Abstract
This study describes the maturation of sirolimus clearance in a cohort of very young pediatric patients with vascular anomalies. The relationship between allometrically scaled in vivo clearance and age was described by the Emax model in patients aged 1 month to 2 years. Consistent with the observed increase, in vitro intrinsic clearance of sirolimus using pediatric liver microsomes showed a similar age-dependent increase. In children older than 2 years, allometrically scaled sirolimus clearance did not show further maturation. Simulated clearance estimates with a sirolimus physiologically based pharmacokinetic model that included CYP3A4/5/7 and CYP2C8 maturation profiles were in close agreement with observed in vivo clearance values. In addition, physiologically based pharmacokinetic model-simulated sirolimus pharmacokinetic profiles predicted the actual observations well. These results demonstrate the utility of a physiologically based pharmacokinetic modeling approach for the prediction of the developmental trajectory of sirolimus metabolic activity and its effects on total body clearance in neonates and infants.
The pharmacokinetics (PK) and pharmacodynamics (PD) of drugs in children are different from those in adults mostly due to anatomical, physiological, and biochemical differences.1,2 In addition, children experience rapid growth and development over the course of their childhood, especially in the first 2 years of life.2,3 There are still a great many unknowns regarding the age related changes in the absorption, distribution, metabolism, and elimination (ADME) of drugs and much research remains to be done on issues such as drug metabolizing enzyme and transporter ontogeny, and factors determining pediatric oral drug absorption and disposition.4,5
Pharmacokinetic parameters for pediatric patients have traditionally been scaled using a linear per kilogram model, despite a long held recognition that metabolic and renal elimination processes are not linearly related to body mass.6,7 This dosing paradigm has resulted in under- and overdosing, depending on the specific age group.7,8 Allometric models based on body weight are increasingly being used to predict drug clearance in children across different age groups.9 Anderson and Holford proposed a “top-down” combined sigmoid Emax maturation model in combination with allometric scaling to model maturation of glomerular filtration rate, and drug clearance of renally excreted and metabolized drugs such as dexmedetomidine, morphine, and acetaminophen in children.9–11
In recent years, there has been renewed interest in the development of pediatric physiologically based pharmacokinetic models. This “bottom-up” approach incorporates developmental physiology and biochemistry into a model structure to predict PK parameters and PK profiles across the pediatric age spectrum.4,12 Using a combined “bottom-up” and “top-down” (classic PK and population PK analyses) approach13 may provide a better understanding of the underlying drivers that contribute to age specific variability in PK.
The objective of this study was to describe the developmental trajectory of sirolimus clearance in pediatric patients, including neonates and young infants, who participated in a concentration controlled phase II sirolimus efficacy and safety trial in patients with vascular anomalies.14 Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor first introduced as an immunosuppressive drug for preventing rejection in organ transplantation which also has shown efficacy in several types of cancer as well as in vascular anomalies.15,16 Vascular anomalies appear within weeks to years after birth while some are acquired by trauma. Because some vascular anomalies are congenital and, therefore, present at birth, very young infants were part of the study cohort. To date, there have been no data published on sirolimus pharmacokinetics in neonates and young infants.
This study was performed with a special focus on the age-related maturation of sirolimus clearance. In addition, the in vitro ontogeny of sirolimus metabolic pathways was assessed using pediatric liver microsomes. CYP3A4, CYP3A5, and CYP2C8 have been reported as the major enzymes involved in the metabolism of sirolimus.17,18 Following our previous report related to CYP3A4 and CYP3A5,17 the relative activity of CYP3A7, known as a fetal CYP3A isoform,19,20 in the metabolism of sirolimus was further investigated. Especially, liver microsomes from neonates might consist of not only CYP3A4 and 3A5 but also CYP3A7 as the expression of CYP3A7 is considered to be replaced by CYP3A4 shortly after birth.19,21 With the in vitro ontogeny data, a pediatric physiologically based pharmacokinetic (PBPK) model was developed to evaluate PBPK model-based predicted systemic exposure in relation to observed in vivo PK profiles.
METHODS
Patients and clinical data
This study was conducted using data collected as part of an ongoing clinical trial “Safety and Efficacy Study of Sirolimus in Complicated Vascular Anomalies.”14 The total patient population included neonates, infants, children, adolescents, and young adults with one of the vascular anomalies, as determined by clinical, radiographic, and histologic criteria,22–25 with complications requiring systemic therapy.
Patients had adequate liver and renal function. Adequate liver function was defined as total bilirubin (sum of conjugated and unconjugated) ≤1.5 × upper limit of normal for age, SGPT (ALT) <5 × upper limit of normal for age, serum albumin greater than or equal to 2 g/dl, and fasting LDL cholesterol of <160 mg/dl, where patients taking a cholesterol lowering agent were on a single medication and on a stable dose for at least 4 weeks. Creatinine clearance was ≥ 70 ml/min/1.73 m2 and patients did not receive CYP3A4 inhibitors or inducers at study entry. In addition, patients on chronic systemic steroid treatment or other immunosuppressive agents were excluded.
