Abstract
Objective: Automated clinical decision support (CDS) has shown promise in improving safe medication use. The authors performed a trial of CDS, given both during computerized physician order entry (CPOE) and in response to new laboratory results, comparing the time courses of clinician behaviors related to digoxin use before and after implementation of the alerts.
Design: Alerts were implemented to notify of the potential risk from low electrolyte concentrations or unknown digoxin or electrolyte concentrations during CPOE. Alerts were also generated in response to newly reported hypokalemia and hypomagnesemia in patients given digoxin.
Measurements: Clinician responses to the alerts for six months were compared with responses to similar situations for six months prior to implementation.
Results: During CPOE, checking for unknown serum values increased after implementation compared with control at one hour: 19% vs. 6% for digoxin, 57% vs. 9% for potassium, and 40% vs. 12% for magnesium as well as at 24 hours (p < 0.01 for all comparisons). Electrolyte supplementation increased with newly reported hypokalemia and hypomagnesemia after implementation at one hour: 35% vs. 6% and 49% vs. 5% for potassium and magnesium, respectively, as well as at 24 hours (p < 0.01 for all comparisons). During CPOE, supplementation for hypokalemia was not improved, whereas supplementation for hypomagnesemia improved at one hour (p < 0.05).
Conclusion: Overall, the alerts improved the safe use of digoxin. During CPOE, alerts associated with missing levels were effective. For hypokalemia and hypomagnesemia, the alerts given during CPOE were not as effective as those given at the time of newly reported low electrolytes.
Adverse drug events (ADEs) have been shown to contribute to the morbidity and mortality associated with the treatment of disease and the cost of the care.1,2,3 Many ADEs are preventable, with estimates in the literature ranging from 20% to 69%.4,5,6,7 Preventable ADEs are often the result of medication errors, defined as errors in drug ordering, transcribing, dispensing, administering, or monitoring.8 Medication errors that adversely affect patient outcomes have been estimated to occur in 0.25% of all hospitalized patients.9 Therefore, efforts to reduce medication errors have the ability to lower the rate of ADEs substantially and improve the overall delivery of health care.
Information and knowledge offered to the clinician to facilitate the best decision and thereby reduce medication errors are termed clinical decision support (CDS). Automated CDS systems transform clinical data gathered in an electronic medical record (EMR) as well as expert- or evidence-based practice guidelines into useful patient-specific knowledge to assist clinical decision making. Recent studies demonstrate that computerized physician order entry (CPOE) in conjunction with basic CDS such as drug–allergy and drug–drug interaction checking decreases the likelihood of serious medication errors.10,11
As CPOE use becomes more prevalent in our health care system, it is likely that clinicians will increasingly interact directly with automated CDS systems at the time of, or synchronous to, the ordering process, providing alerts or information directly to the clinician during ordering. However, alerts may also be generated asynchronously to the ordering process, typically related to recently reported abnormal laboratory results.
In settings without CPOE, decision support alerts must be communicated after, or asynchronous to, the ordering process. Although there is a growing set of data regarding the utility of CDS in affecting clinician behavior,11,12,13 there is a need to understand the comparative efficacy of synchronous alerts associated with CPOE compared with asynchronous alerts that have been used more commonly in the past. An understanding of the efficacy of these different types of alerts will be important in the design of future CDS systems.
Digoxin is a drug used in the treatment of heart failure and common supraventricular arrhythmias, particularly atrial fibrillation. Although effective in treating these conditions, relatively minor changes in the dose or clinical status of a patient may dramatically increase the potential for serious, even life-threatening toxicities. Studies have shown that the clinical and economic impact of digoxin toxicity can be worse than the underlying diseases that it is used to treat.14,15,16 ADEs associated with digoxin are, in most cases, the result of medication errors due to drug interactions, existing electrolyte abnormalities, lack of dose adjustment in patients with renal insufficiency, or a combination of all three.14,16 Thus, these adverse events are often predictable and preventable. Appropriate measurement and awareness of digoxin levels, electrolyte concentrations, and renal function minimize the risk of digoxin toxicity. Because the safe use of digoxin is guided, in part, by these quantifiable parameters, it is an excellent target for automated CDS as a means to prevent medication errors and ADEs.17
Our study simultaneously examined both synchronous and asynchronous alerts associated with inpatient use of digoxin. Because one of the potential promises of synchronous alerts in CPOE is a rapid response, we performed a trial of CDS alerts comparing the time courses of clinician behaviors related to the use of digoxin before and after implementation of the alerts. The efficacies of the synchronous and asynchronous alerts, as measured in this trial, can then be contrasted.
