Abstract
The vitamin D-binding protein (VDBP) genetic polymorphisms have been associated with chronic obstructive pulmonary disease (COPD). A number of studies have been conducted to investigate the combined effects of the VDBP gene (GC) rs7041 and rs4588 polymorphisms on the COPD risk. However, the results obtained are inconclusive. The present meta-analysis aimed to investigate whether GC polymorphisms may be a potential risk factor for COPD. The Web of Science, PubMed, Google Scholar, Embase, Cochrane Library, China National Knowledge Infrastructure and Wanfang Database were searched from inception until June 1, 2014. The meta-analysis was performed using the STATA 12.0 software. Twelve case-control studies, including 2,937 subjects, met the inclusion criteria. Overall, a significantly increased risk was detected in populations of GC*1F homozygotes, whereas no associations between other GC polymorphisms and COPD risk were detected. According to ethnicity, the results demonstrated that the GC*1F homozygotes may be a risk factor for COPD and the GC*2 homozygotes may be a protective factor against COPD in the Asian population. However, similar associations were not observed among the Caucasian population. In conclusion, the current meta-analysis indicates that the GC*1F homozygotes may be a risk factor for COPD and the GC*2 homozygotes may be a protective factors against COPD in the Asian population.
Keywords: vitamin D-binding protein, gene polymorphisms, chronic obstructive pulmonary disease, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide, which is characterized by irreversible or partly reversible progressive limitation of the airflow (1, 2). Cigarette smoking is the major risk factor, but only 10–15% of long-term smokers develop symptomatic airflow obstruction (3, 4). This indicates that the genetic factors may contribute to the individual susceptibility for the development of COPD. Numerous studies have shown that genetic factors are likely to have a role in determining the susceptibility of an individual to COPD (1, 2, 5–8), and genetic variants in the vitamin D-binding protein (VDBP) gene have also been linked to COPD risk (7, 9, 10).
VDBP (also known as Gc-globulin) is expressed in a number of tissues, such as liver, kidney, gonads and fat (11). Notably, VDBP is also expressed by human neutrophils (12), contributes to macrophage activation (13), augments monocyte and neutrophil chemotaxis to C5-derived peptides and acts as a scavenger protein to clear extracellular G-actin released from necrotic cells (14, 15). Additionally, VDBP is the major plasma carrier protein of vitamin D, deficiency of which may also be linked to COPD (7, 16, 17). The human VDBP gene (GC) has been localized to chromosome 4q11-q13 and is highly polymorphic, with three commonly recognized variants (GC*1F, GC*1S and GC*2) caused by non-synonymous single-nucleotide polymorphisms (SNPs; rs7041 and rs4588) and >120 rarer variants (18). GC*1F, GC*1S and GC*2 are not alleles as such, but haplotypes composed of combinations of the SNPs at these loci, so an individual may be homozygous or heterozygous for each variant, depending on the two haplotypes present. The role of GC polymorphisms in COPD has been explored in a number of studies by investigating the association of GC variants and susceptibility of COPD (9, 10, 19–25). However, the results of these studies were inconclusive and may not be powerful enough due to the limited sample size. To evaluate the overall effect of the haplotypic association between the GC polymorphisms (rs7041 and rs4588) and COPD risk, a meta-analysis was conducted in the present study by pooling all the available data together.
Materials and methods
Identification of eligible studies
To identify all the eligible studies that investigated the haplotypic association of the GC rs7041 and rs4588 polymorphisms with COPD risk, a comprehensive electronic search of Web of Science, PubMed, Google Scholar, Embase, Cochrane Library, China National Knowledge Infrastructure and Wanfang Database was performed until June 1, 2014. Various combinations of the following medical subject headings and key words were applied in order to include the maximum number of studies associated as possible: Vitamin D-binding protein, VDBP, GC or Gc-globulin; rs7041 or rs4588; and chronic obstructive pulmonary disease, COPD; polymorphisms, variants or haplotypes. Furthermore, the reference lists of the reviews and retrieved studies were manually screened for additional studies. There were no restrictions for language and only published studies with full-text studies were included.
