Abstract
Background:
Alterations in the innate immune/inflammatory system have been proposed to underlie the pathophysiology of psychotic disease, but the mechanisms implicated remain elusive. The main agents of the innate immunity are the family of toll-like receptors (TLRs), which detect circulating pathogen-associated molecular patterns and endogenous damage-associated molecular patterns (DAMPS). Current antipsychotics are able to modulate pro- and anti-inflammatory pathways, but their actions on TLRs remain unexplored.
Methods:
This study was conducted to elucidate the effects of paliperidone (1mg/Kg i.p.) on acute (6 hours) and chronic (6 hours/day during 21 consecutive days) restraint stress–induced TLR-4 pathway activation and neuroinflammation, and the possible mechanism(s) related (bacterial translocation and/or DAMPs activation). The expression of the elements of a TLR-4-dependent proinflammatory pathway was analyzed at the mRNA and protein levels in prefrontal cortex samples.
Results:
Paliperidone pre-treatment prevented TLR-4 activation and neuroinflammation in the prefrontal cortices of stressed rats. Regarding the possible mechanisms implicated, paliperidone regulated stress-induced increased intestinal inflammation and plasma lipopolysaccharide levels. In addition, paliperidone also prevented the activation of the endogenous activators of TLR-4 HSP70 and HGMB-1.
Conclusions:
Our results showed a regulatory role of paliperidone on brain TLR-4, which could explain the therapeutic benefits of its use for the treatment of psychotic diseases beyond its effects on dopamine and serotonin neurotransmission. The study of the mechanisms implicated suggests that gut-increased permeability, inflammation, and bacterial translocation of Gram-negative microflora and HSP70 and HGMB1 expression could be potential adjuvant therapeutic targets for the treatment of psychotic and other stress-related psychiatric pathologies.
Keywords: antipsychotics, bacterial translocation, damage-associated molecular patterns, stress, toll-like receptor-4
Introduction
Psychosocial and/or physical stress exposure is an environmental risk factor for psychiatric disease (Stahl, 2010). One of the putative mechanisms involved is the stress-induced activation of the innate inflammatory/immune response (rev. in Garcia-Bueno et al., 2008).
The innate immunity is a stereotyped, nonspecific response, and is considered a protective mechanism; however, if excessive in intensity (overexpression or overactivity of several mediators) or time (inefficient resolution), it becomes harmful (Lampron et al., 2013). The main agents of the innate immune response are the family of toll-like receptors (TLRs). TLRs are pattern-recognition receptors that detect circulating pathogen-associated molecular patterns present in pathogens but not in mammalian cells. These patterns trigger a complex pro-inflammatory cascade that can impact the central nervous system (CNS), regulating homeostasis and even promoting pathology (rev. in Medzhitov, 2001). TLRs are highly expressed in immune cells (Akira et al., 2006) and, in the last years, TLR presence has been also found in different CNS-intrinsic cell types, such as neurons, astrocytes, resident microglia, or cells in cerebral microvasculature, plexus choroideus, and leptomeninges (Hanke and Kielian, 2011). This ubiquitous distribution suggests other roles for TLRs in non-pathogen associated CNS diseases or injuries, such as the recognition of a number of endogenous molecules released from damaged tissues (damage-associated molecular patterns [DAMPs]; Piccinini and Midwood, 2010) in a great diversity of processes, such as autoimmunity, neurogenesis, and brain plasticity (Fischer and Ehlers, 2008; Hanke and Kielian, 2011).
The most representative member of the TLR family is TLR-4, which predominantly responds to lipopolysaccharide (LPS) from Gram-negative bacteria (Takeuchi and Akira, 2001) through its co-receptor, myeloid differentiation protein-2 (MD-2), a requisite for LPS signaling of TLR-4 (Shimazu et al., 1999). To achieve specificity of signaling, TLR-4 recruits some other adapter proteins during intracellular signal transduction, such as the myeloid differentiation factor 88 (MyD88). After various consecutive steps in the transduction pathway (that is, specific kinases), the activation of cytokines and the prototypic inflammatory nuclear transcription factor NF-κB (Means et al., 2000) culminate in the production of NF-κB dependent pro-inflammatory mediators, such as the enzymes inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2; Madrigal et al., 2006).
In the last years, alterations on the innate immune/inflammatory system have been implicated in the pathophysiology of psychotic and other psychiatric diseases (Meyer et al., 2011; Kirkpatrick and Miller, 2013). The precise mechanisms are still obscure, but several theories exist: (a) immune challenges before birth (prenatal or maternal infections, diabetes) and obstetric complications (fetal hypoxia, preeclampsia) may prime an immature fetal immune system that will remain impaired for a lifetime (Brown and Derkits, 2010); (b) immune insults in decisive periods of neurodevelopment and maturation (i.e., in adolescence; Meyer, 2013); or (c) inflammatory lifetime changes produced by diverse environmental factors (psychological stress, latent infections, diet, psychotropic drugs abuse, etc.; Suvisaari and Mantere, 2013). In addition, a strong relationship between schizophrenia and autoimmune disorders has been suggested (Benros et al., 2011).
The modulation of uncontrolled inflammation could be a potential therapeutic adjuvant strategy to antipsychotics. Thus, a recent update has reviewed the randomized controlled trials on the efficacy of anti-inflammatory compounds on schizophrenia, including aspirin, celecoxib, davunetide, estrogens, and minocycline (Sommer et al., 2013).
Alterations in the TLRs pathway have been reported in animal models of depression and depressed individuals (Gárate et al., 2011; Hajebrahimi et al., 2014), and also in schizophrenia at a peripheral level (McKernan et al., 2011; Müller et al., 2012). In stress-based animal models of depression in particular, TLR-4 activation could be caused, at least in part, by increased intestinal permeability and a resultant bacterial translocation of intestinal Gram-bacteria (Gárate et al., 2011, 2013). Despite these findings, a role for other TLR-4 endogenous ligands (DAMPs), such as fibrinogen, heat shock proteins (HSP60-70), or high-mobility-group box protein 1 (HGMB-1) has not been studied.
As commented above, the effects of antipsychotics on pro- and anti-inflammatory pathways are well described, but their direct actions on TLRs remain unexplored. This study aimed to elucidate the effects of paliperidone on stress-induced TLR-4 pathway activation and neuroinflammation and the possible mechanism(s) related (bacterial translocation and/or DAMPs activation).
Material and Methods
Animals
72 adult (aged 12 weeks) male Wistar Hannover rats (HsdHan:Wist, Harlan Ibérica) weighing 225–250g were used. All experimental protocols adhered to the guidelines of the Animal Welfare Committee of the Universidad Complutense in accordance with European legislation (D2010/63/UE). The rats were housed with standard temperature and humidity conditions and in a 12h light/dark cycle (lights on at 08:00 hours) with free access to food and water. All the animals were maintained under constant conditions for 7 days prior to the experiment.
