Abstract
Introduction
Frailty is a geriatric syndrome characterised by reductions in muscle mass, strength, endurance and activity level. The frailty syndrome, prevalent in 25–50% of patients undergoing cardiac surgery, is associated with increased rates of mortality and major morbidity as well as function decline postoperatively. This trial will compare a preoperative, interdisciplinary exercise and health promotion intervention to current standard of care (StanC) for elective coronary artery bypass and valvular surgery patients for the purpose of determining if the intervention improves 3-month and 12-month clinical outcomes among a population of frail patients waiting for elective cardiac surgery.
Methods and analysis
This is a multicentre, randomised, open end point, controlled trial using assessor blinding and intent-to-treat analysis. Two-hundred and forty-four elective cardiac surgical patients will be recruited and randomised to receive either StanC or StanC plus an 8-week exercise and education intervention at a certified medical fitness facility. Patients will attend two weekly sessions and aerobic exercise will be prescribed at 40–60% of heart rate reserve. Data collection will occur at baseline, 1–2 weeks preoperatively, and at 3 and 12 months postoperatively. The primary outcome of the trial will be the proportion of patients requiring a hospital length of stay greater than 7 days.
Potential impact of study
The healthcare team is faced with an increasingly complex older adult patient population. As such, this trial aims to provide novel evidence supporting a health intervention to ensure that frail, older adult patients thrive after undergoing cardiac surgery.
Ethics and dissemination
Trial results will be published in peer-reviewed journals, and presented at national and international scientific meetings. The University of Manitoba Health Research Ethics Board has approved the study protocol V.1.3, dated 11 August 2014 (H2014:208).
Trial registration number
The trial has been registered on ClinicalTrials.gov, a registry and results database of privately and publicly funded clinical studies (NCT02219815).
Keywords: PREVENTIVE MEDICINE
Strengths and limitations of this study.
Multicentre, randomised trial.
Does not capture urgent or emergent patients with critical cardiac illness.
Does not capture the very frail older patient.
Introduction
Owing to an ageing demographic, older and increasingly frail patients are now being referred for cardiac surgery. In fact, the proportion of patients aged 75 years and older undergoing cardiac procedures increased from 16% in 1990 to up to 25% in most recent estimates.1 The surgical process in this population is complicated by the multiple comorbidities in addition to the underlying cardiac dysfunction presented by these frail patients. Previous studies have demonstrated that frail patients, typically with higher levels of concurrent medical comorbidities, often experience higher rates of postoperative morbidity, mortality and prolonged hospital length of stay (LOS) with associated increased burden on the healthcare system.2–5
Frailty is a geriatric syndrome, defined as an increased vulnerability to stressors leading to a state of decreased physiological resistance.6 7 It is characterised by a “constellation of symptoms and signs [that] describe the heterogeneous response of older adults to physiological and metabolic challenges.”8 While frailty is not necessarily synonymous with chronological age, it is more prevalent among the older adult population and is associated with up to a threefold increased risk of mortality or major morbidity postsurgery.9 A recent systematic review identified that frailty had a strong positive relationship with major adverse cardiac and cerebrovascular events in patients undergoing cardiac surgery (OR=4.89).10 Thus, it has become critically important for healthcare systems to develop strategies designed to improve clinical outcomes in this high-risk population undergoing cardiac surgery.
Since the phenotype of frailty is characterised by reductions in muscle mass, strength, endurance and activity level,7 cardiac rehabilitation (CR) is ideally suited to counteract these impairments and improve an individual's frailty status. A recent review of randomised trials concluded that multidisciplinary programmes were a promising therapeutic strategy for frail, older adults, although none of these specifically targeted these patients preoperatively.11 Thus, there is a strong biological and epidemiological rationale to investigate CR in this setting.
In Canada, when elective patients require cardiac surgery, they are placed on a waiting list for a period ranging from 1 to 4 months. While the wait period for elective patients is generally considered safe, it has been documented that patients on surgical waiting lists engage in very little physical activity as they wait for their procedure.12–14 A lack of physical activity can be problematic in an already deconditioned, frail cohort of patients because, at present, the current standard of care (StanC) does not provide a formal process to engage patients in physical activity during the waiting period. Therefore, the period of time prior to elective cardiac surgery presents a significant opportunity to optimise preoperative risk factors particularly in vulnerable patients, such as the frail, older adult population. The present study aims to reduce frailty in the high-risk, older adult patient to improve postsurgical outcomes of elective cardiac surgery.
