Abstract
Background
The aim of this study was to identify the incidence of endolymphatic hydrops using 3-dimensional fluid-attenuated inversion recovery (3D-FLAIR) magnetic resonance imaging (MRI) in the contralateral ear in patients with unilateral Ménière’s disease (MD).
Material/Methods
This was a prospective study. 3D-FLAIR MRI was performed with a 3 Tesla (3 T) unit 24 h after the intratympanic administration of gadolinium (Gd) in 30 unilateral MD patients with an asymptomatic contralateral ear. The incidence of contralateral involvement in unilateral MD patients and the potential correlations between the affected and contralateral ears were analyzed.
Results
Endolymphatic hydrops was observed in 7 of the 30 (23.3%) asymptomatic ears. The mean PTA of the asymptomatic ears in the contralateral hydrops patients (33.0±6.1 dB) was significantly higher compared with the non-hydrops patients (17.8±5.7 dB). The patients with observed contralateral hydrops exhibited a significantly longer duration of the disease compared with the non-hydrops patients (6.7±6.3 vs. 2.9±3.1 years, respectively). Furthermore, the patients with contralateral hydrops had a worse hearing level in the affected ears compared with the non-hydrops patients (70.3±7.4 vs. 52.5±3.8 dB, respectively).
Conclusions
Endolymphatic hydrops is closely related to hearing loss but does not necessarily result in Ménière’s symptoms. Patients with a long history of MD and severe hearing loss in the affected ear are more likely to exhibit endolymphatic hydrops in the asymptomatic contralateral ear. Adequate attention should focus on unilateral MD patients with contralateral ear hydrops because of the potential to develop bilateral MD.
MeSH Keywords: Endolymphatic Hydrops; Hearing Loss, Bilateral; Magnetic Resonance Imaging; Meniere Disease
Background
Recurrent attacks of vertigo, fluctuating hearing loss, tinnitus, and ear fullness are the classic quadruple symptoms of Ménière’s disease (MD). According to research from Japan, Finland, and the USA, the prevalence rates of MD range from 3.5 per 100 000 to 513 per 100 000 individuals [1]. From a global perspective, endolymphatic hydrops that result from the dysregulation of endolymph volume are the main pathological characteristics of MD. According to the 1995 American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) criteria [2], the specific diagnosis of MD relies on histopathological confirmation, but histopathological examination of the temporal bones in living patients is impossible. Therefore, the diagnosis predominately relies on a complete history, physical examination, and diagnostic examinations, such as an electrocochleography (EcochG), a glycerol test, and the cervical vestibular-evoked myogenic potential (cVEMP). In recent years, advances in magnetic resonance imaging (MRI) techniques have enabled the visualization of inner ear structures using a 3-dimensional fluid-attenuated inversion recovery (3D-FLAIR) sequence following the intratympanic injection of gadolinium (Gd). The 3D-FLAIR sequence has been successfully used to detect endolymphatic hydrops in patients with MD, sudden sensorineural hearing loss with vertigo, and acute low-frequency hearing loss [3–5]. The in vivo visualization of endolymphatic hydrops in living patients plays a vital role in the evaluation of the degree and localization of the hydrops.
The relationship between endolymphatic hydrops and Ménière’s symptoms has formed the classic “central hypothesis”, which suggests that many potential factors, such as genetic abnormalities, autoimmune reactions, or allergic responses, lead to endolymphatic hydrops, and that the hydrops generate the symptoms of the disease [6]. According to this theory, all patients with MD should exhibit endolymphatic hydrops, and all patients with endolymphatic hydrops should also exhibit Ménière’s symptoms. However, the investigation of endolymphatic hydrops in animal models has indicated that some guinea pigs with hydrops did not exhibit signs of vertigo [7]. This phenomenon was also confirmed in human temporal bone specimen studies [8]. The primary purpose of this study was to explore the relationship between endolymphatic hydrops and the symptoms of MD in living patients using 3D-FLAIR MRI.
Classic MD typically presents with unilateral symptoms [9]. However, several functional studies have reported abnormal changes in the contralateral side in patients with unilateral MD, even if no clinical symptoms, such as vertigo or hearing loss, were observed [10–14]. The histopathological study of temporal bones also confirmed that the loss of spiral ganglion cells and hair cells on the contralateral side in patients with unilateral MD was significantly greater compared with the normal control group [15]. The application of 3D-FLAIR MRI has enabled the in vivo visualization of hydrops in both the involved and uninvolved ears. Therefore, our second goal was to explore the potential factors that contribute to hydrops on the asymptomatic side.
