Abstract
Although there are ongoing intensive research efforts, no effective pharmacological therapies for Parkinson's disease (PD) have been developed thus far. However, with the development of efficient gene delivery systems, gene therapy for PD has become a focus of research and increasing evidence suggests that continuous production of neurotrophic factors play a significant role in the functional restoration of the nigrostriatal dopaminergic (DA) system. Our recent study reported that the transduction of DA neurons with ras homolog enriched in brain, which has an S16H mutation [Rheb(S16H)], protected the nigrostriatal DA projection in a neurotoxin model of PD in vivo. In addition, Rheb(S16H) expression significantly increased the levels of glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor, which contributed to the neuroprotective effects of Rheb(S16H) in DA neurons in the adult brain, indicating that the activation of the signaling pathways involved in cell survival by a specific gene delivery, such as Rheb(S16H) to adult neurons, may be a useful strategy to protect neural systems in the adult brain. In the present study, a brief overview of our recent studies is provided, which demonstrates the neuroprotective mechanisms of Rheb(S16H) on the nigrostriatal DA projection in the adult brain.
Keywords: Rheb(S16H), glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, neuroprotection, Parkinson's disease
1. Introduction
Parkinson's disease (PD) is a chronic and progressive movement disorder that is mediated by the degeneration of nigrostriatal dopaminergic (DA) neurons, which causes motor symptoms, including tremor at rest, rigidity, bradykinesia and postural instability (1–4). Although the etiology of PD is poorly understood and therefore cannot guide the development of knowledge-based targeted therapeutics, investigators have attempted to develop targeted therapies using viral vector technologies to transfer specific genes to neurons, with the aim of improving the function of the degenerating DA system (5, 6). Striatal delivery of adeno-associated virus (AAV) 2-mediated cerebral dopamine neurotrophic factor produced neuroprotective and functional restorative effects in the 6-hydroxydopamine (6-OHDA)-induced animal model of PD (7). In addition, our previous studies (8, 9) demonstrated that AAV1 transduction with a gene encoding the constitutively active form of ras homolog enriched in the brain, with a mutation of the serine to histidine at the 16 position [Rheb(S16H)], induced trophic effects. These effects resulted in the protection and restoration of DA neurons in the 6-OHDA-induced model of PD via activation of the mammalian target of rapamycin complex 1 (mTORC1), indicating that the activation of mTORC1 by a specific gene delivery, such as Rheb(S16H) to DA neurons, may be a useful strategy in protecting the DA systems in the adult brain.
Accumulating evidence suggests that the use of various growth factors, such as glial cell line-derived neurotrophic factor (GDNF) (10–13) and brain-derived neurotrophic factor (BDNF) (10, 12, 14, 15), may have a therapeutic potential against PD. GDNF is a trophic factor involved in the survival of DA neurons (10–13) and has been identified in numerous types of neurons, including DA neurons (16). BDNF is a neurotrophin that can be synthesized by DA neurons in the substantia nigra (SN) of the adult brain (17) and is also involved in the survival of DA neurons (18). The study by Chauhan et al (19) reported that DA neurons in the SN of PD brains express decreased levels of GDNF and BDNF. In animal models of PD, experimental results using GDNF (10–13) and BDNF (10, 18) have consistently shown neuroprotective effects on DA neurons. Thus, replacement strategies to supplement neurotrophic factors are considered potential therapeutic approaches for PD. Our recent study found that the activation of mTORC1 by Rheb(S16H) transduction of DA neurons induced the production of trophic factors, such as GDNF and BDNF, and that the endogenous production of those factors contributed to the neuroprotection by Rheb(S16H) transduction in a neurotoxin model of PD (20). The present study describes the importance of Rheb(S16H) transduction of DA neurons in the adult brain, indicating a potential therapeutic method for PD.
2. mTORC1 activation for survival of DA neurons in the adult brain
Neurotrophic factors, such as GDNF and BDNF, regulate the development, maintenance, function and plasticity of mature neurons and they have emerged as promising therapeutic agents for PD (21). The cellular effects of GDNF and BDNF, which show the most evident reduction in DA neurons in PD brains (19), are initiated by their binding to GNDF family receptor α1 (22) and tropomyosin receptor kinase B (12), respectively. The stimulation of these receptors by treatment with GDNF and BDNF activates the Akt/mTOR signaling pathway, which is involved in the downstream activation of the pro-survival pathway in neurons (23). In addition, consistent with the decreased levels of GDNF and BDNF in PD brains, the decreased levels of Akt phosphorylation, resulting in a loss of mTORC1 activation, are observed in the SN of patients with PD and in a 1-methyl-4-phenylpyridinium (MPP+) model of PD, which mimics the phenotype of patients with PD (24). Numerous studies have also reported that the activation of the Akt/mTOR signaling pathway enhances the activity of intracellular cell survival pathways under a variety of conditions, such as ischemic shock, oxidative stress and the withdrawal of trophic factors (25–27). These results indicate that the activation of mTORC1 signaling pathway may be necessary for the survival of DA neurons and the functional maintenance of the DA system in the adult brain.