Inclusion and exclusion criteria
The studies identified from the above-mentioned databases were screened by two independent investigators according to the following predesigned inclusion criteria: i) Case-control design; ii) evaluating the correlation of GC haplotypes (at position rs7041 and rs4588) with COPD risk; and iii) providing sufficient data to calculate the odds ratio (OR) and its corresponding 95% confidence interval (CI). When several studies with overlapping data were eligible, those with smaller sample size or less reliability were excluded. In addition, the studies without detailed information were excluded, following the efforts to extract data from the original study or failure of contact with the corresponding authors.
Data extraction
Data from the eligible studies were extracted by two investigators independently and in duplicate according to the predesigned data-collection form. The following information was extracted: Last name of the first author, publication year, country of origin, ethnicity, source of controls, number of the two COPD cases, control subjects and phenotypic distribution in the two groups. Different ethnicities were categorized as Asian and Caucasian. Discrepancies occurring during the process of study inclusion and data extraction were resolved by discussion with a third investigator and consensus on each item was achieved eventually.
Data analysis
The statistical analysis was conducted using STATA software (version 12.0; Stata Corporation, College Station, TX, USA). The ORs of COPD were recalculated and compared between GC*1F-1F and non GC*1F-1F, GC*1F-1S and non GC*1F-1S, GC*1S-1S and non GC*1S-1S, GC*2-1S and non GC*2-1S, GC*2-1F and non GC*2-1F, and GC*2–2 and non GC*2–2. Heterogeneity among studies was examined with the χ2-based Q testing and I2 statistics (26). When there was a significant heterogeneity (P<0.10), a random-effects model (the DerSimonian and Laird method) was selected to pool the data (27). Otherwise, a fixed-effects model (the Mantel-Haenszel method) was selected to pool the data (28). Publication bias was examined with funnel plots Begg and Egger tests (29, 30). When no publication bias existed, the funnel plot was considered as symmetrical. The statistical significance of the pooled OR was assessed with the Z test and P<0.05 was considered to indicate a statistically significant difference. For the Egger's tests, the significance level was also set at 0.05. Subgroup analyses were also conducted to assess any moderating effects of ethnicity (Caucasian and Asian) on ORs derived from each study.
Results
Characteristics of the eligible studies
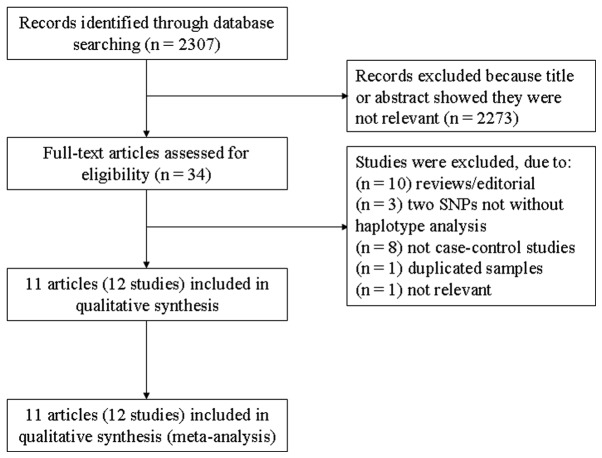
A total of 2,307 studies were obtained with the initial search of databases. Following screening, 12 case-control studies in 11 eligible studies with a total of 2,937 participants (1,190 cases and 1,747 controls) fulfilled the inclusion criteria (9, 10, 19–25, 31, 32), from which genotype data of the GC polymorphisms (rs7041 and rs4588) were obtained (shown in Table I). The flowchart of reviews demonstrates the detailed process of selection (Fig. 1). As for ethnicity, seven studies investigated the Caucasian population (21, 23–25, 31, 32) and five studies investigated the Asian population (9, 10, 19, 20, 22). The qualities of these studies were considered accessible for the meta-analysis.
Table I.