Methods
CPOE and CDS Environment
The University of Illinois Hospital and Medical Center utilizes a commercially available EMR (Millennium; Cerner Corporation, Kansas City, MO), which is used as the primary source of presentation of all results and orders to clinicians. All medication and laboratory orders are placed using CPOE, predominantly by resident physicians. The commercially available automated CDS (Discern Expert, Cerner Corporation) has been previously described.17,18
Development of CDS Alerts
A decision support committee consisting of physicians, clinical pharmacists, nurses, information technology personnel, and a Cerner Corporation consultant developed decision support rules to promote the safe use of digoxin. The rules use patient-specific information maintained in the EMR, including potassium and magnesium concentrations, digoxin concentrations, and concomitant medication orders (e.g., amiodarone, quinidine, electrolyte supplementation) to identify potential medication errors associated with digoxin. The standards for clinician behavior were determined by the committee based on both evidence in the literature and knowledge of prior ADEs at our own institution.16 Although it is well known that renal dysfunction plays a large role in digoxin toxicity,14,16 decision support addressing renal function was not available at the time of the study and was therefore not examined.
Synchronous Alerts
The CDS system can be evoked by ordering a medication (digoxin or interacting drug) or in response to abnormal laboratory results that may increase the risk of digoxin toxicity. When digoxin is ordered using CPOE, the CDS interrogates discrete laboratory values within the EMR to ascertain the existence of specific clinical conditions: untreated hypokalemia or hypomagnesemia (K+ < 3.5 mEq/L or Mg2+ < 1.8 mEq/L and without an order for electrolyte replacement), no recent assessment of a digoxin level in patients with prior digoxin use, a digoxin level >2.2 mg/dL in the past 30 days, and the concurrent use of medications known to increase the digoxin level. If any of these clinical conditions are identified, a real-time alert is presented to the ordering clinician, synchronous to the ordering activity. The alert is a pop-up box that is only informational and does not require a response or include the ability to produce an order automatically. The alert suggests the most likely appropriate action for the given clinical scenario; for instance, for hypokalemia, the alert suggests giving the patient potassium supplementation. Images of these alerts have been previously published.17
Asynchronous Alerts
The CDS system can also be evoked in response to abnormal laboratory results that may increase the risk of digoxin toxicity. This asynchronous decision support is employed when a new potassium, magnesium, or digoxin level is posted to the EMR. The CDS considers the new result as well as the patient's concomitant medication orders and, if necessary, generates alerts warning of untreated hypokalemia or hypomagnesemia or elevated digoxin levels in patients receiving digoxin. The alerts are communicated via printout at designated nursing stations and inpatient pharmacies and sent to the electronic clinical inbox of designated clinicians, which had previously self-declared a clinical relationship with the patients when opening the patient-specific EMR. There is no limit to the number of designated clinicians. Typical examples of these clinicians are attending physicians, housestaff, consulting physicians, and pharmacists, among others, but not nurses. The distribution of the printed asynchronous alerts is based on patient location. The printed alerts contain instructions for both clerical staff and nurses regarding communication of the alert content to clinicians able to act on the alert.
Chart Review for Determination of Alert Compliance
Patient charts were reviewed over a six-month study period to determine whether and when a clinician took appropriate action in response to an alert. This chart review was approved by our institutional review board. Only alerts warning of untreated hypokalemia or hypomagnesemia and no recent digoxin, magnesium, or potassium levels were reviewed for alert compliance because these occurred with sufficient frequency and had clearly defined, appropriate clinician responses to allow analysis. The situations evaluated in this study and the corresponding expected clinician actions are described in ▶. Although the charts and alerts were identified electronically, manual review was used to measure clinician responses.
Table 1.