CR has been shown to decrease morbidity and mortality in patients with established and unrepaired cardiac disease,14–17 and to be safe for patients who are elderly18–22 and suffer heart failure,16 23–25 in both hospital and community-based settings.15 26 27 A meta-analysis of randomised controlled trials reported that patients attending CR have a 20% and 26% relative reduction in all-cause morbidity and cardiac mortality, respectively, as compared with patients not attending CR.28 The proposed study has been designed according to the evidence-based, best practice guidelines published by the Canadian Association of Cardiac Rehabilitation,29 the recently published Canadian Cardiovascular Society Quality Indicators for CR30 and the guidelines for exercise prescription in patients with cardiovascular disease according to the American College of Sports Medicine.31
Although the benefits of postsurgical CR are well documented, less is known about the role that preoperative exercise may have in the trajectory of a patient undergoing heart surgery. A recent systematic review reported on the benefits of preoperative physical therapy in preventing postoperative pulmonary complications in patients undergoing cardiac surgery.32 Eight randomised controlled trials were included in the review, which found that preoperative physical therapy was effective in reducing postoperative pulmonary complications and length of hospital stay. However, the majority of the interventions used inspiratory muscle training and breathing exercises preoperatively and thus, did not address the potential benefit of exercise and education interventions in this population. In a seminal study by Arthur et al,33 249 low-risk patients awaiting elective coronary artery bypass graft (CABG) surgery were randomised to an 8-week preoperative CR programme or to the StanC. Patients in the intervention group completed two exercise sessions per week and attended additional educational sessions on risk factor modifications. Following surgery, these patients experienced shorter intensive care unit (ICU) and hospital LOS postoperatively and an improved quality of life compared with controls.33 Notably, these promising results persisted for 6 months following surgery, indicating the potential long-term benefit of preoperative risk factor reduction. However, this particular study was conducted in relatively low-risk patients and thus, did not address the unique needs of frail, older adult patients that are now commonly being referred for cardiac surgery.
In a pilot, randomised control trial,12 17 preoperative elective CABG surgery patients were randomised to StanC or to an exercise and health education intervention. The intervention involved a 2-day weekly programme consisting of exercise and education classes for 60 min per session. Walking distance was evaluated using a 6 min walk test (6MWT), while gait speed was evaluated using the 5 m gait speed test. Walking distance remained unchanged in the StanC group; however, the intervention group increased their walking distance by 132 and 145 m at the preoperative and 3 months postoperative assessments, respectively. Gait speed was improved in the intervention group, by 27% and 33% preoperatively and 3 months postoperatively, respectively, while remaining unchanged in the control group. This pilot, randomised controlled trial demonstrated the feasibility of implementing a preoperative exercise protocol to improve the patient's functional capacity postsurgery; however, this study was performed for a relatively small number of younger patients and was designed as a simple feasibility and safety trial.
The Pre-operative Rehabilitation for Reduction of Hospitalization After Coronary Bypass and Valvular Surgery (PREHAB) study is a randomised control trial comparing a preoperative, interdisciplinary, exercise intervention and cardiovascular health promotion intervention to current StanC for the purpose of determining if the programme improves 3-month and 12-month clinical outcomes among a population of frail patients waiting for elective cardiac surgery. The primary objectives of the trial are:
To determine if the PREHAB intervention reduces the proportion of frail older adult patients requiring a prolonged hospital LOS greater than 7 days.
To determine if the PREHAB intervention influences frailty, exercise capacity, physical activity behaviour, in-hospital complications and health-related quality of life (HRQoL).
We hypothesise that the performance of an interdisciplinary programme preoperatively in patients awaiting elective cardiac surgery will reduce the proportion of frail, older adult patients requiring a postoperative hospital LOS greater than 7 days. We also hypothesise that the intervention will: (1) reduce frailty; (2) improve exercise capacity; (3) improve self-managed physical activity behaviour; (4) improve in-hospital outcomes; (5) improve clinical outcomes 3 months and 1 year postoperatively; and (6) improve HRQoL.
Methods and analysis
The PREHAB study is a prospective, randomised, open, blinded end point (PROBE)34 controlled trial using assessor blinding and intention-to-treat analysis. To date, no high-quality study has prospectively examined the impact of preoperative CR in the frail-older adult population undergoing cardiac surgery. Thus, the PREHAB trial has been designed to address a noticeable knowledge gap in the current literature. The trial has been registered on National Institutes of Health ClinicalTrials.gov (NCT02219815). The study is funded by an Operating Grant from the Canadian Institutes of Health Research (326290), which was awarded following an independent peer-review process and supported by Technology Evaluation in the Elderly (TVN). We have used the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) guidelines in reporting this clinical trial.35
Setting
The PREHAB trial will be conducted in three centres across Canada: (1) Winnipeg, Manitoba; (2) Halifax, Nova Scotia and (3) Saint John, New Brunswick. The study will recruit a total of 244 patients or 122 per study arm. Surgical procedures in study participants will be conducted at two academic, tertiary care hospitals (Winnipeg, Manitoba and Halifax, Nova Scotia, Canada) and one non-academic hospital (Saint John, New Brunswick, Canada) that perform cardiac surgery. These study sites were strategically chosen based on similar patient demographics and comparable surgical waiting list time.36 37 Additionally, each of these hospitals are partnered with one or more community-based CR centres, which are certified medical fitness facilities dedicated to improving the health of the community through health promotion, disease prevention and rehabilitation services. These facilities offer expert guidance from certified professionals, innovative health enhancement programmes, and provide integrated medical, rehabilitative and fitness services. The CR centres will provide the infrastructure and programme expertise necessary for the implementation and delivery of the PREHAB intervention.
