Abstract
Leptin or obesity receptor (Ob-R) is a member of class I cytokine receptor family. Ob-R, expressed in six isoforms, is the product of alternative RNA splicing of db gene. According to its structural differences, the receptor's isoforms are divided into three classes: long, short, and secretory isoforms. A long, fully active isoform of Ob-Rb is expressed mainly in the hypothalamus, where it takes part in energy homeostasis and in the regulation of secretory organs' activity. Ob-Rb is also present on all types of immune cells, involved in innate and adaptive immunity. Short leptin isoforms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Re) that contain box 1 motif are able to bind JAK kinases (Janus kinases) as well as to activate some other signal transduction cascades. A soluble isoform (Ob-Re) can regulate serum leptin concentration and serve as a carrier protein delivering the hormone to its membrane receptors and is able to transduce the signal into the cell. JAK/STAT pathway plays the major role in leptin signal transduction through membrane receptors. Among all Ob-R isoforms, only full-length isoform (Ob-Rb) is able to fully transduce activation signal into the cell.
Keywords: leptin, leptin receptor, leptin receptor isoforms
Introduction
A search for the biological factor responsible for energy balance and signal transduction to the hypothalamus was initiated by studies on animal models. In 1994, a molecular defect in the obese gene (Ob), a gene responsible for the obesity phenotype in ob/ob mice was identified using a positive cloning method. Ob gene has found on chromosome 6 in mice and on chromosome 7q31.3 in humans and has been shown to encode 4.5 kb-long mRNA. The protein encoded by the ob gene has been isolated and named leptin - from the Greek "leptos" meaning thin [1]. It has been demonstrated that leptin biological activity strongly depends on its proper interactions with Ob-R receptors [2].
The Structure of Leptin Receptors
Ob-R receptor, encoded by db gene has been identified for the first time in a murine vascular plexus using a cloning technique. It is a member of class I cytokine receptors family that, apart from, Ob-R consist of gp130 subunit of IL-2R, IL-3, IL-4, IL-6, IL-7, LIF (leukemia inhibitory factor), G-CSF (granulocytecolony stimulating factor), growth hormone, prolactin, and erythropoietin receptors [3,4]. Ob-R expressed in six isoforms, is the product of alternative RNA splicing of db gene (diabetes gen) [5,6]. According to its structural differences, the receptor's isoforms were divided into three classes: long, short and secretory.
All Ob-R isoforms have a similar, extracellular ligand- binding domain located in N-terminus of the protein. The domain is constituted by 816 amino acids and contains four cysteine residues, WSXWS motif (Trp-Ser-X-Trp-Ser) and a different number of fibronectin III domains. Five isoforms, including the long isoform Ob-Rb and short ones - Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Rf, have a transmembrane domain consisting of 34 amino acids. The intracellular domain of the long isoform consists of 303 amino acids at carboxyl terminus, while intracellular domains of short isoforms are shorter and consists of 32-40 amino acids. Apart from identical extracellular and transmembrane domains, Ob-R isoforms (short and long) have the same sequence of the first intracellular 29 amino acids. This sequence contains a constant box 1 motif and JAK tyrosine kinase. The length of the further intracellular amino acid sequences depends on the alternative mRNA splicing. Ob-Rb isoform contain additional box 2 and SOCS (suppressor of cytokine signaling) motifs. SOCS expression is induced by cytokines [7]. A sixth, secreted Ob-Re isoform, does not contain intracellular and cytoplasmic domains and is secreted to the bloodstream as a soluble receptor (Figure 1) [6,8-10].
Figure 1.