Alerting Situations and Expected Actions
| Alerting Situation | Expected Clinician Action |
|---|---|
| K+ < 3.5 mEq/L, patient receiving digoxin | Order for K+ supplementation |
| Mg2+ < 1.8 mEq/L, patient receiving digoxin | Order for Mg2+ supplementation |
| No recent Mg2+ level, patient receiving digoxin | Order for an Mg2+ level |
| No recent K+ level, patient receiving digoxin | Order for a K+ level |
| No recent digoxin level, patient receiving digoxin currently and in the past | Order for a digoxin level |
K+ = potassium; Mg2+ = magnesium.
A historical cohort was established for the six-month period prior to CDS digoxin alert implementation. For this historical control group, the EMR and an automated searching algorithm were used to identify alerting situations that would have generated an alert if the CDS alerts had been functioning at that time. This algorithm searched through all patients admitted to the hospital during the control period. Identical to the postalert period, manual chart review was used to establish a control rate of clinician responses. The historical control period was from February 15, 2001, through September 7, 2001, and the study period was from September 8, 2001, through March 31, 2002.
Time to clinician action was the primary metric of interest. This time was determined in the same way for the study and control groups. For the synchronous CDS alerts, the time to clinician action was defined as the time between placement of the digoxin order and the appropriate clinician response, if any. For the asynchronous CDS alerts, the time to clinician action was the time between the availability of new results in the EMR and the appropriate clinician response to the clinical situation, if any. Clinician responses were tracked for 24 hours. These response curves are essentially survival curves, typically used in clinical trials. Use of survival curves to measure the effectiveness of alerts has been suggested in the past by Rind et al.19
Statistical Analysis
Kaplan-Meier survival curves were developed describing the proportion of patients with appropriate clinician action taken in response to an alert (or alerting situation) as a function of time. If no clinician action was taken, alerts were censored at 24 hours. These compliance curves were tested for equality using the log-rank statistic.
The proportion of patients for whom an appropriate clinician action occurred was also evaluated at both one and 24 hours, representing both short- and long-term responses to the alerts. This comparison of proportions utilized the χ2 statistic or Fisher's exact test when n was less than 25.
Characteristics of the control and study groups were compared using Student's t-test for continuous variables and the χ2 statistic for proportions. A p-value of 0.05 was chosen for statistical significance of all comparisons.
Results
During the study period, the CDS generated 775 alerts, whereas in the control period, 821 equivalent alerting situations were identified. Characteristics of the control and study groups are shown in ▶. Both the control and study periods included 310 patients, many of whom had more than one alert or alerting situation. The average patient ages in the control and study groups were not statistically different, 61 (standard deviation = 16) years and 59 (standard deviation = 20) years, respectively (p = 0.31). Gender representations in both groups were not statistically different. The average potassium or magnesium levels for the untreated hypokalemia or hypomagnesemia alerts were not statistically different between the control and study groups for either the synchronous or asynchronous alerts.
Table 2.
Control and Study Group Characteristics
| Control Group | Study Group | |
|---|---|---|
| Number of alerts or alerting situations | ||
| Synchronous, no digoxin level | 220 | 169 |
| Synchronous, no K+ level | 35 | 37 |
| Synchronous, no Mg2+ level | 209 | 223 |
| Synchronous, low Mg2+ | 36 | 39 |
| Synchronous, low K+ | 23 | 31 |
| Asynchronous, low Mg2+ | 121 | 136 |
| Asynchronous, low K+ | 177 | 140 |
| Total | 821 | 775 |
| Electrolyte concentrations of alerts or alerting situations | Average (mmol/L) | Average (mmol/L) |
| Synchronous, low Mg2+ | 1.57 ± 0.19 | 1.62 ± 0.12 (NS) |
| Synchronous, low K+ | 3.17 ± 0.32 | 3.24 ± 0.18 (NS) |
| Asynchronous, low Mg2+ | 1.60 ± 0.16 | 1.60 ± 0.16 (NS) |
| Asynchronous, low K+ | 3.17 ± 0.33 | 3.20 ± 0.20 (NS) |
| Critical results for K+ < 3.0 | ||
| Asynchronous, low K+ | 24 (13.5%) | 18 (12.9%) (NS) |
| Number of unique patients | 310 | 310 |
| Age (yr) | 61 ± 16 | 59 ± 20 (NS) |
| % Female | 48% | 53% (NS) |
± correspond to the standard deviation; NS designates no statistically significant difference between the values in the control and study groups.