Data collected included age, sex, race, ethnicity, height, weight, sirolimus concentrations, dosing regimens, and concomitant medications. Sirolimus was started at an oral dose of 0.8 mg/m2 twice a day. The starting dose was based on extrapolation of the recommended sirolimus dose in older children and adult transplant recipients.26 Subsequent dosing was pharmacokinetically guided to achieve a target trough concentration of 10 to 15 ng/ml. Sirolimus concentrations were measured first at 8–14 days after start of treatment and then every 2 weeks until stable trough concentrations were attained. Once the concentration became stable, defined as two consecutive trough concentrations within the target range, trough concentrations were measured every 4–6 weeks. The target concentration range was empirical and based on a small case series of sirolimus-treated patients (ages 3–21 years) with astrocytoma who exhibited lesion regression and tolerated treatment well with trough levels of 10–15 ng/ml.27
Sirolimus concentration measurements in human blood
Clinical sirolimus whole blood concentrations were determined at our institution by a validated tandem mass spectrometry assay performed using electrospray on a Waters Quattro Micro API triple quadrupole mass spectrometer (Milford, MA) interfaced with an Acquity ultra performance liquid chromatography (UPLC) instrument.28 The dynamic range was 0.5–100.0 ng/ml. The LLOQ of the assay was 1.0 ng/ml and within and between-batch variability (CV) was 12.8% and 14.0%, respectively.
Individual clearance estimates by Bayesian estimation
Individual sirolimus clearance estimates were generated by using a Bayesian estimator (MW/Pharm version 3.6, Mediware, Groningen, The Netherlands) as previously described.28,29 Observed sirolimus concentrations during the first 4 weeks of treatment (typically 1–3 measurements per patient) were used for the Bayesian estimation. Population pharmacokinetic model parameters and their distributions used in the MW/Pharm as priors were based on pharmacokinetic data in pediatric patients with neurofibromatosis as follows (means ± SD): 17.3 ± 7.9 L/h/1.85 m2 for clearance, 12.0 ± 5.0 L/kg for volume of distribution, and 2.77 ± 1.33/h for the absorption rate constant in a one-compartment pharmacokinetic model.28 Because concentrations obtained were mostly predose trough measurements, only individual clearances were estimated. Individual clearance estimates were further normalized through allometric scaling (scaling factor of 0.75 and standard adult body weight of 70 kg) by applying the following equation to allow evaluation of the effects of size and age related maturation on sirolimus clearance:9
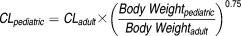 |
1 |
Materials for in vitro assays
Sirolimus and zotarolimus were purchased from LC Laboratories (Woburn, MA) and from Molcan Corporation (Toronto, Canada), respectively. Ketoconazole and montelukast were obtained from Sigma-Aldrich (St. Louis, MO). Potassium phosphate buffer (500 mM, pH 7.4), NADPH regenerating system, CYP3A7 with both reductase and cytochrome b5, and Sf9 insect cell control were obtained from BD Bioscience (Woburn, MA). Individual pediatric liver microsomes were obtained from Xenotech (Lenexa, KS) and were provided with information on the age, gender, ethnicity, microsomal concentration, and metabolic activity of each P450 marker reaction. Other reagents used in this study were commercially available and of analytical grade.
Drug metabolism assays
A substrate depletion assay was performed according to the method of Obach (1999) with slight modifications.30 Sirolimus was incubated with human liver microsomes or recombinant CYP3A7 in 100 mM potassium phosphate buffer (pH 7.4) in a shaking water bath at 37°C. In the recombinant CYP assay, the protein concentration was adjusted to 0.4 mg/ml by adding blank microsomes. In addition, montelukast and ketoconazole were used as specific inhibitors of CYP2C8 and CYP3A, respectively.31,32 A sample aliquot was collected from the reaction mixture at serial time points, where the zero time point corresponded with the time when the NADPH regenerating system was added to the reaction mixture. Samples were extracted with methanol/0.2M ZnSO4 (80/20, v/v) including zotarolimus as an internal standard. After the removal of proteins by centrifugation at 21,200 g for 10 minutes at 4 °C, the supernatant was subjected to high-performance liquid chromatographic separation with tandem mass spectrometric detection (LC-MS/MS).
Analysis of sirolimus in in vitro samples
Sirolimus was quantified by an integrated on-line solid phase extraction–high performance liquid chromatography–tandem mass spectrometry (SPE–HPLC–MS/MS; API 3000, ABSciex, Framingham, MA) system according to the method by Koal et al. with slight modifications.33 The analytical method provided good linearity over a concentration range of 9.1 ng/ml (10 nM) to 2743 ng/ml (3 µM). The intra- and inter-day coefficients of validation were 0.3% to 11% and 1.6% to 7.5%, respectively. The lower limit of quantification was 10 nM. The peak area ratios of sirolimus to an internal standard, zotarolimus, were used for quantification. The area ratios were converted to percentage sirolimus remaining, using the t = 0 min peak area ratio value as 100%. The slope of the linear regression line from the log percentage remaining versus incubation time relationship (-kel) was used in the conversion to in vitro intrinsic clearance (CLint, in units of ml/min/pmol CYP or mg) was according to the following equation:
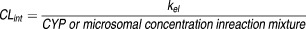 |
2 |
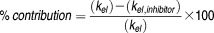
For the inhibition assay, percentage of contribution of each CYP isoform to the in vitro metabolism of sirolimus was calculated by the following equation34:
 |
3 |
where kel and kel,inhibitor were determined in absence and presence of chemical inhibitors, respectively.