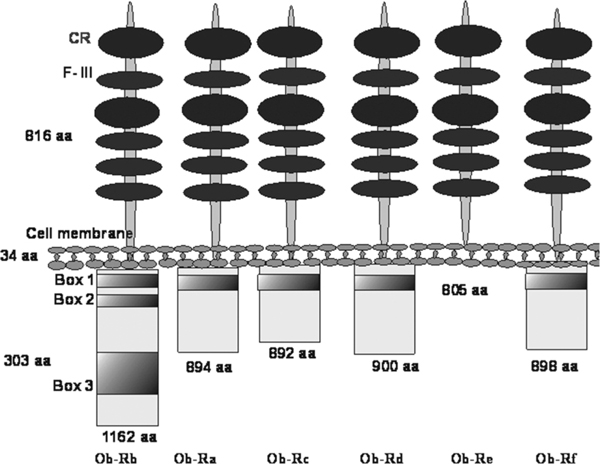
Leptin receptor (Ob-R) isoforms. CR - cytokine receptor domain, F-III - type III fibronectin domains, Box 1, 2, 3 - constant, intracellular motifs [according to 7, 8].
A long, fully active isoform of Ob-Rb is expressed mainly in the hypothalamus, where it takes part in energy homeostasis and in the regulation of secretory organs' activity. Ob-Rb is also present in all types of immune cells, involved in innate and adaptive immunity (Figure 2) [7,9,11-14].
Figure 2.
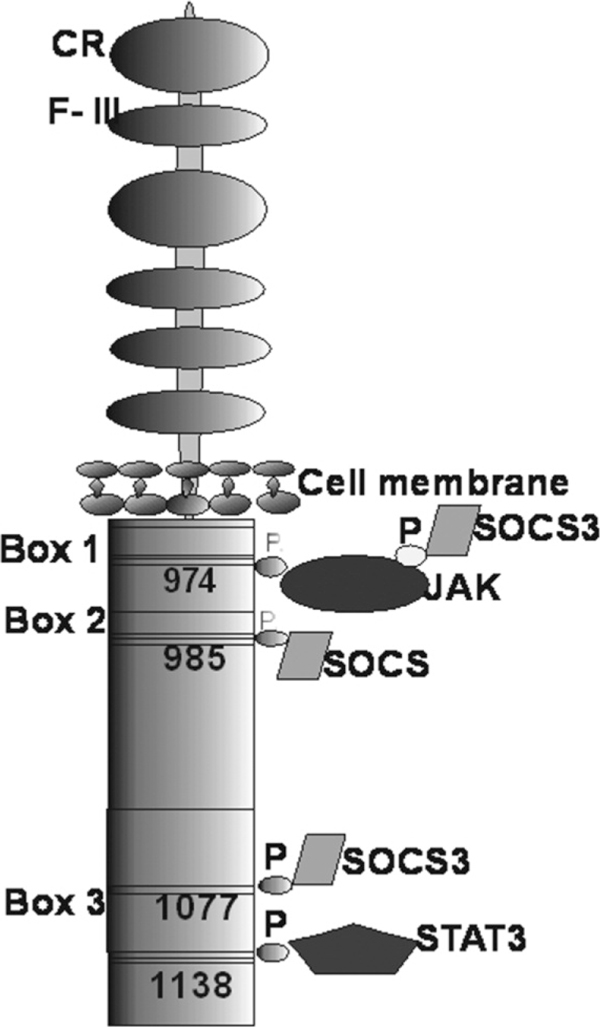
Ob-Rb, long isoform of leptin receptor: JAK-tyrosine kinases, STAT-signal transducer and activator of transcription, SOCS proteins-cytokine signal transduction inhibitors, P - phosphate residues, CR - cytokine receptor domain, F-III-type III fibronectin domains [according to 7, 17].
Lack of a full-length Ob-Rb receptor is responsible for the development of the early obesity phenotype in db/db mice and in obese rats. In db/db mice, a short Ob-Ra isoform with limited activity is synthesized. The condition leads to diabetes, morbid obesity, and pubertal development disorders. The mice strain is also characterized by cold intolerance and elevated concentration of glucosteroid hormones. Moreover, db/db mice phenotype includes a significantly elevated leptin concentration, with no ability to respond to leptin signal [15].