Participant selection
Inclusion criteria
Patients, aged 65 years or older, undergoing elective isolated CABG, aortic valve repair/replacement for moderate aortic stenosis or severe regurgitation, mitral valve repair/replacement for moderate stenosis or severe regurgitation, or combined/valve procedures.
Patients with Clinical Frailty Score (CFS) ≥4 (vulnerable) and <7 (8=very severely frail, approaching end-of-life or 9=terminally ill) at time of acceptance for cardiac surgery.
Patients with an estimated ≥6 week surgical waiting list time.
Exclusion criteria
- Patients who have unstable or recent unstable cardiac syndrome as defined by:
- Severe heart failure (New York Heart Association Class IV) or angina (Canadian Cardiovascular Society Class IV) symptoms.
- Critical left main coronary disease.
- Hospitalisation for arrhythmias, congestive heart failure or acute coronary syndrome prior to randomisation.
- Patients who have severe left ventricular (LV) obstructive disease as defined by:
- Severe aortic or mitral stenosis (aortic or mitral valve area <1.0 cm2 or mean gradient >40 or >10 mm Hg, respectively).
- Dynamic LV outflow obstruction.
Patients who have demonstrated exercise-induced ventricular arrhythmias or have experienced a recent hospitalisation for arrhythmias.
Patients who have cognitive deficits that would preclude rehabilitation.
Patients who have physical limitations that would preclude rehabilitation.
Patients who are unable to attend the PREHAB programme.
Screening
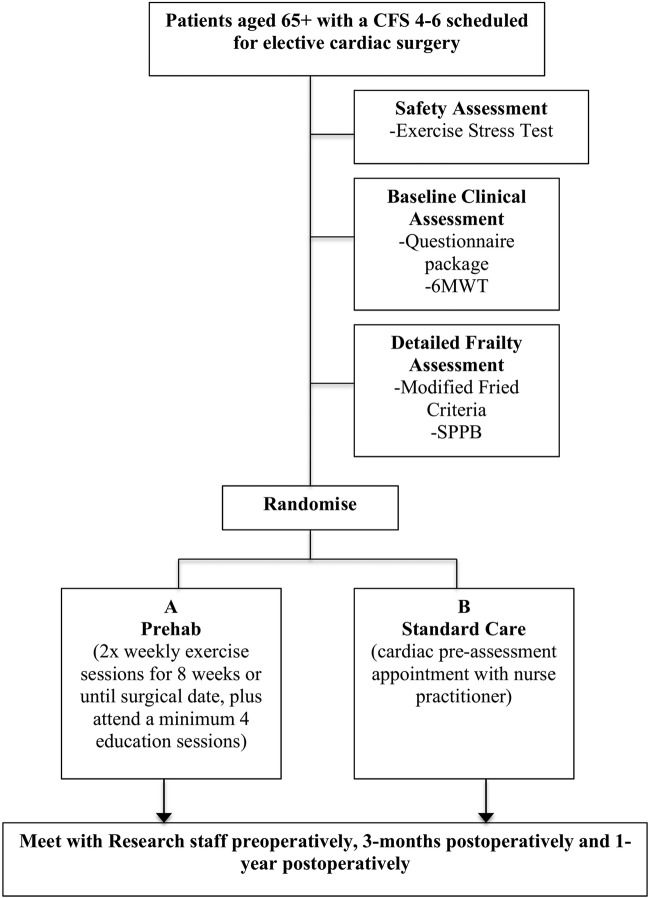
Our strategy for rapid screening of frailty in the large numbers of incoming patients will utilise the nine-point CFS.38 We have defined a CFS of greater than or equal to 4 (classified as ‘vulnerable’) as an initial indicator of frailty. Following training, clinicians will complete a CFS for every new cardiac surgery consult at each of the participating sites. Patients with a CFS of 4–6 will be eligible for enrolment and notification will be sent to the local site coordinator (see figure 1 for patient flow). We will exclude severely frail patients with a CFS greater than or equal to 7 because of the physical limitations in these patients. Furthermore, we anticipate that this criterion will exclude less than 1% of the patients on the elective cardiac surgery wait list.
Figure 1.
Patient flow (CFS, Clinical Frailty Score; 6MWT, 6 min walk test; SPPB, Short Performance Physical Battery Test; PREHAB, Pre-operative Rehabilitation for Reduction of Hospitalization After Coronary Bypass and Valvular Surgery).