Material and Methods
Subject selection
From January 2013 to February 2014, clinically diagnosed unilateral MD patients in the Department of Otolaryngology Head and Neck Surgery, Xinhua Hospital, Shanghai Jiaotong University School of Medicine were enrolled in the study. The diagnostic criteria were based on the AAO-HNS [2]. The selection criteria were as follows: 1) unilateral MD patients without symptoms such as tinnitus, ear fullness, or hearing loss in the contralateral ears; 2) a history of acute vertigo attacks in the previous 2 weeks; 3) no lesions of the central nervous system of the inner ear or cerebellopontine angle, which were ruled out by temporal bone computed tomography (CT) and conventional MRI. The exclusion criteria were as follows: 1) chronic otitis media or other middle or inner ear disease history; 2) history of middle or inner ear surgery; 3) receipt of intratympanic gentamicin or dexamethasone treatment; 4) use of vasodilators or diuretics in the previous 2 weeks; 5) patients with claustrophobia, pregnancy, or Gd allergy. Thirty-one patients met the inclusion criteria, which included 20 males and 11 females, aged 24–69 years, with a mean age of 49.9±13.3 years. Seventeen cases were restricted to the left side and 14 cases were restricted to the right side. The duration of the disease ranged from 2 months to 20 years. All patients voluntarily received a tympanic membrane puncture, a tympanic injection of gadolinium, and inner ear 3D-FLAIR MRI scans.
Methods
The protocol of the study was approved by the Ethics Review Committee of Xinhua Hospital, Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from all patients who participated in our study.
All patients received pure-tone audiometry prior to the intratympanic injection of the Gd contrast agent.
Pure-tone audiometry
Pure-tone audiometry was performed in a soundproof room with the use of an audiometer (Type Madsen, Astera, Denmark). The PTA is the average of the 0.5, 1, 2, and 4 kHz air conduction thresholds.
Intratympanic Gd Injection
The Gd contrast medium (Omniscan, Xudonghaipu Pharmaceutical Co. Ltd, Shanghai, China) was diluted 8-fold with saline (v/v, 1: 7). Approximately 0.4–0.6 ml of the diluted Gd was injected intratympanically through the inferior-posterior quadrant of the tympanic membrane bilaterally using a 23-G needle and a 1-mL syringe. The injection was performed under a microscope. The patient then was placed in the supine position for 60 min.
MRI Scan
Twenty-four hours after the Gd injection, MRI scans were performed with a 3 T MR unit (HDX 3.0T MR, GE, USA) using a receive 8-channel phased-array coil. 3D-FLAIR imaging was subsequently performed. The scan parameters for the 3D-FLAIR sequence were as follows: time of repetition=7000 ms, time of echo=120 ms, time of inversion=2200 ms, flip angle=90°, slice thickness=2 mm, echo train length=23, field of view=24 cm, and matrix size=320*320. The number of excitations was 1, and the scan time was 5 min 40 s.
Image processing
The images were evaluated by an experienced neuroradiologist who was blinded to the patient’s information. The vestibule and cochlea were separately evaluated using the following criteria described by Nakashima et al. in their previous study [16]. In brief, in the vestibule, “significant hydrops” indicates that the ratio of the area of the endolymphatic space to the fluid space (sum of the endolymphatic and perilymphatic spaces) exceeded 50%. “Mild hydrops” indicates that the ratio of the area of the endolymphatic space to the fluid space ranged from 33% to 50%. “No hydrops” indicates that the ratio of the area of the endolymphatic space to the fluid space was less than 33%. In the cochlea, “significant hydrops” indicates that the area of the cochlear duct exceeds the area of the scala vestibuli, which reflects a remarkable enlargement of the endolymphatic space. “Mild hydrops” indicates that the area of the cochlear duct is smaller compared with the area of the scala vestibuli, with a displacement of Reissner’s membrane. “No hydrops” indicates no or very mild enlargement of the endolymphatic space with no displacement of Reissner’s membrane or no endolymphatic space observed.
