Abstract
Acute adrenal failure due to bilateral adrenal haemorrhage is rare and may initially present with non-specific symptoms. It can rapidly progress into a life-threatening condition if not diagnosed promptly. Both traumatic and non-traumatic conditions have been implicated in the aetiology, with the latter been even rarer. We describe the case of a 57-year-old woman presenting with vomiting and epigastric pain and later developing fever and diarrhoea. The patient then deteriorated into shock and primary adrenal insufficiency was identified. A computed tomography scan noted bilateral adrenal haemorrhage. Further investigations showed a sigmoid colon adenocarcinoma and a myelodysplastic syndrome, with monosomy seven. Bilateral non-traumatic adrenal haemorrhage is an infrequent finding and investigating its aetiology can be challenging. In our patient, two simultaneous underlying diseases were identified. To the best of our knowledge, the combination of these two non-traumatic conditions as a cause of acute bilateral adrenal haemorrhage has not been previously reported.
INTRODUCTION
Acute adrenal insufficiency is typically due to stress or non-compliance with medication in those with known Addison's disease. Rarely, it is precipitated by massive destruction of the adrenal cortex, such as in bilateral haemorrhage. The commonest cause of adrenal haemorrhage is trauma [1]. Non-traumatic conditions include adrenal neoplasia, sepsis, for example, meningococcemia (Waterhouse–Friderichsen), and coagulopathies, such as the antiphospholipid syndrome and heparin-induced thrombocytopenia [2, 3]. In these settings, patients typically present in shock, with rapid deterioration, which can often be fatal.
Non-traumatic adrenal haemorrhage is rare; the exact incidence cannot be accurately established as it is often a post-mortem finding [2]. In our case, the patient presented with acute adrenal insufficiency, due to bilateral adrenal haemorrhage.
CASE REPORT
On New Year's Eve, a 57-year-old Caucasian woman was admitted with acute onset vomiting and epigastric pain radiating to the back. The patient had a history of hypertension, well-controlled on enalapril, and hyperlipidaemia, managed with diet alone. There was no relevant family history, recent travel or drug abuse.
On admission, physical examination was unexceptional, with no evidence of acute abdomen. Initial investigations revealed anaemia, with dysplastic granulocyte features in the peripheral blood film, while renal function and electrolytes were unremarkable (Table 1). On Day 4, the patient became febrile, hypotensive and had multiple episodes of diarrhoea. Laboratory findings showed leucocytosis, worsening anaemia, hypotonic hyponatraemia, hypokalaemia and a high C-reactive protein (CRP) (Table 1). On Day 6, the patient was in shock (distributive type), with a blood pressure of 80/60 mmHg.
Table 1:
Laboratory data Day 1 and 4
| Parameter | Day 1 | Day 4 | Reference range |
|---|---|---|---|
| White blood cell | 9.71 | 19 | 4–11 × 109/l |
| Neutrophils (%) | 6.87 (70.7) | 9.95 (79.3) | 2.5–7.5 × 109/l |
| Lymphocytes (%) | 1.20 (12.4) | 1.35 (10.8) | 1.5–3.5 × 109/l |
| Monocytes (%) | 1.57 (16.2) | 1.19 (9.5) | 0.2–0.8 × 109/l |
| Eosinophils (%) | 0.03 (0.3) | 0 (0) | 0.04–0.4 × 109/l |
| Basophils (%) | 0.04 (0.4) | 0.05 (0.4) | 0.01–0.1 × 109/l |
| Haemoglobin | 8.8 | 7.3 | females 11.5–16 g/dl |
| Mean cell volume | 86.5 | 83.1 | 76–96 l |
| Mean corpuscular haemoglobin | 28.2 | 28.1 | 27–33 pg |
| Platelets | 273 | 108 | 150–400 109/l |
| Internationalized normalized ratio | 1.12 | 0.85–1.20 | |
| Urea | 21 | 17 | 17–43 mg/dl |
| Creatinine | 0.42 | 0.58 | 0.51–0.95 mg/dl |
| Sodium | 132 | 122 | 135–145 mmol/l |
| Potassium | 3.3 | 2.7 | 3.5–5 mmol/l |
| CRP | 360 | 0–5 mg/l | |
| Glucose | 119 (random) | 74–106 mg/dl (fasting) |
Extensive workup for microbial organisms was negative, including multiple sets of blood and urine cultures. A plain chest X-ray showed no signs of consolidation or pleural fluid.
