; Tamano et al., 2009; Whittle et al., 2009; Młyniec and Nowak, 2012; Młyniec et al., 2013a, 2013b, 2014a). Clinical studies indicate that zinc is lower in the blood of depressed people (Swardfager et al., 2013b), and that zinc supplementation may produce antidepressant effects alone and in combination with conventional antidepressant therapies (Ranjbar et al., 2013; Siwek et al., 2013; Swardfager et al., 2013a)..
Zinc is an important trace element in the central nervous system and seems to be involved in neurotransmission. As a natural ligand, it was found to activate the metabotropic GPR39 receptor (Holst et al., 2007). Highest levels of GPR39 are found in the brain regions involved in emotion, such as the amygdala and hippocampus (McKee et al., 1997; Jackson et al., 2006). The GPR39 signals with high constitutive activity via Gq, which stimulates transcription mediated by the cyclic adenosine monophosphate (cAMP) following inositol 1,4,5-triphosphate turnover, as well as via G12/13, leading to activation of transcription mediated by the serum response element (Holst et al., 2004). Zinc was found to be a ligand capable of stimulating the activity of the GPR39, which activates the Gq, G12/13, and Gs pathways (Holst et al., 2007). Since zinc shows antidepressant properties and its deficiency leads to the development of depression-like and anxiety-like behaviors (Whittle et al., 2009; Swardfager et al., 2013a), we investigated whether the GPR39 receptor may be involved in the pathophysiology of depression. Recently, we found GPR39 down-regulation in the frontal cortex and hippocampus of zinc-deficient rodents and suicide victims (Młyniec et al., 2014b). On the other hand, we observed up-regulation of the GPR39 after chronic antidepressant treatment (Młyniec and Nowak, 2013). In the present study, we investigated behavior in mice lacking a GPR39 as well as an hypothalamus-pituitary-adrenal axis (HPA) axis and proteins such as CREB, BDNF, and TrkB, all of which are important in the pathophysiology of depression and the antidepressant response.
Methods
Animals
All of the procedures were conducted according to the National Institute of Health Animal Care and Use Committee guidelines, which were approved by the Ethical Committee of the Jagiellonian University Collegium Medicum, Kraków.
CD-1 male mice (~22g) were housed with a natural day-night cycle, a temperature of 22±2°C and humidity at 55±5%. The mice received a zinc-adequate (33.5mg Zn/kg) or zinc-deficient (0.2mg Zn/kg) diet purchased from MP Biomedicals (France) and administered for 6 weeks. The access to food as well as water was ad libitum.
GPR39 (−/−) male mice as described by Holst et al. (2009) were generated through homologous recombination by Deltagen, Inc. by targeting the first exon of GPR39 and replacing the nucleotides from position 278 to 647 of the open reading frame with a neomycin-containing cassette. The chimeric males were crossed with C57BL/6 females and then backcrossed into C57BL/6 mice. The mice were obtained through heterozygous breeding, resulting in wild-type (WT), homozygous, or heterozygous littermates. Genotypes were verified by polymerase chain reaction. Specific primers for the WT and one specific primer for the insert sequences were used.
As with the CD-1 mice, the GPR39 knockouts (KOs) were housed under standard laboratory conditions. GPR39 KO (−/−) and GPR39 WT (+/+) mice received only a standard diet with appropriate zinc amounts.
Forced Swim Test
The forced swim test (FST) was carried out on GPR39 KO and WT mice. In the classical test described by Porsolt et al. (1977), mice were dropped individually into a glass cylinder containing water. The total duration of immobility after adaptation time (the first 2 minutes) was measured during the following 4 minutes of the test.
The immobility time in the FST reflects the level of despair of the mice, prolonged immobility suggesting depressive-like behavior. Because Porsolt et al. (1977) described FST as being a means of evaluating potential antidepressant properties of drugs, we prolonged the test, as described by Młyniec et al. (2014b), from 4 to 6 minutes, during which time the duration of immobility was measured.
Tail Suspension Test
WT and GPR39 KO mice were subjected to the tail suspension test (TST) previously described by Mlyniec and Nowak (2012). Animals were fastened with medical adhesive tape by the tail 30cm below a flat surface and suspended for 6 minutes. During this period, the total immobility time was measured. Immobility time (when mice hung passively without limb movement) was scored manually.