Short leptin isoforms that contain box 1 motif are able to bind JAK kinases (Janus kinases) and to activate some signal transduction cascades. However, the effect of short isoform activation differs from that of long isoform activation [16,17]. Their main function is presumably connected with leptin internalization and degradation [18]. A short isoform, Ob-Ra, is the most common Ob-R isoform that can be found in many various cells and tissues, including kidney, lungs, liver, spleen, and macrophages [6].
A soluble isoform of the receptor, Ob-Re, is probably a result of alternative transcript splicing of db gene or a consequence of transmembrane Ob-R receptor destruction. Circulating Ob-Re is able to bind serum leptin and to inhibit signal transduction pathways. On the other hand, the receptor can regulate serum leptin concentration and serve as a carrier protein delivering the hormone to its membrane receptors able to transduct the signal into the cell [19].
In normal conditions, only 5-25% of all Ob-R isoforms are present on the cell surface, whereas the majority of receptors are localized within the cell. After ligand binding, the receptors are internalized into early endosomes via clathrin-coated vesicles. Next, the receptor is degraded or effectively recycled to the cell membrane. The process concerns mainly Ob-Ra and Ob-Rb isoforms. A decrease in Ob-Rb expression is much higher than changes in Ob-Ra expression, and short isoform Ob-Ra is much faster recycled to the cell membrane. Relatively weak signal transduction through long Ob-Rb isoform observed in obese, hyperleptinemic patients is related to delayed receptor expression on the cell surface, which may explain leptin resistance in these patients [8,20,21].
Signal Transduction Pathways
The major role in leptin signal transduction through membrane receptors is mediated through JAK/STAT (signal transduction and activation of transcription) pathway. Among all Ob-R isoforms, only the fulllength isoform Ob-Rb is able to fully transduce an activation signal into the cell. Ob-Rb is considered a fully active receptor, because it contains 3 intracellular motifs necessary to activate the JAK/STAT pathway. The described motifs are the following: box 1 and box 2 that bind JAK tyrosine kinase, and box 3 motif that binds STAT transcription factor [6,15]. Additionally, in intracellular domain, Ob-Rb contains four tyrosine residues (Tyr974, Tyr985, Tyr1077 and Tyr1138) that activate intracellular signal transduction pathways (Figure 2) [22]. After ligand binding to the Ob-Rb receptor, a cytoplasmic tyrosine kinase JAK2 binds to box-1 and box-2 motifs [23]. The activated kinase is autophosphorylated and activates specific tyrosine residues (Tyr 985 and Tyr1138) in the receptor molecule. Next, after Tyr1138 phosphorylation, STAT proteins bind to box-3 motif. STAT proteins (STAT1, STAT3, STAT5, STAT6), including the key signal transduction molecule STAT3, are phosphorylated on tyrosine residues by JAK2 kinases. The process leads to the dissociation of proteins from the receptor, homo- or heterodimerization and translocation of the proteins to the nucleus. In the nucleus, the described proteins serve as transcription factors activating the expression of a suppressor of cytokine signaling 3 (SOCS-3) or protein tyrosine phosphatase 1B (Figure 3) [17,24-26].
Figure 3.