Statistical analysis
The data were analyzed using SPSS 19.0 for Windows (SPSS, Chicago, IL, USA). Student’s t tests were performed. P < 0.05 was considered significant.
Results
Of the 31 patients initially included, 30 were included in the final data analysis; the contrast medium could not be observed in the membrane labyrinth of both ears in the patient excluded from the final analysis, most likely because of the impermeability of the round window membrane. The clinical characteristics and MRI results of the 30 patients are summarized in Table 1. The 30 MD patients exhibited variable degrees of endolymphatic hydrops in the affected ear. On the contralateral side, vestibular hydrops was observed in 7 of the 30 (23.3%) asymptomatic ears, 6 patients (20.0%, patient no. 8, 11, 17, 20, 23, and 27) exhibited mild hydrops and 1 patient (3.3%, patient no. 2) exhibited severe hydrops (Figure 1). An elevation in PTA (≥25 dB) was observed in the 7 patients. The mean PTA (33.0±6.1 dB) of the asymptomatic ears of these 7 patients was significantly higher compared with the patients without hydrops in the asymptomatic ears (17.8±5.7 dB). Furthermore, mild cochlear hydrops was observed in 3 (10.7%) patients in the asymptomatic ears (no. 2, 17, and 20). The mean PTA of these 3 patients was 38.3±4.0 dB. There was no identification of cochlear hydrops without vestibule hydrops. The correlations between the hydrops in the asymptomatic ears and the current status, which included age, duration of the disease, and mean PTA in the affected ears, were also analyzed. The results were not significant (P=0.051) regarding the age between the patients with hydrops in the asymptomatic ears (56.3±8.8 years) and the patients without hydrops in the asymptomatic ears (47.1±13.5 years). The duration of the disease between the 2 groups of the patients was significantly different (6.7±6.3 vs. 2.9±3.1 years, respectively) (P=0.036). The mean PTA was also significantly different (P=0.04) between the 2 patient groups (70.3±7.4 vs. 52.5±3.8 dB, respectively). Figure 2 shows the Maximum Intensity Projection (MIP) image of the original 3D-FLAIR image of patient no. 20. Mild hydrops and no hydrops can be observed in the right affected side. Mild cochlear hydrops and mild vestibular hydrops can be observed, which indicates the contralateral “asymptomatic” ear is involved.
Table 1.
Clinical characteristics of the Ménière’s disease patients.
| Patient number | Age | Sex | Side | Duration (years) | Hearing level in affected side (dB) | Hearing level in asymptomatic side (dB) |
|---|---|---|---|---|---|---|
| 1 | 35 | Male | Left | 5 | 36 | 11 |
| 2 | 57 | Male | Right | 5 | 69 | 43 |
| 3 | 57 | Female | Left | 1.5 | 22 | 20 |
| 4 | 32 | Male | Right | 4 | 90 | 25 |
| 5 | 45 | Female | Right | 10 | 46 | 15 |
| 6 | 58 | Male | Left | 10 | 71 | 18 |
| 7 | 51 | Female | Left | 1 | 38 | 18 |
| 8 | 64 | Male | Left | 20 | 60 | 33 |
| 9 | 48 | Female | Left | 3 | 78 | 19 |
| 10 | 59 | Female | Left | 0.33 | 38 | 23 |
| 11 | 39 | Male | Right | 4 | 70 | 31 |
| 12 | 51 | Male | Left | 0.25 | 33 | 24 |
| 13 | 52 | Male | Left | 3 | 71 | 21 |
| 14 | 61 | Male | Left | 0.17 | 44 | 21 |
| 15 | 62 | Female | Left | 1.5 | 76 | 15 |
| 16 | 59 | Male | Right | 1.5 | 23 | 19 |
| 17 | 66 | Male | Right | 1 | 76 | 36 |
| 18 | 24 | Male | Right | 1 | 39 | 18 |
| 19 | 35 | Female | Right | 6 | 60 | 10 |
| 20 | 58 | Male | Right | 3 | 75 | 36 |
| 21 | 31 | Male | Right | 0.17 | 21 | 15 |
| 22 | 59 | Male | Left | 3 | 60 | 10 |
| 23 | 54 | Male | Left | 6 | 62 | 27 |
| 24 | 65 | Female | Left | 1 | 50 | 15 |
| 25 | 32 | Female | Right | 0.5 | 95 | 10 |
| 26 | 24 | Male | Right | 3 | 35 | 7 |
| 27 | 56 | Female | Left | 8 | 80 | 25 |
| 28 | 34 | Male | Left | 1 | 74 | 24 |
| 29 | 65 | Male | Left | 2 | 53 | 22 |
| 30 | 44 | Male | Right | 9 | 29 | 29 |
Figure 1.
