Abstract
Gluten is one of the most abundant and widely distributed components of food in many areas. It can be included in wheat, barley, rye, and grains such as oats, barley, spelt, kamut, and triticale. Gluten-containing grains are widely consumed; in particular, wheat is one of the world’s primary sources of food, providing up to 50% of the caloric intake in both industrialized and developing countries. Until two decades ago, celiac disease (CD) and other gluten-related disorders were believed to be exceedingly rare outside of Europe and were relatively ignored by health professionals and the global media. In recent years, however, the discovery of important diagnostic and pathogenic milestones led CD from obscurity to global prominence. In addition, interestingly, people feeding themselves with gluten-free products greatly outnumber patients affected by CD, fuelling a global consumption of gluten-free foods with approximately $2.5 billion in United States sales each year. The acknowledgment of other medical conditions related to gluten that has arisen as health problems, providing a wide spectrum of gluten-related disorders. In February 2011, a new nomenclature for gluten-related disorders was created at a consensus conference in London. In this review, we analyse innovations in the field of research that emerged after the creation of the new classification, with particular attention to the new European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines for CD and the most recent research about non-celiac gluten sensitivity.
Keywords: Celiac disease, Wheat allergy, Gluten sensitivity, Non-celiac gluten sensitivity, Gluten-free diet, Gluten, Anti-gliadin antibodies
Core tip: In recent years, there has been a widespread diffusion of gluten-associated symptoms. Current reactions to gluten include, but are not restricted to, celiac disease. This review analyses this interesting epidemiological worldwide phenomenon by discussing the spectrum of gluten-related disorders and focusing on their clinical features and diagnostic criteria. In particular, this paper will cover the most important news from European Society for Paediatric Gastroenterology, Hepatology and Nutrition guidelines for celiac disease and the state of the art of non-celiac gluten sensitivity.
INTRODUCTION
In recent years, the prevalence of a wide spectrum of gluten-related disorders (GRDs) has increased. This can be attributed to changes in global dietary habits; many countries are experiencing a progressive westernization of diet as well as worldwide diffusion of the Mediterranean diet, which is based on a large number of foods that incorporate gluten (including wheat)[1]. In particular, consumption of wheat is progressively replacing consumption of rice in many countries in North Africa, the Middle East, and Asia[2]. Corn is still the most consumed cereal in the United States; however, a trend toward an increase in consumption of wheat is clearly evident, with an average of $132.50 spent on wheat products per person[3].
In addition, current wheat varieties have an higher content in gluten compared to the past due to changes directed by both technology and nutritional reasons.
Types of wheat cultivated for thousands of years, such as Triticum monococcum and Triticum dicoccum, contained smaller quantities of the highly toxic peptide 33-mer gliadin[4].
The toxic effects of gluten are mediated in humans primarily by immunologic reactions; however, the absence of proper adaptation of gastrointestinal reactions can also play a role[2]. The mechanization of agriculture and the increasing use of industrial pesticides have encouraged the development of new types of wheat with a higher content of toxic peptides of gluten, constituting a further element in the increasing prevalence of GRDs[1]. Furthermore, bread and bakery products currently contain a higher portion of gluten than in the past because of the reduced time of dough fermentation[5].
Diagnostic tools for GRDs have progressively improved over time[6,7]. In the 1980s, classification of GRDs was very simple, because celiac disease (CD) and dermatitis herpetiformis (DH) were the only known diseases with a well-documented role of gluten in their pathogenesis. More recently, gluten and other proteins have been recognized as a possible cause of wheat allergy (WA). In addition, more patients with intestinal and extraintestinal symptoms related to ingestion of gluten but without evidence of CD or WA have been identified as potentially affected by non-celiac gluten sensitivity (NCGS), a disorder aknowledged by the scientific community only in recent times[8]. Increasing complexity in the nomenclature and clinical presentation of GRDs has led to the development of a consensus document by a panel of 15 experts on new classification of five heterogeneous GRDs: CD, NCGS, WA, DH, and gluten ataxia (GA)[9] (Figure 1). Each GRD exhibits a unique pathophysiological response to ingestion of gluten, although there can show considerable overlap in the clinical presentation.
Figure 1.
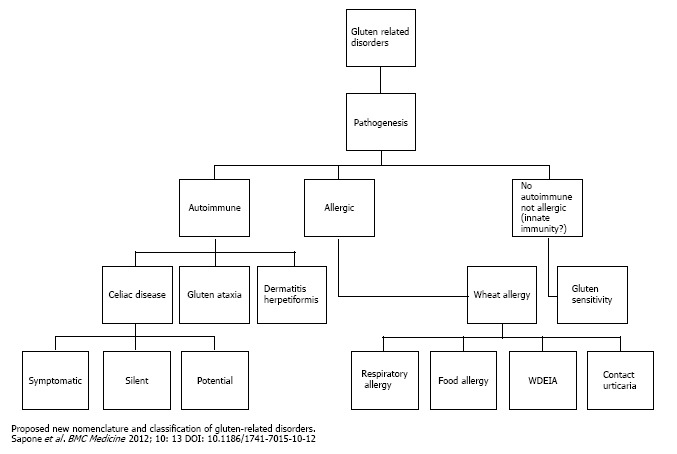
New nomenclature and proposed classification of gluten related disorders according to the the II Consensus Conference on gluten related disorders held in London in February 2011.
The increased prevalence and complexity of GRDs has inevitably sparked growing interest in gluten-free diets (GFDs) in both scientific and non-scientific communities. Although a GFD represents the recommended treatment of GRDs and is believed by many people to be an overall healthier regimen, this is not always the casie. People following a GFD may not meet their nutritional requirements because gluten-free foods may not have the same dietary supplementation as gluten-containing foods[10]. However, a GFD can contribute do meet daily nutritional requirements provided that patients will use a healthful balance of protein, vegetables, fruit, and ancient grains[8]. Even if gluten may not be essential for an healthy diet, an unrequired GFD can be expensive. Furthermore, recognizing gluten-free products can be difficult and time consuming[11,12].
