Abstract
Background
Oxygen transport is optimized at the cellular level, since oxygen serves as the terminal electron acceptor in mitochondrial oxidative phosphorylation and several enzymatic processes require molecular oxygen as substrate. During development and aging, redundant cells and exhausted cells are eliminated, respectively, whereas others can adapt to the stressful environment and survive.
Objective
The study investigated the molecular mechanisms activated in the lung during normal aging, through the expression of hypoxia inducible factor (HIF), vascular endothelial growth factor (VEGF), p53, p66Shc, putative cysteine protease (CPP32) and kinaseB-α phosphorylation (pIkB-α).
Materials and methods
Twelve male Wistar rats divided into two age-groups, each consisting of 6 animals, 3 and 24 months old, were used. The rats were anesthetized with Nembutal (40 mg/kg, ip) and the lungs were excised from each rat and processed for TUNEL and Western blotting analyses.
Results
The expressions of p53, p66Shc and CPP32 were significantly increased in the old normoxic rat lung specimens, when compared with the young ones. In parallel, expressions of VEGF and pIkBα were increased in old rather than young rats.
Conclusions
Aging leads to increased expressions of p53, p66Shc and CPP32, suggesting that apoptosis is in progress. At the same time, the lung tries to counteract apoptosis through the production of VEGF and pIkB-α to adapt itself to a stressful situation. The aging lung creates a life-support system in order to counteract the apoptotic process.
Keywords: lung, apoptosis, aging, p53
Introduction
The lung is the essential respiratory organ in all air-breathing animals and its principal function is to transport oxygen from the atmosphere into the bloodstream and to release carbon dioxide from the bloodstream into the atmosphere. Normally, the partial pressure of oxygen (PO2) decreases from the lung to mitochondria with the changing distance of oxygen diffusion to tissues [1]. To counteract the low availability of oxygen, which is the most physiological stimulus for angiogenesis [2], cells reorganize themselves through the angiogenesis and subsequent remodeling while the oxygen transport is optimized, since oxygen serves as the terminal electron acceptor in mitochondrial oxidative phosphorylation and several enzymatic processes require molecular oxygen as substrate. Functionality of the lung is related to aging, which is a physiological condition characterized by a modification of structure and geometry of tissues which undergo a progressive lost in elasticity (i.e., in both blood vessels and pulmonary tissue) and by a general reduction of the homeostatic capacity along with a decrease in oxygen supply to tissues. During aging the diffusion distance between the lung and mitochondria elongates along with a progressive decrease in maximum consumption of oxygen (VO2max). The regulation of pulmonary function and its adaptive capacity depends on specific feedback mechanisms initiated by chemoreceptors. The molecular mechanism of cell sensitivity to oxygen is still unknown [3]. During development and aging, redundant cells and exhausted cells are eliminated, respectively, whereas other cells can adapt to the stressful environment and survive. The factors that determine the fate of each cell are probably influenced by the main mechanism regulating gene expression [4]. Upon interaction with an external stimulus, such a mechanism involves a series of chemical steps that transduce signals into the nucleus and lead to induction or repression of transcription [5,6]. This can bring about apoptosis [7,8] of exhausted cells during aging [9] or those injured by oxidative stress [10].
Since aging is a condition in which there already is a decrease of oxygen supply to tissue, the hypothesis of this work was that the lung could be able to create a life-support system, adapting to stressful situations caused by reduced oxygen supply to tissue. Among the molecules involved in such a mechanism, a role has been assigned to p53 [11,12]. The p53 tumor suppressor protein triggers cell cycle arrest and apoptosis in response to cellular stresses such as DNA damage, hypoxia and oncogene activation. In particular, this molecule seems to play a role in stabilizing hypoxia-inducible factor-1α (HIF-1α) [13,14]. HIF-1α DNA-binding protein is a heterodimer composed of two subunits: HIF-1β, which can dimerize with different basic helix-loop-helix PAS proteins, and HIF-1α, which is the O2-regulated subunit that determines the biological activity of the molecule. Thus, the aim of this work has been to investigate the molecular mechanism activated during aging by the lung, through the expression of p53 and HIF-1α, but also of vascular endothelial growth factor (VEGF) which gives a signal produced by cells to stimulate the growth of new blood vessels, part of the response aimed at restoring oxygen supply to tissue when blood circulation is inadequate, p66Shc which is a protein that translates oxidative damage into cell apoptosis and increases upon apoptotic stimulation in a p53-dependent way [15], caspase-3 (CPP32) which is a prototypical caspase that becomes activated during apoptosis [16], and kinaseB-α phosphorylation (pIkB-α), an important molecule involved in cellular survival pathways by inhibiting apoptotic processes.