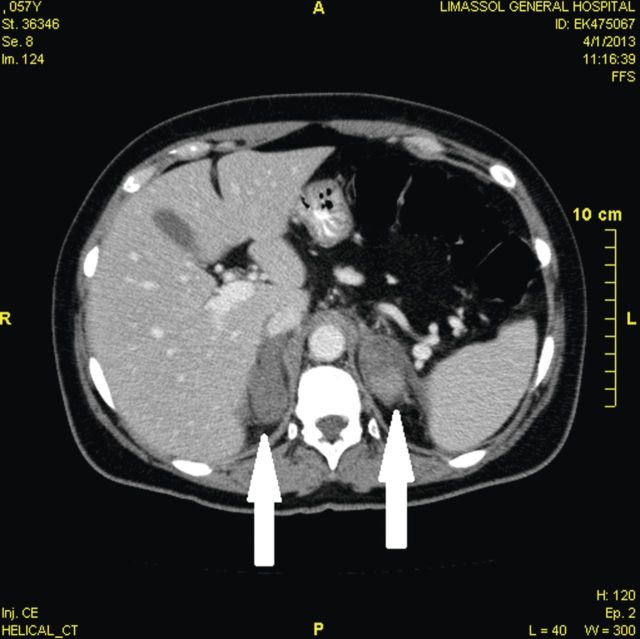
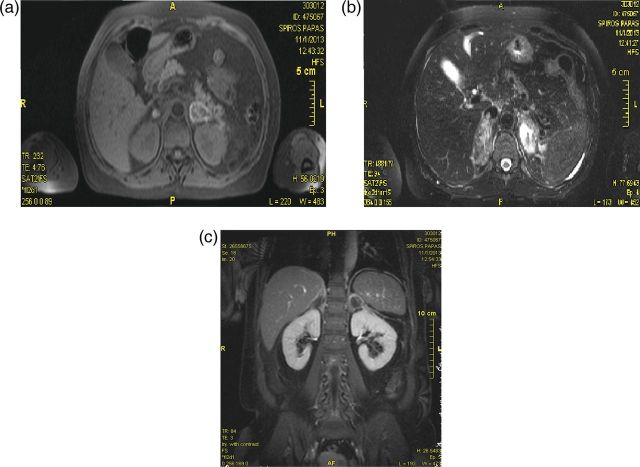
Measurement of early morning cortisol was low, while adrenocorticotropic hormone (ACTH) was high (Table 2), with normal glucose and thyroid function. Tests for clotting, antiphospholipid syndrome and autoimmune disease were negative. A computed tomography scan of the abdomen demonstrated bilateral adrenal enlargement (maximum diameter 23 mm), consistent with haemorrhage (Fig. 1). A magnetic resonance imaging scan confirmed bilateral adrenal haemorrhage (Fig. 2a–c).
Table 2:
Endocrine data (morning samples)
| Cortisol | 58.8 | 138–690 nmol/l |
| Adrenocorticotropic hormone (ACTH) | 530 | 6–57 pg/ml |
Figure 1:
Computer tomography imaging: a transverse contrast-enhanced computed tomography image showing bilateral adrenal enlargement (white arrows), without particular contrast uptake. Maximum diameter 23 mm (left adrenal gland). These findings are consistent with bilateral adrenal haemorrhage.
Figure 2:
Magnetic resonance imaging: performed on Day 7 (subacute phase) (a) Transverse view of a T1-weighted image of the adrenals demonstrating high signal in the periphery (b) Transverse plane of a T2-weighted image showing high signal intensity in the adrenals particularly on the left side (c) Coronal view of a contrast-enhanced image displaying heterogeneous hyperintensity without contrast uptake, excluding metastatic infiltration.
The diagnosis of acute adrenal insufficiency was made and the patient was commenced on hormone replacement therapy as per hospital protocol (hydrocortisone and fludrocortisone).
Further investigations to identify the cause of the adrenal haemorrhage revealed a sigmoid colon adenocarcinoma (Dukes C classification), as well as myelodysplastic syndrome (MDS). The colon adenocarcinoma was surgically removed and the patient received adjuvant chemotherapy (capecitabine and irinotecan).
The first trephine biopsy demonstrated hypercellularity (adipocytes to haematopoietic stem cells 10:90). The erythroids were reduced, megakaryocytes were normal and the myeloid series was hyperplastic, with left shift and 4% blasts.
A bone marrow karyotype analysis revealed the presence of an abnormal clone (45XX monosomy seven) in 11 of the 20 metaphases examined (55%).
A second trephine biopsy performed following chemotherapy demonstrated worsening MDS features (adipocytes to haematopoietic stem cells 40:60). The erythroid lineage was extinct, while the megakaryocytes were small and reduced in number. Hyperplastic myeloid lineage was noted with left shift and 15% blasts.
According to the World Health Organization, this case is categorized as Refractory Anaemia with Excess Blasts-2 (RAEB-2). Based on the International Prognostic Scoring System, the presence of monosomy seven classified the MDS as high risk. The patient was commenced on Azacytidine (Vidaza®). A repeat trephine biopsy showed aplastic anaemia with no blasts.