A medical director at each site will oversee all cases, and ensure patient eligibility and safety for programme initiation and throughout the duration of the study. All eligible and accepted patients will be evaluated at baseline for safety parameters and to ensure angina stability. Patients who are deemed appropriate for enrolment into the study will be randomised, following completion of the baseline data collection and confirmation of eligibility, to one of two trial arms—control (StanC) or treatment (PREHAB). All patients will undergo a baseline exercise stress test supervised by a cardiologist to ensure patient safety. Safety data, including new hospitalisation, worsening angina or heart failure and arrhythmias will be captured and all adverse events will be reportable to an independent data safety monitoring board (DSMB).
Instrumentation
Preoperative patient demographics, cardiac risk profile, procedure data, surgical risk scores (EuroSCORE II and STS-PROMM),39–41 medication profile, cardiac risk factors and cardiac illness severity are routinely collected for all patients in pre-existing site surgical databases. Each site will also collect covariates of comorbidities using the Charleston Co-morbidity Index, Functional Co-morbidity Index,42 Older Americans Resources and Services scale for activities of daily living (ADL) and instrumental ADL,43 as well as postoperative complications and postoperative hospital LOS. Cognitive function will be measured using the Montreal Cognitive Assessment (MoCA).44 45 The Patient Health Questionnaire-9 (PHQ-9) will assess depressive symptoms46 while the Generalized Anxiety Disorder Scale 7-Item (GAD-7) will be used to assess generalised anxiety disorder.47 The Short Form-12 (SF-12 V.2) and the EuroQual-5D (EQ-5D) will be administered as validated measures of HRQoL.48 49 Also included in the data collection package are cardiac-specific questionnaires—the Seattle Angina Questionnaire (SAQ), Kansas City Cardiomyopathy Questionnaire (KCCQ) and the International Physical Activity Questionnaire (IPAQ). Objectively measured physical activity will be quantified using multidirectional accelerometry (Actical Physical Activity Monitors) for a period of 7 days at each data collection time point. This technique is considered the gold standard for assessing daily physical activity accumulation.50 51 Patients will also undergo a standard 6MWT52–56 and will repeat the 6MWT at the preoperative time point in both the control and PREHAB groups to determine the impact of the intervention on physical capacity. The 6MWT was chosen because it requires minimal infrastructure and personnel to complete, has been demonstrated to be a valid prognostic tool for patient outcomes among cardiac surgery patients,12 57 and is comparable to an exercise stress test.52 54–56 The Modified Fried Criteria6 7 58 will be used to assess changes in frailty, in addition to secondary measures of frailty employing the Short Performance Physical Battery Test (SPPB).59
Assessor blinding
Individuals performing the assessments preoperatively, 3 months postoperatively and 1 year postoperatively will be blinded to the study arm in which the patient was assigned. Additionally, hospital staff, including nurses and surgeons, will be blinded to the study arm in which the patient has been assigned.
Randomisation
Randomisation (with concealed allocation) will be stratified by study location and will occur on a 1:1 basis in permuted blocks.
Standard care
Currently, patients awaiting cardiac surgery receive StanC (see table 1) where they are at present given no specific, routine instruction while awaiting their procedure.
Table 1.
Comparison of PREHAB and standard care
| Standard care | PREHAB intervention |
|---|---|
|
|
PREHAB, Pre-operative Rehabilitation for Reduction of Hospitalization After Coronary Bypass and Valvular Surgery.
Intervention
Patients randomised to the intervention group will receive, in addition to the above StanC, an 8-week comprehensive exercise therapy and education programme at a CR facility (see table 1). This programme will target both the physical, and psychological and social cognitive aspects of cardiac disease and frailty. In brief, participants will be required to complete an intake health status assessment by the CR team, including a physiotherapist, cardiovascular nurse and dietitian, and complete a symptom-limited, graded exercise stress test according to the American College of Sports Medicine Guidelines for Exercise Testing and Prescription.31 This assessment will provide the basis for exercise prescription in our population, which will include an individualised, symptom-limited exercise programme. Intervention group patients will be required to complete at least two sessions of supervised, structured exercise sessions per week for a total period of 8 weeks. Attendance will be monitored by the local CR site. Participants will complete a warm-up programme of approximately 15 min at the beginning of each session. Subsequently, aerobic exercise will be prescribed at 40–60% of heart rate reserve (Karvonen Formula) based on baseline exercise stress test data.60 Aerobic exercise will be approximately 10–30 min in duration, depending on individual tolerance and current level of conditioning. Aerobic exercise prescription will progress to high-intensity exercise in the context of symptom-limited, interval training up to 85% of maximal aerobic capacity based on recommendations by a cardiologist and medical fitness facility staff.29 All individual sessions will be concluded with a 10 min cool down period. In addition to the prescribed exercise programme, patients will be required to participate in four educational sessions tailored towards self-management for CR and healthy living practices. These education sessions will be directly relevant to the patient population and will cover a range of topics, including risk factor reduction, medication use, cardiovascular physiology, smoking cessation, healthy eating, stress management and promotion of self-managed care. Principles of shared decision-making will be utilised with the PREHAB intervention, where training for providers and patients involved in the intervention will have shared control of treatment decisions.61–63
Data collection and management
All participants will meet with the research staff at five time points: (1–2) twice at time of enrolment after the patient is referred for a surgical procedure (ie, baseline preintervention); (3) 1 week preoperatively; (4) 3 months postoperatively and (5) 1 year postoperatively. Data collection will occur from both written and electronic medical record sources (ie, patient information systems, where applicable). Existing perioperative surgical, ICU and hospital data will be used to capture patient demographics, procedure urgency, intraoperative procedure and anaesthetic variables, cardiopulmonary bypass information, blood product utilisation, mechanical ventilation, delirium/coma, ICU and hospital LOS, major adverse events, infection and ICU, and hospital recidivism are collected in institutional databases.