Main characteristics of all the eligible studies.
| Genotype frequencies of GC haplotypes (rs7041-rs4588) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Control | |||||||||||||||||
| First author | Year | Country | Ethnicity | Source of controls | No. of case/control | 1F-1F | 1F-1S | 1S-1S | 2-1S | 2-1F | 2–2 | 1F-1F | 1F-1S | 1S-1S | 2-1S | 2-1F | 2–2 | (Refs.) |
| Wood | 2011 | UK | Caucasian | HS | 93/351 | 0 | 2 | 39 | 41 | 1 | 10 | 0 | 0 | 145 | 150 | 0 | 56 | (24) |
| Shen | 2010 | China | Asian | HS | 100/100 | 35 | 20 | 7 | 13 | 22 | 3 | 13 | 26 | 4 | 12 | 29 | 16 | (10) |
| Huang | 2007 | China | Asian | HS | 75/69 | 24 | 21 | 2 | 8 | 18 | 2 | 8 | 18 | 3 | 7 | 20 | 13 | (19) |
| Korytina | 2006 | Rep of Bashk | Caucasian | HS | 131/106 | 8 | 26 | 30 | 29 | 25 | 13 | 12 | 39 | 14 | 25 | 9 | 7 | (21) |
| Korytina | 2006 | Russia | Caucasian | HS | 166/130 | 14 | 31 | 42 | 49 | 20 | 10 | 14 | 29 | 25 | 45 | 12 | 5 | (21) |
| Lu | 2004 | China | Asian | HS | 69/52 | 23 | 15 | 5 | 9 | 16 | 1 | 6 | 16 | 3 | 8 | 14 | 5 | (22) |
| Laufs | 2004 | Iceland | Caucasian | HS | 102/183 | 1 | 11 | 39 | 35 | 5 | 11 | 2 | 24 | 68 | 67 | 8 | 14 | (25) |
| Ito | 2004 | Japan | Asian | HS | 103/88 | 33 | 29 | 3 | 11 | 25 | 2 | 15 | 27 | 5 | 10 | 30 | 1 | (9) |
| Ishii | 2001 | Japan | Asian | HS | 63/82 | 23 | 15 | 1 | 6 | 16 | 2 | 17 | 27 | 5 | 8 | 18 | 7 | (20) |
| Schellenberg | 1998 | UK | Caucasian | HS | 75/64 | 41 | 32 | 2 | 31 | 24 | 9 | (23) | ||||||
| Horne | 1990 | USA | Caucasian | HS | 104/413 | 6 | 24 | 40 | 23 | 3 | 8 | 5 | 66 | 141 | 134 | 25 | 42 | (32) |
| Kueppers | 1977 | USA | Caucasian | HS | 109/109 | 62 | 46 | 1 | 57 | 47 | 5 | (31) | ||||||
GC, vitamin D-binding protein gene; 1F, GC*1F; 1S, GC*1S; 2, GC*2; HS, healthy smokers; Bashk, Bashkortostan.
Figure 1.
Flowchart showing the study selection procedure. SNPs, single-nucleotide polymorphisms.
Meta-analysis of haplotypes and the association with COPD
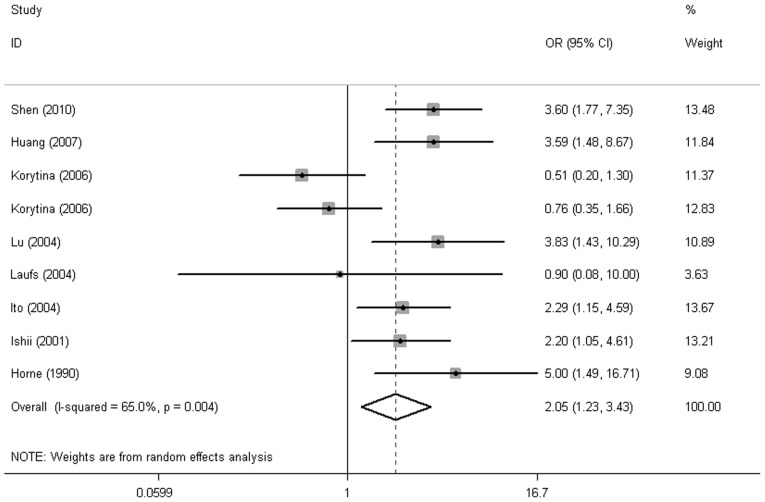
Overall, among the six haplotypes of the VDBP gene (GC*1F-1F, GC*1F-1S, GC*1S-1S, GC*2-1S, GC*2-1F and GC*2–2), a significantly increased risk was observed in GC*1F homozygotes (GC*1F-1F vs. non GC*1F-1F: OR=2.02, 95% CI: 1.23-3.43, P=0.006) and no association between other GC haplotypes and COPD risk were detected (Fig. 2 and Table II).