In 2010, the gluten-free food market was worth an estimated $2.6 billion in the United States, showing a steady increase since 2008. This upward trend is expected to continue in the next years[9,13]. The growing gluten-free market may now be between 15% and 20% of the United States population[14]. The increased awareness and knowledge of CD explains only a small fraction of the development of the GFD market, which is probably sustained also by people with different GRDs, such as NCGS and WA. The remaining section of the market includes people who embark on a GFD as occasional users who do not have a medical necessity but believe that popular cereals are unhealthy because of their composition[15]. Consequently, accurate diagnostic criteria for a GFD are needed to distinguish people with a medical condition from those who simply prefer to avoid gluten, leading to different nutrition and follow-up strategies.
CD
CD is an immune-mediated reaction to gluten; it is characterized by an inappropriate T cell- mediated immune response that causes inflammatory injury to the small intestine in genetically predisposed subjects carrying the HLA-DQ2 and/or -DQ8 haplotypes[9]. CD represents a unique model of autoimmune disease because relevant information is known, including the genetic basis (HLA haplotypes) and the triggering environmental factor (gluten)[9]. The disease epidemiology is also well known, with the worldwide prevalence estimated to be 0.6% to 1% of the general population[16-19]. For each person diagnosed with CD, there are at least another five or six people who have not yet been identified, most of whom are adults without gastrointestinal symptoms (representing the so-called celiac iceberg)[20].
CD is a diagnostic challenge for the clinician because it may develop at any age, even in elderly people, and because of its polymorphic clinical presentation. The clinical spectrum of CD includes symptomatic cases with either intestinal or extraintestinal features as well as silent forms revealed only by serological screening. Intestinal manifestations of CD include diarrhoea, weight loss, abdominal distention, and constipation. Extraintestinal symptoms reflect the systemic nature of the disease and include chronic fatigue, anaemia, reduced bone mineral density, aphthous stomatitis, high aminotransferase levels, joint/muscle pain, and spontaneous abortions, epilepsy, peripheral neuropathy[21].
In 2012, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Working Group decided to revise its classification of CD. In particular, the distinction between classic and atypical CD based on symptoms was removed, because atypical signs and symptoms (e.g., anaemia, reduced bone density, neuropathy) can be substantially more common than classic symptoms (e.g., abdominal pain, chronic diarrhoea)[22].
In such a complex clinical picture, case-finding strategies have to be carefully planned. The most relevant scientific organizations in this field, including ESPGHAN, North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition, and American College of Gastroenterology, have identified groups of patients at particularly high risk that should be investigated for CD. Their recommendations are reported in Table 1[22-24]. Diagnostic algorithms for CD consist of initial screening serological tests followed by a confirmatory small intestinal biopsy.
Table 1.
Populations at risk for celiac disease, in which investigations for celiac disease are indicated, according to the most recent guidelines proposed by European Society for Pediatric Gastroenterology, Hepatology, and Nutrition, North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and American College of Gastroenterology
| ESPGHAN | NASPGHAN | ACG |
| Test children/adolescent with: | Test children/adolescent with: | Test patients with: |
| Chronic or intermittent diarrhoea, growth failure, weight loss | diarrhea and failure to thrive | chronic diarrhea with weight loss |
| Chronic abdominal pain, cramping or distension, nausea or vomiting, chronic constipation | abdominal pain, anorexia, constipation, vomiting | post-prandial abdominal pain, bloating |
| Short stature, delayed puberty | short stature, delayed puberty | other symptoms/signs suspect for CD |
| dermatitis herpetiformis–type rash | dermatitis herpetiformis | |
| unexplained abnormal liver biochemistry | unexplained abnormal liver biochemistry | |
| Iron-deficiency anaemia | Iron-deficient anaemia resistant to oral iron | other laboratory signs suspect for CD |
| repetitive fractures/osteopenia/osteoporosis | osteoporosis | |
| chronic fatigue, ameorrhoea, recurrent aphthous stomatitis | Dental enamel hypoplasia of permanent teeth | |
| First- degree family members | First-degree family members | First- degree family members |
| Type 1-diabetes mellitus | Type 1-diabetes mellitus | Type 1-diabetes mellitus |
| Other associated conditions1 | Other associated conditions1 |
Down syndrome, autoimmune thyroid disease, Turner syndrome, Williams syndrome, IgA deficiency. ESPGHAN guidelines also consider autoimmune liver diseases. CD: Celiac disease; ESPGHAN: European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; NASPGHAN: North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; ACG: American College of Gastroenterology.
Measurement of serum immunoglobulin (Ig) A anti-tissue transglutaminase antibodies (tTG) has the best diagnostic sensitivity for CD[21,25,26]. Measurement of IgA anti-endomysial antibodies (EMA) is nearly 100% specific for CD, but it is also expensive and operator dependent and therefore is better used as a second-line test[22]. Antibodies to deamidated gliadin peptides (DGP) of the IgG class have been shown to be particularly useful in patients with IgA deficiency and children younger than three years of age[26-28]. Even with the most recent advancements in CD serology, it has been reported that up to 2% of patients with CD do not have any circulating markers of gluten sensitivity, defining a condition of seronegative CD[6].