DISCUSSION
The diagnostic workup revealed a colon adenocarcinoma, which is a pro-thrombotic state, and can account for the haemorrhage, despite the absence of metastatic infiltration of the adrenals. To appreciate the mechanisms of thrombophilic conditions causing bleeding, it is essential to acknowledge the anatomy of the adrenal vasculature. The adrenal glands are supplied by three arteries, and are drained by only one adrenal vein. The transition from the rich arterial network to the poor venous circulation creates a ‘vascular dam’ [2, 4]. Therefore, thrombosis of the adrenal vein increases pressures within the gland leading to haemorrhage. Interestingly, adrenal haemorrhage is often bilateral, although the pathophysiology of this is poorly understood.
In addition, the patient was diagnosed with MDS, a condition usually occurring in the older population. The presence of MDS could also attribute to the adrenal haemorrhage as these patients develop quantitative and qualitative platelet abnormalities [5]. Furthermore, some authors claim that MDS is associated with thrombosis, though this remains controversial.
The association of solid tumours and MDS has been reported since the 1990s; the MDS typically develops after chemotherapy of the solid tumour [6]. However, in our patient the two diseases were diagnosed simultaneously. Moreover, monosomy seven was identified, which is an independent predisposing factor for the development of MDS, and carries the worst prognosis of all the chromosomal abnormalities in this condition. It is worth noting that even partial monosomy seven is associated with an unfavourable clinical course.
From our literature review, monosomy seven has been linked to haematological malignancies, aplastic anaemia, Fanconi's anaemia, neurofibromatosis type I and Shwachman syndrome [7]. It has been hypothesized, that chromosome seven may have a cluster of tumour-suppressive genes [7, 8]. Hence, structural abnormalities of this chromosome or monosomy may predispose to malignancies.
We describe the case of a patient presenting with non-specific symptoms that could potentially be managed in the community, who later deteriorated into shock requiring hospitalization and multidisciplinary care. This case illustrates that adrenal insufficiency can present with atypical symptoms and that clinicians need to be vigilant for prompt diagnosis and treatment, as well as for any predisposing factors. In our patient, two concurrent underlying conditions were identified; sigmoid colon adenocarcinoma, and MDS with monosomy seven. To the best of our knowledge, there has been no previous report of a similar case in the literature. It is not entirely clear which one or if both of these disorders predisposed to the adrenal failure. The simultaneous diagnosis of all three diseases makes this case unique and raises the question whether this cluster of conditions may ultimately be linked by the chromosomal defect.
FUNDING
There was no funding received. The authors declare that there is no conflict of interest. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
ACKNOWLEDGEMENTS
We would like to thank Doctor Nikolaos Neokleous of the Haematology Department, Limassol General Hospital, for his assistance. We would also like to thank our patient for providing her written consent to present this case report and related images.
REFERENCES
- 1.Sacerdote MG, Johnson PT, Fishman EK. CT of the adrenal gland: the many faces of adrenal hemorrhage. Emerg Radiol. 2012;19:53–60. doi: 10.1007/s10140-011-0989-9. [DOI] [PubMed] [Google Scholar]
- 2.Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76:161–168. doi: 10.1016/S0025-6196(11)63123-6. [DOI] [PubMed] [Google Scholar]
- 3.Arlt W, Allolio B. Adrenal insufficiency. The Lancet. 2003;361:1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 4.Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: a report of a case and review of the literature. Eur J Endocrinol. 2005;153:507–514. doi: 10.1530/eje.1.02002. [DOI] [PubMed] [Google Scholar]
- 5.Zeidman A, Sokolover N, Fradin Z, Cohen A, Redlich O, Mittelman M. Platelet function and its clinical significance in the myelodysplastic syndromes. Hematol J. 2004;5:234–238. doi: 10.1038/sj.thj.6200364. [DOI] [PubMed] [Google Scholar]
- 6.Sans-Sabrafen J, Buxo-Costa J, Woessner S, Florensa L, Besses C, Malats N, et al. Myelodysplastic syndromes and malignant solid tumors: analysis of 21 cases. Am J Hematol. 1992;41:1–4. doi: 10.1002/ajh.2830410102. [DOI] [PubMed] [Google Scholar]
- 7.Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol. 2008;87:515–526. doi: 10.1007/s00277-008-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Wang XQ, Xu XP, Lin GW. Cytogenetic evolution correlates with poor prognosis in myelodysplastic syndrome. Cancer Genet Cytogenet. 2010;196:159–166. doi: 10.1016/j.cancergencyto.2009.09.015. [DOI] [PubMed] [Google Scholar]