Abstract
Background
Hearing loss is one of the most common symptoms of mitochondrial disorders. However, audiological phenotypes associated with different molecular defects in mtDNA are not yet well characterized.
Material/Methods
A large cohort of 1499 nonconsanguineous patients aged 5–40 years with hearing loss of unknown etiology was screened for mutations in mtDNA. For further analysis, patients harboring m.1555A>G and m.3243A>G were selected.
Hearing status of the patients was assessed by pure tone audiometry. Patterns of audiograms (hearing threshold levels at each examined frequency) were statistically compared among the carriers of the m.1555A>G and the m.3243A>G mutations.
Results
We identified 20 patients positive for m.1555A>G mutation and 16 patients positive for m.3243A>G change. The frequency of the above transitions was calculated in our cohort as 1.33% and 1.06%, respectively. Seventeen affected family members carrying the mutations were included into the study.
Typical shape of the audiograms in patients with m.1555A>G mutation presented a ski-slope pattern, whereas the audiometric curves among the m.3243A>G individuals had a pantonal shape (a flat curve) with slight downward sloping at the higher frequencies. The differences were statistically significant.
The onset of hearing loss was noted earlier among m.1555A>G than m.3243A>G patients (12.5 and 26 years, respectively). Aminoglycoside administration was declared in both groups in 11 and 4 cases respectively, and caused abrupt hearing deterioration in all cases.
Conclusions
A pattern of audiogram in patients with mitochondrial deafness may suggest a localization of mtDNA mutation. The pathogenesis of the audiometric differences needs further study.
MeSH Keywords: Deafness; Genes, Mitochondrial; Mitochondrial Diseases
Background
Hearing loss (HL) is a common symptom of mitochondrial diseases, although its audiological characteristics are not fully known. The typical features of HL caused by mtDNA point mutations are sensorineural type (SNHL), cochlear origin, postlingual onset, and progression [1–3].
One well-known type of HL caused by mtDNA defects is the HL associated with the 1555A>G change in 12S rRNA; this and a few other specific mtDNA mutations cause aminoglycoside susceptibility [4–7]. In these cases the main pathomechanism of HL is related to the ototoxic effect of aminoglycosides due to increased similarity of mtDNA caused by the mutation to bacterial DNA (bacterial 16S rRNA is a target of aminoglycosides) [4]. However, HL may appear in patients carrying the m.1555A>G substitution who have no medical history of aminoglycosides treatment. The pathomechanism of HL in the above cases may complement additional intrinsic factors that have been linked to different mtDNA haplogroups [7–9] or modifier nuclear genes [7,8,10,11].
The mechanism of HL caused by the m.3242A>G mutation has not been satisfactorily explained; it probably results from degeneration of high-energy demand structures of the inner ear [2,12,13]. It is postulated that the accumulation of abnormal mitochondria resulting from impaired process of oxidative phosphorylation and an insufficient elimination of free radicals play the major role in the pathomechanism of that degeneration [1,2,12,14].
The aim of the study was to compare the shape of audiograms and audio profiles in patients with mitochondrial hearing loss caused by 2 mtDNA point mutations: m.1555A>G in 12S rRNA and m.3243A>G in tRNAleu.
Material and Methods
Patients
The 1499 archived blood DNA samples of nonconsanguineous patients with post-lingual hearing loss of unknown etiology diagnosed between the ages of 5 to 40 years underwent molecular screening, identifying the most common mitochondrial DNA mutations. The most common nuclear DNA mutations causing hearing loss in the Polish population are: c.35delG (CD972240), c.167delT (CD972241), c.333delAA (CD982678), c.360delGAG (CD993053), c.313del14 (CD982677), and c.1+1G>A (CS991407) of GJB2 gene, and large deletions of GJB6 gene were previously excluded [15]. The nomenclature of the mutations was made according to the Human Gene Mutation Database (Ref Seq: NM_004004).