Since the advent of the Crosby-Kugler capsule, which enabled tissue sampling and histological examination of intestinal tissue, small intestinal biopsy has been required to confirm the diagnosis in patients with suspected CD and is considered the gold standard for CD[20]. Interestingly, the most recent guidelines from ESPGHAN propose that it may be possible to avoid intestinal biopsy in children who meet the following criteria: (1) symptoms consistent with CD; (2) serum IgA anti-tTG levels > 10 times the upper limit of normal (confirmed with positive anti-EMA in a different blood sample); and (3) positive HLA-DQ2[22]. It remains to be determined whether this new clinical/laboratory standard functions as well as the gold standard of serology plus biopsy, whether different cutoff values for serology kits should be used, and whether including HLA typing is necessary[29]. Nevertheless, with this notable exception, biopsy is still a required and essential element for the diagnosis of CD according to all of the guidelines[22-24].
Histology alone, however, is not specific for the diagnosis of CD, particularly if villous atrophy is absent. For instance, an increase in the number of lymphocytes in the intestinal epithelium can be found in a number of different conditions, including small bowel bacterial overgrowth, drug-associated enteropathy, infectious enteritis (e.g., giardiasis and Whipple disease), Crohn’s disease, autoimmune enteropathy, and enteropathy associated with acquired immunodeficiency syndrome[24]. Genetic studies have identified HLA-DQ2 and -DQ8 as the major determinants of susceptibility to CD. Because these haplotypes are common in the general population, determination of HLA is better suited for ruling out the presence of CD in suspicious cases[22].
In light of the aforementioned complexities, the final diagnosis of CD must be based on a comprehensive evaluation of clinical, serological, histological, and, when indicated, genetic elements. This consideration is known as the “4 out of 5 rule”.
As such, four of these five criteria should be satisfied for diagnosing CD: (1) presence of symptoms associated with CD; (2) presence of CD-associated autoantibodies (i.e., tTG, IgA EMA); (3) presence of HLA-DQ2 or -DQ8 alleles; (4) duodenal biopsy demonstrating blunting or absence of villi (Marsh III) with > 25 lymphocytes/100 enterocytes (with cluster of differentiation 3+ staining); and (5) melioration of symptoms after a GFD[30].
As we improve our understanding of the pathogenesis of CD, the interplay of genetic, epigenetic, and environmental factors may need to be considered as part of the diagnostic process[29]. In this regard, in recent years, more studies have investigated possible novel biomarkers of CD. Intriguing studies have identified CD4+ gluten-DQ2 tetramers in the peripheral blood of patients with CD after a short gluten challenge[31]. These cells were not present in controls or in patients with CD while on a GFD. More recently, Galatola et al[32] reported that a small gene expression panel from peripheral blood monocytes could discriminate between patients with active CD and healthy controls. The intestinal microbiome is another novel field of interest in CD, potentially leading to further understanding of its pathogenic mechanism and to the discovery of new markers of disease. An overall lack of Bifidobacteria and high abundance of Firmicutes were found in children with genetic susceptibility for CD who had early exposure to gluten in a study by Sellitto et al[33], which examined a small cohort of at-risk infants and controls up to 24 mo of age. The investigators suggest that there may be predictive ability in measuring such biomarkers. Viitasalo et al[34] found significant evidence of high levels of antibodies to ASCA (anti-Saccharomyces cerevisiae antibodies), OmpW (Bacteroides caccae TonB-linked outer membrane protein), and I2 (Pseudomonas fluorescens-associated sequence) in patients with early-stage lesions. Because antibody titres decreased after introduction of a GFD, the investigators proposed that Bacteroides and Pseudomonas species may play a part in the pathogenesis of CD. These results should encourage studies of novel biomarkers as we advance toward the possibility of a biopsy-free diagnosis of CD because they may add further security to the diagnosis of CD.
DH
DH is a skin disease characterized by a blistering rash and pathognomonic cutaneous IgA deposits[35]. Duhring’s original description also included patients with different conditions, such as erythema multiforme and pemphigus.
Differently from other GRDs, the prevalence of DH is higher in men than in women (1.5 to 1.9:1).
DH shares an high prevalence of HLA alleles DQ2 (90%) and DQ8 (5%) with CD[36]. Even if a skin rash or other dermatologic manifestations can be common in untreated CD[37], DH presents with unique characteristics. The usual clinical presentation of DH consists of diffuse, symmetrical, grouped polymorphic lesions consisting of erythema, urticarial plaques, papules, herpetiform vesiculae, and blisters followed by erosions, excoriations, and hyperpigmentation[38,39]. DH most frequently involves the extensor surfaces of the elbows (90%), knees (30%), shoulders, sacral region, buttocks, and face. Itching of variable intensity, scratching, and burning sensation immediately preceding the development of lesions are common.
Gastrointestinal symptoms in patients with DH are uncommon (affecting approximately 10% of patients) and usually mild[38,39]. However, 65% to 75% of patients show a celiac-type intestinal atrophy[9]. Even in patients with apparently normal biopsy specimens, an increased number of intraepithelial lymphocytes can be found consistently with a gluten sensitization[9]. Although often asymptomatic in adults, small bowel involvement in patients with DH can be associated with abdominal pain, diarrhoea, iron deficiency, and reduced growth rates in children[38,39].
Celiac-type serological markers (anti-tTG, anti-EMA, anti-DGP antibodies) can be typically found in DH patients. In a recent study, Borroni et al[40] identified IgA anti-epidermal transglutaminase autoantibodies as a promising marker for the serological diagnosis of DH[40].
The revelation of IgA by immunofluorescence staining on biopsy specimens of uninvolved skin analysed is another key diagnostic element[41]. Differently from other autoimmune disorders such as pemphigus, which can display homogeneous linear IgA deposits, IgA are detected as granular or fibrillar deposits in the dermal papillae in DH. Sometimes IgA can be found in linear granular deposits along the basement membrane as well[41].
Final diagnosis of Duhring disease can be made according to the histopathologic findings on skin specimen and presence of celiac related antibodies[41]. Once diagnosis have been formulated duodenal biopsies can be avoided, as it is commonly recognized that DH represents the skin counterpart of CD[41].