Figure 2.
Forest plot for the COPD risk associated with VDBP gene polymorphisms in total analysis (GC*1F-1F vs. non GC*1F-1F). COPD, chronic obstructive pulmonary disease; VDBP, vitamin D-binding protein; OR, odds ratio; CI, confidence interval.
Table II.
Results of the meta-analysis for haplotypes (rs7041-rs4588).
| Meta-analysis | Heterogeneity | ||||
|---|---|---|---|---|---|
| Genetic model | Pooled OR (95% CI) | POR | I2, % | P-value | Bias P-value |
| 1F-1F vs. non 1F-1F | 2.052 (1.227–3.431)a | 0.006a | 65.0 | 0.004 | 0.883 |
| 1.115 (0.402–3.090)b | 0.834 | 67.7 | 0.026 | 0.667 | |
| 2.922 (2.064–4.136)a, c | 1.47E-09a | 0.0 | 0.760 | 0.287 | |
| 1F-1S vs. non 1F-1S | 0.808 (0.651–1.003) | 0.054 | 0.0 | 0.629 | 0.427 |
| 0.830 (0.617–1.117)b | 0.220 | 28.6 | 0.246 | 0.671 | |
| 0.784 (0.572–1.074)c | 0.130 | 0.0 | 0.801 | 0.563 | |
| 1S-1S vs. non 1S-1S | 1.174 (0.947–1.456) | 0.144 | 0.0 | 0.604 | 0.327 |
| 1.223 (0.974–1.536)b | 0.083 | 0.0 | 0.566 | 0.059 | |
| 0.837 (0.434–1.615)c | 0.595 | 0.0 | 0.470 | 0.088 | |
| 2-1S vs. non 2-1S | 0.864 (0.707–1.056) | 0.152 | 0.0 | 0.935 | 0.463 |
| 0.835 (0.666–1.047)b | 0.118 | 0.0 | 0.554 | 0.853 | |
| 0.980 (0.633–1.515)c | 0.927 | 0.0 | 0.995 | 0.540 | |
| 2-1F vs. non 2-1F | 0.937 (0.726–1.209) | 0.616 | 30.0 | 0.179 | 0.739 |
| 1.340 (0.869–2.067)b | 0.185 | 44.8 | 0.142 | 0.269 | |
| 0.774 (0.563–1.062)c | 0.113 | 0.0 | 0.753 | 0.174 | |
| 2–2 vs. non 2–2 | 0.610 (0.308–1.207) | 0.155 | 71.5 | 0.000 | 0.112 |
| 1.033 (0.505–2.129)b | 0.929 | 68.1 | 0.004 | 0.443 | |
| 0.212 (0.105–0.428)a, c | 1.51E-05a | 3.5 | 0.387 | 0.285 | |
Bold print denotes significant results of pooled ORs.
Studies with Caucasian samples were included.
Studies with Asian samples were included. OR, odds ratio; CI, confidence interval; 1F, GC*1F; 1S, GC*1S; 2, GC*2.