In the group of 24 carriers of the m.1555A>G transition (including 20 probands), there were 15 females and 9 males. The age of the patients ranged from 5 to 52 years, mean age was 26 years, and there were 9 individuals under 18 years of age. The mean age at onset of hearing loss – 12.5 years – was equal for both sexes.
Among 29 subjects harboring the m.3243A>G mutations (16 probands), 11 were males and 18 were females, aged 5–40 years, mean age 31 years. The mean age at the beginning of hearing loss was 18 for females and 28 for males, respectively.
The clinical characteristics of patients having the m.3243A>G mutation identified from this cohort have already been described (16). Detailed clinical characteristics of the examined patients are shown in Table 1.
Table 1.
Characteristics of the whole examined group of patients with hearing loss (HL) and both identified subgroups: 29 carriers of the m.3243A>G mutation, and 24 subjects positive for the m.1555A>G mutation.
| Parameter | Examined group of HL patients | m.3243A>G mutation carriers | m.1555A>G mutation carriers |
|---|---|---|---|
| Number | 1499 | 29 (16 probands) | 24 (20 probands) |
| Males | 652 (44%) | 11 (35%) | 9 (38%) |
| Females | 844 (56%) | 18 (65%) | 15 (62%) |
| Mean age at the study (years) | 27 | 31 | 26 |
| Mean age of onset of HL (years), males and females, respectively | 15 13/16 SD=10.4 |
26** 18/28 SD=13.7 |
12.5 12.5/12.5 SD=9 |
| Profound hearing loss* | 4% | 3% | 25% |
| Severe hearing loss* | 6% | 14% | 17% |
| Moderate hearing loss* | 44% | 52% | 37% |
| Mild hearing loss* | 46% | 31% | 21% |
| Tinnitus | 399 (27%) | 12 (35%) | 7 (30%) |
| Vertigo | 103 (7%) | 5 (15%) | 0 |
| Progression of hearing impairment | ND | 14 (48%) | 11 (46%) |
| Aminoglycosides administration | ND | 4 (14%) | 11 (46%) |
| Positive family history of hearing loss | 528 (36%) | 33*** (97%) | 16 (67%) |
| Other clinical symptoms | ND | 15 (63%) | 6 (25%) |
| Number of 35delG GJB2 heterozygote | 80 (6%) | 1/16 (6%) | 2/24 (8%) |
Arithmetic mean of 0.5, 1, 2, 4, and 8 kHz for the better-hearing ear; ND=no data;
p<0.000001 vs. patients without m.3243A>G (t-test);
p<0.00001 vs. patients without m.3243A>G (t-test).
Hearing tests
Audiograms of the patients harboring the m.1555A>G mutation in 12S rRNA and the m.3243A>G change in tRNAleu were assessed, analyzed, and compared.
Hearing levels were determined by pure tone audiometry. Hearing threshold level (air and bone conduction) at 500 Hz, 1, 2, 4, and 8 kHz for each ear were analyzed. Evaluation of the degree of hearing loss was based on ANSI (American National Standards Institute) and ISO (International Standards Organization) scale introduced in 1965 (mild hearing loss is up to 40 dB HL, moderate is 41–70 dB HL, severe is HL 71–90 dB HL, and profound is higher than 90 dB HL).
The character of hearing loss – sensorineural hearing loss of the cochlear origin, retro-cochlear, or conductive – was assessed on the basis of the battery of hearing tests: transient evoked otoacoustic emissions, speech audiometry, tympanometry with stapedial reflexes, and brainstem auditory evoked potentials.
Molecular investigations
The search for the m.1555A>G mutation was accomplished using TaqMan Assay on Demand from Life Technology, Foster City, CA, according to the manufacturer’s instructions. Analysis was made on Viia7 Life Technology apparatus. All positive samples were directly sequenced to confirm the presence of the mutation. The comparable technology of searching the m.3243A>G mutation was performed and was previously described with details [16].
