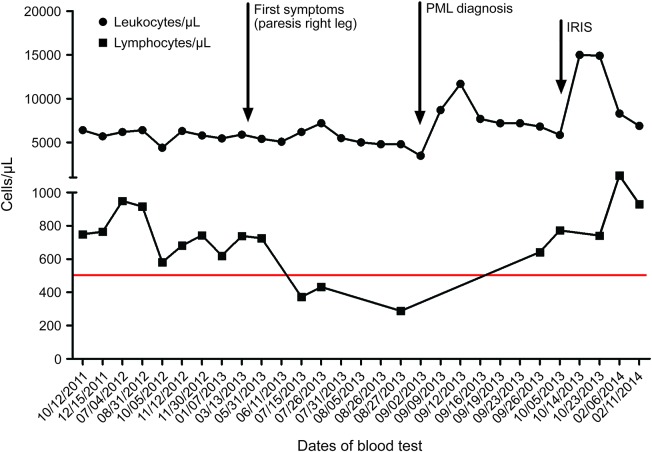
In September 2013, a 69-year-old Caucasian man who was anti–JC virus (JCV) antibody positive was admitted to our hospital with slowly progressing right hemiparesis and aphasia lasting for approximately 6 months. Medical history revealed arterial hypertension, biological aortic valve replacement, and psoriasis vulgaris, treated with 3–6 tablets daily of dimethylfumarate (DMF; 120 mg)/ethylhydrogenfumarate (EHF; 95 mg) (Fumaderm, Biogen Idec, Ismaning, Germany) since December 2008 (table e-1 at Neurology.org/nn). No other immunosuppressive pretreatment had been given. In April/May 2013, the patient recognized a steadily progressing weakness of the right leg. In June 2013, an external diagnosis of ischemic stroke was made. An MRI scan (figure e-1), which was performed after deterioration of clinical symptoms, revealed a subcortical left hemispheric lesion; biopsy demonstrated macrophage-dominated inflammation, dysmorphic astrocytes, simian virus 40 positivity, and several p53- and MiB1-positive cells, suggestive of a JCV encephalitis (figure e-2). JCV DNA was detected in CSF at 2 different time points using a highly sensitive PCR protocol (September 24, 2013, 16 copies/mL; October 7, 2013, 42 copies/mL),1 leading to the diagnosis of progressive multifocal leukoencephalopathy (PML) in September 2013. Further diagnostic workup (table e-2) unmasked toxic bone marrow damage (figure e-3) and an increased excretion of kappa light chains in urine without any evidence for a plasmocytoma. Taking into account the patient's initial presentation with a slowly progressive paresis since April/May 2013 as well as the initial MRI scan (figure e-1), which is compatible with the PML diagnosis, we believe that the onset of PML was in April/May without preexisting leukopenia and only moderate lymphopenia (grade 2 lymphopenia: 724–738 cells/µL, figure). Several weeks later, white blood cell count dropped to a minimum of 4,800 cells/µL with 288 cells/µL lymphocytes under continuous Fumaderm treatment. Fumaderm was discontinued, and treatment with mirtazapine (45 mg/day; Remergil, MSD Sharp und Dohme GmbH, Haar, Germany), mefloquine (250 mg/week; Lariam, Roche Pharma AG, Grenzach-Wyhlen, Germany), and levetiracetam (1,000 mg/day; Keppra, UCB Pharma GmbH, Monheim, Germany) was initiated.2
Figure. Leukocyte and lymphocyte counts.
IRIS = immune reconstitution inflammatory syndrome; PML = progressive multifocal leukoencephalopathy.
One month later, a mild immune reconstitution inflammatory syndrome (IRIS) occurred, with deterioration of the hemiparesis (Karnofsky index [KI] 50%) accompanied by gadolinium enhancement on MRI. Two IV methylprednisolone treatments (each 500 mg/day for 3 days) were given. In June 2014, hemiparesis and aphasia had improved (KI 90%), JCV CSF PCR was negative, and leukocyte and lymphocyte counts had normalized (8,310 cells/µL and 1,240 cells/µL, respectively). Cerebral MRI scan was stable.