Locomotor Activity
Locomotor activity was measured by photoresistor actometers. The number of times the light beams were crossed by GPR39 or WT mice was counted by placing them individually in an actometer, with the test duration being between 2 and 8 minutes.
Light/Dark Test
WT and GPR39 KO mice were subjected to the light/dark test as previously described by Whittle et al. (2009). The fully automated light/dark box apparatus (Stoelting) consisted of white and black compartments, which were connected by a small opening located in the center of the common partition. Mice were individually placed in the apparatus for 10 minutes and allowed to freely explore. The following parameters were quantified in the test: 1) time spent in lit compartment (seconds), 2) entries into the lit compartment (number), 3) line crossing (number), 4) immobility time (seconds), 5) freezing (seconds), and 6) overall distance travelled (meters).
Zinc Concentration
The serum zinc concentration in both GPR39 KO and WT mice was measured by total reflection X-ray fluorescence as described by Młyniec et al. (2014b). This method is based on the same physical principles as energy dispersive X-ray fluorescence. Galium was added to the serum sample as an internal standard (20mL) to achieve the final concentration of 5mg/L. For all measurements, the total reflection X-ray fluorescence spectrometer Nanohunter (Rigaku) was used as well as a single measurement time of 2000 seconds and a Mo X-ray tube (50kV, 0.8 mA). The detection limits for Zn were about 0.4mg/L.
Corticosterone Assay
The serum corticosterone concentration was determined by a radioimmunological method as described by Młyniec et al. (2013a). Corticosterone was extracted from the serum by ethanol. This extract (ethanol-serum) was dried under a nitrogen stream and then dissolved in 0.1mL of 0.05mM phosphate buffer. Extracts were incubated with a 0.1-mL solution of 1,2,6,7-[3H]-corticosterone and with a 0.1-mL solution of a corticosterone antibody (Chemicon) for 16 hours at 4°C. Bound and free corticosterone were separated using dextran-coated charcoal. Incubation time for the samples was established for 10 minutes at 4°C with 0.2mL of 0.05% dextran and 0.5% charcoal suspension. After centrifugation, the supernatant was placed in a scintillator. The radioactivity was measured in a counter (Beckmann LS 335). The corticosterone content was calculated using a log-logit transformation.
Western-Blot Analysis
Glucocorticoid receptor (GR) levels were determined in the frontal cortex and hippocampus of zinc-adequate and -deficient mice after administration of the diet for 6 weeks. In the GPR39 KO and WT mice, in addition to GR, the levels of such proteins as CREB, BDNF, and TrkB were also determined, as described by Młyniec et al. (2014b). All mice were previously subjected to the FST. After rapid decapitation of the mice (24 hours after FST procedure), tissues were immediately isolated on dry ice and then frozen at −80°C until analysis took place.
The frontal cortex and hippocampus were homogenized in 2% sodium dodecyl sulphate. After centrifugation, the total amount of protein was determined in the supernatant (BCA Protein Assay Kit, Pierce Biotechnology). The samples were separated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Bio-Rad) under a constant voltage and then transferred (in a semi-dry transfer process) to nitrocellulose membranes. To avoid nonspecific binding, membranes were blocked for 60 minutes at room temperature with blocking solution (Roche). Then the membranes were incubated overnight at 4°C with primary antibodies: anti-GR (1/1000, Santa Cruz Biotechnology), anti-CREB (1/1000), anti-BDNF (1/1000), and anti-TrkB (1/400) (Abcam, Cambridge, UK). After washing (3×10 minutes in Tris-buffered saline with Tween 20), the membranes were incubated in a secondary antibody with a horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (Western Blotting Kit, Roche) for 60 minutes at room temperature. Blots were developed using an enhanced chemiluminescence reaction (BM Chemiluminescence Western Blotting Kit, Roche). The GR, CREB, BDNF, and TrkB signals were visualized and quantified with the Gel Doc XR+ system and Image Lab 4.1 software (both Bio-Rad). To confirm equal loading of the samples on the gel, the membranes were incubated with a loading control antibody and then processed as described above. The density of each GR, CREB, BDNF, or TrkB protein band was normalized to the density of the loading control band.