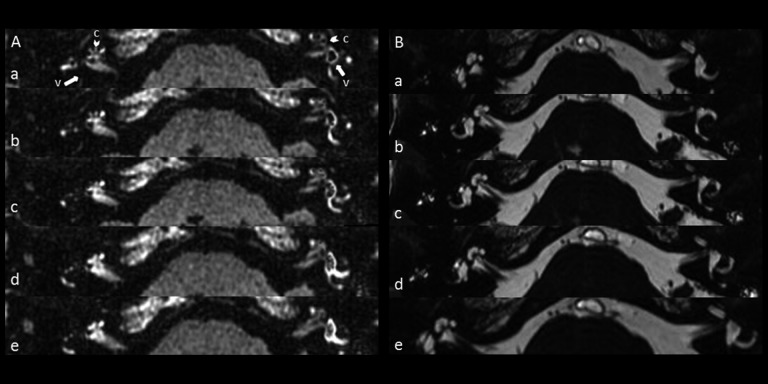
3D-FLAIR MRI and 3D-FIESTA MRI of patient no. 2. (A – a–e) Five consecutive 3D-FLAIR image of patient no. 2. Arrow with letter c represents cochlear. Arrow with letter v represents vestibule. The vestibular part of the perilymph is not enhanced by the contrast agent in the right affected side. The basal and middle turns of the cochlea are partially enhanced. Severe hydrops in the vestibule and mild hydrops in the cochlea can be observed on the left side, which indicates that the “asymptomatic” ear is involved. (B – a–e) Five consecutive 3-dimensional Fast Imaging Employing Steady-State Acquisition (3D-FIESTA) image of the same patients, which indicates a normal inner ear structure.
Figure 2.
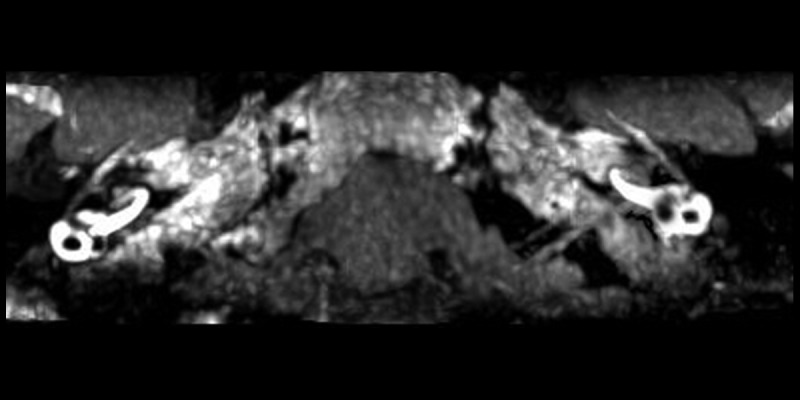
MIP of 3D-FLAIR MRI of patient no. 20. Maximum Intensity Projection (MIP) of original 3D-FLAIR images. The vestibular part of the perilymph is partially enhanced by contrast agent in the right affected side. The cochlea showed no hydrops. Mild hydrops in the vestibular and cochlea can be observed on the left side, which indicates the “asymptomatic” ear is involved.
Discussion
In our study, the imaging of endolymphatic hydrops of the contralateral “asymptomatic ear” in unilateral MD patients was performed. Both vestibular and cochlear endolymphatic hydrops were observed using 3T MRI when assessed 24 h following the intratympanic injection of Gd. Our results revealed that endolymphatic hydrops was observed in 23.3% (7 of 30) of the “asymptomatic ears”. Previous examination of temporal bone autopsies demonstrated a similar proportion of bilateral involvement in MD patients [17]. None of these patients complained of classic Ménière’s symptoms (e.g., fluctuating hearing loss, tinnitus, and ear fullness) in the unaffected side when vertigo occurred. However, a variable degree of endolymphatic hydrops was observed in the affected ear with Ménière’s symptoms in all 30 patients. These results were consistent with the MRI studies conducted by Fiorino and Gürkov [18,19]. Our study found that although endolymphatic hydrops was present in both ears in a patient with unilateral MD, the contralateral ear had not yet displayed clinical signs of MD.