An independent manual critical laboratory reporting system is used for potassium levels < 3.0 mEq/L. Thus, the CDS system may generate alerts duplicating the efforts of the existing reporting system when the potassium was < 3.0 mEq/L. The proportions of alerts or alerting situations that also engaged the critical reporting system were not different in the control and study periods (13.5% vs. 12.9%, respectively, p = 0.99). Because the critical reporting system was in place and fired with similar frequencies in the control and study periods, the critical reporting system was not considered in this analysis.
The synchronous CDS alerts were displayed to a variety of types of clinicians. Based on the first 115 generated alerts, the receiving clinicians were primarily housestaff physicians (80%); the remainder were pharmacists (9%), nurses (5%), or medical students (5%) placing orders under the supervision of a physician. These data are consistent with those of the operations at our institution, where housestaff physicians place the majority of the orders.
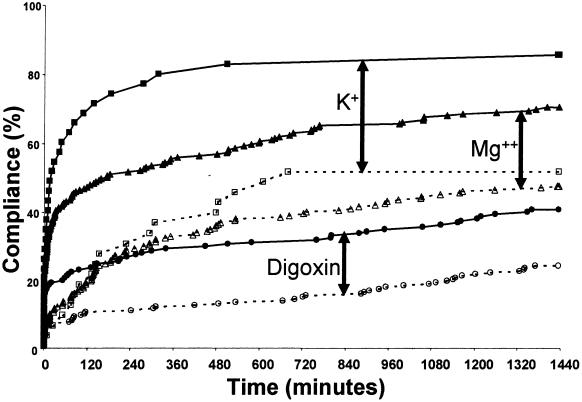
▶ displays the control and study group response rates for each alert at one and 24 hours as well as the compliance curve comparisons. Digoxin and electrolyte concentrations were monitored more frequently after the CDS alerts were instituted than in the control period. Within one hour of ordering digoxin in the control period, only 9% of clinicians had ordered potassium levels and only 12% had ordered magnesium levels compared with 57% and 40%, respectively, after CDS implementation (p < 0.01 for both). At 24 hours, less than half of the control patients had potassium (49%) or magnesium (44%) levels ordered compared with 81% and 66%, respectively, after implementing the CDS alerts (p < 0.01 for both). Ordering of digoxin levels increased from 6% to 19% at one hour and 22% to 38% at 24 hours (p < 0.01 for both). These results are represented as a function of time in ▶. The compliance curves for digoxin, magnesium, and potassium were all improved after alert implementation (p < 0.001 for all).
Table 3.
Response Rates at 1 and 24 Hours
| Compliance (1 hr) |
Compliance (24 hr) |
||||
|---|---|---|---|---|---|
| Alert or Alerting Situation | Control Group (%) | Study Group (%) | Control Group (%) | Study Group (%) | Comparison of Curves* (p-value) |
| Synchronous, no digoxin level | 6 | 19† | 22 | 38† | 0.0003 |
| Synchronous, no K+ level | 9 | 57† | 49 | 81† | 0.0001 |
| Synchronous, no Mg2+ level | 12 | 40† | 44 | 66† | 0.0000 |
| Synchronous, low Mg2+ | 6 | 23‡ | 47 | 56 (NS) | < 0.2 |
| Synchronous, low K+ | 22 | 39 (NS) | 74 | 65 (NS) | < 0.2 |
| Asynchronous, low Mg2+ | 5 | 49† | 70 | 87† | 0.0000 |
| Asynchronous, low K+ | 6 | 35† | 77 | 93† | 0.0000 |
NS designates no statistically significant difference between the values in the control and study groups.
K+ = potassium;
Mg2+ = magnesium.
Comparisons of compliance curves made using log-rank statistic.
p < 0.01 using χ2 test.
p < 0.05 using Fisher's exact test.
Figure 1.