Statistical Analysis
The data are presented as the mean±SEM and were evaluated with the Student t test using GraphPad Prism software (San Diego, CA). P<.05 was considered to be statistically significant.
Results
Behavioral Studies of Gpr39 KO Mice
Before experiments, mice were weighed. There were no differences between WT and GPR39 KO groups [t(10)=0.2715, P=.7916].
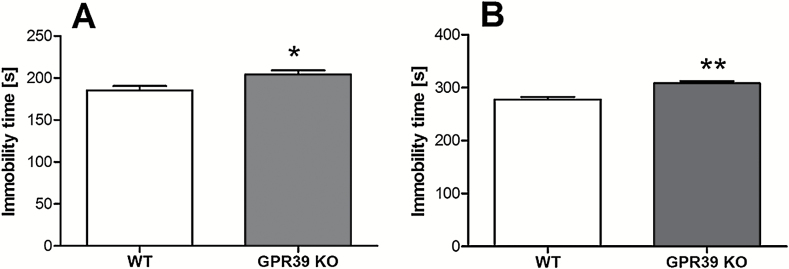
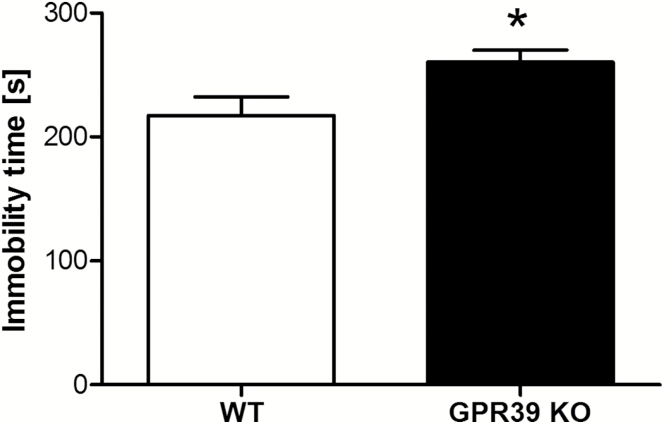
The effect of deletion of the GPR39 on immobility time in the FST is shown in Figure 1A. GPR39 KO mice showed an increased immobility time in the FST designed by Porsolt et al. (1977) in comparison with WT mice [t(15)=2.563, P=.0217]. We found a more significant increase in immobility time in GPR39 KO vs WT using a modified FST (Młyniec et al., 2014b) [t(15)=4.571, P=.0004] (Figure 1B). We also found an increased immobility time in the TST in GPR39 KO mice [t(10)=2.415, P=.0363] (Figure 2).
Figure 1.
The effect of GPR39 knockout (KO) on immobility time in the standard (A) and prolonged (B) forced swim test in GPR39 KO mice. Values are the means ± SEM of 6 to 7 animals per group. * p < 0.05, ** p < 0.001 vs wild-type control.
Figure 2.
The effect of GPR39 knockout (KO) on immobility time in the tail suspension test in GPR39 KO mice. Values are the means ± SEM of 6 animals per group. p* < 0.05 vs wild-type control.
There were no differences in locomotor activity between GPR39 KO and WT mice after 2 [t(15)=0.004016, P=.9968], 4 [t(15)=0.1016, P=.9205], 6 [t(15)=.04298, P=.9663], and 8 [t(15)=0.05586, P=.9562] minutes (Table 1).
Table 1.
The Effect of GPR39 KO on Spontaneous Locomotor Activity in GPR39 KO Mice. Values are the means ± SEM of 6 to 7 animals per group.
| 2 min | 4 min | 6 min | 8 min | |
|---|---|---|---|---|
| WT | 158.1±15.08 | 307.8±24.42 | 458.5±29.09 | 609.3±35.23 |
| GPR39 KO | 158.2±18.45 | 311.9±31.65 | 456.5±39.81 | 605.9±47.21 |
In the light/dark box test, GPR39 KO mice displayed decreased entries into the lit compartment, line crossing, and enhanced immobility time compared with WT control mice (Table 2).