Therefore, our results indicate that endolymphatic hydrops is not necessarily associated with classic Ménière’s symptoms. The results are consistent with the opinion that endolymphatic hydrops should be considered a histologic marker for Ménière’s syndrome rather than directly responsible for its symptoms [6].
Abundant evidence obtained from functional studies has confirmed a high incidence of abnormalities in the contralateral ear in unilateral MD, with reports that range from 15% to 50% [11,14,20,21].
These abnormalities have also been confirmed using temporal bone histopathology [15]. The application of 3D-FLAIR MRI has made the in vivo visualization of the hydrops possible. Many previous studies have concentrated on the affected ears of unilateral MD patients. The correlations between hydrops images and an audiometry functional test of the affected ears had been widely studied [22–24]. However, the in vivo observation of endolymphatic hydrops in the contralateral ear of unilateral MD patients using 3D-FLAIR MRI images has not been previously discussed. These results represent the first study that has focused on the current status of the contralateral ear in unilateral MD patients.
In our study, an elevation of PTA was observed in the “asymptomatic ear” in all 7 patients with endolymphatic hydrops demonstrated by MRI. Three of the 7 patients exhibited vestibular and cochlear hydrops, whereas the other 4 patients only exhibited vestibular hydrops. Previous studies have revealed that endolymphatic hydrops were rarely present segmentally in only the vestibular system, but was often present in only the cochlear portion [25]. One potential rationale for the phenomenon previously discussed may be that 3D-FLAIR MRI using the method presented in the current study could not detect the minor hydrops in the cochlea. However, none of the 7 patients complained of a history of hearing loss. A reasonable explanation for the lack of awareness of hearing change may be that these patients lacked a healthy comparison because the hearing in the affected ears was significantly compromised. Cochlear hydrops had a close correlation with hearing loss, but not necessarily the fluctuating type. Merchant et al. [6] summarized previous animal studies and speculated the mechanism of cochlear hydrops as a dysfunction of type II fibrocytes of the spiral ligament that interfere with the intercellular ionic channels, primarily the recycling of potassium. Potassium plays a vital role in cochlear homeostasis; it is the main ion responsible for the generation of the endocochlear potential. The perturbation of the potassium recycling pathway may result in disordered cochlear homeostasis via osmotic imbalance and eventually lead to auditory dysfunction. These findings suggest that hydrops is the result of disordered cochlear homeostasis rather than the cause. However, the limitation of the previously described animal models is that very limited vestibular dysfunction, which represents a vital feature of MD, has been demonstrated. Takumida and Kakigi [26,27] proposed that only rapid changes in the ionic imbalance accompanied by the dysfunction of the endolymphatic sac (ES) for the regulation of endolymph fluid volume would eventually result in vestibular dysfunction. The existence of endolymphatic hydrops alone may not necessarily result in vestibular dysfunction. Therefore, we hypothesized that a potential explanation for the existence of an “asymptomatic ear” that is accompanied by hearing loss with EH is that the imbalance of ionic exchange that results from many potential factors led to auditory dysfunction. Moreover, the regulatory function of ES at this stage can still compensate for rapid changes in the endolymph fluid volume, which reflects the lack of symptoms.