Local research staff will enter de-identified study data onto a trial-specific electronic case report form through REDCap, a secure, web-based application for managing online surveys and databases.64 The data will be housed on a secure server located at the University of Manitoba Faculty of Medicine.
Statistical analysis
The sample size calculation was based on the primary objective of the study—hospital LOS. Preliminary data collected by our group demonstrated the proportion of frail patients with a hospital LOS greater than 7 days to be 58.3%. For the intervention group, based on improvements in functional capacity in our pilot trial12 and previous work by Arthur et al,33 we believe an absolute 20% reduction in the proportion of patients that require prolonged hospital LOS greater than 7 days is feasible. A sample size of 194 individuals (97 per study arm) will be required for a two-tailed test at an α of 0.05 and power of 80%. We are estimating a 20% dropout rate given the target population of the study and thus, plan to recruit 244 patients (122 in each study arm) to achieve an eventual sample size of 194 after dropout.
Objective 1: To determine if PREHAB reduces the proportion of frail older adult patients requiring a prolonged hospital LOS of greater than 7 days. A univariable analysis will be undertaken to determine if hospital LOS greater than 7 days is associated with frailty and other patient-level factors. A stepwise multivariable logistic regression model will then be developed to determine if PREHAB attendance is independently associated with a decreased risk of prolonged hospital LOS greater than 7 days. As a secondary analysis, hospital LOS will also be compared as a continuous variable between PREHAB and the StanC groups using a Mann-Whitney test. A multivariable model for hospital LOS will also be developed utilising generalised linear models with Poisson or Negative Binomial distribution.
Objective 2: Does attending PREHAB influence frailty, exercise capacity, physical activity behaviour, in-hospital complications, and HRQoL? The Modified Fried Criteria will be the primary frailty outcome over time from baseline to preoperative or from baseline to the long-term follow-up assessments. The primary measure of exercise capacity will be the 6MWT and will be analysed with univariable linear regression models to evaluate the association between the 6MWT and PREHAB attendance. Accelerometers will be used to objectively measure the number of minutes spent performing moderate to vigorous physical activity and the total physical activity per day for a period of 7 days and compared between groups. A composite outcome for a major adverse cardiac event (ie, in hospital stroke, myocardial infarction, renal failure requiring dialysis and death) and delirium will be compared between groups using a χ2 or Fisher's exact test. A multivariable logistic regression will be used to identify if PREHAB is independently associated with in-hospital delirium. The SF-12V.2 and EQ-5D scores, after normative standardisation, will be analysed through use of a repeated measures analysis of variance to allow for a standardised group and group/time interaction effect estimation.
Ethics
Ethics approval will be obtained at each individual study site. Any substantial protocol amendments will be sent to the local ethics committee for approval as per standard regulatory requirements at the institution. All study sites will then be informed of the amendments.
Informed consent
During an initial meeting with a cardiac surgeon, eligible and interested patients will be approached by a research assistant (RA) who will provide the patient with further details of the study. The patient will be informed about the trial by the RA and provided with a copy of the patient information and consent form. Patients will be provided with adequate amount of time to consider their participation in the trial and will be given an opportunity to ask questions. If the patient decides to participate in the study, they will be asked to provide written consent, which will then be countersigned by the RA. All participants are free to withdraw from the study at any time, without any prejudice to future medical treatment.
End of trial
The trial will end when 244 patients have been enrolled and the final patient has completed their 1-year follow-up appointment.
Trial monitoring and oversight
The study management and steering committee is responsible for ensuring that the study meets the proposed milestones and deadlines. They will also be responsible for all aspects of the study design, management, ethical conduct, analysis and dissemination of results.
Safety data, including new hospitalisation, worsening angina or heart failure and arrhythmias, will be captured and all adverse events will be reportable to the DSMB. The DSMB is an independent group of experts that advises study investigators. They are responsible to periodically evaluate the study data for participant safety and study conduct, in addition to making recommendations concerning the modification and/or termination of the trial.