Table 2.
Behavioral Parameters Quantified in the Light/Dark Test in WT and GPR 39 KO mice. Values are the means ± SEM of 6 animals per group. *p < 0.05, **p < 0.01 vs proper control.
| Wild type | GPR39 knockout | Statistics | |
|---|---|---|---|
| Time spent in lit compartment (s) | 300.5±30.47 | 249.0±26.24 | [t(10) = 1.281, p = 0.2291] |
| Entries into the lit compartment (number) | 18.67±1.978 | 13.17±0.543 * | [t(10) = 2.628, p = 0.0230] |
| Line crossing (number) | 41.17±2.713 | 25.67±1.116 ** | [t(10) = 5.284, p = 0.0004] |
| Immobility time (s) | 103.5±15.86 | 146.9±10.42 * | [t(10) = 2.287, p = 0.0452] |
| Freezing (s) | 128.0±12.95 | 164.3±11.03 | [t(10) = 2.130, p = 0.0590] |
| Overall distance travelled (m) | 23.75±2.049 | 23.42±1.540 | [t(10) = 0.131, p = 0.8982] |
Serum Zinc Concentration in GPR39 KO Mice
There was no difference between GPR39 KO (1.707±0.1606mg/L) and WT mice (1.848±0.1130mg/L) in terms of serum zinc concentration [t(11)=0.7328, P=.4790].
CREB, BDNF, and TrkB Protein Levels in GPR39 KO Mice
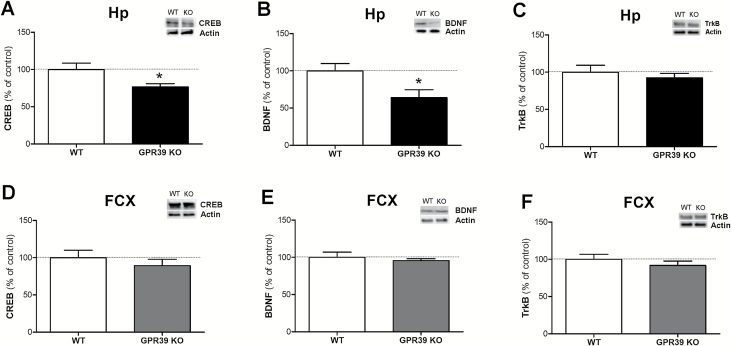
The effect of deletion of the GPR39 on CREB, BDNF, and TrkB levels in mice is shown in Figure 3. GPR39 KO mice show reduced CREB levels in the hippocampus [t(12)=2.427, P=.0319] but not in the frontal cortex [t(12)=0.8192, P=.4286] in comparison with WT mice (Figures 3A and D, respectively).
Figure 3.
The effect of GPR39 knockout (KO) on CREB, BDNF, and TrkB levels in the hippocampus (A, B, and C, respectively) and in the frontal cortex (D, E, and F, respectively) of GPR39 KO mice. Values are the means ± SEM of 6 to 7 animals per group. *p < 0.05 vs wild-type control.
In a similar way to the CREB levels, GPR39 KO mice also have reduced BDNF levels in the hippocampus [t(10)=2.510, P=.0309] (Figure 3B), but not in the frontal cortex, in comparison with WT control mice [t(12)=0.6015, P=.5587] (Figure 3E). There was no difference in TrkB levels between GPR39 KO and WT mice in the hippocampus [t(12)=0.6861, P=.5057] or frontal cortex [t(12)=0.9219, P=.3747] (Figure 3C and F, respectively).
Serum Corticosterone Concentration in Zinc-Deficient and GPR39 KO Mice
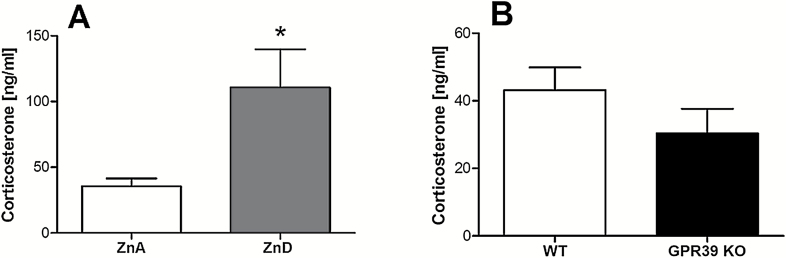
The effects of zinc deficiency on the serum corticosterone level are shown in Figure 4A. A 6-week zinc-deficient diet causes a significant increase in serum corticosterone concentration in comparison with control mice [t(8)=2.547, P=.0343]. There were no significant differences between GPR39 KO and WT [t(9)=1.298, P=.2266] (Figure 4B).