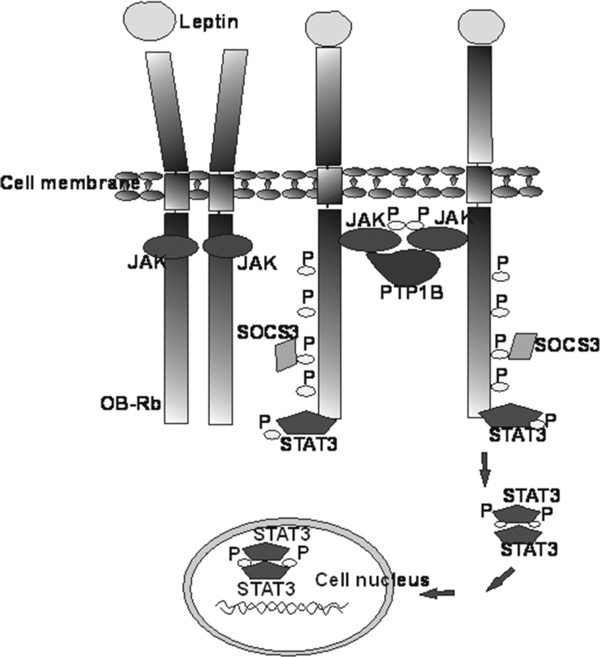
JAK/STAT pathway activation by Ob-Rb leptin receptor mechanism. After leptin binding, the Ob-Rb receptor changes its conformation that leads to JAK translocation. JAK proteins become activated and start to exert their kinase activity and phosphorylate tyrosine residues of other JAK proteins and of Ob-Rb receptor. Tyr1138 phosphorylation allows STAT3 protein binding and next, STAT proteins become the substrate for JAK proteins related to Ob-R receptor. STAT3 protein phosphorylation leads to dissociation of the protein from the receptor, dimerization and translocation to the nucleus. After the translocation STAT proteins stimulate specific genes expression [according to [17,24]].
STAT proteins are activated by leptin and depending on the target tissue; the type of activated protein may differ between various types of target cells [24]. JAK/STAT pathway activation also requires SOCS proteins, mainly SOCS3. SOCS proteins serve as a negative regulator of cytokines' activity. Leptin induces SOCS3 expression by binding to the SOCS3 SH2 domain with Tyr985 or through the inhibition of SOCS3 dependent JAK2 phosphorylation activity [21,27]. In addition, PTP1B (protein tyrosine phosphatase 1B) is another negative leptin signal transduction modulator that regulates signal transduction through dephosphorylation of JAK2. PTPB1 overexpression decreases JAK2 phosphorylation and inhibits leptin-induced SOCS3 and c-fos (proto-oncogene) transcription [28].
Apart from the above described, leptin signaling also is mediated by other pathways. MAPK kinases (mitogen-activated protein kinase), IRS1 (insulin receptor substrate-1), and phosphatidylinositol kinase (PI3K) are important pathways responsible for Ob-Rb receptor activation by leptin in various cells, e.g., T-cells [28,29]. The MAPK kinase signal transduction pathway is mediated by ERK (estrogen receptor) and p38 kinases. It has been demonstrated in osteoblasts that leptin, after activation of Ob-Rb, induces apoptosis through the activation of MAPK, ERK1/2-dependent activation of cytoplasmic phospholipase A, and subsequent cytochrome c release and activation of caspases 3 and 9 [30]. Osmotic stress, heat shock, and cytokines are able to activate another MAPK family protein, namely p38. In mononuclear cells, after binding to Ob-Rb, leptin increases the level of p38MAPK phosphorylation. In LPS (lipopolysaccharide)-stimulated Kupffer cells, leptin significantly increases TNFα secretion through the activation of p38 and JNK/MAPK (c-Jun N-terminal kinase), whereas in smooth muscle cells it is able to induce hypertrophy through the activation of p38 MAPK [31].
The majority of insulin-mediated biological effects are caused by PI3K activation. PI3K is believed to constitute an important common element for signal transduction pathways activated by insulin and leptin and its receptor. in the central nervous system, adipose tissue, liver, and pancreas, leptin induces a similar pathway to that activated by insulin, including PI3Kdependent activation of PDE3B (phosphodiesterase 3B) and cAMP (cyclic adenosine monophosphate) reduction [32]. It seems that PI3K/PDE3B/cAMP pathway cooperates with the JAK2/STAT cascade and is an important element of leptin signal transduction pathways in the hypothalamus [28].