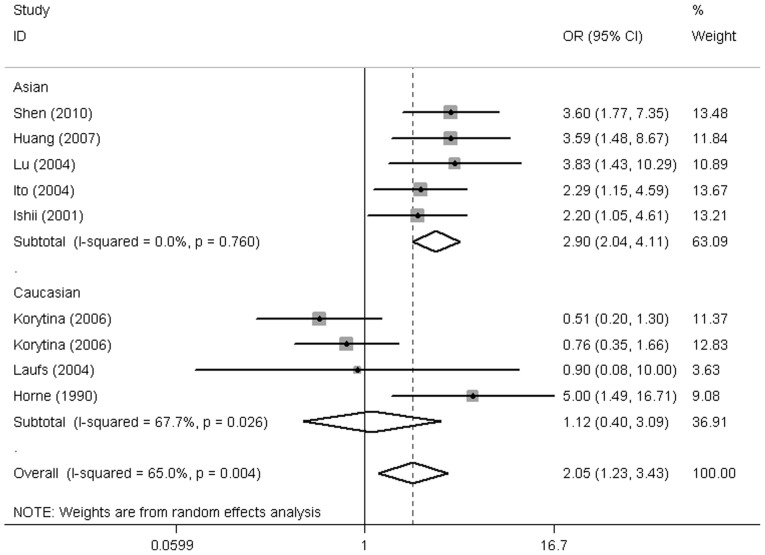
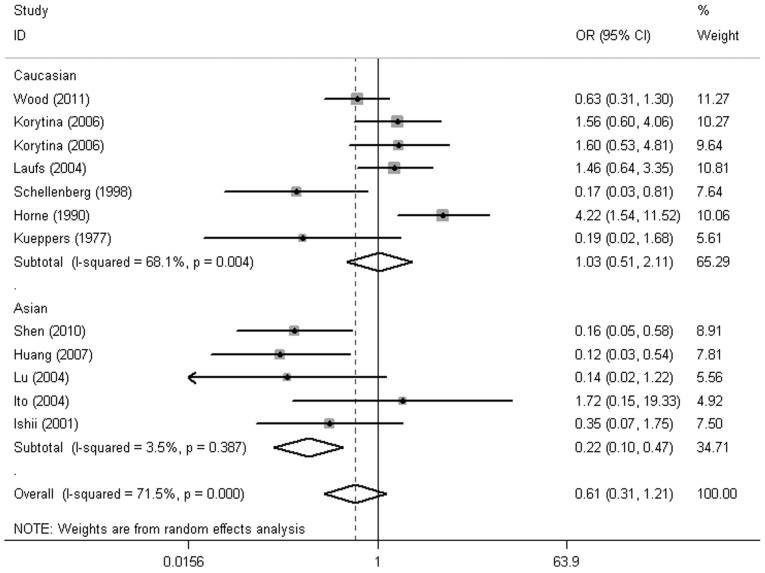
According to ethnicity, the results demonstrated a markedly increased risk in Asians with GC*1F homozygotes, but not in Caucasians (GC*1F-1F vs. non GC*1F-1F; OR, 2.92; 95% CI, 2.06-4.14; P=1.47E-09; OR, 1.12; 95% CI, 0.40-3.09; P=0.834, respectively) (Fig. 3 and Table II). In addition, GC*2 homozygotes in Asians were at lower risk of COPD (GC*2–2 vs. non GC*2–2: OR, 2.21; 95% CI, 0.11-0.43; P=1.51E-05) (Fig. 4). No significant association was observed in the other haplotypes (Table II).
Figure 3.
Forest plot for the COPD risk associated with VDBP gene polymorphisms stratified by ethnicity (GC*1F-1F vs. non GC*1F-1F). COPD, chronic obstructive pulmonary disease; VDBP, vitamin D-binding protein; OR, odds ratio; CI, confidence interval.
Figure 4.
Forest plot for risk the COPD risk associated with VDBP gene polymorphisms stratified by ethnicity (GC*2–2 vs. non GC*2–2). COPD, chronic obstructive pulmonary disease; VDBP, vitamin D-binding protein; OR, odds ratio; CI, confidence interval.
Sensitivity analysis
Sensitivity analysis was also performed to explore the potential influence of each individual study on the overall results by deleting one single study each time from the pooled analysis. No substantial change was demonstrated in the overall studies, indicating that no individual study affected the pooled OR significantly (data not shown).
Heterogeneity and publication bias
Among the six haplotypes, significant heterogeneity was observed in the GC*1F homozygotes and GC*2 homozygotes with P<0.10; subsequent to stratifying for populations, no significant heterogeneity was observed in Asians, but the heterogeneity remained significant in Caucasians. For publication bias, no significant results were observed with all P>0.05 of Egger's test. Additionally, funnel plots of haplotypes did not show significant publication bias either. Results of heterogeneity and publication bias are shown in Table II.
Discussion
In the present study, the results indicated that the GC*1F-1F homozygote carriers have an increased risk of COPD. However, following subgroup analysis by ethnicity, a significant increased risk of COPD was found in Asians, but not in Caucasians. The GC*2–2 homozygote carriers in Asians may have a decreased risk of COPD.