Figure 4.
The effect of a zinc-deficient diet (A) or GPR39 knockout (B) on serum corticosterone level in mice. Values are the means ± SEM of 6 to 7 animals per group. *p < 0.05 vs proper control.
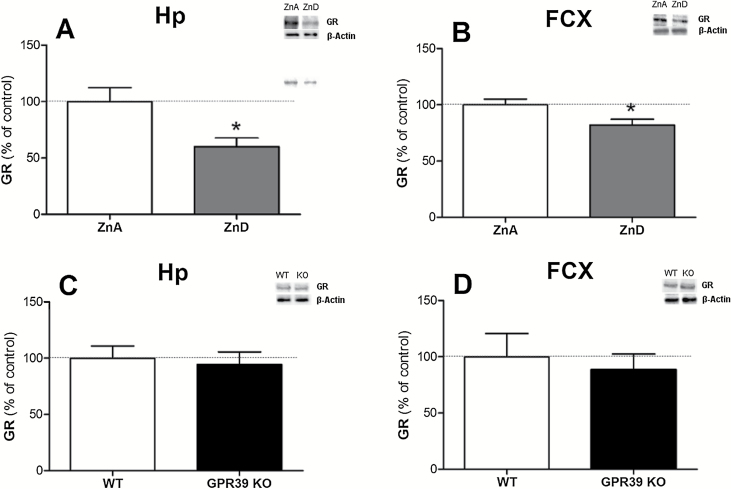
GR Protein Levels in Zinc-Deficient and GPR39 KO Mice
Administration of a zinc-deficient diet for 6 weeks causes a reduction in glucocorticoid receptor levels in the hippocampus [t(11)=2.649, P=.0226] and frontal cortex [t(12)=2.475, P=.0292] (Figure 5A and B, respectively). There were no changes in the GR levels in the hippocampus [t(12)=0.3628, P=.7231] or the frontal cortex [t(12)=0.4638, P=.6511] of GPR39 KO mice in comparison with WT control mice (Figure 5C and D, respectively).
Figure 5.
The effect of a zinc-deficient diet (A,B) or GPR39 knockout (C,D) on glucocorticoid receptor levels in the hippocampus (A,C) and frontal cortex (B,D) of mice. Values are the means ± SEM of 6 to 7 animals per group. * p < 0.05 vs. proper control.
Discussion
In the present study, we found that elimination of GPR39 leads to the development of depressive-like behavior, as measured by the FST and TST. Additionally, we found decreased entries into the lit compartment and line crossing and increased immobility time in the light/dark test, indicating anxiety-like phenotype in GPR 39 KO mice. Although not statistically significant, we also observed some tendencies towards less time spent in the lit compartment (decreased by 17%) and increased and freezing behavior (28%) in GPR39 KO compared to WT mice.
The GPR39 was found to be activated by zinc ions (Holst et al., 2007; Yasuda et al., 2007), and the link between zinc and depression is well known (Maes et al., 1997, 1999; Szewczyk et al., 2011; Swardfager et al., 2013a, 2013b). Ishitobi et al. (2012) did not find behavioral abnormalities in the FST after administering antisense of DNA for GPR39-1b. In our present study, mice with general GPR39 KO were used. GPR39-1a full-length isoform was found to be a receptor for zinc ions, whereas GPR39-1b, corresponding to the 5-transmembrane truncated form (Egerod et al., 2007), did not respond to zinc stimulation, which means that the GPR39-1b splice variant is not a receptor of Zn2+ (Yasuda and Ishida, 2014).