Materials and methods
Anvimals
Two groups, each composed of 6 male Wistar rats, three-months (weight 200-250 g) and twenty-four (weight 350-400 g) months old, were used according to the guidelines of the Declaration of Helsinki for care and use of experimental animals. Only animals free of acute and chronic illness were employed. The rats were anesthetized with Nembutal (40 mg/kg, i.p.) and the lungs, excised from each rat, was processed for TUNEL and Western blotting analysis. In parallel we have also applied a hypoxic condition (8% O2) for the same number of animals for the same days (data and results not shown in this article).
Tunel Staining
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) is a method of choice for a rapid identification and quantification of apoptotic cells. DNA strand breaks, yielded during apoptosis, can be identified by labeling free 3'-OH terminals with modified nucleotides in an enzymatic reaction. Paraffin-embedded tissue sections (dewaxed, re-hydrated, and preincubated with proteinase K 20 μg/ml in 10 mmol/l Tris-HCl at pH 7.6) were exposed to the TUNEL mixture according to the manufacturer's instructions (Boehringer Mannheim, Germany). After two rinses in phosphate-buffered saline, the slides were mounted by using a propidium iodide-glycerol solution (10 μg/ml) and analyzed under a fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Negative controls were prepared by omitting incubation in the presence of the enzymatic mixture (data not shown).
Western Blotting
Total cell lysates or immunoprecipitated proteins was electrophoresed and transferred to nitrocellulose. Nitrocellulose, blocked in 5% nonfat milk, 10 mmol/l Tris at pH 7.5, 100 mmol/l NaCl, and 0.1% Tween20, was probed with HIF-1α, VEGF, p53, p66Shc, CPP32, and pIkB-α antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and the incubated in the presence of specific IgG horse-radish peroxidase conjugate. Immunoreactive bands were detected by means of an ECL detection system (Amersham International, Little Chalfont, UK) and analyzed by densitometry.
Image Processing
Quantitative analysis of protein expression was performed by using a Sony video camera connected to a Leica Quantimet 500 plus software (Leica Cambridge Ltd., Cambridge, UK) determining the change in integrated optical intensity (IOI) using ISO transmission density Kodak CAT 152-3406 (Eastman Kodak Company, Rochester, NY) as standard.
Statistical Evaluation
Results were expressed as means ± SD of eight different experiments. The values obtained from 12-15 electron micrographs were averaged for any given lung. The mean values were calculated from the morphometric measurements of cell parameters. Statistical comparison between the young group and the corresponding age-matched control group, was performed using an unpaired t-test, with the values of P < 0.01 considered significant (GraphPad Prism Software, Version 5.0). The same analysis was performed for the old group.
Results
TUNEL analysis revealed an increase in the number of apoptotic cells in 3 normoxic specimens of old lungs compared with the young ones. HIF-1α levels were almost the same in both young and old rats (Figure 1 and 2A). Since HIF-1α can bind to the tumor suppressor factor p53 and promote p53-dependent apoptosis by inducing pro-apoptotic proteins, the p53 detected by Western blotting was increased in old compared with young rats (Figure 1 and 2A). Oxidative stress is closely related to the aging processes [17]; therefore, expression of p66Shc, the molecule involved in controlling the life-span in mammals, was evaluated. The p66 levels were significantly increased in old compared with young rats (Figure 1 and 2B). As described for p53, the p66Shc protein could also have a role in HIF-1α stabilization [18-21].
Figure 1.
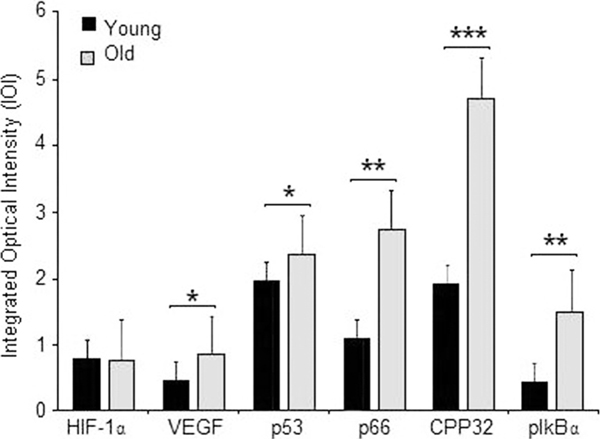
Expressions of the studied proteins involved in the aging process in young and old rat lungs. Quantitative analysis determining the change in integrated optical intensity (IOI) was normalized against the mouse α-sarcomeric actin. Results are means ± SD (n = 3) for all groups. Normoxic young vs. normoxic old - *P < 0.01 for VEGF and p53; **P < 0.001 for p66Shc and pIkB-α; and ***P < 0.0001 for CPP32.