Dissemination
Results will be presented at scientific meetings and published in peer-reviewed journals. All publications and presentations related to the study will be authorised and reviewed by the study investigators. Authorship will be determined based on internationally agreed on criteria and journal guidelines for authorship. A major component of the proposed project is to collect information that is appropriate to enable knowledge users to obtain the information they need to inform new initiatives within their own organisations. The integrated knowledge translation (iKT) approach enhances the likelihood that the PREHAB intervention will be implemented in the Canadian healthcare system and be sustainable over the long term. By using an iKT approach and best practice evidence to inform a model of care for frail elderly persons requiring cardiac surgery, it is anticipated that the project will influence the healthcare system in a variety of ways. The study protocol also includes a process for sharing study outcomes with participants. Specifically, study participants will have the option at the time of consent to indicate their interest in receiving a summary of study findings. This summary will then be sent to participants on completion of the study.
Trial status
The study is not yet recruiting patients. The first patient will be recruited in January 2015, and we expect recruitment to be complete in March of 2016. The expected completion date of the project, including all 1-year follow-up appointments, is May 2017.
Acknowledgments
The authors would like to acknowledge the infrastructure support provided by the St. Boniface Hospital Research Centre, and the Health Leisure and Human Performance Research Institute at the University of Manitoba.
Footnotes
Contributors: JA, LJA, SMB, HPG, J-FL, SL, CM, TN, KR, JS, J-AS, NT, NG, AH, TAD and RCA were involved in conception and design of research. NG, AH, TAD and RCA co-lead investigators. ANS, DSK, TAD and RCA obtained ethics approval. ANS and DSK prepared figures. ANS, DSK, TAD and RCA drafted manuscript. ANS, DSK, JA, HPG, SL, J-AS, NT, TAD and RCA edited and revised the manuscript. ANS, DSK, JA, LJA, SMB, HPG, J-FL, SL, CM, TN, KR, JS, J-AS, NT, NG, AH, TAD and RCA approved the final version of the manuscript.
Funding: The work was supported by an Operating Grant from the Canadian Institutes of Health Research, grant number 326290. It is also supported by Technology Evaluation in the Elderly (TVN). TAD was supported by a Manitoba Health Research Council (MHRC) Establishment Grant. ANS was supported by a CIHR Canada Graduate Scholarship. DSK was supported by an MHRC Studentship, a CIHR Strategic Training Initiative in Health Research (STIHR) Knowledge Translation Canada Student Fellowship, a CIHR STIHR Population Intervention for Chronic Disease Prevention Fellowship, and the Heart and Stroke Foundation Dr Dexter Harvey Award.
Competing interests: None.
Ethics approval: The trial protocol V.1.3 and participant information sheets have been reviewed by the University of Manitoba Health Research Ethics Board and have been approved (H2014:208).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1.Afilalo J, Mottillo S, Eisenberg MJ et al. . Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes 2012;5:222–8. 10.1161/CIRCOUTCOMES.111.963157 [DOI] [PubMed] [Google Scholar]
- 2.Green P, Woglom AE, Genereux P et al. . The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv 2012;5:974–81. 10.1016/j.jcin.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DH, Buth KJ, Martin B-J et al. . Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010;121:973–8. 10.1161/CIRCULATIONAHA.108.841437 [DOI] [PubMed] [Google Scholar]
- 4.Singh M, Rihal CS, Lennon RJ et al. . Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes 2011;4:496–502. 10.1161/CIRCOUTCOMES.111.961375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sündermann S, Dademasch A, Rastan A et al. . One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg 2011;13:119–23; discussion 123 10.1510/icvts.2010.251884 [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Ferrucci L, Darer J et al. . Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63. 10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J et al. . Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 8.Quinlan N, Marcantonio ER, Inouye SK et al. . Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc 2011;59(Suppl 2):S262–8. 10.1111/j.1532-5415.2011.03674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afilalo J, Eisenberg MJ, Morin J-F et al. . Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 2010;56:1668–76. 10.1016/j.jacc.2010.06.039 [DOI] [PubMed] [Google Scholar]
- 10.Sepehri A, Beggs T, Hassan A et al. . The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg 2014;148:3110–17. 10.1016/j.jtcvs.2014.07.087 [DOI] [PubMed] [Google Scholar]
- 11.Bibas L, Levi M, Bendayan M et al. . Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis 2014;57:134–43. 10.1016/j.pcad.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Sawatzky J-AV, Kehler DS, Ready AE et al. . Prehabilitation program for elective coronary artery bypass graft surgery patients: a pilot randomized controlled study. Clin Rehabil 2014;28:648–57. 10.1177/0269215513516475 [DOI] [PubMed] [Google Scholar]