PBPK model development and simulations
Simcyp Pediatric version 12 (Release 2) and version 13 (Release 1) were used as the pediatric PBPK modeling platform with input of drug specific data as used in the sirolimus PBPK model previously developed for adults (see Supplementary Table S1, which is available online).17 The elimination pathways of sirolimus in the adult PBPK model consisted of P450 metabolism and non-P450 elements, the latter defined as representing residual elimination, such as biliary excretion and/or sirolimus degradation through hydrolysis or open ring isomer formation.35,36 Simcyp default ontogeny profiles of CYP2C8, CYP3A4, and CYP3A7 were used, where the times to 50% maturation (Age50) of hepatic and intestinal CYP3A4 maturation were 0.9 and 2.36 years, respectively. The ontogeny profile of CYP3A5 was modified from the default setting to an expression pattern according to reported data.37,38 In both the hepatic and intestinal CYP3A5 ontogeny profiles in Simcyp (equation below), Age50 and Fbirth were set to 0, and Adultmax and n were set to 1. This eliminates an age-dependent expression pattern of CYP3A5 in a manner as described.37,38
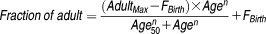 |
4 |
The ontogeny profile of the residual elimination pathway was determined from three typical maturation patterns predefined in Simcyp version 12, as fast, middle, and late, and according to fitting of the observed clearances. For the residual elimination pathway, the predefined pattern of “fast ontogeny” was the most suited among the three options in Simcyp with settings for Adultmax, Age50, n, and Fbirth of 1, 0.02, 1, and 0, respectively.
Sirolimus clearance was simulated in Simcyp Pediatric with the developed PBPK model using 300 virtual healthy pediatric subjects who were zero to 3 years of age. In the PBPK model simulations, sirolimus was administrated as an oral dose of 1.0 mg/m2 twice a day for 30 days, in accordance with the actually administered average dose of 1.0 mg/m2 between start and 1 month of therapy (0.8 ± 0.1 and 1.3 ± 0.4 mg/m2, respectively).
Statistical analyses
Data were analyzed using linear and nonlinear regression analysis (Emax model) in Graphpad Prism (version 5.0, San Diego, CA). A linear regression analysis was performed to assess the relationship between in vitro sirolimus CLint and testosterone 6β-hydroxylation. A nonlinear regression analysis was performed using an Emax model to describe the relationship between individual clearances and age. Individual in vivo sirolimus clearance estimates were obtained with the Bayesian estimator as described. Individual in vivo sirolimus clearance estimates were standardized to a 70 kg patient by using allometric scaling and used with the Emax model to estimate CLmatured (clearance at the fully matured adult level) and Age50 (age at 50% of CLmatured) values. The relationships of clearance with age, sex, or race was analyzed by Wilcoxon's test in JMP software (version 8, Cary, NC).
RESULTS
Age-dependent sirolimus in vivo clearance in patients
Sirolimus oral clearance was estimated in 44 pediatric patients with vascular anomalies using concentration-time data collected as part of a concentration controlled trial. Patient's demographics and diagnoses are summarized in Table 1. Age ranged from 1 month to 18 years. Median (range, Min to Max) of body weight (BW), height, and body surface area (BSA) were 19.5 kg (4.0–100.6), 115 cm (53–183), and 0.79 m2 (0.23–2.2), respectively. Patients with vascular anomalies in this study population represented a spectrum of vascular anomalies22,23 (Table 1).
Table 1.
Demographic data in pediatric patients with vascular anomalies
| Parameter | Median | Min-Max | 25–75 percentiles |
|---|---|---|---|
| Age (years) | 5.0 | 0.08–18 | 1.6–12.0 |
| Body weight (kg) | 19.5 | 4.0–100.6 | 10.7–44.2 |
| Height (cm) | 115 | 53–183 | 76–156 |
| Body surface area (m2) | 0.79 | 0.23–2.20 | 0.46–1.37 |
| Sex | Male, 18 (41%); Female, 26 (59%) | ||
| Race | White, 34 (77%); Black, 5 (11%); Asian, 1 (2%); Others, 3 (7%); Not-reported, 1 (2%) | ||
| Ethnicity | Non-Hispanic, 42 (95%); Hispanic, 1 (2%); Not-reported, 1 (2%) | ||
| Stratified diagnosis | Kaposiform hemangioendothelioma or tufted angioma with/without Kasabach-Merritt phenomenon (With, 23%; Without, 9%), | ||
| Capillary venous lymphatic malformation (18%), | |||
| General lymphatics abnormalities (16%), | |||
| Kaposiform lymphangiomatosis (14%), | |||
| Abnormalities of the central conducting lymphatic channels (5%), | |||
| Phosphatase and tensin homologue (PTEN) with overgrowth and hamartoma/venous malformation (5%), | |||
| PTEN with arteriovenous malformation (2%), | |||
| Gorham (5%), | |||
| Microcystic lymphatic malformation (2%), | |||
| Venous lymphatic malformation (2%) | |||
When normalized for body size using allometric scaling with a coefficient of 0.75, clearance increase with age leveled off after the age of 2 years (Figure 1). However, in patients younger than 1 year, the mean of allometrically scaled sirolimus clearance was significantly lower than that observed in older patients (>3 years) indicating immaturity of clearance in these young patients (Figure 2a). Mean sirolimus oral clearance was 11 ± 3 L/h, 17 ± 4 L/h, 21 ± 3 L/h, and 18 ± 6 L/h for the age groups of 1–8 months (<1), 1 year (≥1, <2), 2 years (≥2, <3), and 3–18 years (≥3), respectively. Sex, ethnicity, or race did not show a statistically significant association with sirolimus clearance in this small patient cohort.