Figure 2.
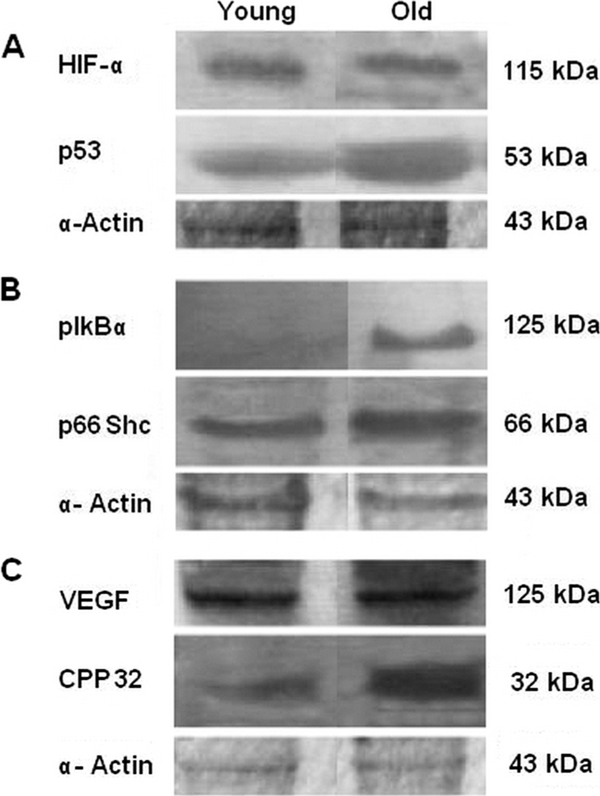
Western blots demonstrating the studied proteins involved in the aging process in young and old rat lungs.
Furthermore, in the old rats a substantially increased CPP32 expression was observed (Figure 1 and 2C). VEGF, detected by Western blotting, can be induced in cells that are not receiving enough oxygen. When a cell is deficient in oxygen, it produces HIF which stimulates the release of VEGF among other functions (including modulation of erythropoiesis). The expression of VEGF was also increased in the old rats (Figure 1 and 2C). At the same time there also was a significantly higher expression of pIkB-α in the old rats (Figure 1 and 2B).
Discussion
Apoptosis is a tightly regulated endogenous cell death program requiring executing proteases. Among the events that determine apoptosis, development, aging, and reduction in oxygen tension are included. During development, apoptosis consists of three types of cell death: phylogenetic, histogenetic, and morphogenetic cell death [8]. During adult and aged life, apoptosis serves to maintain homeostasis by counterbalancing mitosis and deleting vestigial cells which are potentially immunoreactive, malignant, or virus infected. In addition, with age, a progressive decline in cell function can be observed, related either to a genetic program or to accumulation of stochastic errors in somatic cells [22]. However, lipid peroxidation, protein modification, enzyme inactivation, DNA strand breaks, and base modifications are induced, and oxidative damage results [23,24].
Obviously, the degree to which a pathway is activated depends on the nature and duration of stress as well as on the cell type. Thus, here we tried to match the responses of a rat lung during aging in terms of expression and activation of HIF-1α, p53, p66Shc, CPP32, VEGF, and pIkB-α. Aging and reduced oxygen supply to tissue are two stressful events involved in the induction of HIF-1α expression, activation, and apoptosis [25]. HIF-1α is a key cellular modulator controlled by many different pathways [19]. In the old lungs, HIF-1α expression was not modified compared with young ones. Aging led to increased expression of p53, p66Shc and CPP32 in lungs. These data suggest that apoptosis was in progress. At the same time, the lung tries to counteract apoptosis through the production of VEGF and pIkB-α to adapt itself to a stressful situation and subsequent low availability of oxygen for tissues. The lung may thus attempt to create a life-support system to counteract the apoptotic process and free radical production in the course of the life span.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
The first author wish to thank Profs. Camillo Di Giulio, Amelia Cataldi, and Dr. Vittore Verratti for their moral support and unconditional help during the three years of a Ph.D. course.