Drug Administration and Experimental Designs
The atypical antipsychotic paliperidone (3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one; MW 426.48, ref. P0099), LPS (Escherichia coli serotype 0111:B4, ref. L2630), and other chemicals were purchased from Sigma-Aldrich or as indicated. LPS was dissolved in 0.9% saline and paliperidone was suspended in a saline solution with 0.1% Tween 20 by sonication for 1min (Veh).
Three experimental designs were performed (see Figure S1 in Supplementary Materials).
The first consisted of an acute model of LPS-induced neuroinflammation (MacDowell et al., 2013). Experimental groups were: (1) Veh + no injection; (2) 1mg/Kg paliperidone (P1) + no injection; (3) 0.3mg/Kg paliperidone (P0.3) + no injection; (4) Veh + 0.5mg/Kg LPS; (5) P1 + 0.5mg/Kg LPS; and (6) P0.3 + 0.5mg/Kg LPS. Animals were sacrificed 120min after first injection.
The second experiment performed was an acute restraint-stress model (6 hours of immobilization; Leza et al., 1998) using a plastic rodent restrainer that allowed for a close fit to rats. Control animals were not subjected to stress, but were handled for a few seconds, and food and water were removed during the period of time that the stressed rats were kept in the restrainer. Veh or paliperidone was given by oral gavage immediately before placing the animal into the restrainer. Groups were: (1) Veh + no stress; (2) P1 + no stress; (3) Veh + stress; and (4) P1 + stress.
The last experiment conducted consisted of a chronic restraint-stress model (6h/day over 21 days; Goldwater et al., 2009). The experimental groups were as follows: (1) Veh + no stress; (2) P1 + no stress; (3) Veh + stress; and (4) P1 + stress.
All protocols started at 09:00h to avoid circadian changes in the stress response. All groups in experiment 1 contained n = 5 animals, in experiment 2 contained n = 4 animals, and in experiment 3 there were n = 6 animals in the control groups and n = 7 in the stress groups.
Animals were killed 120min after the first injection in experiment 1 and immediately after the restraint session in experiments 2 and 3, using sodium pentobarbital (320mg/Kg, i.p. Vetoquinol®). All i.p. drugs (or saline) were administered at 2mL/Kg. The doses of paliperidone (0.3mg/Kg and 1mg/Kg) were chosen on the basis of previous in vivo determinations of signaling pathways related to oxidative stress, cytokines, and synaptic plasticity in the prefrontal cortex of Wistar rats (Corena-McLeod et al., 2008).
Preparation of Biological Samples
After decapitation, the brain was removed from the skull and, after careful removal of the meninges and blood vessels, the prefrontal cortical areas (PFC) from both brain hemispheres were excised and frozen at -80ºC until assayed. In some groups of animals, a portion of the descending colon (approx. 2cm proximal from rectum) was removed and stored at -80°C (see Supplementary Material for more details).
Preparation of Nuclear and Cytosolic Extracts from Tissue Samples
A modified procedure based on the method of Schreiber et al. (1989) was used (see Supplementary Material for more details).
Western Blot Analysis
To determine expression levels of TLR-4 and its adapter proteins MyD88 and MD-2, the oxidative/nitrosative and inflammatory mediators iNOS, COX-2, mPGES-1, and TLR-4, the endogenous ligands HMGB1, HSP60, and HSP70, and the brain PFC were homogenized by sonication in 400 µL of PBS (pH = 7) mixed with a protease inhibitor cocktail (Complete, Roche Farma) and were centrifuged at 12.000g for 10 minutes at 4ºC. The same protocol was used to determine the protein levels of the intestinal epithelial cell-derived chemokine CCL28 in colonic samples. In the case of the NF-κB subunit p65, analyses were carried out in nuclear extracts; for its inhibitory subunit IκBα, cytosolic extracts were used. Detailed information about sample preparation, protocol, and primary and secondary antibodies can be found in the Supplementary Material.
RT-PCR Analysis
Total cytoplasmic RNA was prepared from samples of brain PFC using the TRIZOL reagent (Invitrogen); aliquots were converted to complementary DNA using random hexamer primers. Quantitative changes in messenger RNA (mRNA) levels were estimated by real time-polymerase chain reaction (RT-PCR; see Table S1 in Supplementary Material for details).
PGE2 Levels
PGE2 levels were measured by a commercially available enzyme immunoassay following the manufacturer’s instructions (Prostaglandin E2 EIA Kit-Monoclonal; Cayman Chemical; see Supplementary Material for details).
Nitrites (NO2-) Levels
As the stable metabolites of the free radical nitric oxide, NO2 - were measured using the Griess method (see Supplementary Material references for details).
Lipid Peroxidation
Lipid peroxidation was measured by the thiobarbituric acid test for malondialdehyde (MDA) following the method described by Das and Ratty (1987) with some modifications (see Supplementary Material references for details).
Plasma LPS Levels
LPS levels were determined using a commercially available kit, following the manufacturer’s instructions (Hycult Biotech; see Supplementary Material for more details).
Immunoglobulin A Determination
Colonic immunoglobulin A (IgA) levels were determined using a commercially available enzyme immunoassay kit (Bethyl Laboratories; see Supplementary Material for more details).
Protein Assay
Protein levels were measured using the Bradford method based on the principle of protein-dye binding (see Supplementary Material references for details).
Statistical Analyses
Data in text and figures are expressed as mean ± standard error of the mean. For multiple comparisons, a one-way analysis of variance (ANOVA) followed by the Newman–Keuls post hoc test was used to compare all pairs of means between groups. A p-value < 0.05 was considered statistically significant. All the results of the one-way ANOVA analyses (F values and dfs) are included in Table S2 in the Supplementary Material.