The analysis of heteroplasmy rate of the m.3243A>G and m.1555A>G mutations was checked for each patient from the study group by PCR-RFLP. Primers and ApaI (Fermentas, Lithuania) enzyme (a mutation creates a restriction site for this enzyme) were used. Digested products were analyzed on an ABI 3130 capillary sequencer. The heteroplasmy level of the mutation was assessed as the ratio of the counted area under the peak of the mutated/non-mutated DNA. The homoplasmic status of the m.1555A>G mutation was confirmed in all our cases.
Statistical analysis
The Mann-Whitney U test and the t-test for independent samples were applied. All analyses were done using the Statistica 9 software package.
Ethics statement
The bioethics commission of the Institute of Physiology and Pathology of Hearing in Warsaw, Poland approved the study and all subjects or their parents/guardians gave signed consent.
Results
Prevalence of m.1555A>G
The frequency of the m.1555A>G mutation was established in our cohort at 1.3% (20 probands out of 1499) and the prevalence of the m.3243A>G was slightly above 1% (16/1499).
Audiological characteristics of m.1555A>G
Audiometric curves of the m.1555A>G patients revealed a ski-slope pattern. The air and bone conduction hearing threshold in PTA were within the range of 15 dB in all individuals. Brainstem auditory evoked potentials did not reveal retro-cochlear pathology in any case and confirmed audiometric hearing thresholds. Otoacoustic emissions were lost at the audiometric frequencies at which hearing level was above 30 dB HL. Tympanogram type A was detected in each ear, and stapedial reflexes were not registered when hearing threshold exceeded 70 dB HL. The battery of audiometric tests performed in all patients confirmed bilateral SNHL of the cochlear origin in every case. We have not observed any spontaneous progression of hearing threshold of the patients for the period of our observations up to 10 years.
Eleven of the patients with hearing loss caused by the m.1555A>G mutation received therapy with aminoglycosides at any time during the patient’s lifetime. After the treatment, an abrupt onset of hearing deterioration was observed. In 3 patients who were diagnosed with hearing loss before the treatment, the shape of audiometric curve had a ski-slope pattern within the high frequencies. After the treatment with aminoglycosides, the level of hearing decreased in all frequencies. Despite the deterioration of the average hearing threshold, the audiogram remained ski-slope shaped. Interestingly, we noticed a profound hearing loss in 5 treated females and only moderate hearing impairment in 5 males.
The pattern of audiograms of the m.3243A>G mutation carriers presented a pantonal shape (the flat curve) with slight downward sloping at the higher frequencies, as already described [16].
Comparison of HL associated with m.1555A>G and m.3243A>G mutation
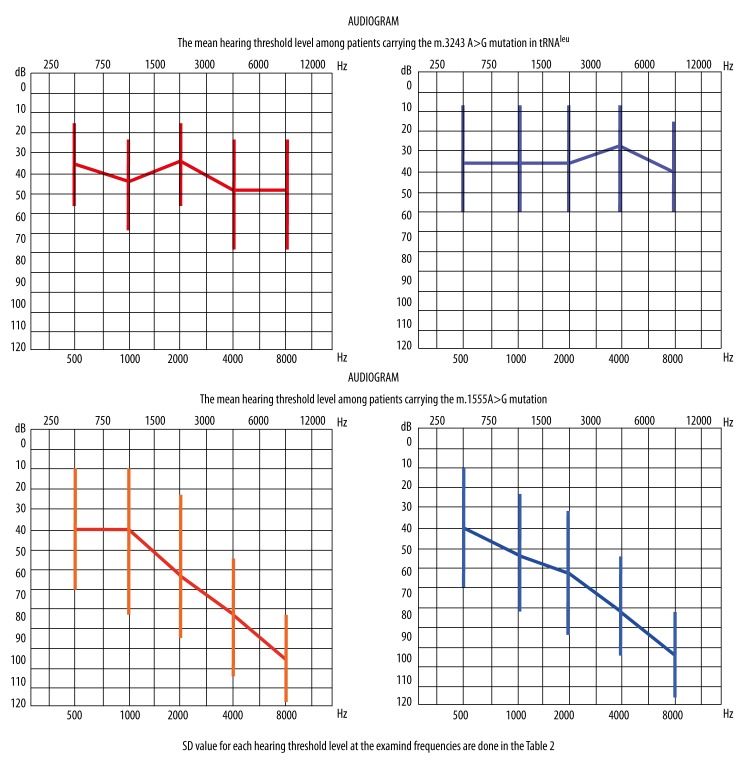
We analyzed and subsequently compared audiograms of the patients by dividing them into 2 groups: individuals harboring m.1555A>G and individuals harboring m.3243A> G. The average hearing threshold level in the groups was established for each frequency and are presented as the arithmetic mean of the audiometric values in dB HL for each member in the group, for the right and left ear separately. The audiograms presented in Figure 1 show the average hearing threshold for each group.