3. Rheb(S16H) transduction of DA neurons for mTORC1 activation
Rheb, a key upstream regulator of mTORC1 activation, is regulated via GTPase activity of tuberous sclerosis complex (TSC)1/TSC2 and it is involved in cellular processes, such as protein synthesis, cell growth, proliferation, survival and synaptic plasticity (8, 28). Furthermore, the serine at position 16 of Rheb has sensitivity to TSC activation and Rheb(S16H), a mutation of the serine to histidine, exhibits resistance to GTPase activation by TSC (8, 29), indicating that Rheb(S16H) is a strong activator of mTORC1. Our recent studies demonstrated that Rheb(S16H) expression in DA neurons induced an increase in phosphorylation of the mTORC1 substrates, 4E-BP1 and p70S6K, indicating activation of mTORC1 and showed apparent neurotrophic effects in the nigrostriatal DA system in the adult brains (8, 20). Additionally, the activation of mTORC1 by Rheb(S16H) transduction of DA neurons resulted in neuroprotective effects on the nigrostriatal DA projection in rat and mouse models of PD, indicating that Rheb(S16H) transduction of SN pars compacta neurons may have a common effect against different neurotoxins and models associated with the degeneration of the nigrostriatal DA projection in the adult brain.
4. GDNF and BDNF against PD
GDNF and BDNF are important trophic factors involved in the survival of neurons (10–15). The neurotrophic properties of GDNF were first reported by Lin et al (11), in a study that demonstrated that GDNF promotes cell survival and increases dopamine uptake in the DA neuron cultures derived from the embryonic rodent midbrain. Subsequent studies have demonstrated that treatment with GDNF has neurotrophic and protective effects in animal models of PD (10, 12, 13) and conditional ablation of GDNF in adult mice results in a delayed and progressive loss of DA neurons (30). Similar to GDNF, the experimental results have shown that BDNF has neuroprotective effects in animal models of PD (10, 18) and the infusion of an antisense oligonucleotide specific to BDNF results in anatomical, neurochemical and behavioral deficits that are characteristic of neurotoxic models of PD (31). Although these results indicate that neurotrophic factors are indispensable for the survival and protection of DA neurons and the support of diverse neurotrophic factors may mediate the survival of DA neurons, the use as a treatment for PD is impeded due to one particular factor. GDNF and BDNF do not cross the blood-brain barrier; therefore, direct application of these factors to the brain is essential. However, clinical trials of intracerebroventricular injection and intraputaminal infusion of GDNF failed in patients with PD (32, 33), possibly due to the limited penetration and distribution to the target brain areas. Thus, despite the potential clinical importance of these factors, the utilization in clinical pharmacology and other therapeutics for PD is dependent upon sustained delivery of the appropriate amounts of the factors to the target areas in a safe and efficacious manner, without producing adverse effects.
5. Mechanisms of Rheb(S16H)-induced neuroprotection
As reported in our previous studies (8, 9), the activation of mTORC1 by Rheb(S16H) transduction of DA neurons resulted in the SN-induced neurotrophic effects in the DA neurons, as well as in the protection and restoration of the nigrostriatal DA projection in a neurotoxin model of PD. These results indicate that an alternative to delivering neurotrophic molecules to the brain extracellular spaces, such as viral vector approaches to transduction of neurons, is to directly activate the intracellular signaling pathways responsible for their effects. However, it is largely unknown whether the activated mTORC1 induced the production of neurotrophic factors, such as GDNF and BDNF, thereby contributing to the protection of the nigrostriatal DA projection by intracellular signaling pathways in neurons of the adult brain. Our recent study found that Rheb(S16H) expression induced GDNF and BDNF, which contributed to the protection of the nigrostriatal DA projection in adult DA neurons in vivo and the induction of those factors was significantly attenuated by treatment with rapamycin, a specific mTORC1 inhibitor (20). Additionally, Rheb(S16H) expression induced an increase in the levels of the phospho-cAMP response element-binding protein (p-CREB) in DA neurons, which may be involved in the production of GDNF and BDNF (20). In addition to the induction of neurotrophic factors, our results using neutralizing antibodies against GDNF and BDNF showed that the Rheb(S16H)-induced trophic factors contributed to the protection of the nigrostriatal DA projection through synergistic effects in the MPP+-treated rat model of PD (20). These observations indicate that the observed upregulation of neurotrophic factors may be mediated by the Rheb(S16H)/mTORC1/CREB signaling pathway in DA neurons, and Rheb(S16H) expression in DA neurons protects the nigrostriatal DA projections through synergistic trophic effects mediated by GDNF and BDNF (Fig. 1).