Figure 1.
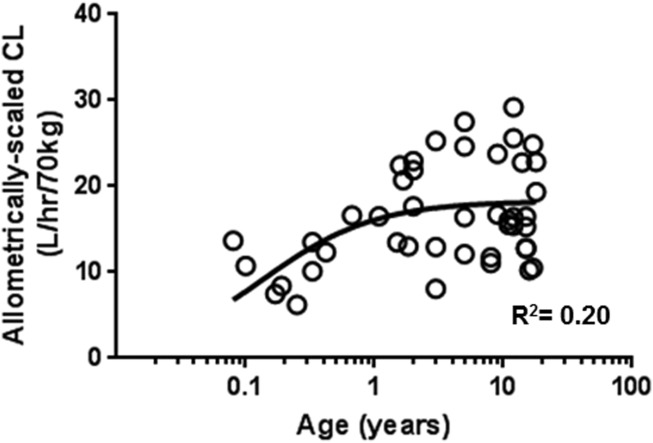
Allometrically scaled individual sirolimus clearance (CL) vs. age in 44 patients with vascular anomalies. Individual clearances were estimated using concentration at trough with MW-Pharm. The nonlinear regression analysis was conducted with GraphPad Prism. Solid line indicates the nonlinear regression line following Emax model. Each data point represents the clearance estimate of one patient.
Figure 2.
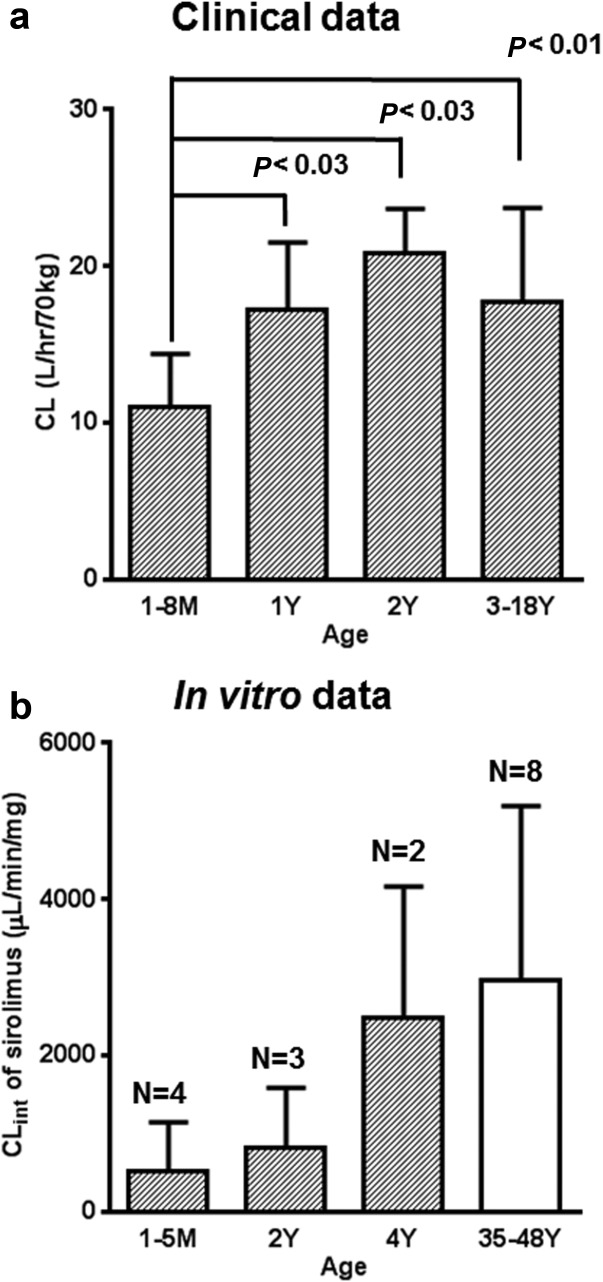
Age-dependent change of allometrically scaled sirolimus clearance from pediatric patients and in vitro intrinsic clearance of sirolimus in individual pediatric liver microsomes. a: The number of patients are 9, 5, 3, and 27 for the age groups: 1–8 months, 1 year (≥1, <2), 2 years (≥2, <3), and 3–18 years, respectively. Statistical P-values are based on nonparametric analysis (Wilcoxon's test). b: Age ranges of donors of liver microsomes were 1-month- to 4-year-old for pediatric (hatched column) and 35- to 48-year-old for adults liver microsomes (open column). N represents the number of individual human liver microsomes in each age group.