Figure 1.
Mean hearing threshold levels among patients carrying the m.1555A>G and the m.3243A>G mutation.
The average hearing level for each examined frequency was measured in dB and compared to the values obtained for 500 Hz using the non-parametric Mann-Whitney U test for 2 independent groups. Statistically significant results at p<0.05 occurred only in the m.1555A>G group and confirmed the ski-slope audiogram shape (Table 2, statistically significant results marked as *a,*b compared with a,b). An analogous analysis carried out for the hearing thresholds in the group m.3243A>G did not show statistically significant differences, confirming the rather flat pattern of the audiometric curve.
Table 2.
Comparison of the mean hearing threshold level (dBHL) at 0.5; 1; 2; 4 and 8 kHz of the right and the left ear of patients carrying the m.3243A>G mutation and the m.1555A>G mutation.
| 0.5 kHz (dB) | 1 kHz (dB) | 2 kHz (dB) | 4 kHz (dB) | 8 kHz (dB) | ||
|---|---|---|---|---|---|---|
| Right ear | m.1555A>G (n=24) | 39±32a | 48±36 | 61±36*a | 79±27c,*a | 92±20d,*a |
| m.3243A>G (n=29) | 42±21 | 44±25 | 45±26 | 50±27*c | 55±28*d | |
| Left ear | m.1555A>G (n=24) | 33±28b | 44±33 | 60±33*b | 76±27e,*b | 88±23f,*b |
| m.3243A>G (n=29) | 43±27 | 45±29 | 47±29 | 50±29*e | 50±25*f |
The mean ±SD values are given. n – number of examined ears, Mann Whitney U Test from Statistica 9 package was used to check a significant difference between analysed groups.
*a*b*c*d*e*f– statistically significant difference (p<0.05) in comparison to control marked as abcdef.
Average audiometric thresholds for each frequency, separately for the right and left ear, were also compared between the 2 examined groups (m.1555A>G and m.3243A>G). Implementation of the Mann-Whitney U test for 2 independent samples (p<0.05) allowed us to demonstrate similar statistically significant differences for the 4 and 8 kHz frequencies for both ears (Table 2, statistically significant results marked as *c,*d,*e,*f compared with c,d,e,f). There were no statistically significant differences in values measured in dB for the remaining frequencies (0.5, 1, and 2 kHz).
Discussion
This study reveals the differences in audiological phenotype associated with 2 relatively common mtDNA point mutations – m.1555A>G in 12S rRNA and m.3243A>G in tRNAleu.
Audiograms of the patients with the m.1555A>G mutation revealed partial deafness. The curve at low frequencies displayed normal or slightly impaired threshold, but at mild and high frequency hearing was much more impaired. These observations are consistent with previous findings showing audiograms with a downward sloping shape in all examined HL patients carrying the m.1555A>G mutation [9,17]. Similarly, another research group reported hearing impairment at high frequencies in the m.1555A>G mutated individuals [18]. However, the study of a large cohort of 65 Chinese children with hearing loss and harboring the m.1555A>G mutation [7] revealed typical, ski-slope shaped audiometric configurations in only 47 of the examined patients. The remaining 18 audiograms displayed different profile shapes: a flat-shaped pattern in 11 cases, U-shaped pattern in 5, and rising-shaped pattern in 2. It is postulated that the effect of modifier genes responsible for SNHL may explain the discrepancy among audiograms [8,9,11,19]. Audiograms of the patients with a point mutation in the position 3243 in mtDNA were remarkably different. We noticed a rather pantonal shape of the curve, with only slight downward sloping at the highest frequencies [16].