Results
Effects of Paliperidone on Brain TLR-4 Signaling Pathway Activation and Neuroinflammation After Acute LPS Exposure
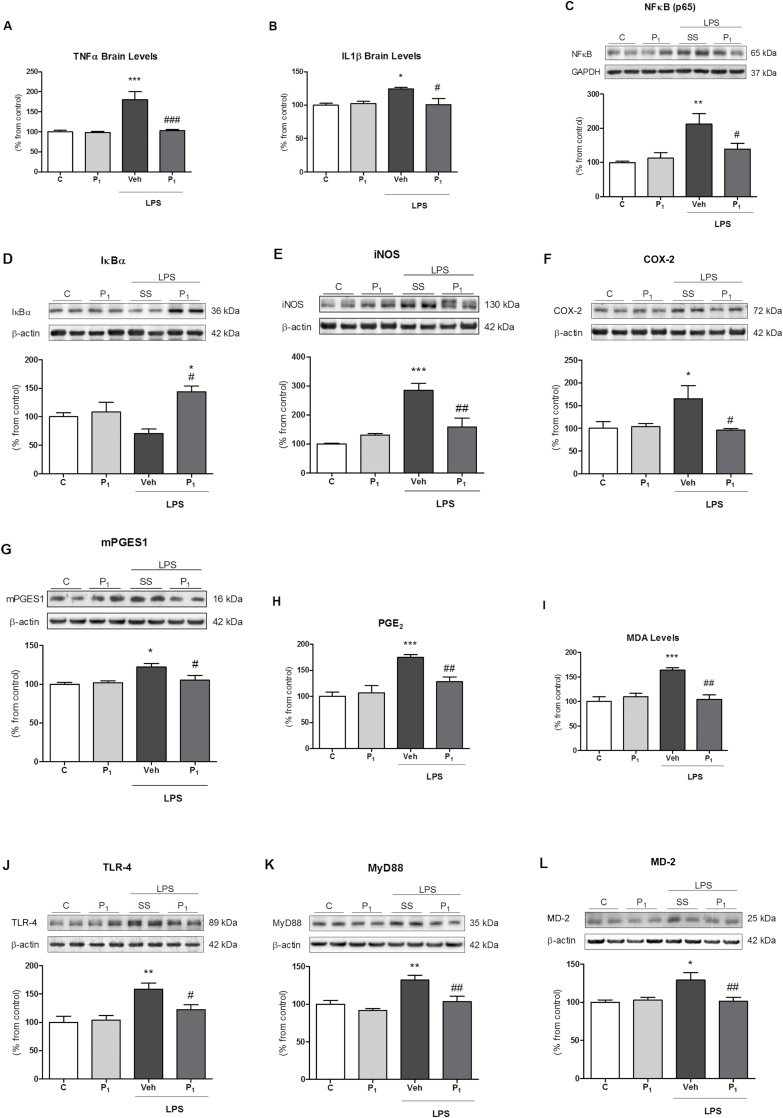
Previously, we described the anti-inflammatory effects of risperidone in a LPS model of neuroinflammation (MacDowell et al., 2013). Now we aimed to corroborate whether its active metabolite, paliperidone, also presents this anti-inflammatory profile. LPS elicited a proinflammatory response characterized by increased levels of TNF-α and IL-1β (Figure 1A and B) and nuclear NF-κB (Figure 1C) and degradation of the NF-κB inhibitory protein IκBα in cytosol (Figure 1D). As a direct consequence of NF-κB activation, there was an increase of proinflammatory enzymes iNOS, COX-2, and m-PGES-1 (Figure 1E–G), whose activity lead to the over-accumulation of proinflammatory and oxidative/nitrosative stress mediators, such as prostaglandin E2 (PGE2) and MDA (index of lipid peroxidation; Figure 1H and I). As in the case of risperidone (MacDowell et al., 2013), the pre-administration of paliperidone (1mg/Kg) fully prevented the activation of this proinflammatory/oxidant pathway in rat PFCs by LPS (Figure 1A–I). All these results were paralleled at the mRNA level by RT-PCR (see Figure S2 in Supplementary Material).
Figure 1.
Paliperidone effects on lipopolysaccharide (LPS)-induced neuroinflammation and toll-like receptor-4 (TLR-4) pathway upregulation in rat prefrontal cortices. Analysis of the pro-inflammatory cytokines levels of tumour necrosis factor TNFα (A) and interleukin IL1β (B); protein levels of NF-κB (p65; C), NF-κB inhibitory protein IκBα (D), iNOS (E), COX-2 (F), m-PGES-1 (G); pro-inflammatory PGE2 levels (H) and lipid peroxidation levels (malondialdehyde [MDA]; I); protein levels of TLR-4 (J), MyD88 (K), and MD-2 (L) in the prefrontal cortices of vehicle (Veh) + no injection (C), 1mg/Kg paliperidone (P1) + no injection, Veh + LPS, and 1mg/Kg paliperidone (P1) + LPS. The densitometric data of the respective bands of interest are normalized by β-actin or GAPDH (lower band). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. LPS. One-way analysis of variance followed by Newman–Keuls post-hoc test. Data represent the mean ± standard error of the mean.
Considering that LPS is the main ligand of TLR-4, we checked whether paliperidone was able to interact with the elements of its signaling pathway. LPS exposure upregulated TLR-4, MyD88, and MD-2 at the protein (Figure 1J–L) and mRNA levels (see Figure S2 in Supplementary Material). Paliperidone prevented the activation of the TLR-4 signaling pathway (Figure 1A–I) in rat PFC. All these results were paralleled at the mRNA level by RT-PCR (see S2 in Supplementary Material). None of the parameters measured changed between control and control animals pre-treated with paliperidone (Figure 1 and Supplementary Figure S2).
In a parallel study, we checked whether a lower dose of paliperidone (0.3mg/Kg) was able to produce similar anti-inflammatory/antioxidant actions in the LPS-induced proinflammatory response. In general, paliperidone effects on the TLR-4 pathway were well conserved, and only some of the inflammatory (IκBα, COX-2, and m-PGES-1 expression) and oxidative (MDA levels) parameters were not altered (see Table S3 for details in Supplementary Material).
Finally, we studied whether there are differences in the most representative inflammatory parameters (TLR-4, NFκB, and COX-2 expression, and TNF-α levels) between a single injection control group (Veh; n = 5) and a control group receiving two injections (Veh+saline; n = 5), trying to check an effect of the dual injections in the LPS groups as compared to the single injection used for the control groups. As can be seen in Table S4 in Supplementary Material, there were no differences between groups, suggesting that the effect of LPS is not dependant on whether animals have been injected once or twice.
Effects of Paliperidone on Acute Restraint Stress-Induced Activation of the TLR-4 Signaling Pathway and Neuroinflammation
Our next objective was to evaluate whether the effects of paliperidone could be extended to other inflammatory/immune challenges also related to innate immune system activation, such as restraint stress exposure (Gárate et al., 2011, 2013). Considering that stress contributes to the aetiology of psychotic disease, the study of possible effects of paliperidone in stress models could be relevant for therapeutic purposes.
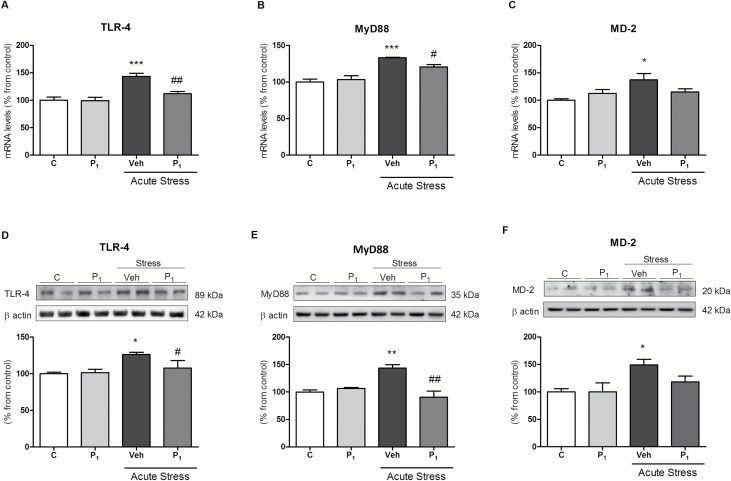
Acute-restraint stress upregulated TLR-4, MyD88, and MD-2 proteins and mRNA in rat PFCs, and these effects were normalized to control levels by paliperidone (Figure 2A–F), except for the stress induced MD-2 increase. Paliperidone did not alter the TLR-4 signaling pathway in control conditions (Figure 2A–F).