GA
GA is perhaps the most dramatic representation of neurological involvement in the setting of an immune response to gluten-containing foods. It has been defined as an otherwise unexplained sporadic ataxia with presence of serological antibodies consistent with a condition of sensitization to gluten[42]. Its predominant clinical manifestations include dysarthria, dysphonia, pyramidal signs, nystagmus and other ocular signs of cerebellar dysfunction (in up to 80% of cases), progressive ataxia of gait associated with myoclonus, palatal tremor, or opsoclonus[43]. Only very few subjects with GA experience any gastrointestinal symptoms; however, almost one-third of these patients have histological evidence for small bowel villous atrophy on biopsy specimens[9]. The precise pathogenic mechanism of GA is not clear, but a number of factors probably play different roles. Vitamin deficiency (in particular, vitamin E and vitamin B1) secondary to malabsorption in the small intestine may play a role; however, there is also evidence for a toxic and immune-mediated response to gluten[44]. Different studies suggest that some subjects with GA have both anti-gliadin antibodies and antibodies reacting with Purkinje cells. The latter marker is not present in patients with other causes of ataxia, resulting a pathognomonic feature of GA[44].
It is believed that the Purkinje cells of the cerebellum share epitopes with gliadin proteins and, as further evidence for the role of the immune system, intravenous immune globulin therapy has been reported to improve ataxia[44]. Recently, tTG6 has been identified in specimens of nervous tissue from patients with GA[45]. The molecular structure of tTG6 is similar to the tTG2 molecule involved in the development of CD and to the tTG3 molecule linked to DH[45]. Interestingly, anti-tTG6 deposits of the IgA class have also been found around brain vessels of patients with GA[45] and circulating anti-tTG6 antibodies appear to be a specific and sensitive marker for GA[46]. In light of these considerations, any subject with an history of progressive cerebellar ataxia of unknown origin should be regarded as possibly affected by GA, and tested for anti-gliadin antibodies (AGA), classical anti-tTG and possibly anti-tTG6 IgG and IgA antibodies[9]. Patients displaying positivity for any of these antibodies should be followed up for one year on a GFD. Stabilization or improvement of the ataxia associated with the negativity of antibodies should also be evaluated after the first year on a GFD[9].
WA
WA can be defined as an adverse reaction of the immune system to the the proteins contained in wheat.
In the vast field of WA a further classification can be made, distinguishing: (1) a classical form of food allergy with involvement of gastrointestinal tract, skin and possibly respiratory tract; (2) a typical form of inhalant allergy (baker’s asthma and rhinitis); (3) wheat- dependent, exercise-induced anaphylaxis (WDEIA); and (4) more rarely, a form of contact urticaria. The role of IgE-mediated reactions in the pathogenesis of baker’s asthma and rhinitis has been demonstrated as early as the beginning of the 20th century[47].
Symptoms consisting with baker’s asthma have been referred by 4.2% of bakery workers after a single year, increasing after 8.6% after the second working year[48]. Symptoms include rhinitis, skin itching/rash, ocular symptoms (including tearing, itching, and conjunctival injection), respiratory symptoms (including coughing, wheezing, shortness of breath, and sputum production), and “grain fever”[49-51].
Alfa-amylase inhibitors are the considered the most prominent allergen ivolved in the generation of symptoms, but different wheat proteins can play a role[52].
Alimentary WA, in its turn, is a less common disease, however in its most severe forms it can lead to serious reactions, including anaphylaxis and death[53].
In contrast to CD, symptoms of WA are typical for an IgE-mediated allergy, including itching and swelling in the mouth, nose, eyes, and throat; skin rash or swelling; wheezing in the respiratory tract; gastrointestinal symptoms such as cramps, bloating, and diarrhoea; and life-threatening anaphylaxis[54]. Differently from CD, WA does not cause permanent gastrointestinal damage[54].
WDEIA is a separate form of WA that is induced by physical exercise after ingestion of wheat. A specific type of grain protein, ω5-gliadin, acts as a trigger factor. Exercise within three hours of wheat consumption can induce an adverse reaction in susceptible people. In some cases, this can also occur when wheat is consumed directly after exercise[9,53]. Patients with WDEIA can refer different symptoms, ranging from urticaria to anaphylaxis and other severe reactions[55]. Although the mechanism of physical exercise-induced anaphylaxis is indistinguishable, an immediate-type hypersensitivity to water/salt-insoluble fraction of gluten has been considered to underlie this disease[53].
Skin prick tests and IgE assays are considered first-level studies for diagnosis of WA[9]. However, interpretation of these tests can be difficult because different confounding factors should be considered. First, a number of commercial kits for skin prick tests lack in sensitivity because they do not include allergens deriving from the insoluble gliadin fraction. Second, cross reactions with grass pollens may be present (especially in adults), resulting in lower specificity[9]. Testing prick by prick with raw material potentially overcomes these problems; nevertheless a definite diagnosis still requires an oral food challenge as a final test in a number of cases[9].
NCGS
NCGS is the “youngest” member of the GRD family and is characterized by intestinal and extraintestinal symptoms that occur after the ingestion of gluten-containing food in subjects in whom CD and WA have been ruled out. Rapid disappearance of symptoms with a GFD and recurrence a few hours/days after gluten reintroduction are also characteristic of this condition[56].
The existence of NCGS was firstly postulated in 1980 by Cooper et al[57], who described an evident amelioration of symptoms (bloating, diarrhoea and abdominal pain) in 6 out of 8 women after gluten withdrawal, in absence of the diagnostic criteria for CD.
After 20 years without further mention of this condition, Kaukinen et al[58] reported in 2000 that the majority of patients complaining gluten-related symptoms were non actually classificable as as being affected by CD or WA. Since they had a clear benefit from gluten withdrawal, their condition was called NCGS.