Regarding the hearing thresholds, we observed stable levels in 14 patients carrying the m.1555A>G mutation, in contrast to 10 subjects in whom hearing deterioration strongly corresponded to aminoglycosides administration. Our observations are consistent with those of others who did not find differences in comparing the rate of progression of hearing loss between the m.1555A>G carriers and presbyacusis [8]. Hearing status definitely worsened in 14 out of the 29 m.3243A>G carriers after the following triggers: severe stress, aminoglycosides (in 4 treated cases), pregnancy, noise, or acute inflammatory diseases [16]. The remaining patients with the m.3243A>G mutation presented slow progression of their hearing thresholds. Similar observations were reported in 2 Finnish studies, one by Uimonen et al. who assessed 181 consecutive audiograms from 24 carriers of the m.3243A>G mutation, and another by Majamaa-Voltti et al. presenting a 3-year clinical follow-up study [20,21].
Hearing impairment was the main health problem in every patient from our study group. Among the carriers of the m.1555A>G mutation, 6 patients presented with other clinical symptoms, in our opinion unrelated to hearing loss: 3 of them had hypothyroidism, 2 were diagnosed with high-grade myopia, and the last had a duodenal ulcer. Among individuals with the m.3243A>G mutation, 18 out of 29 patients (62%) were oligosymptomatic; hearing impairment remained their major complaint. Twelve of them presented with isolated hearing loss and 6 others had 1 or 2 additional symptoms, as described previously [16].
The age at onset of hearing impairment was different between our groups. The beginning of hearing impairment among m.1555A>G carriers was 12.5 years of age, much earlier than in subjects having the m.3243A>G transition (26 years of age). Del Castillo et al. failed to estimate the beginning of high-frequency hearing loss in most of the examined carriers of the m.1555A>G mutation, since the probands were not aware of partial deafness [22]. Subsequently, they distinguished 2 subgroups of patients harboring the m.1555A>G, depending on heteroplasmy ratio of the mutation. Interestingly, the majority of those with homoplasmic status, comparable with our group, demonstrated early onset of hearing loss at between 1 and 5 years of age. Analogous results were presented by Hakli et al. [23[. Chen Chi Wu et al. reported 30 individuals with hearing loss caused by the m.1555A>G mutation [9] and found early onset of hearing loss in 23 of them (in 8 individuals under 10 years of age, in the remaining 15 between 10 and 20 years of age). Estivil et al., following Kokotas et al. [4], observed the early beginning of hearing loss (before the age of 5 years) of the m.1555A>G patients treated with aminoglycosides, and later onset (about 20 years of age) of non-treated individuals [24]. This phenomenon was quite distinctive in our group. Interestingly, the age at the beginning of hearing loss among the carriers of the m.3243A>G was much higher. Sue et al. described 14 subjects, including 4 teenagers, with the m.3243A>G, in whom the onset of hearing loss was at 12–65 years of age [2]. In Chinnery’s group, consisting of 17 patients, the age at onset of hearing loss slightly varied between sexes [14]: it was 28 years among women, like in our group, and 26 years among men, manifesting much later than among our males. The results of Chinnery et al. are consistent with the data published by Majamaa-Voltti and Uimonen [20,21].
Aminoglycosides treatment of the carriers of the m.1555A> G point mutation usually caused abrupt hearing deterioration. Ten of 11 of our m.1555A>G patients have confirmed worsening of hearing threshold after administration of aminoglycosides. These antibiotics caused deafness in all treated females from our cohort and partial deafness in an equal number of males. We considered this phenomenon of sex-related hearing phenotype after aminoglycosides treatment as an incidental observation, probably due to other independent factors (e.g., the dose of used antibiotic or the time of treatment). In fact, we do not have not conclusive data on treatment of our patients having the m.1555A>G transition because most patients were treated in childhood in different regions of Poland.