Figure 2.
Paliperidone effects on acute restraint stress–induced toll-like receptor-4 (TLR-4) signaling pathway activation in rat prefrontal cortices. Messenger RNA (mRNA) relative levels of TLR-4 (A), MyD88 (B), and MD-2 (C). Protein levels of TLR-4 (D), MyD88 (E), and MD-2 (F) in prefrontal cortices of vehicle (Veh) + no stress (C), 1mg/Kg Paliperidone (P1) + no stress, Veh + stress (6 hrs), and 1mg/Kg Paliperidone (P1) + stress. The densitometric data of the respective bands of interest are normalized by β-actin (lower band). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; # p < 0.05, ## p < 0.01 vs. stress. One-way analysis of variance followed by Newman–Keuls post-hoc test. Data represent the mean ± standard error of the mean.
Next, we explored whether paliperidone-blocking effects on the TLR-4 signaling pathway were extensive to cytokines and NF-κB-driven proinflammatory pathways in rat prefrontal cortices. As in the case of LPS, acute restraint stress elicits a brain increase of TNF-α and IL-1β (Figure S3A and B) and nuclear NF-κB (Figure S3C). There is an increase of iNOS, COX-2, and m-PGES-1 (Figure S3E–G), whose activity augmented the levels of proinflammatory PGE2 and the oxidative/nitrosative stress markers MDA and NO2 - (Figure S3H–J). Pre-treatment with paliperidone (1mg/Kg) prior to acute restraint-stress exposure fully prevented the activation of this proinflammatory/oxidant pathway in rat brains (Figure S3A–J). Even paliperidone increased IκBα expression in cytosol (Figure S3D).
None of the proinflammatory/oxidant elements measured were modified in control animals receiving paliperidone (Figure S3A–J).
These results were paralleled at the mRNA level (see Figure S4 in Supplementary Material), with the exception of COX-2.
Effects of Paliperidone on Chronic Restraint Stress-Induced Activation of the TLR-4 Signaling Pathway and Neuroinflammation
The effects of long-lasting antipsychotic medication on the inflammatory/innate immune system are partly unknown. Thus, our last objective was to explore the effects of daily paliperidone in a standardized protocol of chronic restraint stress (6 hours/day during 21 consecutive days).
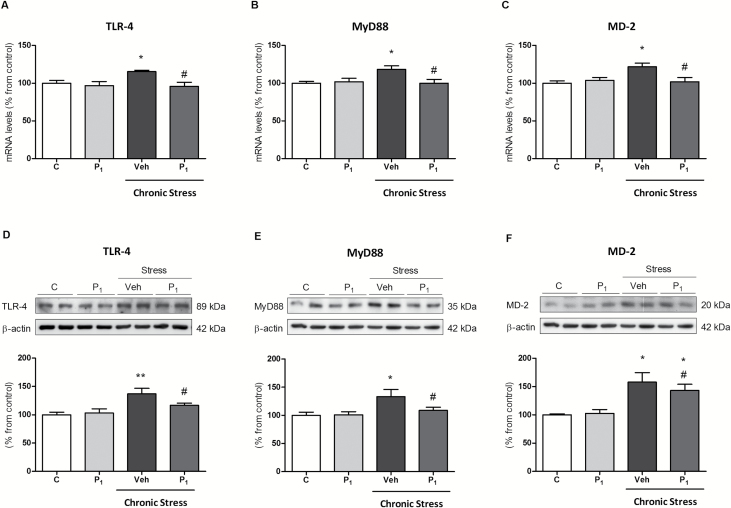
Chronic restraint stress increased TLR-4, MyD88, and MD-2 mRNA and protein (Figure 3A–F) levels compared to control conditions. Daily paliperidone completely blocked stress-induced stimulatory effects. Chronic paliperidone did not alter the TLR-4 signaling pathway in control conditions.
Figure 3.
Paliperidone effects on chronic restraint stress–induced toll-like receptor-4 (TLR-4) signaling pathway activation in rat prefrontal cortices. Messenger RNA (mRNA) relative levels of TLR-4 (A), MyD88 (B), and MD-2 (C). Protein levels of TLR-4 (D), MyD88 (E), and MD-2 (F) in the prefrontal cortices of vehicle (Veh) + no stress (C), 1mg/Kg Paliperidone (P1) + no stress, Veh + stress (6 hrs/day during 21 days), 1mg/Kg Paliperidone (P1) + stress. The densitometric data of the respective bands of interest are normalized by β-actin (lower band). *p < 0.05, **p < 0.01 vs. control; # p < 0.05 vs. stress. One-way analysis of variance followed by Newman–Keuls post-hoc test. Data represent the mean ± standard error of the mean.
We also checked whether paliperidone’s blocking effects on the TLR-4 signaling pathway also extended to cytokines and the NF-κB-driven proinflammatory/oxidant pathway in brain rat PFCs in chronic conditions of stress and treatment.
Repeated restraint stress enhanced TNF-α and IL-1β (Figure S5A and B) levels and nuclear NF-κB p65 subunit expression (Figure S5C), as well as the degradation of IκBα (Figure S5D). Also, there was an increase of iNOS and COX-2 (Figure S5E and F), whose activity augmented PGE2 and MDA levels, but not an increase of NO2 - (Figure S5H–J). Daily administration of paliperidone inhibited chronic stress-induced proinflammatory/oxidant effects, except for the case of m-PGES-1 protein expression and PGE2 levels in rat prefrontal cortices. m-PGES-1 did not appear to be modified by chronic stress (Figure S5G).
At the mRNA level, chronic stress induced the expression of NF-κB, COX-2, and m-PGES-1. Paliperidone blocked the stress effects on NF-κB and m-PGES-1 (see Figure S6), and induced a marked increase of IκBα in stress conditions.