In the past 10 years, NCGS has received growing attention as patients have reported more severe nonspecific symptoms with intake of gluten by accident than seen with classic CD[59]. The recent Consensus Conference on GRD[9] is a clear sign of the scientific interest surrounding this clinical entity, even if little is still known about NCGS, especially when compared with current advancements in CD. For instance, reliable studies regarding the actual prevalence of this condition are still lacking. In current studies, prevalence of NCGS ranges from 0.63% in a primary care program[60] to 6% in a tertiary care centre[9].
The pathogenesis of NCGS is another hot topic. Since it was first described, a link between gluten and symptoms was suggested in subjects with NCGS[57]. Indeed, gluten itself has opioid-like activity because gluten proteins can alter the intestinal transit time in healthy volunteers and its action is reverted by naloxone[61]. Furthermore, an experimental model with transgenic mice gliadin-sensitized demonstrated increased secretion of acetylcholine from the myenteric plexus, with consequential enhancement of muscle contractility and increase in epithelial secretion. Gluten withdrawal was able to revert these abnormalities[62]. However, recent studies suggested that gluten may not be the only triggers of NCGS, with different wheat proteins likely playing relevant roles in this condition. For example, some grains and cereals (such as wheat, rye, and barley) are known to be particularly rich in fermentable oligosaccharides, disaccharides, and monosaccharides and polyols (FODMAPs). In turn, FODMAPS are known to provoke gastrointestinal symptoms in patients with irritable bowel syndrome through mechanisms involving gut microbiota, gas production, and fermentation[63]. Similarly to irritable bowel syndrome, FODMAPs can possibly play a role in generating both intestinal and extraintestinal manifestations in subjects with NCGS. Recent studies have shown that a diet low in FODMAPs results in improved symptoms in patients with self-reported gluten intolerance, supporting the hypothesis of a major role of FODMAPs compared to gluten[64,65]. Furthermore, wheat amylase and trypsin inhibitors, a complex of proteins in innate immunity, could contribute to the origination of symptoms in NCGS[7].
Because it is unclear which component of wheat-based products is responsible for an individual’s symptoms, it may be premature to assign all of the blame to gluten. From a clinical point of view, patients with NCGS may display great variability in gastrointestinal (bloating, abdominal pain, diarrhoea, nausea, aerophagia, aphthous stomatitis, constipation) and extraintestinal symptoms (lack of well-being, tiredness, headache, anxiety, foggy mind, numbness, joint or muscle pain, skin rash, anaemia, dermatitis)[66].
Interestingly, mood disorders in NCGS may recognize similar pathophysiological mechanisms to other neurological manifestations observed in gluten-related disorders such as GA, as reiterated toxic insults might an impaired immunological tolerance (i.e., the so-called “toxicant induced loss of tolerance”)[67].
Some papers also suggested a relationship between NCGS and neuropsychiatric diseases, with a particular regard to autism and schizophrenia, however the responsibility of gluten in conditions affecting the nervous system remains hot topic requiring additional studies[68].
Other important clinical aspects of NCGS are its frequent occurrence in first-degree relatives of patients with CD and a straightforward prevalence in female subjects (6:1)[66]. Heterogeneity in clinical presentation with identification of various subgroups of patients has been noticed by some investigators[65,69], possibly reflecting different pathogenic roles for the various proteins and carbohydrates contained in wheat and other gluten-rich cereals. Consequently, it has been speculated that NCGS only provides the best current description of a heterogeneous group of conditions with the common feature of improvement in symptoms on withdrawal of gluten[70]. At this time, it appears that NCGS is not associated with malabsorption or nutritional deficiencies or with any increased risk of autoimmune disorders or intestinal malignancy[24]. Therefore, differently from subjects affected by CD, patients with NCGS should not fear contaminations due to inadvertently introduced traces of gluten.
As noted in the preceding text, before diagnosing NCGS other conditions such as WA or CD should be excluded with appropriate tests during a gluten-containing diet.
WA should be excluded by testing for serum IgE antibodies to gluten and wheat fractions and by skin prick tests, while CD must be ruled out by the negativity of celiac-specific antibodies, i.e., IgA tTG, IgA EMA, and IgG DGP. A duodenal biopsy is also highly recommended because of the possibility of seronegative CD, occuring in 1%-2% of all patients with CD[6].
HLA-DQ2 and -DQ8 aplotypes are present in 50% of subjects with NCGS, representing a low prevalence compared with CD (95%), only slightly higher than in the general population (30%)[9]. Genetic studies investigating non-HLA regions are still lacking and, in general, the immunogenetics of NCGS are still non-existent[71].
Once identified negative criteria for the diagnosis of NCGS, the double-blind, placebo-controlled challenge (DBPCC) was proposed as a first positive diagnostic criterion. DBPCC trials have been highly as a confirmatory test for NCGS because of a possible placebo effect generated by gluten withdrawal[9]. However, a DBPCC is time-consuming and requires a close follow-up of patients and thus is currently used only in research[70].
The identification of other positive criteria and diagnostic markers is of great interest in NCGS. Recently, an elegant retrospective study by Kabbani et al[72] analysed 238 patients with symptoms responsive to GFD without prior diagnosis or exclusion of CD, demonstrating that patients with CD and patients with NCGS may have different clinical presentations. In particular, patients with NCGS were less likely to have malabsorptive symptoms, nutrient deficiency, and a personal history of autoimmune diseases, which is consistent with previous reports[24]. Consequently, the investigators concluded that patients who improve with a GFD but have negative findings on serology, lack of malabsorptive symptoms, and absence of risk factors for CD are likely to have NCGS and may not need to routinely undergo diagnostic endoscopy[72]. Nonetheless, these interesting findings need to be validated by future prospective studies.