Age-dependent sirolimus in vitro intrinsic clearance in human liver microsomes
To assess the mechanism of the observed in vivo maturation effect, sirolimus in vitro CLint was examined in pediatric liver microsomes from donors aged 1 month to 4 years of age, as well as from adult donors in the age range of 35–48 years (Figure 2b). The sirolimus CLint in pediatric liver microsomes tended to be lower compared with that in adult microsomes although this difference did not reach statistical significance most likely due to small sample size. CYP3A contribution to sirolimus metabolism was estimated to be 95.8%, while that of CYP2C8 was less than 10% based on the activity assays using pediatric liver microsomes and specific chemical inhibitors for each CYP isoform (Table 2). In addition, a linear correlation was observed between sirolimus CLint and testosterone 6β-hydroxylation; the latter being used as a marker of CYP3A activity (R2 = 0.95; P < 0.0001; n = 9 individual liver microsomal preparations). As for CYP3A7, the estimated CLint value for recombinant CYP3A7 was 0.30 µl/min/pmol CYP protein, which was 30 and 13-fold lower than that of reported CYP3A4 and CYP3A5 activity, respectively.17
Table 2.
CYP contributions on sirolimus metabolism in individual pediatric liver microsomes aged from 1 month to 4 years
| CYP isoform (chemical inhibitor) | % of Contribution (mean ± SD from nine individual liver microsomes) |
|---|---|
| CYP2C8 (montelukast at 1 µM) | 8.5±4.3 |
| CYP3A (ketoconazole at 1 µM) | 95.8±4.8 |
PBPK model prediction of sirolimus clearance
Estimates of CLmatured and Age50 predicted with the pediatric PBPK model (300 simulations) were 15.2 ± 0.8 L/h/70 kg (mean ± S.E.) and 0.19 ± 0.06 year (or 2.3 ± 0.7 months), respectively. As can be seen in Figure 3, almost all of sirolimus Bayesian clearance estimates based on the observed concentrations fall within the 5–95 percentiles of clearance estimates simulated by the PBPK model. Observed CLmatured and Age50 using the clinical clearance estimates and actual patient age (range from 1 months to 3 years; n = 20, R2 = 0.17) were 19.4 ± 2.2 L/h/70 kg and 0.21 ± 0.10 year (or 2.5 ± 1.2 months), respectively.
Figure 3.
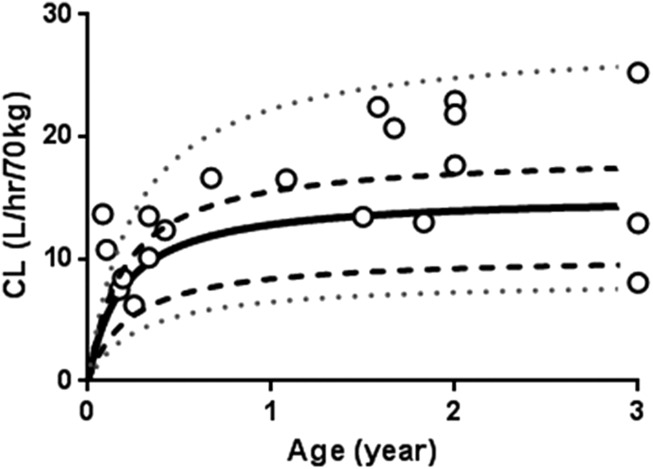
Age-dependent changes in simulated and observed allometrically scaled sirolimus clearance (CL). Individual clearance of sirolimus was simulated in Simcyp version 13/Release 1 with established pediatric physiologically based pharmacokinetic model using 300 virtual healthy pediatric subjects aged from after birth to 3-year-old presented as three independent age groups with 100 virtual subjects aged birth to 1 year, 1–2 years, or 2–3 years. Each line shows the median (bold line), 25 to 75 percentiles (dashed line), and 5 to 95 percentiles (dotted line) data simulations. Each symbol represents the observed clearance value of each patient estimated in MW Pharm (1 months to 3 years, n = 20) according to the method by Scott et al.28
Figure 4 illustrates the concentration-time profiles of sirolimus at steady-state simulated with the PBPK model for three different pediatric age groups (Figure 4: a, birth to 1 year; b, 1-2 year; and c, 2-3 year, 100 virtual healthy pediatric subjects for each age group). The observed sirolimus concentrations fall within the 5–95 percentiles of simulated concentration-time curves, although the 5–95 percentiles ranges indicated substantial predicted between-patient variability.
Figure 4.