- 13.Nery RM, Barbisan JN. Effect of leisure-time physical activity on the prognosis of coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc 2010;25:73–8. 10.1590/S0102-76382010000100016 [DOI] [PubMed] [Google Scholar]
- 14.Mooney M, Fitzsimons D, Richardson G. ‘No more couch-potato!’ Patients’ experiences of a pre-operative programme of cardiac rehabilitation for those awaiting coronary artery bypass surgery. Eur J Cardiovasc Nurs 2007;6:77–83. 10.1016/j.ejcnurse.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Goel K, Lennon RJ, Tilbury RT et al. . Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011;123:2344–52. 10.1161/CIRCULATIONAHA.110.983536 [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Buschmann I, Jaureguizar KV, Calero MJ et al. . Programming exercise intensity in patients on beta-blocker treatment: the importance of choosing an appropriate method. Eur J Prev Cardiol 2014;21:1474–80. 10.1177/2047487313500214 [DOI] [PubMed] [Google Scholar]
- 17.Cote AT, Bredin SSD, Phillips AA et al. . Left ventricular mechanics and arterial-ventricular coupling following high-intensity interval exercise. J Appl Physiol 2013;115:1705–13. 10.1152/japplphysiol.00576.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Audelin MC, Savage PD, Ades PA. Exercise-based cardiac rehabilitation for very old patients (>=75 years): focus on physical function. J Cardiopulm Rehabil Prev 2008;28:163–73. 10.1097/01.HCR.0000320066.58599.e5 [DOI] [PubMed] [Google Scholar]
- 19.Ades PA, Ballor DL, Ashikaga T et al. . Weight training improves walking endurance in healthy elderly persons. Ann Intern Med 1996;124:568–72. 10.7326/0003-4819-124-6-199603150-00005 [DOI] [PubMed] [Google Scholar]
- 20.Suaya JA, Stason WB, Ades PA et al. . Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009;54:25–33. 10.1016/j.jacc.2009.01.078 [DOI] [PubMed] [Google Scholar]
- 21.Rengo G, Galasso G, Vitale DF et al. . An active lifestyle prior to coronary surgery is associated with improved survival in elderly patients. J Gerontol A Biol Sci Med Sci 2010;65:758–63. 10.1093/gerona/glp216 [DOI] [PubMed] [Google Scholar]
- 22.Busch JC, Lillou D, Wittig G et al. . Resistance and balance training improves functional capacity in very old participants attending cardiac rehabilitation after coronary bypass surgery. J Am Geriatr Soc 2012;60:2270–6. 10.1111/jgs.12030 [DOI] [PubMed] [Google Scholar]
- 23.Chrysohoou C, Tsitsinakis G, Vogiatzis I et al. . High intensity, interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. QJM 2014;107:25–32. 10.1093/qjmed/hct194 [DOI] [PubMed] [Google Scholar]
- 24.Zoll J, N'Guessan B, Ribera F et al. . Preserved response of mitochondrial function to short-term endurance training in skeletal muscle of heart transplant recipients. J Am Coll Cardiol 2003;42:126–32. 10.1016/S0735-1097(03)00499-6 [DOI] [PubMed] [Google Scholar]
- 25.Nilsson BB, Westheim A, Risberg MA. Effects of group-based high-intensity aerobic interval training in patients with chronic heart failure. Am J Cardiol 2008;102:1361–5. 10.1016/j.amjcard.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 26.Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005;103:113–17. 10.1159/000083433 [DOI] [PubMed] [Google Scholar]
- 27.Aamot I-L, Forbord SH, Gustad K et al. . Home-based versus hospital-based high-intensity interval training in cardiac rehabilitation: a randomized study. Eur J Prev Cardiol 2014;21:1070–8. 10.1177/2047487313488299 [DOI] [PubMed] [Google Scholar]
- 28.Taylor RS, Brown A, Ebrahim S et al. . Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004;116:682–92. 10.1016/j.amjmed.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Stone JA, Arthur HM; Canadian Association of Cardiac Rehabilitation Guidelines Writing Group. Canadian guidelines for cardiac rehabilitation and cardiovascular disease prevention, second edition, 2004: executive summary. Can J Cardiol 2005;21(Suppl D):3D–19D. [PubMed] [Google Scholar]
- 30.Grace SL, Poirier P, Norris CM et al. . Pan-Canadian development of cardiac rehabilitation and secondary prevention quality indicators. Can J Cardiol 2014;30:945–8. 10.1016/j.cjca.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 31.Thompson PD, Arena R, Riebe D et al. . ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep 2013;12:215–17. 10.1249/JSR.0b013e31829a68cf [DOI] [PubMed] [Google Scholar]
- 32.Hulzebos EHJ, Smit Y, Helders PPJM et al. . Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev 2012;11:CD010118 10.1002/14651858.CD010118.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arthur HM, Daniels C, McKelvie R et al. . Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med 2000;133:253–62. 10.7326/0003-4819-133-4-200008150-00007 [DOI] [PubMed] [Google Scholar]
- 34.Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press 1992;1:113–19. 10.3109/08037059209077502 [DOI] [PubMed] [Google Scholar]
- 35.Chan A-W, Tetzlaff JM, Altman DG et al. . SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamarche Y, Sirounis D, Arora RC et al. . A survey of standardized management protocols after coronary artery bypass grafting surgery in Canadian intensive care units. Can J Cardiol 2011;27:705–10. 10.1016/j.cjca.2011.08.123 [DOI] [PubMed] [Google Scholar]