References
- Law R, Bukwirwa H. The Physiology of Oxygen Delivery. Update in Anaesthesia. 1999;10:3. [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Kummer W, Yamamoto Y. Cellular distribution of oxygen sensor candidates-oxidases, cytochromes, K+-channels in the carotid body. Microsc Res Tech. 2002;59:234–42. doi: 10.1002/jemt.10197. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Di Giulio C, Rapino C, Rapino M, Antonucci A, Cataldi A. p53 and p66 proteins compete for hypoxiainducible factor-1α stabilization in young and old rat hearts exposed to intermittent hypoxia. Gerontology. 2006;52:17–23. doi: 10.1159/000089821. [DOI] [PubMed] [Google Scholar]
- Ostadal B, Ostadalova I, Dhalla NS. Development of cardiac sensitivity to oxygen deficiency: comparative and ontogenetic aspects. Physiol Rev. 1999;79:635–59. doi: 10.1152/physrev.1999.79.3.635. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–85. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- van den Hoff MJ, van den Eijnde SM, Viragh S, Moor-man AF. Programmed cell death in the developing heart. Cardiovasc Res. 2000;45:603–20. doi: 10.1016/S0008-6363(99)00401-0. [DOI] [PubMed] [Google Scholar]
- Centurione L, Di Giulio C, Cacchio M, Rapino M, Bosco D, Grifone G, Sabatini N, Bianchi G, Castorina S, Antonucci A, Cataldi A. Correlations between protein kinase C ζ signaling and morphological modifications during rat heart development and aging. Mech Ageing Dev. 2003;124:957–66. doi: 10.1016/S0047-6374(03)00168-4. [DOI] [PubMed] [Google Scholar]
- Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. 2000;301:125–32. doi: 10.1007/s004419900156. [DOI] [PubMed] [Google Scholar]
- Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89:279–86. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82:55–84. doi: 10.1016/s0065-230x(01)82002-9. [DOI] [PubMed] [Google Scholar]
- Pan Y, Oprysko PR, Asham AM, Koch CJ, Simon MC. p53 cannot be induced by hypoxia alone but responds to the hypoxic microenvironment. Oncogene. 2004;23:4975–83. doi: 10.1038/sj.onc.1207657. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Tomida A, Tsuruo T. Dephosphorylated hypoxia-inducible factor 1-alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene. 2001;20:5779–88. doi: 10.1038/sj.onc.1204742. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–71. doi: 10.1016/S0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P, Giorgio M. p66Shc signals to age. Aging. 2009;1:503–10. doi: 10.18632/aging.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kägi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, Senaldi G, Howard T, Lowe SW, Mak TW. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–19. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci USA. 1997;94:9585–9. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Abe J, Berk BC. Hypoxia and HIF-1α stability: another stress-sensing mechanism for Shc. Circ Res. 2002;91:4–6. doi: 10.1161/01.RES.0000026654.65882.55. [DOI] [PubMed] [Google Scholar]
- Piret JP, Mottet D, Raes M, Michiels C. Is HIF-1α a pro-or an anti-apoptotic protein? Biochem Pharmacol. 2002;64:889–92. doi: 10.1016/S0006-2952(02)01155-3. [DOI] [PubMed] [Google Scholar]
- Jung F, Haendeler J, Hoffmann J, Reissner A, Dernbach E, Zeiher AM, Dimmeler S. Hypoxic induction of the hypoxia-inducible factor is mediated via the adaptor protein Shc in endothelial cells. Circ Res. 2002;91:38–45. doi: 10.1161/01.RES.0000024412.24491.CA. [DOI] [PubMed] [Google Scholar]
- Camougrand N, Rigoulet M. Aging and oxidative stress: studies of some genes involved both in aging and in response to oxidative stress. Respir Physiol. 2001;128:393–401. doi: 10.1016/S0034-5687(01)00314-0. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Michiels C, Minet E, Mottet D, Raes M. Regulation of gene expression by oxygen: NF-κB and HIF-1, two extremes. Free Radic Biol Med. 2002;33:1231–42. doi: 10.1016/S0891-5849(02)01045-6. [DOI] [PubMed] [Google Scholar]
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1-alpha. Nature. 1998;392:405–8. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]