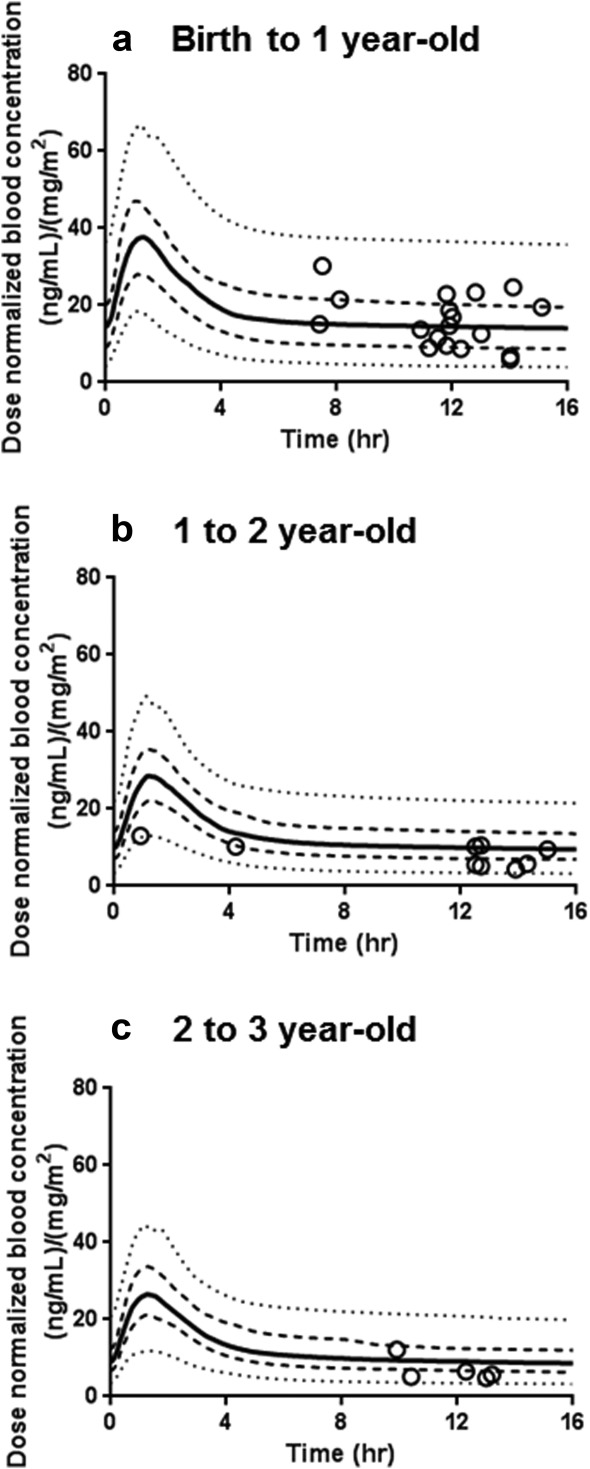
Comparison of observed sirolimus concentrations with the simulated concentration-time profiles with the pediatric physiologically based pharmacokinetic model using virtual healthy pediatric population aged birth to 1 year (a), 1–2 years (b), and 2–3 years (c). In the simulations with the physiologically based pharmacokinetic model, sirolimus was administrated orally at a dose of 1.0 mg/m2 twice a day for 30 days. Each line shows the median (bold line), 25 to 75 percentiles (dashed line), and 5 to 95 percentiles (dotted line) of the simulated data. Each symbol represents the observed concentration in each patient.
DISCUSSION
This is the first study to demonstrate a maturation effect on sirolimus clearance in very young pediatric patients under the age of 2 years. Sirolimus clearance estimates were obtained from a concentration-controlled clinical trial and were analyzed by Bayesian estimation. In our analysis, we separated effects of growth (increase in body size) by allometric scaling after which age related maturation (development of organ function/metabolism) could be explored.9 The relation of allometrically scaled clearance versus age was best described with an Emax model thus allowing the capture of the maturation of sirolimus clearance. This clinical observation was confirmed by in vitro metabolism studies using pediatric liver microsomes. The in vitro metabolism studies indicated that in vitro intrinsic clearance of sirolimus increases in an age-dependent manner and also confirmed that the metabolism of sirolimus is highly dependent on the CYP3A pathway.15 The CYP3A pathway ontogeny as observed for sirolimus was comparable to what has been reported for other CYP3A substrates such as midazolam, alfentanil, and cisapride in neonates.39,40
The pediatric PBPK model was conceptualized and successfully developed as an extension of the adult sirolimus model previously described.17 The pediatric PBPK model was created with the pediatric platform in Simcyp (Version 13 Release 1) which has recently incorporated pediatric physiological parameters. The PBPK model execution using the built-in pediatric populations provided reasonable sirolimus pharmacokinetic profile simulations as judged by the fact that the observed sirolimus concentrations all were within the 5 to 95th prediction interval of PK profile simulations. However, substantial predicted between-patient variability in PK remained. This between-patient variability might be associated not only with sirolimus elimination but also with sirolimus distribution.
Integrating CYP3A ontogeny was essential to adequately describe the observed PK data in patients younger than 2 years of age for the PBPK model. The most recent isoform-dependent ontogeny profiles implemented in Simcyp was used in the current study with the exception of a modified CYP3A5 profile based on reported data (see the Methods section). This appeared to be more relevant than the default setting in the software with the CYP3A5 ontogeny profile assumed to be the same as that of CYP3A4. Interestingly, there is no apparent increase in the hepatic CYP3A5 expression level as a function of age after birth, only a very high degree of interindividual variability,37,38 while CYP3A4 expression gradually increases after birth in an age-dependent manner. Regarding the ontogeny of intestinal CYP3A4, several studies have shown significant increases in CYP3A4 expression and activity as measured by the testosterone 6β-hydroxylase biomarker reaction in fetuses, neonates and children, and relatively low expression in neonates and absence of expression in the fetal duodenum as measured by Western blotting.41 Although the pediatric PBPK model with currently available data provided reasonably good PK parameter estimates, sirolimus clearance estimates simulated with the PBPK model slightly under-predicted the observed clearance values. To address this, further ontogeny investigation is needed specifically for CYP3A5 as well as for intestinal CYP3As. Incorporation of this will likely further improve the model and its predictive performance and address the slight under-prediction of the clearance in the PBPK simulations. Other possible reasons for the under-prediction include the effect of disease status, age-dependent changes in gastric emptying, intestinal transit time in this very young population, and potential contribution of transporter effects related to sirolimus absorption and distribution, which all have not yet been taken into account in the current pediatric PBPK model. Actually, sirolimus has been reported as a substrate of P-glycoprotein (MDR1/ABCB1).35 Underrepresentation of transporter activity and ontogeny in this area as well as further understanding of physiological changes related to the absorption process may explain some of the clearance under-prediction.