As already mentioned, other intrinsic factors may play the key role in different phenotypes of hearing loss in the m.1555A>G carriers. Lu et al. [8], following Bykhovskaya [25], connected the phenotypic expression of hearing loss of the m.1555A>G carriers with the nuclear region 8p23.1. Another study linked the shape of audiometric curve with 2 mitochondrial tRNA modifier proteins [19]. Fishel-Ghodsian suggested the possible impact of mutations in connexin 26 (GJB2 gene) on the configuration of the audiogram in the m.1555A>G subjects [11]. The above candidates, as is probably the case with many others, may certainly correlate with the audiogram shape of patients harboring the m.1555A>G mutation, regardless of aminoglycosides exposure.
Conclusions
The shape of audiometric curves may be helpful at the early stages of the HL molecular diagnostic procedures. Patients with partial deafness require testing for m.1555A>G, whereas those with slowly progressive hearing deterioration of the flat curve configuration should be tested for the m.3243A>G. The age at diagnosis of hearing loss may also be helpful in the diagnostic process, and it is usually earlier among 1555A>G carriers. The phenomenon of hearing deterioration after aminoglycosides treatment does not allow discriminating between 2 pathogenic mtDNA mutations: m.1555A>G in 12S rRNA and m.3243A>G in tRNA leucine.
Footnotes
Source of support: The study was supported by a grant from the Polish Ministry of Science and High Level Education No. NN 403130 636, NCN 2011/03/D/NZ5/05592
References
- 1.Karkos PD, Waldron M, Johnson IJ. The MELAS syndrome. Review of the literature; the rol of the otologist. Clin Otolaryngol. 2004;29:1–4. doi: 10.1111/j.1365-2273.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 2.Sue CM, Lipsett LJ, Crimmins DS, et al. Cochlear origin of sensorineural deafness in MELAS syndrome. Ann Neurol. 1998;43:350–59. doi: 10.1002/ana.410430313. [DOI] [PubMed] [Google Scholar]
- 3.Sawada S, Takeda T, Kakigi A, et al. Audiological findings of sensorineural deafness associated with a mutation in the mitochondrial DNA. Am J Otol. 1997;18:332–35. [PubMed] [Google Scholar]
- 4.Kokotas H, Petersem MB, Wilems PJ. Mitochondrial deafness. Clin Genet. 2007;71:379–91. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 5.Guan M-X. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion. 2011;11:237–45. doi: 10.1016/j.mito.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Rydzanicz M, Wróbel M, Pollak A, et al. Mutation analysis of mitochondrial 12S rRNA gene in Polish patients with non-syndromic and aminoglycoside-induced hearing loss. Biochem Biophys Res Commun. 2010;395:116–21. doi: 10.1016/j.bbrc.2010.03.149. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Li Z, Zhu Y, et al. Mitochondrial 12S rRNA variants in 1642 Han Chinese pediatric subjects with aminoglycoside-induced and nonsyndromic hearing loss. Mitochondrion. 2010;10:380–90. doi: 10.1016/j.mito.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu SY, Nishio S, Tsukada K, et al. Factors that affect hearing level in individuals with the mitochondrial 1555A>G mutation. Clin Genet. 2009;75:480–84. doi: 10.1111/j.1399-0004.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Chiu YH, Chen PJ, Hsu CJ. Prevalence and clinical features of the mitochondrial m.1555A>G mutation in Taiwanese patients with idiopatic sensorineural hearing loss and association of haplogroup F with low penetrance in three families. Ear Hear. 2007;28:332–42. doi: 10.1097/AUD.0b013e318047941e. [DOI] [PubMed] [Google Scholar]