Figure 1.
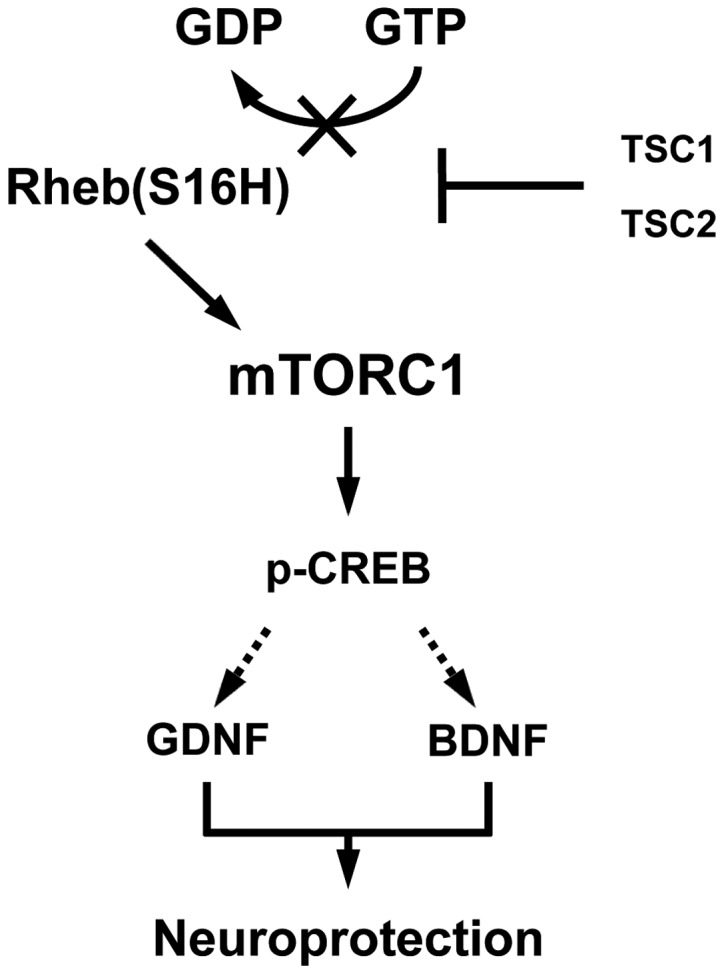
Schematic representation of the Rheb(S16H)/mTORC1/p-CREB, GDNF and BDNF signaling pathways. The serine at position 16 of Rheb has sensitivity to TSC GTPase activation (20, 29) and Rheb(S16H) results in the persistence of the GTP-bound Rheb as an activated from. The accumulation of GTP-bound Rheb by hRheb(S16H) stimulates mTORC1 to produce p-CREB, GDNF and BDNF, resulting in protection of the nigrostriatal DA projection. The dotted arrows indicate that p-CREB may mediate the production of GDNF and BDNF. Rheb(S16H), ras homolog enriched in brain, which has a S16H mutation; mTORC1, mammalian target of rapamycin complex 1; p-CREB, phospho-cAMP response element-binding protein; GDNF, glial cell line-derived neurotrophic factor; BDNF, brain-derived neurotrophic factor; TSC, tuberous sclerosis complex.