Of note, this study efficiently leveraged sirolimus concentration data from a concentration-controlled phase II clinical trial in patients with vascular anomalies14 and clearly illustrated the utility of precise concentration-time data, even though sampling was limited in terms of the number of samples across the dosing interval. A population PK model-based approach allowed estimation of individual apparent clearances to explore the maturation pattern of sirolimus clearance. A similar approach was recently described to facilitate drug development as well as personalized medicine.42
In conclusion, this is the first study to demonstrate a maturation effect on sirolimus clearance. The maturation effect could be explained by the ontogeny of CYP3A pathways. The pediatric sirolimus PBPK model including a maturation function as well as an allometrically scaled body size descriptor allowed for exploration of age-dependent developmental changes in sirolimus clearance. The PBPK model provides new insights into ontogeny mechanisms in pediatric patients and can be used in the design of prospective clinical studies.
Acknowledgments
This study was in part supported by FDA 1R01FD003712. We thank Paula Mobberley-Schuman for facilitating access to the clinical information, Shareen Cox for analytical assistance, and Dr. Tomoyuki Mizuno for valuable scientific input.
Author Contributions
C.E., T.F., T.N.J., and A.A.V. wrote manuscript. C.E., T.F., D.M.A., and A.A.V. designed research. C.E., T.F., D.M.A., and A.A.V. performed research. C.E., T.F., D.M.A., and A.A.V. analyzed data. C.E. contributed new reagents/analytical tools. C.E. and T.F. contributed equally to this work.
Conflict of Interest
T.N.J. is an employee of Simcyp Ltd.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ There is an unmet clinical need for the development of the age-appropriate dosing guidelines for m-TOR inhibitors such as sirolimus in pediatric patients with cancer and blood related diseases.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ We studied the age-related maturation of sirolimus clearance using pediatric in vivo data, demonstrated age-dependent in vitro sirolimus metabolism associated with CYP3A metabolic activity, and developed a pediatric PBPK model to predict sirolimus exposure across the pediatric age spectrum.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ This is the first study to demonstrate a maturation effect on sirolimus clearance in pediatric patients younger than 2 years old. The maturation effect can be explained by the ontogeny of CYP3A pathways. The pediatric sirolimus PBPK model allowed the exploration of age-dependent sirolimus oral clearance.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
✓ This study will help generate sirolimus dosing guidance in very young patients with vascular anomalies. The PBPK approach leverages limited clinical observations will serves as a practical application for the establishment of age-appropriate dosing of sirolimus in pediatric patients.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- Johnson TN, Rostami-Hodjegan A. Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet. 2006;45:931–956. doi: 10.2165/00003088-200645090-00005. [DOI] [PubMed] [Google Scholar]
- Alcorn J. McNamara PJ. Pharmacokinetics in the newborn. Adv. Drug Deliv. Rev. 2003;55:667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS. Kauffman RE. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- Barrett JS, Della Casa Alberighi O, Laer S. Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin. Pharmacol. Ther. 2012;92:40–49. doi: 10.1038/clpt.2012.64. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman SM, Amidon GL, Kaul A, Lukacova V, Vinks AA. Knipp GT. Summary of the National Institute of Child Health and Human Development-best pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop-Pediatric Biopharmaceutics Classification System Working Group. Clin. Ther. 2012;34:S11–S24. doi: 10.1016/j.clinthera.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Takahashi H. Echizen H. Developmental changes in the liver weight- and body weight-normalized clearance of theophylline, phenytoin and cyclosporine in children. Int. J. Clin. Pharmacol. Ther. 2002;40:485–492. doi: 10.5414/cpp40485. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Allegaert K. Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur. J. Pediatr. 2006;165:819–829. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- Holford NH. A size standard for pharmacokinetics. Clin. Pharmacokinet. 1996;30:329–332. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- Anderson BJ. Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- Anderson BJ. Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug. Metab. Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- Holford N. Dosing in children. Clin. Pharmacol. Ther. 2010;87:367–370. doi: 10.1038/clpt.2009.262. [DOI] [PubMed] [Google Scholar]
- Bouzom F. Walther B. Pharmacokinetic predictions in children by using the physiologically based pharmacokinetic modelling. Fundam. Clin. Pharmacol. 2008;22:579–587. doi: 10.1111/j.1472-8206.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Tsamandouras N, Rostami-Hodjegan A. Aarons L. Combining the “bottom-up” and “top-down” approaches in pharmacokinetic modelling: Fitting PBPK models to observed clinical data. Br. J. Clin. Pharmacol. 2015;79:48–55. doi: 10.1111/bcp.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinicaltrials.gov . A phase 2 study - clinical trial assessing efficacy and safety of the mTOR inhibitor sirolimus in the treatment of complicated vascular anomalies ( NCT00975819). < http://clinicaltrials.gov/ct2/show/NCT00975819 > (2014)