Leptin Receptor Gene Polymorphism
LEPR gene (leptin receptor gene) mutations are extremely rare in human and animals. A single nucleotide substitution (G to A) in exon 6 leads to a deficiency in intracellular and transmembrane domain of the receptor [33]. The mutation caused by premature stop codon insertion at the 3' terminus of LEPRb mRNA was found in db/db mice [15], whereas in Zucker rats, the mutation was caused by amino acid substitution (Gln to Pro) at position 269 of the extracellular domain of the receptor. The described mutation resulted in a severe reduction of Ob-R expression on the cell surface and limitation in the leptin-receptor binding. Obese Koletsky rats had a point mutation at 763 position resulting in premature stop codon introduction in the intracellular domain of the receptor that led to a total deficiency in all Ob-R isoforms on the cell surface. Both Zucker and Koletsky rats are characterized by morbid obesity, hyperphagia, hyperlipidemia, and numerous hormonal disorders [34,35]. In humans, a rare mutation caused by a single substitution of G to A in exon 16 was identified. It leads to an abnormal expression of transmembrane and intracellular domains of receptors. As in case of laboratory animals, humans with LEPR gene defects demonstrate obesity, hyperphagia, pubertal development disorders, and endocrine system abnormalities [33].
LEPR gene polymorphisms seem to be much better described. It has been demonstrated that the polymorphisms may lead to the impairment of signal transduction from the receptor into the cell through premature Ob-Ra, instead of Ob-Rb isoform production, or through a decrease in Ob-R expression on the cell surface and limitation in leptin-receptor interactions. LEPR gene defects influence the development of hyperphagia, obesity, pubertal development disorders, neuroendocrine system regulation impairment, and diabetes caused by β-cell apoptosis in the pancreas [8,15,33,36]. The most commonly seen polymorphism is Gln223Arg, encoding the extracellular domain of the receptor responsible for leptin binding. Glutamine to arginine change may be responsible for an impaired signal transducing capacity of the leptin receptor [36]. A connection between Gln223Arg polymorphism and breast cancer development and progression has been suggested [37]. Genetic interactions between leptin and Gln223Arg polymorphisms of LEPR gene can increase the risk of the development of non-Hodgkin lymphoma in obese patients [38]. It also is suggested that the discussed polymorphisms are related to hemodynamic and metabolic disorders found in obese patients [39]. Overweight and obesity commonly observed after the management of childhood acute lymphoblastic leukemia also may have to do with the Gln223Arg polymorphism of LEPR gene [40].
Conflicts of interest
The authors declare that they have no competing interests.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Brunner I, Nick HP, Cumin F, Chiesi M, Baum H-P, Whitebread S, Stricker-Krongrad A, Levens N. Leptin is a physiologically important regulator of food intake. Int J Obesity. 1997;21:1152–60. doi: 10.1038/sj.ijo.0800529. [DOI] [PubMed] [Google Scholar]
- Bazan JF. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor β-chain. Biochem Biophys Res Commun. 1989;164:788–95. doi: 10.1016/0006-291X(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin induced signal transduction pathways. Cell Biol Int. 2004;28:159–69. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Wang X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, Ob-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Sweeney G. Leptin signaling. Cell Signal. 2002;14:655–63. doi: 10.1016/S0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–41. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Bjorbak C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–95. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. Novel B219/Ob receptor isoforms: Possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–9. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- Barr VA, Lane K, Taylor S. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem. 1999;274:21416–24. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- Caldefie-Chezer F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414–18. [PubMed] [Google Scholar]
- Li Q, Li JY, Li Y, Zhang JC. Expression of long isoform leptin receptor and shortest membrane bound variant in peripheral blood mononuclear cells from the obese and normal individuals. Zhonghua Yi Xue Za Zhi. 2007;87:3288–99. [PubMed] [Google Scholar]