In regard to serological markers, it was recently shown that 56% of patients with NCGS have circulating IgG AGA antibodies[73], which is consistent with the results from other investigators[69]. Interestingly, after starting a GFD, almost all of the patients with NCGS had normalization of AGA IgG levels, whereas these antibodies were still present in 40% of patients with CD after gluten withdrawal[74]. Strict compliance with and a good response to a GFD, with significant improvement in symptoms, were significantly related to the disappearance of AGA IgG in patients with NCGS[74]. Still, AGA IgG cannot be considered a reliable biomarker of NCGS because it can be detected in multiple different disorders, including autoimmune diseases, as well as in healthy subjects. As the cost of DNA sequencing is spectacularly reducing, it could be interesting for the clinicians to characterize the different subgroups of patients with this condition[71]. Consequently, further research on biomarkers of NCGS is strongly encouraged.
CONCLUSION
Based on the data presented in this report, it is clear that clinical manifestations of GRDs cover a wide variety of medical specialties, ranging from gastroenterology to allergology and from neurology to dermatology. Within the large family of GRDs, two opposite trends seem evident. First, we have a great deal of information about the clinical presentation, pathogenesis, and diagnostic markers of some diseases (with CD as a prototypic example). Second, there are some more recently accepted conditions, such as NCGS, with many clinical and diagnostic aspects still to be investigated. Consequently, different priorities are required for different situations. In regard to CD, identification of new early microbial or non-microbial markers represents a promising field of interest, eventually leading to an early and non-invasive diagnosis in the future. In the case of NCGS, first we have to understand whether it constitutes a singular entity or only provides the best description of a heterogeneous group of conditions attributable to different wheat-related food constituents. Only studies with more homogeneous groups of patients will be able to provide relevant information on this apparently frequent but still elusive condition.
Footnotes
P- Reviewer: Butterworth J, Dickey W, Pavlovic M, Rostami K, Torres MI S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Conflict-of-interest: None to declare by any author.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 30, 2014
First decision: September 30, 2014
Article in press: December 19, 2014
References
- 1.Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. 2013;10:383–392. doi: 10.1038/cmi.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catassi C, Cobellis G. Coeliac disease epidemiology is alive and kicking, especially in the developing world. Dig Liver Dis. 2007;39:908–910. doi: 10.1016/j.dld.2007.07.159. [DOI] [PubMed] [Google Scholar]
- 3.Wheat’s role in the US diet. US Department of Agriculture. Available from: http: //www.ers.usda.gov/[homepage on the Internet]
- 4.Molberg O, Uhlen AK, Jensen T, Flaete NS, Fleckenstein B, Arentz-Hansen H, Raki M, Lundin KE, Sollid LM. Mapping of gluten T-cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology. 2005;128:393–401. doi: 10.1053/j.gastro.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Gobbetti M, Giuseppe Rizzello C, Di Cagno R, De Angelis M. Sourdough lactobacilli and celiac disease. Food Microbiol. 2007;24:187–196. doi: 10.1016/j.fm.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Volta U, Villanacci V. Celiac disease: diagnostic criteria in progress. Cell Mol Immunol. 2011;8:96–102. doi: 10.1038/cmi.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inomata N. Wheat allergy. Curr Opin Allergy Clin Immunol. 2009;9:238–243. doi: 10.1097/ACI.0b013e32832aa5bc. [DOI] [PubMed] [Google Scholar]
- 8.Leonard MM, Vasagar B. US perspective on gluten-related diseases. Clin Exp Gastroenterol. 2014;7:25–37. doi: 10.2147/CEG.S54567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallert C, Grant C, Grehn S, Grännö C, Hultén S, Midhagen G, Ström M, Svensson H, Valdimarsson T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. 2002;16:1333–1339. doi: 10.1046/j.1365-2036.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 11.Verrill L, Zhang Y, Kane R. Food label usage and reported difficulty with following a gluten-free diet among individuals in the USA with coeliac disease and those with noncoeliac gluten sensitivity. J Hum Nutr Diet. 2013;26:479–487. doi: 10.1111/jhn.12032. [DOI] [PubMed] [Google Scholar]
- 12.Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. J Hum Nutr Diet. 2007;20:423–430. doi: 10.1111/j.1365-277X.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 13.Lundin KE, Alaedini A. Non-celiac gluten sensitivity. Gastrointest Endosc Clin N Am. 2012;22:723–734. doi: 10.1016/j.giec.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Biesiekierski JR, Muir JG, Gibson PR. Is gluten a cause of gastrointestinal symptoms in people without celiac disease? Curr Allergy Asthma Rep. 2013;13:631–638. doi: 10.1007/s11882-013-0386-4. [DOI] [PubMed] [Google Scholar]
- 15.Lundin KE. Non-celiac gluten sensitivity - why worry? BMC Med. 2014;12:86. doi: 10.1186/1741-7015-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 17.Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, Murray L, Metzger MH, Gasparin M, Bravi E, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–595. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 18.Gandolfi L, Pratesi R, Cordoba JC, Tauil PL, Gasparin M, Catassi C. Prevalence of celiac disease among blood donors in Brazil. Am J Gastroenterol. 2000;95:689–692. doi: 10.1111/j.1572-0241.2000.01847.x. [DOI] [PubMed] [Google Scholar]