Compliance curves for unknown digoxin, potassium (K+), and magnesium (Mg2+) levels. Proportions of patients with orders checking digoxin (○), K+ (□), and Mg2+ (▵) levels after digoxin was ordered with no recent levels recorded. The x-axis represents time to clinician order for the laboratory level. Clinician response for the control group is represented by the open symbols (○, □, ▵) and dashed lines, and the response for the study group is indicated by the solid symbols (•, ▪, ▴) and solid lines. Responses for the study group were significantly improved for all three alerts (p < 0.001 using the log-rank statistic). Arrows are included for easier identification of each pair of control/study curves.
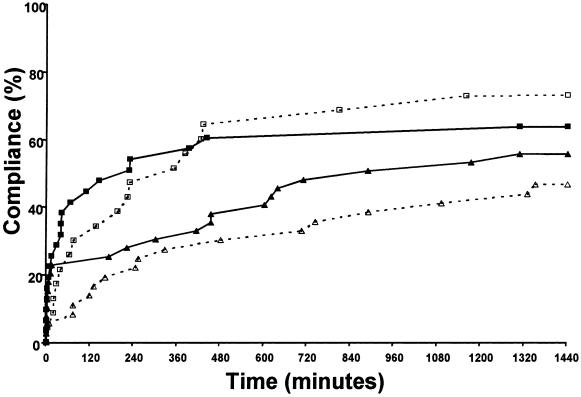
▶ shows the compliance curves for the synchronous alerts associated with untreated hypokalemia and hypomagnesemia. Overall clinician compliance, defined as potassium and/or magnesium supplementation, was not statistically improved by the alerts. However, as indicated in ▶, the proportion of patients with magnesium supplementation at one hour was statistically improved after CDS alert intervention (6% vs. 23%, p < 0.05), although not at 24 hours (47% vs. 56%, p = 0.57).
Figure 2.
Compliance curves for synchronous alerts. Proportion of patients with orders for electrolyte supplementation after digoxin was ordered with a low potassium (K+) (□) or magnesium (Mg2+) (▵) levels. The x-axis represents time to clinician order for supplementation. Clinician response is indicated for the control groups with open symbols (□, ▵) and dotted lines and for the study group with solid symbols (▪, ▴) and solid lines. The respective control versus study group curves are not statistically different, but the supplementation of Mg2+ was higher at one hour using Fisher's exact test.
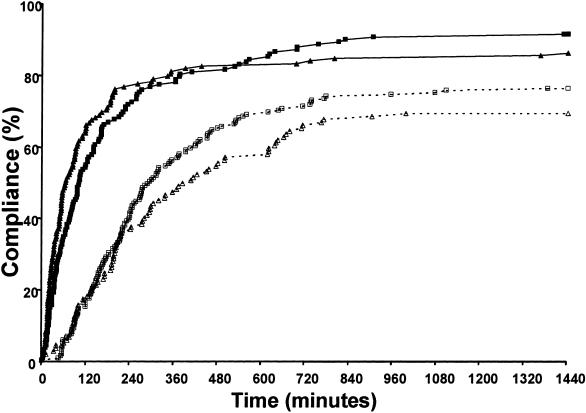
▶ shows the compliance curves for the asynchronous alerts associated with newly reported hypokalemia and hypomagnesemia. Compliance was significantly greater in the CDS study group in both time periods. At one hour, both magnesium and potassium supplementation occurred more frequently in the CDS study group compared with the control group (49% vs. 5% for magnesium and 35% vs. 6% for potassium; p < 0.01 for both). These differences remained significant at 24 hours (87% vs. 70% for magnesium and 93% vs. 77% for potassium; p < 0.01 for both). The overall curves were also improved for magnesium and potassium (p < 0.0001 for both).
Figure 3.
Compliance curves for asynchronous alerts. Proportions of patients with orders for electrolyte supplementation after newly reported low potassium (K+) (□) or magnesium (Mg2+) (▵) levels. The x-axis represents time to clinician order for supplementation. Clinician response is indicated for the control groups with open symbols (□, ▵) and dotted lines and for the study group with solid symbols (▪, ▴) and solid lines. Clinician response significantly improved in both situations with p < 0.0001 using the log-rank statistic.