- Martin-Romero C, Santoz-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (Ob-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71:187–95. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- Frühbeck G. Intracellular signaling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uotani S, Bjorbaek C, Tornoe J, Flier JS. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;96:279–86. doi: 10.2337/diabetes.48.2.279. [DOI] [PubMed] [Google Scholar]
- Chan JL, Blüher S, Yannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–12. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- Münzberg H, Björnholm M, Bates SH, Myers MG Jr. Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–65. doi: 10.1007/s00018-004-4432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzberg H, Myers MG Jr. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;5:566–70. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost G-H, Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–19. doi: 10.1111/j.1432-1033.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–55. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- Bendinelli P, Maroni P, Pecori-Giraldi F, Piccoletti R. Leptin activates Stat3, Stat1 and AP-1 in mouse adipose tissue. Mol Cell Endocrinol. 2000;168:11–20. doi: 10.1016/S0303-7207(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Cheng A, Uetani N, Simoncic PD, Chaubery VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/S1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Muraoka O, Xu B, Tsurumaki T, Akira S, Yamaguchi T, Higuchi H. Leptin-induced transactivation of NPY gene promoter mediated by JAK1, JAK2 and STAT3 in the neural cell lines. Neurochem Int. 2003;42:591–601. doi: 10.1016/S0197-0186(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Dunn S, Björnholm M, Bates SH, Chen Z, Seifert M, Myers MG Jr. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin Eeceptor and suppressor of cytokine signaling 3. Mol endocrinol. 2005;19:925–38. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–53. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Martin-Romero C, Sanchez-Margarel V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam 68. Cell immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- Kim GS, Hong JS, Kim SW, Koh JM, An CS, Choi JY, Cheng SL. Leptin induces apoptosis via ERK/cPLA2/cytochrome c pathway in human bone morrow stromal cells. J Biol Chem. 2003;278:21920–29. doi: 10.1074/jbc.M204598200. [DOI] [PubMed] [Google Scholar]
- Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–63. doi: 10.1016/S0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–8. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte J-M, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- White DW, Wang D, Chua SC Jr, Morgenstern JP, Leibel RI, Bauman H, TARTAGLIA LA. Constitutive and impaired signaling of leptin receptors containing the Gln→Pro extracellular domain fatty mutation. Proc Natl Acad Sci USA. 1997;94:10657–62. doi: 10.1073/pnas.94.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Peng XS, Chua SC Jr, Okada N, Liu SM, Nicolson M, Leibel RL. Phenotype of the obese Koletsky (f) rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor (Lepr): evidence for deficient plasma-to-CSF transport of leptin in both the Zucker and Koletsky obese rat. Diabetes. 1997;46:513–8. doi: 10.2337/diabetes.46.3.513. [DOI] [PubMed] [Google Scholar]
- Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated wit obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–9. doi: 10.1210/jc.86.9.4434. [DOI] [PubMed] [Google Scholar]
- Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Helal AN, Chouchane L. Leptin and leptin receptor polymorphism are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6:2407–17. doi: 10.1186/1471-2407-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibola CF, Holly EA, Forrest MS, Hubbard A, Bracci PM, Skibola DR, Hegedus C, Smith MT. Body mass index, leptin and leptin receptor polymorphism, and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:779–86. [PubMed] [Google Scholar]
- Guizar-Mendoza JM, Amador-Licona N, Flores-Martinez SE, Lopez-Gardona MG, Ahuatzin-Tremary R, Sanchez-Corona J. Association analysis of the Gln223Arg polymorphism in the human leptin receptor gene, and traits related to obesity in Mexican adolescents. J Hum Hypertens. 2005;19:341–6. doi: 10.1038/sj.jhh.1001824. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kevin C, Oeffinger KC, Davies SM, Mertens AC, Langer EK, Kiffmeyer WR, Sklar CA, Stovall M, Yasui Y, Robison LL. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: A Report from the childhood cancer survivor study. J Clin Oncol. 2004;22:3558–62. doi: 10.1200/JCO.2004.11.152. [DOI] [PubMed] [Google Scholar]