- MacDonald A, Scarola J, Burke JT. Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin. Ther. 2000;22 (SupplB):B101–B121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- Hammill AM, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediat Blood Cancer. 2011;57:1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- Emoto C, Fukuda T, Cox S, Christians U. Vinks AA. Development of a physiologically-based pharmacokinetic model for sirolimus: predicting bioavailability based on intestinal CYP3A content. CPT: Pharmacometrics Syst. Pharmacol. 2013;2:e59. doi: 10.1038/psp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen W, Serkova N, Hausen B, Morris RE, Benet LZ. Christians U. Comparison of the in vitro metabolism of the macrolide immunosuppressants sirolimus and RAD. Transplant. Proc. 2001;33:514–515. doi: 10.1016/s0041-1345(00)02116-3. [DOI] [PubMed] [Google Scholar]
- Komori M, et al. Fetus-specific expression of a form of cytochrome P-450 in human livers. Biochemistry 1990. 1990;29:4430–4433. doi: 10.1021/bi00470a024. [DOI] [PubMed] [Google Scholar]
- Nelson DR, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Lacroix D, Sonnier M, Moncion A, Cheron G. Cresteil T. Expression of CYP3A in the human liver–evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur. J. Biochem. 1997;247:625–634. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- Mulliken JB. Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Enjolras O. Mulliken JB. Vascular tumors and vascular malformations (new issues) Adv. Dermatol. 1997;13:375–423. [PubMed] [Google Scholar]
- Redondo P. [Vascular malformations (I). Concept, classification, pathogenesis and clinical features] Actas Dermosifiliogr. 2007;98:141–158. [PubMed] [Google Scholar]
- Adams DM. Wentzel MS. The role of the hematologist/oncologist in the care of patients with vascular anomalies. Pediatr. Clin. North Am. 2008;55:339–355. doi: 10.1016/j.pcl.2008.01.007. viii ( ) [DOI] [PubMed] [Google Scholar]
- Zimmerman JJ. Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J. Clin. Pharmacol. 1997;37:405–415. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Franz DN, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- Scott JR, et al. Population pharmacokinetics of sirolimus in pediatric patients with neurofibromatosis Type 1. Ther. Drug Monit. 2013;35:332–337. doi: 10.1097/FTD.0b013e318286dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost JH. Meijer DK. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput. Biol. Med. 1992;22:155–163. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]
- Walsky RL, Obach RS, Gaman EA, Gleeson JP. Proctor WR. Selective inhibition of human cytochrome P4502C8 by montelukast. Drug Metab. Dispos. 2005;33:413–418. doi: 10.1124/dmd.104.002766. [DOI] [PubMed] [Google Scholar]
- Walsky RL. Obach RS. Validated assays for human cytochrome P450 activities. Drug. Metab. Dispos. 2004;32:647–660. doi: 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- Koal T, Deters M, Casetta B. Kaever V. Simultaneous determination of four immunosuppressants by means of high speed and robust on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;805:215–222. doi: 10.1016/j.jchromb.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Youdim KA, et al. Application of CYP3A4 in vitro data to predict clinical drug-drug interactions; predictions of compounds as objects of interaction. Br. J. Clin. Pharmacol. 2008;65:680–692. doi: 10.1111/j.1365-2125.2007.03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins CL, Jacobsen W, Christians U. Benet LZ. CYP3A4-transfected Caco-2 cells as a tool for understanding biochemical absorption barriers: studies with sirolimus and midazolam. J. Pharmacol. Exp. Ther. 2004;308:143–155. doi: 10.1124/jpet.103.058065. [DOI] [PubMed] [Google Scholar]
- Paine MF, et al. Identification of a novel route of extraction of sirolimus in human small intestine: roles of metabolism and secretion. J. Pharmacol. Exp. Ther. 2002;301:174–186. doi: 10.1124/jpet.301.1.174. [DOI] [PubMed] [Google Scholar]
- Blake MJ, Castro L, Leeder JS. Kearns GL. Ontogeny of drug metabolizing enzymes in the neonate. Semin. Fetal Neonat. Med. 2005;10:123–138. doi: 10.1016/j.siny.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol. Ther. 2008;118:250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Bjorkman S. Prediction of cytochrome p450-mediated hepatic drug clearance in neonates, infants and children: how accurate are available scaling methods? Clin. Pharmacokinet. 2006;45:1–11. doi: 10.2165/00003088-200645010-00001. [DOI] [PubMed] [Google Scholar]
- Kearns GL, et al. Cisapride disposition in neonates and infants: in vivo reflection of cytochrome P450 3A4 ontogeny. Clin. Pharmacol. Ther. 2003;74:312–325. doi: 10.1016/S0009-9236(03)00225-X. [DOI] [PubMed] [Google Scholar]
- Johnson TN. Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J. Pediatr. Gastroenterol. Nutr. 2008;47:3–10. doi: 10.1097/MPG.0b013e31816a8cca. [DOI] [PubMed] [Google Scholar]
- Momper JD. Wagner JA. Therapeutic drug monitoring as a component of personalized medicine: applications in pediatric drug development. Clin. Pharmacol. Ther. 2014;95:138–140. doi: 10.1038/clpt.2013.227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