Discussion
This study evaluated the effectiveness of automated decision support alerts in changing clinician behavior in a CPOE environment. Certain alerts were displayed in real time, directly to the clinician, synchronous to the act of ordering digoxin. These synchronous alerts recommended internally developed appropriate guidelines of care for safe use of digoxin. Asynchronous alerts based on newly reported laboratory results were also generated. Statistical analysis clearly demonstrates that five of the CDS alerts increased the speed of clinician response to the clinical situation and enhanced overall clinician compliance at 24 hours. These results are consistent with the literature, with Rind et al.12 finding a more rapid response to worsening renal dysfunction with asynchronous alerts. A study on synchronous corollary orders also demonstrated improved compliance with institutional practice guidelines for a variety of order types.13
Compliance curves for the control group represent the response of clinicians to the clinical situations prior to alert implementation. The control group curves for the five synchronous alerts (▶ and ▶) indicate that at the time of ordering digoxin, only a limited number of clinicians placed orders appropriate to address the relevant clinical situation. This may be due to the fact that the clinician was not always aware of the clinical situation. Over the successive 24 hours, a more linear trend evolves as more clinicians took appropriate action. However, the curves for the study group demonstrate distinctly different clinician behavior after alert implementation. While ordering digoxin, the clinician was alerted to the clinical situation and was able to take immediate action in response to the alert. The curves for the study group clearly indicate a higher rate of initial compliance after alert generation, with the exception of untreated hypokalemia. It is not clear why the response to hypokalemia did not improve with the alerts.
The synchronous alerts associated with no recent levels of digoxin, magnesium, or potassium (▶) demonstrated improved clinician response and compliance; however, the magnitudes of the responses were not equal. It is interesting to note that clinicians ordered digoxin levels less frequently than potassium and magnesium levels both before and after alert implementation (p < 0.001). In fact, clinician response to the CDS alerts indicating no digoxin level for the study group was actually no better than the clinician response to unknown potassium and magnesium levels in the control group before the CDS alerts were even employed. Clinically, digoxin concentrations are of limited use unless toxicity or medication noncompliance is suspected. Therefore, routine monitoring of digoxin levels may not always be appropriate and could explain the lower rate of clinician compliance with this recommendation.
The control curves for the asynchronous alerting situations (▶) indicate a very limited initial response in the first hour, with gradual increases in compliance over time. Early response is probably related to the likelihood that a clinician will access the EMR and recognize the low electrolyte value. However, our data indicate that clinicians are more likely to treat electrolyte deficiencies and do so much more rapidly after alert implementation. CDS alerts for newly reported hypokalemia in patients being given digoxin have been previously studied, using an automatic paging system to deliver the alert to an appropriate clinician.20 Unfortunately, the study did not provide specific analysis of this hypokalemia alerting system that can be compared with the findings of our study.
The clinician response to low electrolytes prior to alert implementation shows an interesting pattern. The responses to newly reported low magnesium or low potassium were roughly the same (70% vs. 77%, p = 0.2, at 24 hours; control curves in ▶). At the time of ordering digoxin, however, the control responses to low levels of the two electrolytes were very different; there was a higher attentiveness to low potassium than to low magnesium (74% vs. 47%, p = 0.02) after 24 hours (control curves in ▶). It is interesting to speculate on the reason for this difference. While ordering a medication, a clinician needs to actively review the patient's clinical data. If the clinician does not realize that a certain element of data is relevant, the information will not be considered when placing the order. In this case, the compliance curves indicate that clinicians were less aware of the importance of hypomagnesemia in digoxin use than the importance of hypokalemia. However, responding to a newly reported low electrolyte level does not necessarily require a full contextual clinical understanding but rather an appropriate reaction to the new result.
The above discussion emphasizes the importance of showing clinicians all relevant clinical information on the same visual interface used to order medications. It may have been possible to improve the supplementation of magnesium at the time of ordering digoxin by proactively displaying the low magnesium level to the clinician at the initial stages of the ordering process. This proactive approach may be preferred to the annoyance that may be experienced when clinicians are forced to respond to CDS-generated alerts. Users may prefer to be given the data that they need to make good decisions rather than to be criticized or asked to respond when they make poor decisions. Proactive decision support must be further studied to better understand the impact on physician practice patterns.