- 10.Guan MX, Yan Q, Li X, et al. Mutation in TRMU Related to Transfer RNA Modification Modulates the Phenotypic Expression of the Deafness-Associated Mitochondrial 12S Ribosomal RNA Mutations. Am J Hum Gen. 2006;79(2):291–303. doi: 10.1086/506389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischel-Ghodsian N. Mitochondrial deafness. Ear Hear. 2003;24(4):303–13. doi: 10.1097/01.AUD.0000079802.82344.B5. [DOI] [PubMed] [Google Scholar]
- 12.Nadol JB, Merchant SN. Histopathology and molecular genetics of hearing loss in the human. Int J Pediatr Otorhinolaryngol. 2001;61:1–15. doi: 10.1016/s0165-5876(01)00546-8. [DOI] [PubMed] [Google Scholar]
- 13.Forli F, Passetti S, Mancuso M, et al. Mitochondrial Syndromic Sensorineural Hearing Loss. Biosci Rep. 2007;27:113–23. doi: 10.1007/s10540-007-9040-5. [DOI] [PubMed] [Google Scholar]
- 14.Chinnery PF, Elliott C, Green GR, et al. The spectrum of hearing loss due to mitochondrial DNA defects. Brain. 2000;123:82–92. doi: 10.1093/brain/123.1.82. [DOI] [PubMed] [Google Scholar]
- 15.Pollak A, Skórka A, Mueller-Malesińska M, et al. M34T and V37I mutations in GJB2 associated hearing impairment: evidence for pathogenicity and reduced penetrance. Am J Med Genet. 2007;143A(21):2534–43. doi: 10.1002/ajmg.a.31982. [DOI] [PubMed] [Google Scholar]
- 16.Iwanicka-Pronicka K, Pollak A, Skórka A, et al. Post-lingual hearing loss as a mitochondrial 3243A>G mutation phenotype. PloS ONE. 2012;7(10):e44054. doi: 10.1371/journal.pone.0044054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yelverton JC, Arnos K, Xia XJ, et al. The clinical and audiologic features of hearing loss due to mitochondrial mutations. Otolaryngol Head Neck Surg. 2013;148(6):1017–22. doi: 10.1177/0194599813482705. [DOI] [PubMed] [Google Scholar]
- 18.Lehtonen MS, Uimonen S, Hassinen I, Majamaa K. Frequency of mitochondrial DNA point mutation among patients with familial sensorineural hearing impairment. Eur J Hum Genetics. 2000;8:315–18. doi: 10.1038/sj.ejhg.5200455. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Guan MX. A human mitochondrial GTP binding protein related to tRNA modification may modulate phenotypic expression of the deafness-associated mitochondrial 12S rRNA mutation. Molecular Cell Biol. 2002;22:7364–74. doi: 10.1128/MCB.22.21.7701-7711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uimonen S, Moilanen JS, Sorri M, et al. Hearing impairment in patients with 3243A>G mtDNA mutation: phenotype and rate of progression. Hum Genet. 2001;108:284–89. doi: 10.1007/s004390100475. [DOI] [PubMed] [Google Scholar]
- 21.Majamaa-Voltti KA, Winqvist S, Remes AM, et al. A 3-year clinical follow-up of adult patients with 3243 A>G in mitochondrial DNA. Neurology. 2006;66:1470–75. doi: 10.1212/01.wnl.0000216136.61640.79. [DOI] [PubMed] [Google Scholar]
- 22.del Castillo FJ, Rodríguez-Ballesteros M, Martín Y, et al. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non-syndromic hearing loss. J Med Genet. 2003;40(8):632–36. doi: 10.1136/jmg.40.8.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Häkli S, Luotonen M, Sorri M, Majamaa K. Audiological follow-up of children with the m.1555A>G mutation in mitochondrial DNA. Audiol Neurootol. 2013;18(1):23–30. doi: 10.1159/000342905. [DOI] [PubMed] [Google Scholar]
- 24.Estivill X, Govea N, Barceló E, et al. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet. 1998;62(1):27–35. doi: 10.1086/301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bykhovskaya Y, Mengesha E, Fischel-Ghodsian N. Phenotypic expression of maternally inherited deafness is affected by RNA modification and cytoplasmic ribosomal proteins. Mol Genet Metab. 2009;97(4):297–304. doi: 10.1016/j.ymgme.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]