Activation of the GPR39 triggers diverse neuronal pathways (Holst et al., 2004, 2007; Popovics and Stewart, 2011) that may be involved in neuroprotection (Depoortere, 2012). Zinc stimulates GPR39 activity, which activates the Gαs, Gαq, and Gα12/13 pathways (Holst et al., 2007). The Gαq pathway triggers diverse downstream kinases and mediates CREB activation and cyclic adenosine monophosphate response element-dependent transcription. Our previous study showed decreased CREB, BDNF, and TrkB proteins in the hippocampus of mice under zinc-deficient conditions (Młyniec et al., 2014b). Moreover, disruption of the CaM/CaMKII/CREB signaling pathway was found after administration of a zinc-deficient diet for 5 weeks (Gao et al., 2011). Since GPR39 was discovered to be a receptor for zinc, we previously investigated whether GPR39 may be involved in the pathophysiology of depression and suicide behavior. In our postmortem study, we found GPR39 down-regulation in the hippocampus and the frontal cortex of suicide victims (Młyniec et al., 2014b). In the present study, we investigated whether GPR39 KO would decrease levels of such proteins as CREB, BDNF, and TrkB, which were also found to be impaired in depression in suicide victims (Dwivedi et al., 2003; Pandey et al., 2007). Indeed, we found that lack of the GPR39 gene causes CREB and BDNF reduction in the hippocampus, but not in the frontal cortex, suggesting that the hippocampus might be a specific region for CREB and BDNF down-regulation in the absence of a zinc receptor. The CA3 region of the hippocampus seems to be strongly involved in zinc neurotransmission. Besser et al. (2009) found that the GPR39 is activated by synaptically released zinc ions in the CA3 area of the hippocampus. This activation triggers Ca2+ and Ca2+/calmodulin kinase II, suggesting that it has a role in neuron survival/neuroplasticity in this brain area (Besser et al., 2009), which is of importance in antidepressant action. In this study, we did not find any changes in TrkB levels in either the hippocampus or frontal cortex; in the case of the hippocampus, this may be a compensatory mechanism, and it needs further investigation.
There is strong evidence that zinc deficiency leads to hyperactivation of the HPA axis (Watanabe et al., 2010; Takeda and Tamano, 2012; Młyniec et al., 2012, 2013a), which is activated as a reaction to stress. The activity of the HPA axis is regulated by negative feedback through GR receptors that are present in the brain, mainly in the hippocampus (Herman et al., 2005). This mechanism was shown to be impaired in mood disorders. In the present study, we compared corticosterone and GR receptor levels in zinc-deficient and GPR39 KOs. We found an elevated corticosterone concentration in the serum and decreased GR levels in the hippocampus and frontal cortex of mice receiving a zinc-deficient diet. However, there were no changes in corticosterone or GR levels in GPR39 KO mice in comparison with WT mice. This suggests that the depressive-like behavior observed in mice lacking the GPR39 gene is not due to higher corticosterone concentrations and that there is no link between GPR39 and the HPA axis. In the present study, we did not find any changes in the serum zinc level in GPR39 KO mice in comparison with WT mice, which indicates a possible correlation between serum zinc and serum corticosterone.
Depressive-like changes with component of anxiety observed in GPR39 KO mice may result of glutamatergic abnormalities that were found in cases of zinc deficiency, but this requires further investigation. Zinc as an NMDA antagonist modulates the glutamatergic system, which is overexcited during depression. Zinc co-released with glutamate from “gluzinergic” neurons modulates excitability of the brain by attenuating glutamate release (Frederickson et al., 2005). The GPR39 zinc receptor seems to be involved in the mechanism regulating the amount of glutamate in the brain (Besser et al., 2009). Activation of the GPR39 up-regulates KCC2 and thereby enhances Cl− efflux in the postsynaptic neurons, which may potentiate γ-aminobutyric acidA-mediated inhibition (Chorin et al., 2011).
Our present study shows that deletion of GPR39 leads to depressive-like behaviors in animals, which may be relevant to depressive disorders in humans. Decreased levels of CREB and BDNF proteins in the hippocampus of GPR39 KO mice support the involvement of GPR39 in the synthesis of CREB and BDNF, proteins that are important in neuronal plasticity and the antidepressant response.