Effects of Paliperidone on the Potential Regulatory Mechanisms of Stress-Induced TLR-4 Activation in Brain PFC (I)
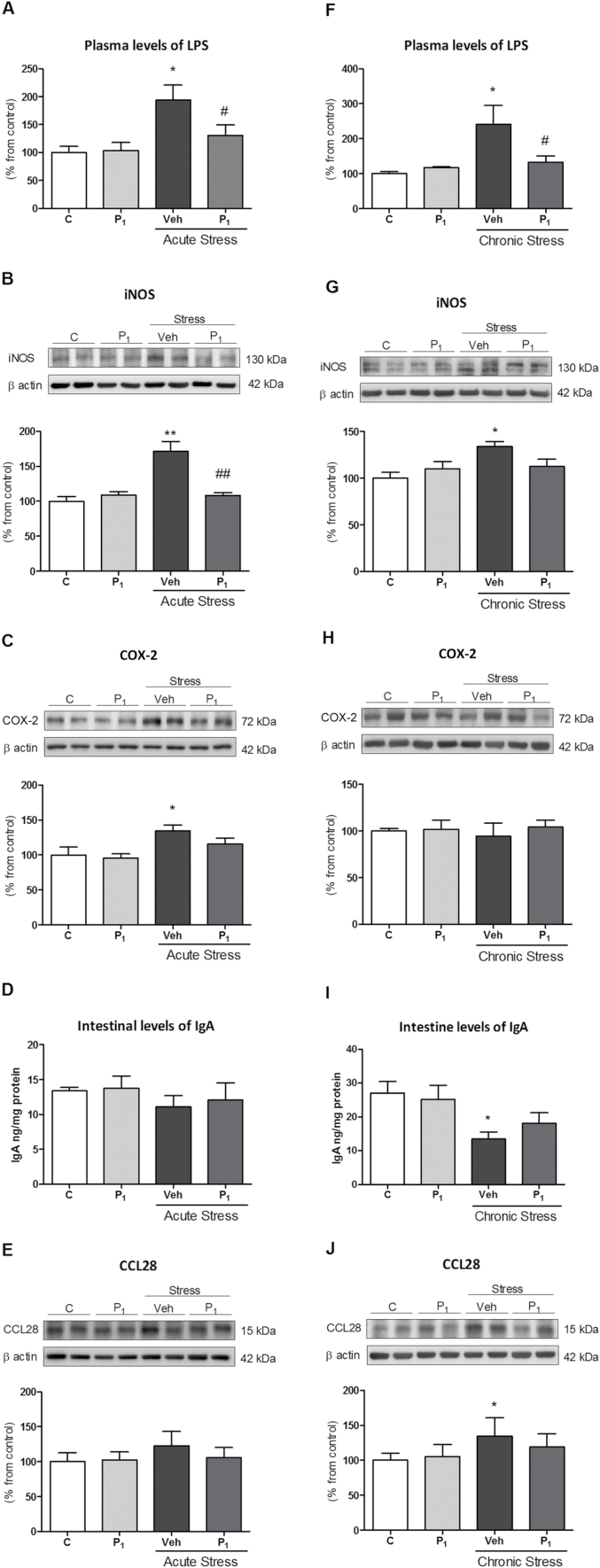
As previously shown in other stress models (Gárate et al., 2011, 2013), increased systemic levels of LPS from translocation of colonic bacteria is a mechanism responsible, at least in part, for the TLR-4 activation and neuroinflammation found. Our last objective was to explore whether the inhibitory effects of paliperidone on central TLR-4 activation were related to the prevention of stress-induced increased inflammation and intestinal permeability, and the resultant bacterial translocation to systemic circulation.
Acute restraint stress increased LPS plasma levels, but this was prevented by paliperidone pre-treatment (Figure 4A). To clarify whether the bacterial translocation observed after acute stress exposure was related to the intestinal dysfunction caused by inflammation, we decided to study some inflammatory-related parameters in this organ. Stress exposure upregulated iNOS and COX-2 protein expression in colon samples (Figure 4B and C). Paliperidone treatment prevented iNOS activation and showed a tendency to decrease COX-2 levels (Figure 4B and C).
Figure 4.
Paliperidone effects on the potential regulatory mechanisms of acute and chronic stress-induced toll-like receptor-4 (TLR-4) activation I. Lipopolysaccharide (LPS) plasma levels (A); intestinal levels of inflammatory proteins iNOS (B) and COX-2 (C); intestinal levels of immunoglobulin A (IgA; D); and protein levels of CCL28 (E) in vehicle (Veh) + no stress (C), 1mg/Kg paliperidone (P1) + no stress, and animals exposed to acute restraint stress (6h) with (P1) or Veh paliperidone pre-treatment. LPS plasma levels (F); intestinal levels of inflammatory proteins iNOS (G) and COX-2 (H); intestinal levels of immunoglobulin A (IgA; I); and protein levels of CCL28 (J) in Veh + no stress (C), 1mg/Kg paliperidone (P1) + no stress, and animals exposed to chronic restraint stress (6 hrs/day during 21 days) with (P1) or Veh paliperidone pre-treatment. The densitometric data of the respective bands of interest are normalized by β-actin (lower band). *p < 0.05, **p < 0.001 vs. control; # p < 0.05, ## p < 0.001 vs. stress. One-way analysis of variance followed by Newman–Keuls post-hoc test. Data represent the mean ± standard error of the mean.
In addition, we quantified the chemokine CCL28, also known as mucosae-associated epithelial chemokine, which regulates the migration of Immunoglobulin-expressing cells. Immunoglobulin A represents a first line of defence against pathogens, and a decrease in its amount in the colon contributes to bacterial translocation. Neither IgA levels nor CCL28 protein expression in colonic samples were modified after acute stress exposure or by paliperidone treatment (Figure 4D and E).
Next, we explored the effects of daily paliperidone administration in chronic restraint-stress exposure (6 hours/day during 21 consecutive days). As in acute conditions, chronic stress induced an increase in the LPS plasma levels (Figure 4F) and iNOS expression in the colon (Figure 4G). COX-2 expression was not modified in any condition (Figure 4H). Paliperidone prevented stress-induced LPS level increases and normalized iNOS protein to control levels (Figure 4F–H).
Contrarily to acute stress conditions, chronic stress decreased IgA levels and increased CCL28 protein in colonic samples (Figure 4I and J). Paliperidone did not significantly alter both stress-induced effects (Figure 4I and J).
Effects of Paliperidone on the Potential Regulatory Mechanisms of Stress-Induced TLR-4 Activation in Brain PFC (II)
TLR-4 recognizes a number of unspecific endogenous molecules released from damaged tissues, such as heat shock proteins 60 and 70 (HSP60–70) and High-mobility group protein box-1 (HGMB-1). The activation of DAMPs could be a complementary regulatory mechanism suitable to be regulated by the treatment with paliperidone in our stress conditions.