Analysis of the compliance curves associated with low magnesium or potassium demonstrates that asynchronous alerts were successful in changing clinician behavior, whereas the synchronous alerts generally were not. The reason for this discrepancy is not certain, but several hypotheses are plausible. When an alert reporting a new low electrolyte value is printed to the nursing station, it is verbally communicated by a nurse to the houseofficer. This resulting nurse-to-resident human interaction may be a more effective communication method than an alert window presented to the resident on the computer screen in CPOE. Implicit in this discussion is the culture of an academic teaching hospital. Houseofficers quickly learn to respond to nurse requests, whereas there are no immediate consequences of ignoring an on-screen computer-generated CDS alert. This discrepancy may also be related to the fact that nurses sometimes accept verbal orders for electrolyte supplementation, thus enabling easier compliance with the asynchronous alert recommendation. Thoughtful synchronous CDS alert design may be able to remedy the ease of alert recommendation compliance by enabling ordering directly from the alert window. By including easily initiated orders for supplementation directly in the alert interface, we may find that the compliance with the recommendations for electrolyte supplementation will improve.
Comparing the effectiveness of all the synchronous alerts also provides information regarding how the clinical content of the alert may influence its effectiveness. All the synchronous alerts interacted with the ordering clinician in a visibly identical manner, with a pop-up window of identical size and color and text of identical font and color. The only difference was the clinical content provided by the alert. Of the five synchronous alerts, three were found to be effective in changing behavior, one was minimally effective, and one was not effective at all. One can conclude that the clinical content of the alerts affects their effectiveness.
Although this finding may seem obvious, it is possible that clinicians may ignore the content of these pop-up windows because they have become desensitized to repeated alerts and grow accustomed to ignoring pop-up messages while browsing the Internet and using many different applications that use them. Our data clearly demonstrate that these alerts were at least sometimes read and were efficacious, but obviously we cannot measure the effect of future desensitization, and studies at our institution and others need to address this important question.
The data presented here suggest that the CDS alerts improved both the speed and magnitude of clinician responses. This is clearly evidenced by the asynchronous alerts indicating a low potassium or low magnesium level for patients receiving digoxin. Collectively, a sevenfold improvement in the proportion of patients with an order for electrolyte supplementation was demonstrated at one hour. This dramatic improvement at one hour likely represents the ability for automation to speed up the presentation of the relevant information to clinicians. Laboratory alerts have been previously demonstrated as a means to reduce the time to result notification.12,20,21,22 The 20%–25% improvement noted after 24 hours likely represents the ability of CDS alerts to educate clinicians of the relevance of the new information. By improving clinician response to potentially adverse clinical situations, medication errors and ADEs may be reduced, but more studies are necessary to confirm this hypothesis.
Limitations
The control group was historical rather than concurrent and prospective. The hospital-wide implementation of CDS did not allow a prospective, randomized trial design. Because many houseofficers were associated with both the control and study groups and because no other quality improvement or educational programs were developed related to digoxin use, this limitation in study design is unlikely to mitigate the results.
Another issue with the control group was that the two time periods were different compared with the academic schedule. New housestaff physicians typically begin in July and finish in June, implying they are more experienced clinicians in June than in July. If we count the days of the academic year starting in July, the control period would have an average of 204 days in the academic year and the trial period would be 177 days. Although we do not know a priori what the effect of training would be on clinical response to the situations studied, it is difficult to assume that a reduction in training of this slight amount is the cause of the improved measured response.
The study did not consider CDS alert effectiveness based on the clinician role. As additional studies concerning CDS are published, we may find the effect of alerts on clinician practice may be related to the type of clinician and the level of training (i.e., attending, housestaff, nurse).
Renal dysfunction is also a critical consideration in the safe use of digoxin.1,4,16 CDS addressing renal function was not available and was therefore not examined in this study. We have since implemented CDS for renal dysfunction and are currently studying its effect on digoxin dosing.
Although we were able to show that the asynchronous alerts for electrolyte deficiency were effective and the synchronous alerts were not, the study was not designed to know for certain which aspect of the synchronous versus asynchronous mode of communication caused the difference. Because the verbiage, mode of communication, and activity of the clinicians at the time of the alerts varied slightly, we cannot state for certain which aspects of the alerts account for the differences noted here.