Statement of Interest
None.
Acknowledgments
This study was supported by a grant from the National Science Centre K/PBO/000106 (contract DEC-2011/03/B/NZ7/01999).
References
- Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. (2009). Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci 29:2890–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Lucki I. (2013). Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorin E, Vinograd O, Fleidervish I, Gilad D, Herrmann S, Sekler I, Aizenman E, Hershfinkel M. (2011). Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J Neurosci 31:12916–12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere I. (2012). GI functions of GPR39: novel biology. Curr Opin Pharmacol 12:647–652. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. (2003). Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60:804–815. [DOI] [PubMed] [Google Scholar]
- Egerod KL, Holst B, Petersen PS, Hansen JB, Mulder J, Hökfelt T, Schwartz TW. (2007). GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol Endocrinol 21:1685–1698. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG. (1996). Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 19:179–200. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. (2008). Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry 165:342–351. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. (2005). The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462. [DOI] [PubMed] [Google Scholar]
- Gao H-L, Xu H, Xin N, Zheng W, Chi Z-H, Wang Z-Y. (2011). Disruption of the CaMKII/CREB signaling is associated with zinc deficiency-induced learning and memory impairments. Neurotox Res 19:584–591. [DOI] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ, De La Garza R, Charney DS, Newton TF, Mathew SJ. (2014). Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–1213. [DOI] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. (2004). Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem 279:53806–53817. [DOI] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach L-O, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. (2007). GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 148:13–20. [DOI] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Jin C, Petersen PS, Østergaard MV, Hald J, Sprinkel a ME, Størling J, Mandrup-Poulsen T, Holst JJ, Thams P, Orskov C, Wierup N, Sundler F, Madsen OD, Schwartz TW. (2009). G protein-coupled receptor 39 deficiency is associated with pancreatic islet dysfunction. Endocrinology 150:2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitobi Y, Akiyoshi J, Honda S, Ninomiya T, Kanehisa M, Tanaka Y, Tsuru J, Isogawa K, Kitamura H, Fujikura Y. (2012). Administration of antisense DNA for GPR39-1b causes anxiolytic-like responses and appetite loss in rats. Neurosci Res 72:257–262. [DOI] [PubMed] [Google Scholar]
- Jackson VR, Nothacker H-P, Civelli O. (2006). GPR39 receptor expression in the mouse brain. Neuroreport 17:813–816. [DOI] [PubMed] [Google Scholar]
- Lara DR, Bisol LW, Munari LR. (2013). Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol 16:2111–2117. [DOI] [PubMed] [Google Scholar]
- Maes M, Vandoolaeghe E, Neels H, Demedts P, Wauters A, Meltzer HY, Altamura C, Desnyder R. (1997). Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol Psychiatry 42:349–358. [DOI] [PubMed] [Google Scholar]
- Maes M, De Vos N, Demedts P, Wauters A, Neels H. (1999). Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J Affect Disord 56:189–194. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, Cui Z, Young WS, Nakazawa K, Zarate CA, Manji HK, Chen G. (2012). Acute D-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 15:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW. (2012). Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 26:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LH. (1997). Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 46:426–434. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Davies CL, Budziszewska B, Opoka W, Reczyński W, Sowa-Kućma M, Doboszewska U, Pilc A, Nowak G. (2012). Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol Rep 64:567–575. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Budziszewska B, Reczyński W, Doboszewska U, Pilc A, Nowak G. (2013a) Zinc deficiency alters responsiveness to antidepressant drugs in mice. Pharmacol Rep 65:579–592. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Budziszewska B, Reczyński W, Sowa-Kućma M, Nowak G. (2013b) The role of the GPR39 receptor in zinc deficient-animal model of depression. Behav. Brain Res 238:30–35. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Davies CL, de Agüero Sánchez IG, Pytka K, Budziszewska B, Nowak G. (2014a) Essential elements in depression and anxiety. Part I. Pharmacol Rep 4:534–544. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Doboszewska U, Szewczyk B, Sowa-Kućma M, Misztak P, Piekoszewski W, Trela F, Ostachowicz B, Nowak G. (2014b) The involvement of the GPR39-Zn(2+)-sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology 79:290–297. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Nowak G. (2012). Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol Rep 64:249–255. [DOI] [PubMed] [Google Scholar]