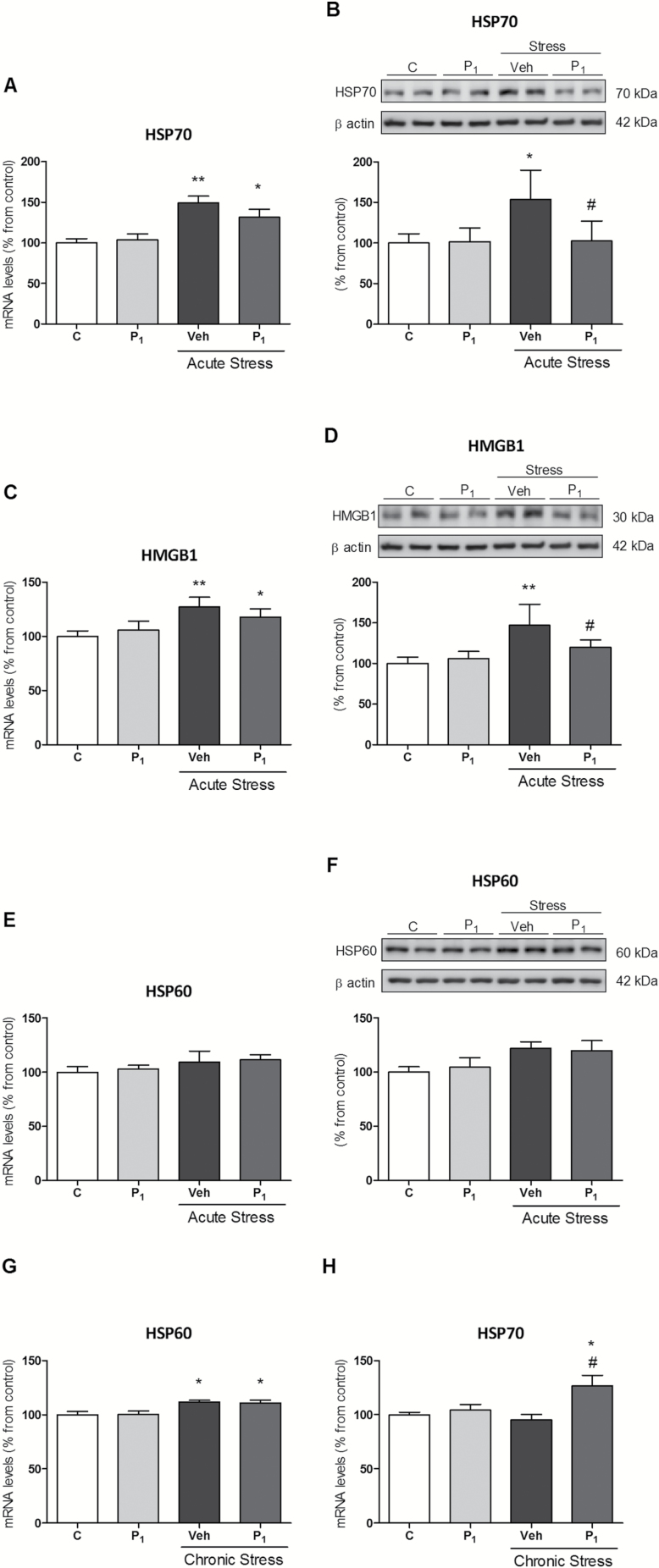
Acute restraint stress elicited an increase in HSP70 and HGMB-1 mRNA and protein, respectively (Figure 5A–D). Paliperidone treatment prevented stress-induced upregulation of HSP70 and HGMB-1 only at protein level (Figure 5B and D). HSP60 was not affected in any condition (Figure 5E and F).
Figure 5.
Paliperidone effects on the potential regulatory mechanisms of acute and chronic stress-induced toll-like receptor-4 (TLR-4) activation II. Messenger RNA (mRNA) and protein levels of HSP70 (A and B), HMGB1 (C and D), and HSP60 (E and F) in vehicle (Veh) + no stress (C), 1mg/Kg paliperidone (P1) + no stress, and animals exposed to acute restraint stress (6 hrs) with (P1) or Veh paliperidone pre-treatment. Messenger RNA (mRNA) relative levels of HSP60 (G) and HSP70 (H) in Veh + no stress (C), 1mg/Kg Paliperidone (P1) + no stress, and animals exposed to chronic restraint stress (6 hrs/day during 21 days) with (P1) or Veh Paliperidone pre-treatment. The densitometric data of the respective bands of interest are normalized by β-actin (lower band). *p < 0.05, **p < 0.01 vs. control; # p < 0.05 vs. stress. One-way analysis of variance followed by Newman–Keuls post-hoc test. Data represent the mean ± standard error of the mean.
Finally, we studied the effects of daily paliperidone treatment in chronic restraint stress conditions (6 hours/day during 21 consecutive days). Chronic stress upregulated the mRNA levels of HSP60 but it had no effect on HSP70 and HGMB-1. The chronic administration of paliperidone did not prevent the stress-induced HSP60 levels, but increased HSP70 mRNA levels compared to control and stress conditions (Figure 5G and H).
Discussion
To our knowledge, the results of the present study provide the first evidence supporting a regulatory role of the antipsychotic paliperidone in the activation of the innate immune receptor TLR-4 and the subsequent neuroinflammatory response in the PFCs of rats submitted to LPS challenge and to acute/chronic restraint stress. A deeper study of the possible mechanisms implicated shows that paliperidone pre-treatment regulates stress-induced increased intestinal inflammation/dysfunction and plasma LPS levels. In addition, paliperidone also prevents the activation of the endogenous, unspecific ligands of TLR-4 HSP70 and HGMB-1, at least in acute stress conditions.
Although the archetypical point of view considers schizophrenia as a neuropsychiatric pathology characterized by increased dopamine function, the potential of paliperidone as a regulator of the innate immune system/inflammation at the CNS level could be relevant to explain the therapeutic benefits of its use for the treatment of psychosis beyond its classical effects on dopamine and serotonin neurotransmission. Our results agree with previous evidence, which has pointed to an anti-inflammatory effect of antipsychotics as one of the beneficial effects of these drugs in patients with schizophrenia. In particular, the stimulation of anti-inflammatory cytokines seems to be a mechanism elicited by several antipsychotics to regulate uncontrolled and potentially deleterious inflammation in schizophrenia (Sugino et al., 2009). It has been shown that risperidone normalizes elevated inflammatory mediators (cytokines and PGs) and restores anti-inflammatory pathways in murine models of neuroinflammation (MacDowell et al., 2013). Chronic administration of olanzapine or clozapine also reduces proinflammatory PGE2 concentration in the rat brain (Cheon et al., 2011). A recent study using a SNP-based analysis of neuroactive pathways implicated PGE2 as a mediator of the effects of risperidone (Adkins et al., 2012).
There is not much evidence clarifying which receptor binding profile of risperidone/paliperidone leads to its anti-inflammatory actions. In this vein, some authors have tested the effects of several specific agonists and antagonists on neurotransmitter receptors that were related to the basic mechanism of paliperidone. These investigations showed that only ketanserin (5-HT2 antagonist) and prazosin (α1 antagonist) had anti-inflammatory effects in the same LPS induced-inflammation model (Sugino et al., 2009). Further studies are required to explain how the chemical structure of paliperidone could affect these immune targets, or if the effect is actually indirect and mediated by the dopaminergic blockade.
Our results acquire special relevance considering that alterations in the TLR pathway have been previously reported in schizophrenia at the peripheral level (McKernan et al., 2011; Müller et al., 2012). Furthermore, there is some evidence of discordant patterns of bacterial translocation markers in schizophrenia, possibly related to an activated innate immune state throughout the stimulation of TLRs (Severance et al., 2013). However, a direct nexus between gut barrier dysfunction, bacterial translocation, and TLR-4 pathway activation has not been demonstrated yet in neuropsychiatric patients.