- 37.Martin B-J, Buth KJ, Arora RC et al. . Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care 2010;14:R171 10.1186/cc9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockwood K, Song X, MacKnight C et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahian DM, O'Brien SM, Filardo G et al. . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3—valve plus coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S43–62. 10.1016/j.athoracsur.2009.05.055 [DOI] [PubMed] [Google Scholar]
- 40.Shahian DM, O'Brien SM, Filardo G et al. . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S2–22. 10.1016/j.athoracsur.2009.05.053 [DOI] [PubMed] [Google Scholar]
- 41.O'Brien SM, Shahian DM, Filardo G et al. . The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg 2009;88:S23–42. 10.1016/j.athoracsur.2009.05.056 [DOI] [PubMed] [Google Scholar]
- 42.Groll DL, To T, Bombardier C et al. . The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595–602. 10.1016/j.jclinepi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 43.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 1981;36:428–34. 10.1093/geronj/36.4.428 [DOI] [PubMed] [Google Scholar]
- 44.Cameron J, Worrall-Carter L, Page K et al. . Screening for mild cognitive impairment in patients with heart failure: Montreal cognitive assessment versus mini mental state exam. Eur J Cardiovasc Nurs 2013;12:252–60. 10.1177/1474515111435606 [DOI] [PubMed] [Google Scholar]
- 45.Dong Y, Lee WY, Basri NA et al. . The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr 2012;24:1749–55. 10.1017/S1041610212001068 [DOI] [PubMed] [Google Scholar]
- 46.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herr NR, Williams JW, Benjamin S et al. . Does this patient have generalized anxiety or panic disorder? The Rational Clinical Examination systematic review. JAMA 2014;312:78–84. 10.1001/jama.2014.5950 [DOI] [PubMed] [Google Scholar]
- 48.Lacson E, Xu J, Lin S-F et al. . A comparison of SF-36 and SF-12 composite scores and subsequent hospitalization and mortality risks in long-term dialysis patients. Clin J Am Soc Nephrol 2010;5:252–60. 10.2215/CJN.07231009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res 1998;7:155–66. 10.1023/A:1008809610703 [DOI] [PubMed] [Google Scholar]
- 50.TROST SG, MCIVER KL, PATE RR. Conducting accelerometer-based activity assessments in field-based research. [Miscellaneous Article]. Med Sci Sports Exerc 2005;37(11 Suppl):S531–43. 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 51.Colley RC, Garriguet D, Janssen I et al. . Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep 2011;22:15–23. [PubMed] [Google Scholar]
- 52.Ross RM, Murthy JN, Wollak ID et al. . The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med 2010;10:31 10.1186/1471-2466-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers J, Prakash M, Froelicher V et al. . Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. 10.1056/NEJMoa011858 [DOI] [PubMed] [Google Scholar]
- 54.Opasich C, De Feo S, Pinna GD et al. . Distance walked in the 6-minute test soon after cardiac surgery: toward an efficient use in the individual patient. Chest 2004;126:1796–801. 10.1378/chest.126.6.1796 [DOI] [PubMed] [Google Scholar]
- 55.Beatty AL, Schiller NB, Whooley MA. Six-minute walk test as a prognostic tool in stable coronary heart disease: data from the heart and soul study. Arch Intern Med 2012;172:1096–102. 10.1001/archinternmed.2012.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enright PL. The six-minute walk test. Respir Care 2003;48:783–5. [PubMed] [Google Scholar]
- 57.Rasekaba T, Lee AL, Naughton MT et al. . The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J 2009;39:495–501. 10.1111/j.1445-5994.2008.01880.x [DOI] [PubMed] [Google Scholar]
- 58.Bergman H, Ferrucci L, Guralnik J et al. . Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol A Biol Sci Med Sci 2007;62:731–7. 10.1093/gerona/62.7.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guralnik JM, Ferrucci L, Pieper CF et al. . Lower extremity function and subsequent disability consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31. 10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 60.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med 1988;5:303–11. 10.2165/00007256-198805050-00002 [DOI] [PubMed] [Google Scholar]
- 61.Glasgow RE, Funnell MM, Bonomi AE et al. . Self-management aspects of the improving chronic illness care breakthrough series: implementation with diabetes and heart failure teams. Ann Behav Med 2002;24:80–7. 10.1207/S15324796ABM2402_04 [DOI] [PubMed] [Google Scholar]
- 62.Glasgow RE, Nutting PA, King DK et al. . A practical randomized trial to improve diabetes care. J Gen Intern Med 2004;19:1167–74. 10.1111/j.1525-1497.2004.30425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681–92. 10.1016/S0277-9536(96)00221-3 [DOI] [PubMed] [Google Scholar]
- 64.Obeid JS, McGraw CA, Minor BL et al. . Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform 2013;46:259–65. 10.1016/j.jbi.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]