- Młyniec K, Nowak G. (2013). GPR39 up-regulation after selective antidepressants. Neurochem Int 62:936–939. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. (2007). Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol 10:621–629. [DOI] [PubMed] [Google Scholar]
- Pilc A, Wierońska JM, Skolnick P. (2013). Glutamate-based antidepressants: preclinical psychopharmacology. Biol Psychiatry 73:1125–1132. [DOI] [PubMed] [Google Scholar]
- Pochwat B, Pałucha-Poniewiera A, Szewczyk B, Pilc A, Nowak G. (2014). NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin Investig Drugs 1–12. [DOI] [PubMed] [Google Scholar]
- Popovics P, Stewart AJ. (2011). GPR39: a Zn(2+)-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol Life Sci 68:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn thérapie 229:327–336. [PubMed] [Google Scholar]
- Ranjbar E, Kasaei MS, Mohammad-Shirazi M, Nasrollahzadeh J, Rashidkhani B, Shams J, Mostafavi S-A, Mohammadi MR. (2013). Effects of zinc supplementation in patients with major depression: a randomized clinical trial. Iran J Psychiatry 8:73–79. [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Sinner C, Hetzenauer A, Sartori SB, Murck H. (2004). Magnesium-deficient diet alters depression- and anxiety-related behavior in mice--influence of desipramine and Hypericum perforatum extract. Neuropharmacology 47:1189–1197. [DOI] [PubMed] [Google Scholar]
- Siwek M, Szewczyk B, Dudek D, Styczeń K, Sowa-Kućma M, Młyniec K, Siwek A, Witkowski L, Pochwat B, Nowak G. (2013). Zinc as a marker of affective disorders. Pharmacol Rep 65:1512–1518. [DOI] [PubMed] [Google Scholar]
- Skolnick P. (2002). Modulation of glutamate receptors: strategies for the development of novel antidepressants. Amino Acids 23:153–159. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. (2009). Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30:563–569. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. (2013a) Zinc in depression: a meta-analysis. Biol Psychiatry 74:872–878. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, McIntyre RS, Mazereeuw G, Goldberger K, Cha DS, Schwartz Y, Lanctôt KL. (2013b) Potential roles of zinc in the pathophysiology and treatment of major depressive disorder. Neurosci Biobehav Rev 37:911–929. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Kubera M, Nowak G. (2011). The role of zinc in neurodegenerative inflammatory pathways in depression. Prog Neuropsychopharmacol Biol Psychiatry 35:693–701. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tamano H. (2012). Proposed glucocorticoid-mediated zinc signaling in the hippocampus. Metallomics 4:614–618. [DOI] [PubMed] [Google Scholar]
- Tamano H, Kan F, Kawamura M, Oku N, Takeda A. (2009). Behavior in the forced swim test and neurochemical changes in the hippocampus in young rats after 2-week zinc deprivation. Neurochem Int 55:536–541. [DOI] [PubMed] [Google Scholar]
- Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. (2008). Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav 95:365–369. [DOI] [PubMed] [Google Scholar]
- Ustun TB. (2004). Global burden of depressive disorders in the year 2000. Br J Psychiatry 184:386–392. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tamano H, Kikuchi T, Takeda A. (2010). Susceptibility to stress in young rats after 2-week zinc deprivation. Neurochem Int 56:410–416. [DOI] [PubMed] [Google Scholar]
- Whittle N, Lubec G, Singewald N. (2009). Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids 36:147–158. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Miyazaki T, Munechika K, Yamashita M, Ikeda Y, Kamizono A. (2007). Isolation of Zn2+ as an endogenous agonist of GPR39 from fetal bovine serum. J Recept Signal Transduct Res 27:235–246. [DOI] [PubMed] [Google Scholar]
- Yasuda S-I, Ishida J. (2014). GPR39-1b, the 5-transmembrane isoform of GPR39 interacts with neurotensin receptor NTSR1 and modifies its function. J Recept Signal Transduct Res 9893:1–6. [DOI] [PubMed] [Google Scholar]