In our stress conditions, the treatment with paliperidone produces anti-inflammatory effects at a gastrointestinal level. These specific actions deserve further investigation, considering that patients with schizophrenia quite often present gut problems, and irritable bowel syndrome commonly occurs in mental disorders (Wei and Hemmings, 2005). Some risk factors for schizophrenia are related to the gastrointestinal system, such as gluten and milk casein hypersensitivity or Toxoplasma gondii infection (Dickerson et al., 2010; Severance et al., 2013). Indeed, there is a clear association between schizophrenia and the autoimmune condition of celiac disease (Kalaydjian et al., 2006): patients with schizophrenia present complement C1q formation of immune complexes with milk casein and wheat gluten that could be considered markers for gastrointestinal inflammation (Dickerson et al., 2010). Based on these findings, gut conditions should be taken into account in future pharmacologic and patho-physiological studies of schizophrenia, but always considering the myriad of gastrointestinal and metabolic alterations produced by some antipsychotics as possible confounding factors. As a recent example, chronic olanzapine administration altered gut microbiota in female rats in a mechanism related to olanzapine-induced weight gain and associated metabolic syndrome (Davey et al., 2013). In light of current knowledge, schizophrenia and other psychosis-related pathologies could not be considered as predominantly immune or gut disorders, but our results and others suggest that adjuvant strategies dealing with these alterations could present therapeutic benefits to explore in the future.
Other potential TLR-4 regulatory molecules modulated by paliperidone, especially in acute stress conditions, are HSP70 and HGMB1. HSP70 is a chaperone involved in neurodevelopment and neuroprotection, and its defective production, caused by stress during neuronal development, could have a role in the pathophysiology of psychotic disease (Bates et al., 1996). Furthermore, an association between HSP70 gene polymorphisms and clinical variations of schizophrenia (Kim et al., 2008; Pae et al., 2009) and in first-psychotic-episode, drug-naïve schizophrenic patients (Bozidis et al., 2014) also exists. Our results are in agreement with previous findings showing an inhibitory effect of risperidone and haloperidol on HSP70 protein upregulation in MK-801-treated rat C6 glioma cells (Roh et al., 2008). Other authors have found differential levels of antibodies against HSP60-70 in schizophrenic subjects (Kim et al., 2001). The ability of paliperidone to upregulate HSP70 mRNA levels in chronic stress conditions is a putative mechanism of neuroprotection that deserves further investigation. On the other hand, this activation could be considered a CNS homeostatic mechanism of response against chronic paliperidone treatment in stress conditions. In this vein, some authors have recently reported that the hydroxylamine HSP co-inducer BGP-15 can prevent the metabolic side effects of the atypical antipsychotics (Literáti-Nagy et al., 2012).
HGMB-1 is a chromatin-binding protein that facilitates the transcription on genes involved in neurite outgrowth and cell migration (Thomas and Travers, 2001). In addition, HGMB-1 has been recognized as a danger signal that, once activated, induces TLR-4 derived neuroinflammation after injury (i.e., alcohol abuse or ischaemic stroke; Yang et al., 2010; Crews et al., 2013). Taking into account that schizophrenia is considered by some authors as a neurodevelopmental and inflammation-related disorder; the original regulatory effects of paliperidone on increased HGMB-1 levels in stressed rats are especially remarkable in the search for novel therapeutic targets, considering that HGMB-1 expression is a risk factor for memory impairment, chronic neurodegeneration, and progression of neuroinflammation (Fang et al., 2012).
The use of stress-based animal models is relevant to study possible mechanisms involved in the patho-physiology of psychotic disease. Stress is a major contributor to the aetiology (as a risk factor) and progression of psychotic illness in its multiple clinical and subclinical manifestations (Bradley and Dinan, 2010). In fact, stress exposure is present in almost all psychiatric diseases, and its effects on the immune/endocrine system need to be elucidated and controlled (García-Bueno et al., 2008). Future translational research should corroborate the effects of paliperidone on the TLR-4 pathway and its regulatory mechanisms in animal models of schizophrenia and, finally, in schizophrenic or psychotic patients.
There has been renewed interest in immune/inflammatory changes and their associated oxidative/nitrosative consequences as key pathophysiological mechanisms in schizophrenia. In particular, there is growing evidence from animal models supporting a decisive role for pre-perinatal infections in inducing maternal immune activation and oxidative/nitrosative stress that can lead to neurodevelopmental damage and behavioral abnormalities in progeny (Venkatasubramanian and Debnath, 2013). Toll-like receptors could participate decisively in these alterations of the immune system. Some of the current pharmacological approaches try to deal with these processes (mainly treatments with antioxidants or anti-inflammatory drugs as add-ons to antipsychotics; Sommer et al., 2013). Our results support the idea that some of the neuroprotective effects afforded by paliperidone may be related to its ability to regulate stress-induced alterations on the innate immune/inflammatory system. Further investigation is needed to determinate the state of the innate immune system in the different states of psychotic disease and whether the pharmacological modulation of the molecular mechanisms regulating the TLR-4 dependent neuroinflammation (bacterial translocation of gut Gram-negative microflora, DAMPs) is useful for the management of the symptomatology of psychotic pathologies.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
None.
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation (SAF07-63138), the Instituto de Salud Carlos III (FIS 10/00123 & 13/1102), Centro de Investigación en Red de Salud Mental, CIBERSAM, Foundation Santander-UCM (GR 58/08), and Fundación Mutua Madrileña 2011. Dr Caso is a Juan de la Cierva post-doctoral fellow (MEC). Dr García-Bueno is a Ramón y Cajal post-doctoral fellow (MEC).