Several studies have focused on the correlation between the hearing levels in the affected and contralateral ears in unilateral MD patients. Takumida et al. [21] concentrated their attention on the correlation between changes in hearing loss in the affected and contralateral ears of patients with unilateral MD. In their study, patients were followed up from 16 to 161 months. Takumida et al. concluded that the hearing levels in the affected and contralateral ears exhibited a significant correlation. Specifically, the more profound the hearing level in the affected ears, the worse the hearing level in the contralateral ears, and this correlation was more prominent in patients with more severe hearing loss. Perez et al. [28] revealed that the incidence of contralateral audiometric change and even the development of bilateral MD increases with time. In their study, 101 unilateral MD patients were followed up from 2 to 45 years; 4our of the 101 (4%) patients exhibited contralateral audiometric changes at the initial diagnosis. The proportion increased to 5 of 101 (5%) at 2 years; 6 of 96 (6%) at 5 years; 4 of 61 (7%) at 10 years; and 3 of 14 (21%) at 20 years. These tendencies demonstrated that the involvement of the contralateral ear in unilateral MD is closely related to the duration of the disease, as well as the hearing level of the affected ear. In our study, the patients with observed contralateral hydrops exhibited a significantly longer duration of MD compared with the non-hydrops group. Furthermore, the patients with contralateral hydrops had a worse hearing level in the affected ears. House et al. [29] suggested that the mechanism could be analogous to the development of delayed endolymphatic hydrops or sympathetic ophthalmia. Events that lead to the damage of the primary affected ear activate a feedback system between antigens and their receptors. Initially, immunologic tolerance against these antigens can maintain the inner ear homeostasis for a prolonged period of time. However, after many years, the tolerance may be disturbed, and the immunologic response may be reactivated against the inner ear antigens, which leads to the onset of endolymphatic hydrops in the contralateral healthy ear [30–32]. The autoimmune changes ultimately lead to the dysfunction of inner ear fluid homeostasis.
With the advances in the application of intratympanic Gd MRI, clinicians should not only rely on the absence of complaints regarding hearing loss, tinnitus, or aural fullness to exclude the potential involvement of the contralateral side. 3D-FLAIR MRI has revealed that a subset of unilateral MD patients have hydrops in the “asymptomatic” contralateral ear. We suggest that when a diagnosis of unilateral MD is confirmed, the status of the contralateral ear should be routinely evaluated by the examination of symptoms, audiometry, and 3D-FLAIR MRI. One limitation is that 3D-FLAIR MRI with a 3-Tesla unit is only available in a tertiary center. Thus, we recommend that patients with unilateral MD be comprehensively evaluated at specialized institutions. Furthermore, the patients should also be fully informed of the necessity of the examination and the possibility of high costs. Patients should be informed regarding the possibility of developing bilateral MD, particularly when endolymphatic hydrops is observed using 3D-FLAIR MRI. A therapeutic regimen should be carefully developed, especially when endolymphic hydrops is observed in the contralateral ear. In general, ablative surgery, such as vestibular neurectomy or labyrinthectomy, is contraindicated because of the future risks of bilateral vestibular hypofunction. Thus, treatment options are limited. According to a national survey in the USA, the Meniett device was reported as the most common second-line treatment if initial conservative therapy failed [33]. Endolymphatic sac surgery or triple semicircular occlusion surgery may also be considered when conservative therapy is ineffective, because of the preservation of the vestibular nerve in these non-destructive surgeries [34,35]. However, the best therapy for unilateral MD patients with bilateral endolymphatic hydrops involvement has not been determined in the literature. Belichon et al. [36] proposed that unilateral MD patients should be followed for at least 7 years to exclude bilateral involvement before destructive treatment is performed.
One limitation of our study is the small number of patients enrolled. Moreover, the patients were not followed for subsequent observations to determine whether or when the contralateral hydrops ears eventually exhibit symptoms and progress to the development of bilateral MD over time. A close follow-up of these patients is currently ongoing to address these issues. Another limitation of this study is that the obtained images could not attain the highest standard, especially regarding the slice thickness and the scanning time, because of the restricted parameter settings of the MRI machine available at our hospital. We also note that when the images were exported from the workstation, the clarity of the images was worse compared with when they were presented using professional medical monitors in the Department of Radiology. Thus, these drawbacks may affect our results to some extent, and we will improve our methodology to ensure the image quality is improved in future studies.
Conclusions
Based on our findings, endolymphatic hydrops was observed using 3D-FLAIR MRI in the asymptomatic contralateral ear in 23.3% of unilateral MD patients. Endolymphatic hydrops is closely related to hearing loss, but it does not necessarily result in Ménière’s symptoms. Patients with a long history of MD and severe hearing loss in the affected ear are more likely to exhibit endolymphatic hydrops in the asymptomatic contralateral ear. Adequate attention should focus on the potential development of bilateral MD in unilateral MD patients with contralateral ear hydrops prior to selecting the therapeutic regimen.