6. Conclusion
Our recent studies showed that Rheb(S16H) transduction of DA neurons induced the sustained production of GDNF and BDNF, which contributed to the neuroprotective effect of Rheb(S16H) in a neurotoxin model of PD. Additionally, the decreased levels of GDNF, BDNF and Akt phosphorylation, which resulted in a loss of mTORC1 activation, are observed in the SN of PD patients. Therefore, even though the optimum gene delivery system without any side-effects for the clinical trial remains to be found, our results indicate that Rheb(S16H) transduction may be a useful strategy to protect the nigrostriatal DA pathway in the adult brain, particularly considering that the activation of mTORC1 by a specific gene delivery to DA neurons in the SN results in the sustained production of diverse neurotrophic factors, such as GDNF and BDNF, involved in the maintenance and protection of the nigrostriatal DA system in the adult brain.
Acknowledgements
The present study was supported by the National Research Foundation of Korea grant funded by the Korean government (nos. 2008-0061888 and 2012R1A1A1039140).
References
- 1.Burke RE, O'Malley K. Axon degeneration in Parkinson's disease. Exp Neurol. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung UJ, Leem E, Kim SR. Naringin: a protector of the nigrostriatal dopaminergic projection. Exp Neurobiol. 2014;23:124–129. doi: 10.5607/en.2014.23.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leem E, Nam JH, Jeon MT, et al. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson's disease. J Nutr Biochem. 2014;25:801–806. doi: 10.1016/j.jnutbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartus RT, Weinberg MS, Samulski RJ. Parkinson's disease gene therapy: success by design meets failure by efficacy. Mol Ther. 2014;22:487–497. doi: 10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coune PG, Schneider BL, Aebischer P. Parkinson's disease: gene therapies. Cold Spring Harb Perspect Med. 2012;2:a009431. doi: 10.1101/cshperspect.a009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren X, Zhang T, Gong X, Hu G, Ding W, Wang X. AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp Neurol. 2013;248:148–156. doi: 10.1016/j.expneurol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim SR, Kareva T, Yarygina O, Kholodilov N, Burke RE. AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol Ther. 2012;20:275–286. doi: 10.1038/mt.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C, During M, Kholodilov N, Burke RE. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011;70:110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 12.Manfredsson FP, Okun MS, Mandel RJ. Gene therapy for neurological disorders: challenges and future prospects for the use of growth factors for the treatment of Parkinson's disease. Curr Gene Ther. 2009;9:375–388. doi: 10.2174/156652309789753400. [DOI] [PubMed] [Google Scholar]
- 13.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/S0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 15.Studer L, Spenger C, Seiler RW, Othberg A, Lindvall O, Odin P. Effects of brain-derived neurotrophic factor on neuronal structure of dopaminergic neurons in dissociated cultures of human fetal mesencephalon. Exp Brain Res. 1996;108:328–336. doi: 10.1007/BF00228106. [DOI] [PubMed] [Google Scholar]
- 16.Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9:463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 17.Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- 18.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson's disease brain. J Chem Neuroanat. 2001;21:277–288. doi: 10.1016/S0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 20.Nam JH, Leem E, Jeon MT, et al. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson's disease. Mol Neurobiol. 2014 May 25; doi: 10.1007/s12035-014-8729-2. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Aron L, Klein R. Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci. 2011;34:88–100. doi: 10.1016/j.tins.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Kholodilov N, Kim SR, Yarygina O, Kareva T, Cho JW, Baohan A, Burke RE. Glial cell line-derived neurotrophic factor receptor-α1 expressed in striatum in trans regulates development and injury response of dopamine neurons of the substantia nigra. J Neurochem. 2011;116:486–498. doi: 10.1111/j.1471-4159.2010.07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 26.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/S0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 27.Chang N, El-Hayek YH, Gomez E, Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–586. doi: 10.1016/j.tins.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Karassek S, Berghaus C, Schwarten M, et al. Ras homolog enriched in brain (Rheb) enhances apoptotic signaling. J Biol Chem. 2010;285:33979–33991. doi: 10.1074/jbc.M109.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan L, Findlay GM, Jones R, Procter J, Cao Y, Lamb RF. Hyperactivation of mammalian target of rapamycin (mTOR) signaling by a gain-of-function mutant of the Rheb GTPase. J Biol Chem. 2006;281:19793–19797. doi: 10.1074/jbc.C600028200. [DOI] [PubMed] [Google Scholar]
- 30.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 31.Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol. 2005;192:226–234. doi: 10.1016/j.expneurol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Nutt JG, Burchiel KJ, Comella CL, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/WNL.60.1.69. [DOI] [PubMed] [Google Scholar]
- 33.Peterson AL, Nutt JG. Treatment of Parkinson's disease with trophic factors. Neurotherapeutics. 2008;5:270–280. doi: 10.1016/j.nurt.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