Last, our results do not comment on the clinical impact of CDS alerts. Although clinician compliance was improved by the alerts, we did not measure the impact this had on medication errors or ADEs related to digoxin use. As this technology evolves, outcome analyses must be performed to better assess the clinical utility of such systems.
Conclusion
We developed and implemented CDS for the inpatient use of digoxin in a CPOE environment. Both synchronous CDS alerts generated in real time during CPOE and asynchronous alerts influenced clinician behavior. The alerts generally acted to improve both the speed and overall magnitude of appropriate clinician response to a clinical situation. We were able to measure the effectiveness of both synchronous and asynchronous alerts in a similar clinical scenario, namely, hypomagnesemia or hypokalemia in patients being given digoxin, and found that the asynchronous alerts were effective in promoting electrolyte supplementation, whereas the synchronous alerts were not. Thus, response to the alerts depended on both the manner in which the alert was communicated to the clinician, synchronous or asynchronous, and the clinical content of the alert.
Special thanks to Amy Looi, RN, for technical assistance in the CDS alert development. Portions of this work were presented at the annual meeting of the Society of General Internal Medicine, Atlanta, GA, May 2002.
Dr. Polikaitis was an employee of Cerner Corporation at the time that the research was performed.
References
- 1.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. JAMA. 1997;277:307–11. [PubMed] [Google Scholar]
- 2.Classen DC, Pestotnik SL, Evans S, et al. Adverse drug events in hospitalized patients. JAMA. 1997;277:301–6. [PubMed] [Google Scholar]
- 3.Johnson JA, Bootman JL. Drug-related morbidity and mortality: a cost-of-illness model. Arch Intern Med. 1995;155:1949–56. [PubMed] [Google Scholar]
- 4.Bates DW, Boyle DL, Vander Vliet MD, et al. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10:199–205. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Cullen D, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 6.Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993;8:289–94. [DOI] [PubMed] [Google Scholar]
- 7.Leape LL, Lawthers AG, Brennan TA, Johnson WG. Preventing medical injury. Qual Rev Bull. 1993;19:144–9. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric patients. JAMA. 2001;285:2114–20. [DOI] [PubMed] [Google Scholar]
- 9.Bond CA, Raehl CL, Franke T. Medication errors in United States hospitals. Pharmacotherapy. 2001;21:1023–36. [DOI] [PubMed] [Google Scholar]
- 10.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–6. [DOI] [PubMed] [Google Scholar]
- 11.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. [DOI] [PubMed] [Google Scholar]
- 12.Rind DM, Safran C, Phillips RS, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154:1511–7. [PubMed] [Google Scholar]
- 13.Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of “corollary orders” to prevent errors of omission. J Am Med Inform Assoc. 1997;4:364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G–19G. [DOI] [PubMed] [Google Scholar]
- 15.Roberts SA, Diaz C, Nolan PE, et al. Effectiveness and costs of digoxin treatment for atrial fibrillation and flutter. Am J Cardiol. 1993;72:567–73. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi AJ, Vlasses PH, Morton DJ, Bauman JL. Economic impact of digoxin toxicity. Pharmacoeconomics. 1997;12:175–81. [DOI] [PubMed] [Google Scholar]
- 17.Galanter WL, DiDomenico J, Polikaitis A. Preventing exacerbation of an ADE with automated decision support. J Healthc Inf Manage. 2002;16(4):44–9. [PubMed] [Google Scholar]
- 18.Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events. JAMA. 1998;280:1317–20. [DOI] [PubMed] [Google Scholar]
- 19.Rind DM, Davis R, Safran C. Designing studies of computer-based alerts and reminders. MD Comput. 1995;12:122–6. [PubMed] [Google Scholar]
- 20.Kuperman GJ, Teich JM, Tanasijevic MJ, et al. Improving response to critical laboratory results with automation: results of a randomized controlled trial. J Am Med Inform Assoc. 1999;6:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tate KE, Gardner RM, Weaver LK. A computerized laboratory alerting system. MD Comput. 1990;7:296–301. [PubMed] [Google Scholar]
- 22.Shabot MM, LoBue M, Leyerle BJ, Dubin SB. Decision support alerts for clinical laboratory and blood gas data. Int J Clin Monit Comput. 1990;7(1):27–31. [DOI] [PubMed] [Google Scholar]