References
- Adkins DE, Khachane AN, McClay JL, Aberg K, Bukszár J, Sullivan PF, van den Oord EJ. (2012). SNP-based analysis of neuroactive ligand-receptor interaction pathways implicates PGE2 as a novel mediator of antipsychotic treatment response: data from the CATIE study. Schizophr Res 135:200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. (2006). Pathogen recognition and innate immunity. Cell 124:783–801. [DOI] [PubMed] [Google Scholar]
- Bates PR, Hawkins A, Mahadik SP, McGrath JJ. (1996). Heat stress lipids and schizophrenia. Prostaglandins Leukot Essent Fatty Acids 55:101–107. [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. (2011). Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psych 168:1303–1310. [DOI] [PubMed] [Google Scholar]
- Bozidis P, Hyphantis T, Mantas C, Sotiropoulou M, Antypa N, Andreoulakis E, Serretti A, Mavreas V, Antoniou K. (2014). HSP70 polymorphisms in first psychotic episode drug-naïve schizophrenic patients. Life Sci 100:133–137. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Dinan TG. (2010). A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol 24:91–118. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. (2010). Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psych 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon Y, Park JY, Modi HR, Kim HW, Lee HJ, Chang L, Rao JS, Rapoport SI. (2011). Chronic olanzapine treatment decreases arachidonic acid turnover and prostaglandin E(2) concentration in rat brain. J Neurochem 119:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corena-McLeod Mdel P, Oliveros A, Charlesworth C, Madden B, Liang YQ, Boules M, Shaw A, Williams K, Richelson E. (2008). Paliperidone as a mood stabilizer: a pre-frontal cortex synaptoneurosomal proteomics comparison with lithium and valproic acid after chronic treatment reveals similarities in protein expression. Brain Res 1233:8–19. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. (2013). High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das NP, Ratty AK (1987) Studies on the effects of the narcotic alkaloids, cocaine, morphine, and codeine on nonenzymatic lipid peroxidation in rat brain mitochondria. Biochem Med Metab Biol 37:258–264. [DOI] [PubMed]
- Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF, O’Mahony SM. (2013). Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry 3:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. (2010). Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry 68:100–104. [DOI] [PubMed] [Google Scholar]
- Fang P, Schachner M, Shen YQ. (2012). HMGB1 in development and diseases of the central nervous system. Mol Neurobiol 45:499–506. [DOI] [PubMed] [Google Scholar]
- Fischer M, Ehlers M. (2008). Toll-like receptors in autoimmunity. Ann NY Acad Sci 1143:21–34. [DOI] [PubMed] [Google Scholar]
- Gárate I, García-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, Micó JA, Leza JC. (2011). Origin and consequences of brain toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflammation 8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC. (2013). Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry 73:32–43. [DOI] [PubMed] [Google Scholar]
- García-Bueno B, Caso JR, Leza JC. (2008). Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 32:1136–1151. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. (2009). Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 164:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajebrahimi B, Bagheri M, Hassanshahi G, Nazari M, Bidaki R, Khodadadi H, Arababadi MK, Kennedy D. B, Bagheri M, Hassanshahi G, Nazari M, Bidaki R, Khodadadi H, et al. (2014). The adapter proteins of TLRs, TRIF and MYD88, are upregulated in depressed individuals. Int J Psychiatry Clin Pract 18:41–44. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Kielian T. (2011). Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci 121:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjian AE, Eaton W, Cascella N, Fasano A. (2006). The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr Scand 113:82–90. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee SJ, Toh KY, Lee CU, Lee C, Paik IH. (2001). Identification of antibodies to heat shock proteins 90kDa and 70kDa in patients with schizophrenia. Schizophr Res 52:127–135. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Mandelli L, Lim S, Lim HK, Kwon OJ, Pae CU, Serretti A, Nimgaonkar VL, Paik IH, Jun TY. (2008). Association analysis of heat shock protein 70 gene polymorphisms in schizophrenia. Eur Arch Psychiatry Clin Neurosci 258:239–244. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ. (2013). Inflammation and schizophrenia. Schizophr Bull 39:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron A, Elali A, Rivest S. (2013). Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron 78:214–232. [DOI] [PubMed] [Google Scholar]
- Leza JC, Salas E, Sawicki G, Russell JC, Radomski MW. (1998). The effects of stress on homeostasis in JCR-LA-cp rats: the role of nitric oxide. J Pharm Exp Ther 286:1397–1403. [PubMed] [Google Scholar]
- Literáti-Nagy Z1, Tory K, Literáti-Nagy B, Kolonics A, Török Z, Gombos I, Balogh G, Vígh L, Horváth I, Mandl J, Sümegi B, Hooper PL, Vígh L. (2012). The HSP co-inducer BGP-15 can prevent the metabolic side effects of the atypical antipsychotics. Cell Stress Chaperones 17:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDowell KS, García-Bueno B, Madrigal JL, Parellada M, Arango C, Micó JA, Leza JC. (2013). Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychop 16:121–135. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, García-Bueno B, Caso JR, Pérez-Nievas BG, Leza JC. (2006). Stress-induced oxidative changes in brain. CNS Neurol Disord Drug Targets 5:561–568. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Dennison U, Gaszner G, Cryan JF, Dinan TG. (2011). Enhanced peripheral toll-like receptor responses in psychosis: further evidence of a pro-inflammatory phenotype. Transl Psychiatry 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Golenbock DT, Fenton MJ. (2000). The biology of toll-like receptors. Cytokine Growth Factor Rev 11:219–232. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. (2001). Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145. [DOI] [PubMed] [Google Scholar]
- Meyer U. (2013). Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 42:20–34. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Muller N. (2011). Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 132:96–110. [DOI] [PubMed] [Google Scholar]
- Müller N, Wagner JK, Krause D, Weidinger E, Wildenauer A, Obermeier M, Dehning S, Gruber R, Schwarz MJ. (2012). Impaired monocyte activation in schizophrenia. Psychiatry Res 198:341–346. [DOI] [PubMed] [Google Scholar]
- Pae CU, Drago A, Kim JJ, Mandelli L, De Ronchi D, Serretti A. (2009). The impact of heat shock protein 70 gene variations on clinical presentation and outcome in schizophrenic inpatients. Neuropsychobiology 59:135–141. [DOI] [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS. (2010). DAMPening inflammation by modulating TLR signaling. Mediators Inflamm. Advance online publication. Retrieved 2010. 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh K, Roh S, Yang BH, Lee JS, Chai YG, Choi MR, Park YC, Kim DJ, Kim D, Choi J, Kim SH. (2008). Effects of haloperidol and risperidone on the expression of heat shock protein 70 in MK-801-treated rat C6 glioma cells. Prog Neuropsychopharmacol Biol Psychiatry 32:1793–1797. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W (1989) Rapid detection of octamer binding proteins with ‘mini-extracts’ prepared from a small number of cells. NucleicAcids Res 17:6419. [DOI] [PMC free article] [PubMed]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. (2013). Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res 148:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. (1999). MD-2, a molecule that confers lipopolysaccharide responsiveness on toll like receptor 4. J Exp Med 189:1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. (2013). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 40:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. (2009). Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry 33:303–307. [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2010). Psychiatric stress testing: novel strategy for translational psychopharmacology. Neuropsychopharmacology 35:1413–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvisaari J, Mantere O. (2013). Inflammation theories in psychotic disorders: a critical review. Infect Disord Drug Targets 13:59–70. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. (2001). Toll-like receptors: their physiological role and signal transduction system. Int Immunopharmacol 1:625–635. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Travers AA. (2001). HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci 26:167–174. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Debnath M. (2013). The TRIPS (toll-like receptors in immuno-inflammatory pathogenesis) hypothesis: a novel postulate to understand schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 44:301–311. [DOI] [PubMed] [Google Scholar]
- Wei J, Hemmings GP. (2005). Gene, gut and schizophrenia: the meeting point for the gene-environment interaction in developing schizophrenia. Med Hypotheses 64:547–52. [DOI] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. (2010). Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Front Biosci (Schol Ed) 2:1081–1091. [DOI] [PubMed] [Google Scholar]