- 19.Sood A, Midha V, Sood N, Kaushal V, Puri H. Increasing incidence of celiac disease in India. Am J Gastroenterol. 2001;96:2804–2805. doi: 10.1111/j.1572-0241.2001.04150.x. [DOI] [PubMed] [Google Scholar]
- 20.Bizzaro N, Tozzoli R, Villalta D, Fabris M, Tonutti E. Cutting-edge issues in celiac disease and in gluten intolerance. Clin Rev Allergy Immunol. 2012;42:279–287. doi: 10.1007/s12016-010-8223-1. [DOI] [PubMed] [Google Scholar]
- 21.Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419–2426. doi: 10.1056/NEJMcp1113994. [DOI] [PubMed] [Google Scholar]
- 22.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 23.Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676; quiz 677. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaukinen K, Lindfors K, Collin P, Koskinen O, Mäki M. Coeliac disease--a diagnostic and therapeutic challenge. Clin Chem Lab Med. 2010;48:1205–1216. doi: 10.1515/CCLM.2010.241. [DOI] [PubMed] [Google Scholar]
- 26.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520–2524. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 27.Villalta D, Tonutti E, Prause C, Koletzko S, Uhlig HH, Vermeersch P, Bossuyt X, Stern M, Laass MW, Ellis JH, et al. IgG antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem. 2010;56:464–468. doi: 10.1373/clinchem.2009.128132. [DOI] [PubMed] [Google Scholar]
- 28.Amarri S, Alvisi P, De Giorgio R, Gelli MC, Cicola R, Tovoli F, Sassatelli R, Caio G, Volta U. Antibodies to deamidated gliadin peptides: an accurate predictor of coeliac disease in infancy. J Clin Immunol. 2013;33:1027–1030. doi: 10.1007/s10875-013-9888-z. [DOI] [PubMed] [Google Scholar]
- 29.Zevit N, Shamir R. Diagnosis of celiac disease: where are we heading after the ESPGHAN 2012 guidelines? J Pediatr Gastroenterol Nutr. 2014;59 Suppl 1:S13–S15. doi: 10.1097/01.mpg.0000450396.76521.b0. [DOI] [PubMed] [Google Scholar]
- 30.Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691–693. doi: 10.1016/j.amjmed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Ráki M, Fallang LE, Brottveit M, Bergseng E, Quarsten H, Lundin KE, Sollid LM. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc Natl Acad Sci USA. 2007;104:2831–2836. doi: 10.1073/pnas.0608610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galatola M, Izzo V, Cielo D, Morelli M, Gambino G, Zanzi D, Strisciuglio C, Sperandeo MP, Greco L, Auricchio R. Gene expression profile of peripheral blood monocytes: a step towards the molecular diagnosis of celiac disease? PLoS One. 2013;8:e74747. doi: 10.1371/journal.pone.0074747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellitto M, Bai G, Serena G, Fricke WF, Sturgeon C, Gajer P, White JR, Koenig SS, Sakamoto J, Boothe D, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS One. 2012;7:e33387. doi: 10.1371/journal.pone.0033387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viitasalo L, Niemi L, Ashorn M, Ashorn S, Braun J, Huhtala H, Collin P, Mäki M, Kaukinen K, Kurppa K, et al. Early microbial markers of celiac disease. J Clin Gastroenterol. 2014;48:620–624. doi: 10.1097/MCG.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmi TT, Hervonen K, Kautiainen H, Collin P, Reunala T. Prevalence and incidence of dermatitis herpetiformis: a 40-year prospective study from Finland. Br J Dermatol. 2011;165:354–359. doi: 10.1111/j.1365-2133.2011.10385.x. [DOI] [PubMed] [Google Scholar]
- 36.Holmes G, Catassi C, Fasano A. Dermatitis Herpetiformis in Celiac disease. Oxford: Health Press; 2009. pp. 83–90. [Google Scholar]
- 37.Bonciolini V, Antiga E, Fabbri P, Caproni M. Skin manifestations of celiac disease: not always dermatitis herpetiformis. Int J Dermatol. 2014;53:e352–e353. doi: 10.1111/ijd.12350. [DOI] [PubMed] [Google Scholar]
- 38.Nicolas ME, Krause PK, Gibson LE, Murray JA. Dermatitis herpetiformis. Int J Dermatol. 2003;42:588–600. doi: 10.1046/j.1365-4362.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 39.Fry L. Dermatitis herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523–531. [PubMed] [Google Scholar]
- 40.Borroni G, Biagi F, Ciocca O, Vassallo C, Carugno A, Cananzi R, Campanella J, Bianchi PI, Brazzelli V, Corazza GR. IgA anti-epidermal transglutaminase autoantibodies: a sensible and sensitive marker for diagnosis of dermatitis herpetiformis in adult patients. J Eur Acad Dermatol Venereol. 2013;27:836–841. doi: 10.1111/j.1468-3083.2012.04586.x. [DOI] [PubMed] [Google Scholar]
- 41.Caproni M, Antiga E, Melani L, Fabbri P. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633–638. doi: 10.1111/j.1468-3083.2009.03188.x. [DOI] [PubMed] [Google Scholar]
- 42.Hadjivassiliou M, Grünewald RA, Chattopadhyay AK, Davies-Jones GA, Gibson A, Jarratt JA, Kandler RH, Lobo A, Powell T, Smith CM. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582–1585. doi: 10.1016/s0140-6736(98)05342-2. [DOI] [PubMed] [Google Scholar]
- 43.Hadjivassiliou M, Grünewald R, Sharrack B, Sanders D, Lobo A, Williamson C, Woodroofe N, Wood N, Davies-Jones A. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685–691. doi: 10.1093/brain/awg050. [DOI] [PubMed] [Google Scholar]
- 44.Hadjivassiliou M, Boscolo S, Davies-Jones GA, Grünewald RA, Not T, Sanders DS, Simpson JE, Tongiorgi E, Williamson CA, Woodroofe NM. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002;58:1221–1226. doi: 10.1212/wnl.58.8.1221. [DOI] [PubMed] [Google Scholar]
- 45.Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM. Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxia. Amino Acids. 2010;39:1183–1191. doi: 10.1007/s00726-010-0554-y. [DOI] [PubMed] [Google Scholar]