Footnotes
Source of support: Departmental sources
References
- 1.Harris JP, Alexander TH. Current-day prevalence of Meniere’s syndrome. Audiol Neurootol. 2010;15:318–22. doi: 10.1159/000286213. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:181–85. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima T, Naganawa S, Sugiura M, et al. Visualization of endolymphatic hydrops in patients with Meniere’s disease. Laryngoscope. 2007;117:415–20. doi: 10.1097/MLG.0b013e31802c300c. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Zhang XD, Gu X, et al. Endolymphatic space imaging in idiopathic sudden sensorineural hearing loss with vertigo. Laryngoscope. 2012;122:2265–68. doi: 10.1002/lary.23452. [DOI] [PubMed] [Google Scholar]
- 5.Shimono M, Teranishi M, Yoshida T, et al. Endolymphatic hydrops revealed by magnetic resonance imaging in patients with acute low-tone sensorineural hearing loss. Otol Neurotol. 2013;34:1241–46. doi: 10.1097/MAO.0b013e3182990e81. [DOI] [PubMed] [Google Scholar]
- 6.Merchant SN, Adams JC, Nadol JB., Jr Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Horner KC. Functional changes associated with experimentally induced endolymphatic hydrops. Hear Res. 1993;68:1–18. doi: 10.1016/0378-5955(93)90059-a. [DOI] [PubMed] [Google Scholar]
- 8.Cureoglu S, Schachern PA, Paul S, et al. Cellular changes of Reissner’s membrane in Meniere’s disease: human temporal bone study. Otolaryngol Head Neck Surg. 2004;130:113–19. doi: 10.1016/j.otohns.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Sajjadi H, Paparella MM. Meniere’s disease. Lancet. 2008;372:406–14. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 10.Salvinelli F, Trivelli M, Greco F, et al. Unilateral endolymphatic hydrops: what about the contralateral ear? Rev Laryngol Otol Rhinol (Bord) 2002;123:71–75. [PubMed] [Google Scholar]
- 11.Conlon BJ, Gibson WP. Meniere’s disease: the incidence of hydrops in the contralateral asymptomatic ear. Laryngoscope. 1999;109:1800–2. doi: 10.1097/00005537-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Friedrichs I, Thornton AR. Endolymphatic hydrops in asymptomatic ears in unilateral Meniere’s disease. Laryngoscope. 2001;111:857–60. doi: 10.1097/00005537-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Rauch SD, Zhou G, Kujawa SG, et al. Vestibular evoked myogenic potentials show altered tuning in patients with Meniere’s disease. Otol Neurotol. 2004;25:333–38. doi: 10.1097/00129492-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Brookes GB, Morrison AW, Richard R. Unilateral Meniere’s disease: is the contralateral ear normal? Am J Otol. 1985;6:495–99. [PubMed] [Google Scholar]
- 15.Kariya S, Cureoglu S, Fukushima H, et al. Histopathologic changes of contralateral human temporal bone in unilateral Meniere’s disease. Otol Neurotol. 2007;28:1063–68. doi: 10.1097/MAO.0b013e31815a8433. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima T, Naganawa S, Pyykko I, et al. Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol Suppl. 2009;(560):5–8. doi: 10.1080/00016480902729827. [DOI] [PubMed] [Google Scholar]
- 17.Yazawa Y, Kitahara M. Bilateral endolymphatic hydrops in Meniere’s disease: review of temporal bone autopsies. Ann Otol Rhinol Laryngol. 1990;99:524–28. doi: 10.1177/000348949009900705. [DOI] [PubMed] [Google Scholar]
- 18.Fiorino F, Pizzini FB, Beltramello A, et al. Reliability of magnetic resonance imaging performed after intratympanic administration of gadolinium in the identification of endolymphatic hydrops in patients with Meniere’s disease. Otol Neurotol. 2011;32:472–77. doi: 10.1097/MAO.0b013e31820e7614. [DOI] [PubMed] [Google Scholar]
- 19.Gurkov R, Flatz W, Louza J, et al. In vivo visualization of endolyphatic hydrops in patients with Meniere’s disease: correlation with audiovestibular function. Eur Arch Otorhinolaryngol. 2011;268:1743–48. doi: 10.1007/s00405-011-1573-3. [DOI] [PubMed] [Google Scholar]