To the best of our knowledge, this is the first meta-analysis of the VBDP gene polymorphsims and COPD susceptibility. In the present meta-analysis, 12 eligible studies were combined with 1,190 cases and 1,747 controls to yield summary statistics, indicating that the GC*1F-1F homozygotes of the VDBP gene were associated with the COPD risk among the overall population (Table II). According to ethnicity, the GC*1F homozygotes were considered as a risk factor for COPD in Asian populations, while the presence of the GC*2 homozygotes may be one of the protective factors against COPD in Asians (Table II). These findings on the correlation between GC polymorphisms and COPD were indirectly supported by the evidence that the VDBP gene influenced vitamin D concentrations and was associated to forced expiratory volume in one second (7, 24). However, the results from the present analyses failed to reveal the significant association between the GC polymorphisms and COPD susceptibility among Caucasians, this was extremely different from the findings by Horne et al (32) and Schellenberg et al (23), but consistent with the findings by other studies (21, 25, 31, 33). This may be due to the fact that other factors, such as the differences in age, gender, lifestyle factors, clinical characteristics or the analysis method of the VDBP genotype may have influenced the results. The frequency difference between Caucasians and Asians may be a cause of variable association with COPD between these two ethnic groups and studies based on larger well-designed populations are required to clarify the associations between the VDBP gene polymorphism and COPD susceptibility.
The heterogeneity is of note when interpreting the meta-analysis results. For the significant and stable results of the GC polymorphisms in the majority of models, there was no or low-to moderate heterogeneity (indicated by I2-statistics) among studies (see Table II). However, relatively clear heterogeneity was observed in the models of GC*1F-1F and GC*2–2 in Caucasians, which may have been responsible for the negative results.
The current meta-analysis focused on the combined effects of the VDBP gene rs7041 and rs4588 polymorphisms rather than one single SNP on COPD risk (7, 34), which may help to derive a precise estimation of the roles of VDBP SNPs in the development of COPD episodes. However, several limitations should be considered when interpreting the results. First, the limited number of COPD cases and control subjects may lead to a relatively small power. Second, the heterogeneity was not resolved following subgroup analyses in certain cases, indicating that other factors, such as the differences in age, gender, lifestyle factors or clinical characteristics, may have caused heterogeneity. Third, only published studies with sufficient data were included, and therefore, publication bias may have occurred even though results of the Begg's and Egger's tests did not detect it.
In conclusion, the present meta-analysis indicated that the GC*1F homozygotes of the VDBP gene may be a risk factor for COPD among Asian populations, while the presence of the GC*2 homozygotes may be one of the protective factors against COPD in Asians. Larger and well-designed studies based on different ethnic groups are required to confirm our results.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81070045) and the Key Clinical Project for the Affiliated Hospital of the Ministry of Public Health of China (grant no. 111). The authors would like to acknowledge the reviewers for their helpful comments on the study.
References
- 1.Vijayan VK. Chronic obstructive pulmonary disease. Indian J Med Res. 2013;137:251–269. [PMC free article] [PubMed] [Google Scholar]
- 2.Barczyk A, Pierzchala W. Diagnostic and treatment of chronic obstructive pulmonary disease based on GOLD statement 2011. Pol Merkur Lekarski. 2012;33:187–192. (In Polish) [PubMed] [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faner R, Gonzalez N, Cruz T, Kalko SG, Agusti A. Systemic inflammatory response to smoking in chronic obstructive pulmonary disease: evidence of a gender effect. PLoS One. 2014;9:e97491. doi: 10.1371/journal.pone.0097491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. (PRISMA Group). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Issac MS, Ashur W, Mousa H. Genetic polymorphisms of surfactant protein D rs2243639, Interleukin (IL)-1beta rs16944 and IL-1RN rs2234663 in chronic obstructive pulmonary disease, healthy smokers, and non-smokers. Mol Diagn Ther. 2014;18:343–354. doi: 10.1007/s40291-014-0084-5. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Liu X, Xu Y, et al. The correlation of vitamin D level and vitamin D-binding protein gene polymorphism in chronic obstructive pulmonary disease. Zhonghua Nei Ke Za Zhi. 2014;53:303–307. (In Chinese) [PubMed] [Google Scholar]