- 46.Hadjivassiliou M, Aeschlimann P, Sanders DS, Mäki M, Kaukinen K, Grünewald RA, Bandmann O, Woodroofe N, Haddock G, Aeschlimann DP. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology. 2013;80:1740–1745. doi: 10.1212/WNL.0b013e3182919070. [DOI] [PubMed] [Google Scholar]
- 47.Houba R, Doekes G, Heederik D. Occupational respiratory allergy in bakery workers: a review of the literature. Am J Ind Med. 1998;34:529–546. doi: 10.1002/(sici)1097-0274(199812)34:6<529::aid-ajim1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 48.Walusiak J, Hanke W, Górski P, Pałczyński C. Respiratory allergy in apprentice bakers: do occupational allergies follow the allergic march? Allergy. 2004;59:442–450. doi: 10.1111/j.1398-9995.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 49.Matsumura Y, Niitsuma T, Ito H. A study of factors contributing to bakers’ allergy symptoms. Arerugi. 1994;43:625–633. [PubMed] [Google Scholar]
- 50.Merget R, Sander I, van Kampen V, Beckmann U, Heinze E, Raulf-Heimsoth M, Bruening T. Allergic asthma after flour inhalation in subjects without occupational exposure to flours: an experimental pilot study. Int Arch Occup Environ Health. 2011;84:753–760. doi: 10.1007/s00420-011-0617-8. [DOI] [PubMed] [Google Scholar]
- 51.Baldo BA, Krilis S, Wrigley CW. Hypersensitivity to inhaled flour allergens. Comparison between cereals. Allergy. 1980;35:45–56. doi: 10.1111/j.1398-9995.1980.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 52.Tatham AS, Shewry PR. Allergens to wheat and related cereals. Clin Exp Allergy. 2008;38:1712–1726. doi: 10.1111/j.1365-2222.2008.03101.x. [DOI] [PubMed] [Google Scholar]
- 53.Pasha I, Saeed F, Sultan MT, Batool R, Aziz M, Ahmed W. Wheat allergy & amp; intolerence; recent updates and perspectives. Crit Rev Food Sci Nutr. 2013:Sep 2; Epub ahead of print. doi: 10.1080/10408398.2012.659818. [DOI] [PubMed] [Google Scholar]
- 54.Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36:68S–75S. doi: 10.1177/0148607111426276. [DOI] [PubMed] [Google Scholar]
- 55.Palosuo K, Varjonen E, Kekki OM, Klemola T, Kalkkinen N, Alenius H, Reunala T. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J Allergy Clin Immunol. 2001;108:634–638. doi: 10.1067/mai.2001.118602. [DOI] [PubMed] [Google Scholar]
- 56.Troncone R, Jabri B. Coeliac disease and gluten sensitivity. J Intern Med. 2011;269:582–590. doi: 10.1111/j.1365-2796.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 57.Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology. 1980;79:801–806. [PubMed] [Google Scholar]
- 58.Kaukinen K, Turjanmaa K, Mäki M, Partanen J, Venäläinen R, Reunala T, Collin P. Intolerance to cereals is not specific for coeliac disease. Scand J Gastroenterol. 2000;35:942–946. doi: 10.1080/003655200750022995. [DOI] [PubMed] [Google Scholar]
- 59.Mulder CJ, van Wanrooij RL, Bakker SF, Wierdsma N, Bouma G. Gluten-free diet in gluten-related disorders. Dig Dis. 2013;31:57–62. doi: 10.1159/000347180. [DOI] [PubMed] [Google Scholar]
- 60.DiGiacomo DV, Tennyson CA, Green PH, Demmer RT. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. Scand J Gastroenterol. 2013;48:921–925. doi: 10.3109/00365521.2013.809598. [DOI] [PubMed] [Google Scholar]
- 61.Corazza GR, Frazzoni M, Strocchi A, Prati C, Sarchielli P, Capelli M. Alimentary exorphin actions on motility and hormonal secretion of gastrointestinal tract. In Fraioli F, Isidori A, Mazzetti M (ed.). Opioid Peptides in the Periphery. Amsterdam: Elsevier Sciences Publisher; 1984. pp. 243–247. [Google Scholar]
- 62.Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, McKay DM, Murray JA. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G217–G225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 63.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 64.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–8.e1-320-8.e3. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 65.Zanini B, Baschè R, Ferraresi A, Lanzarotto F, Marullo M, Ricci C, Lanzini A. PTH-111 “non Celiac Gluten Sensitivity” (ncgs) Is Uncommon In Patients Spontaneously Adhering To Gluten Free Diet (gfd), And Is Outnumbered By “fodmaps Sensitivity”. Gut. 2014;63:A260. [Google Scholar]
- 66.Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85. doi: 10.1186/1741-7015-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Genuis SJ, Lobo RA. Gluten sensitivity presenting as a neuropsychiatric disorder. Gastroenterol Res Pract. 2014;2014:293206. doi: 10.1155/2014/293206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, et al. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839–3853. doi: 10.3390/nu5103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–1906; quiz 1907. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 70.Mooney PD, Aziz I, Sanders DS. Non-celiac gluten sensitivity: clinical relevance and recommendations for future research. Neurogastroenterol Motil. 2013;25:864–871. doi: 10.1111/nmo.12216. [DOI] [PubMed] [Google Scholar]
- 71.Pena AS. Immunogenetics of non celiac gluten sensitivity. Gastroenterol Hepatol Bed Bench. 2014;7:1–5. [PMC free article] [PubMed] [Google Scholar]
- 72.Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvez J, Pallav K, Hansen J, Mukherjee R, Dennis M, Kelly CP. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol. 2014;109:741–746; quiz 747. doi: 10.1038/ajg.2014.41. [DOI] [PubMed] [Google Scholar]
- 73.Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, Fiorini E, Caio G. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–685. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 74.Caio G, Volta U, Tovoli F, De Giorgio R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014;14:26. doi: 10.1186/1471-230X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]