- 20.Seo T, Saka N, Sakagami M. Furosemide-loading vestibular evoked myogenic potential testing can suggest developing bilateral involvement of unilateral Meniere’s disease. Acta Otolaryngol. 2012;132:632–36. doi: 10.3109/00016489.2011.653443. [DOI] [PubMed] [Google Scholar]
- 21.Takumida M, Kakigi A, Takeda T, Anniko M. Meniere’s disease: a long-term follow-up study of bilateral hearing levels. Acta Otolaryngol. 2006;126:921–25. doi: 10.1080/00016480500535204. [DOI] [PubMed] [Google Scholar]
- 22.Seo YJ, Kim J, Choi JY, Lee WS. Visualization of endolymphatic hydrops and correlation with audio-vestibular functional testing in patients with definite Meniere’s disease. Auris Nasus Larynx. 2013;40:167–72. doi: 10.1016/j.anl.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Fiorino F, Pizzini FB, Beltramello A, Barbieri F. MRI performed after intratympanic gadolinium administration in patients with Meniere’s disease: correlation with symptoms and signs. Eur Arch Otorhinolaryngol. 2011;268:181–87. doi: 10.1007/s00405-010-1353-5. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Teranishi M, Naganawa S, et al. Relationship between the degree of endolymphatic hydrops and electrocochleography. Audiol Neurootol. 2010;15:254–60. doi: 10.1159/000258681. [DOI] [PubMed] [Google Scholar]
- 25.Paparella MM. The cause (multifactorial inheritance) and pathogenesis (endolymphatic malabsorption) of Meniere’s disease and its symptoms (mechanical and chemical) Acta Otolaryngol. 1985;99:445–51. doi: 10.3109/00016488509108936. [DOI] [PubMed] [Google Scholar]
- 26.Takumida M, Akagi N, Anniko M. Effect of inner ear blood flow changes in Meniere’s model mice. Acta Otolaryngol. 2009;129:244–53. doi: 10.1080/00016480802241980. [DOI] [PubMed] [Google Scholar]
- 27.Kakigi A, Salt AN, Takeda T. Effect of artificial endolymph injection into the cochlear duct on perilymph potassium. ORL J Otorhinolaryngol Relat Spec. 2010;71(Suppl 1):16–18. doi: 10.1159/000265118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez R, Chen JM, Nedzelski JM. The status of the contralateral ear in established unilateral Meniere’s disease. Laryngoscope. 2004;114:1373–76. doi: 10.1097/00005537-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 29.House JW, Doherty JK, Fisher LM, et al. Meniere’s disease: prevalence of contralateral ear involvement. Otol Neurotol. 2006;27:355–61. doi: 10.1097/00129492-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Kitahara T, Maekawa C, Kizawa K, et al. Plasma vasopressin and V2 receptor in the endolymphatic sac in patients with delayed endolymphatic hydrops. Otol Neurotol. 2009;30:812–19. doi: 10.1097/MAO.0b013e3181b11db5. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Hanamitsu M, Kitanishi T, et al. Autoantibodies against inner ear proteins in patients with delayed endolymphatic hydrops and unilateral juvenile deafness. Acta Otolaryngol. 2006;126:117–21. doi: 10.1080/00016480500266008. [DOI] [PubMed] [Google Scholar]
- 32.Harris JP, Aframian D. Role of autoimmunity in contralateral delayed endolymphatic hydrops. Am J Otol. 1994;15:710–16. [PubMed] [Google Scholar]
- 33.Peterson WM, Isaacson JE. Current management of Meniere’s disease in an only hearing ear. Otol Neurotol. 2007;28:696–99. doi: 10.1097/mao.0b013e3180577963. [DOI] [PubMed] [Google Scholar]
- 34.Kitahara T, Kubo T, Okumura S, Kitahara M. Effects of endolymphatic sac drainage with steroids for intractable Meniere’s disease: a long-term follow-up and randomized controlled study. Laryngoscope. 2008;118:854–61. doi: 10.1097/MLG.0b013e3181651c4a. [DOI] [PubMed] [Google Scholar]
- 35.Yin S, Chen Z, Yu D, et al. Triple semicircular canal occlusion for the treatment of Meniere’s disease. Acta Otolaryngol. 2008;128:739–43. doi: 10.1080/00016480701730000. [DOI] [PubMed] [Google Scholar]
- 36.Belinchon A, Perez-Garrigues H, Tenias JM. Evolution of symptoms in Meniere’s disease. Audiol Neurootol. 2012;17:126–32. doi: 10.1159/000331945. [DOI] [PubMed] [Google Scholar]