In contrast to the previously described cases of Fumaderm- and Psorinovo-associated PML in psoriasis,3,4 we present a case without a preexisting, long-standing, and severe leukopenia/lymphopenia (figure 1) or immunosuppressive pretreatment. In the absence of other discernable myelotoxic factors, bone marrow damage might have been related to Fumaderm treatment. Despite the diagnostic delay, disease course, including IRIS, was mild.3–5 Further studies on potential myelotoxic effects of fumarates and specific effects of DMF vs EHF are warranted. Physicians treating multiple sclerosis patients with DMF should be vigilant for PML as a possible but rare side effect. A long-lasting and presumably severe lymphopenia may especially predispose patients to PML.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank Prof. Dr. med. Anke Reinacher-Schick for her great support in hemato-oncologic diagnostics and Prof. Dr. med. Andrea Tannapfel/Dr. med. M. Gruener for providing pictures of the bone marrow biopsy.
Footnotes
Supplemental data at Neurology.org/nn
Author contributions: R. Hoepner: collected and interpreted the data, drafted and revised the manuscript. S. Faissner: collected and interpreted the data, drafted the manuscript. A. Klasing: interpreted the data, revised the manuscript. R. Schneider: interpreted the data, revised the manuscript. I. Metz: interpreted the neuropathologic findings, critically reviewed the manuscript. B. Bellenberg: interpreted the radiologic data, revised the manuscript. C. Lukas: interpreted the radiologic data, revised the manuscript. P. Altmeyer: interpreted the data, revised the manuscript. R. Gold: interpreted the data, drafted and revised the manuscript. A. Chan: interpreted the data, drafted and revised the manuscript.
Study funding: Research support from the German Ministry for Education and Research (BMBF, German Competence Network Multiple Sclerosis [KKNMS], 01GI0914).
Disclosure: R. Hoepner has received travel funding and speaker honoraria from Biogen Idec and Novartis. S. Faissner has received travel grants from Biogen Idec and Genzyme. A. Klasing and R. Schneider report no disclosures. I. Metz has received speaker honoraria and travel grants from Biogen Idec, Bayer Healthcare, Teva, Serono, and Novartis; and has received research support from Biogen Idec and German Ministry for Education and Research. B. Bellenberg reports no disclosures. C. Lukas is on the scientific advisory board for Biogen Idec, Novartis, and Sanofi; has received travel funding and/or speaker honoraria from Bayer Schering, Novartis, Biogen Idec, Teva, Genzyme, and Sanofi; and has received research support from Merck Serono, Federal Ministry of Education and Research of the Federal Republic of Germany, and Novartis Foundation. P. Altmeyer reports no disclosures. R. Gold is on the scientific advisory board for Teva, Biogen Idec, Bayer Schering, and Novartis; has received speaker honoraria from Biogen Idec, Teva, Bayer Schering, and Novartis; is an editor for Therapeutic Advances in Neurological Disorders; is on the editorial boards for American Journal of Pathology, Journal of Neuroimmunology, and Experimental Neurology; has consulted for Biogen Idec, Elan, Teva, and Chugai Inc; and has received research support from Teva, Biogen Idec, Bayer Schering, Merck Serono, and Novartis. A. Chan has served on the scientific advisory board for Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis Pharma, Sanofi Aventis, and Teva Neuroscience; has received speaker honoraria from Allmirall, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi Aventis, and Teva Neuroscience; was a guest editor for the International Journal of Endocrinology; holds a patent for proteomic profiles of NMO; has consulted for Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi Aventis, and Teva Neuroscience; has received research support from Biogen Idec, Novartis Pharma, Genzyme, German Ministry for Education and Research, and Ruhr University Bochum; and has consulted for Sanofi Aventis for legal proceedings. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004;121:217–221. [DOI] [PubMed] [Google Scholar]
- 2.Hoepner R, Dahlhaus S, Kollar S, et al. Prophylactic antiepileptic treatment reduces seizure frequency in natalizumab-associated progressive multifocal leukoencephalopathy. Ther Adv Neurol Disord 2014;7:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med 2013;368:1657–1658. [DOI] [PubMed] [Google Scholar]
- 4.van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med 2013;368:1658–1659. [DOI] [PubMed] [Google Scholar]
- 5.Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry 2013;84:1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.