- 8.Kauffmann F, Kleisbauer JP, Cambon-De-Mouzon A, et al. Genetic markers in chronic air-flow limitation. A genetic epidemiologic study. Am Rev Respir Dis. 1983;127:263–269. doi: 10.1164/arrd.1983.127.3.263. [DOI] [PubMed] [Google Scholar]
- 9.Ito I, Nagai S, Hoshino Y, et al. Risk and severity of COPD is associated with the group-specific component of serum globulin 1F allele. Chest. 2004;125:63–70. doi: 10.1378/chest.125.1.63. [DOI] [PubMed] [Google Scholar]
- 10.Shen LH, Zhang XM, Su DJ, et al. Association of vitamin D binding protein variants with susceptibility to chronic obstructive pulmonary disease. J Int Med Res. 2010;38:1093–1098. doi: 10.1177/147323001003800337. [DOI] [PubMed] [Google Scholar]
- 11.McLeod JF, Cooke NE. The vitamin D-binding protein, alpha-fetoprotein, albumin multigene family: detection of transcripts in multiple tissues. J Biol Chem. 1989;264:21760–21769. [PubMed] [Google Scholar]
- 12.Kew RR, Sibug MA, Liuzzo JP, Webster RO. Localization and quantitation of the vitamin D binding protein (Gc-globulin) in human neutrophils. Blood. 1993;82:274–283. [PubMed] [Google Scholar]
- 13.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci USA. 1991;88:8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder R, Kress A, Kan G, Herrmann K, Kirschfink M. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36:885–892. doi: 10.1016/S0161-5890(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 15.McLeod JF, Kowalski MA, Haddad JG., Jr Interactions among serum vitamin D binding protein, monomeric actin, profilin, and profilactin. J Biol Chem. 1989;264:1260–1267. [PubMed] [Google Scholar]
- 16.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 17.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 18.Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang. 1988;54:215–225. doi: 10.1111/j.1423-0410.1988.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang P, Ma Y, Du X, Chen J, Song B, Hong Y. The vitamin D-binding protein gene polymorphism in chronic obstructive pulmonary disease patients. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30:780–781. (In Chinese) [Google Scholar]
- 20.Ishii T, Keicho N, Teramoto S, et al. Association of Gc-globulin variation with susceptibility to COPD and diffuse panbronchiolitis. Eur Respir J. 2001;18:753–757. doi: 10.1183/09031936.01.00094401. [DOI] [PubMed] [Google Scholar]
- 21.Korytina GF, Akhmadishina LZ, Ianbaeva DG, Viktorova TV. Genotypes of vitamin-D-binding protein (DBP) in patients with chronic obstructive pulmonary disease and healthy population of Republic Bashkortostan. Mol Biol (Mosk) 2006;40:231–238. doi: 10.1134/S002689330602004X. (In Russian) [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Yang B, Cai YY. The relationship between vitamin D binding protein gene polymorphism and chronic obstructive pulmonary disease. Zhonghua Nei Ke Za Zhi. 2004;43:117–120. (In Chinese) [PubMed] [Google Scholar]
- 23.Schellenberg D, Pare PD, Weir TD, Spinelli JJ, Walker BA, Sandford AJ. Vitamin D binding protein variants and the risk of COPD. Am J Respir Crit Care Med. 1998;157:957–961. doi: 10.1164/ajrccm.157.3.9706106. [DOI] [PubMed] [Google Scholar]
- 24.Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66:205–210. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 25.Laufs J, Andrason H, Sigvaldason A, et al. Association of vitamin D binding protein variants with chronic mucus hypersecretion in Iceland. Am J Pharmacogenomics. 2004;4:63–68. doi: 10.2165/00129785-200404010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 29.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kueppers F, Miller RD, Gordon H, Hepper NG, Offord K. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med. 1977;63:336–342. doi: 10.1016/0002-9343(77)90270-4. [DOI] [PubMed] [Google Scholar]
- 32.Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990;40:173–176. doi: 10.1159/000153926. [DOI] [PubMed] [Google Scholar]
- 33.Wood AM, Needham M, Simmonds MJ, Newby PR, Gough SC, Stockley RA. Phenotypic differences in alpha 1 antitrypsin-deficient sibling pairs may relate to genetic variation. COPD. 2008;5:353–359. doi: 10.1080/15412550802522320. [DOI] [PubMed] [Google Scholar]
- 34.Bakke PS, Zhu G, Gulsvik A, et al. Candidate genes for COPD in two large data sets. Eur Respir J. 2011;37:255–263. doi: 10.1183/09031936.00091709. [DOI] [PubMed] [Google Scholar]