Abstract
Atrial natriuretic peptide (ANP) is extensively expressed in the cochlea, including the strial vascularis (StV). ANP may participate in the regulation of the water-electrolyte balance. However, the functional significance of ANP in the cochlea is less understood and little is known regarding the exact mechanisms. Studies suggest that the epithelial sodium channel (ENaC) is important for regulating sodium transport across epithelia. ENaC may be involved in the clearance of endolymphatic Na+ and maintenance of a K-rich and Na-poor composition in the endolymph. Whether ANP has a regulatory effect on the Na+ channel in the StV remains unknown. The aim of the present study was to evaluate whether ANP affects the expression of the α-subunit of the ENaC (α-ENaC) mRNA in the mouse StV, using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) technique. The mouse StV tissues were incubated with 10−6 mol/1 ANP for different times (2, 6, 12, 24 and 48 h) and were subsequently harvested. α-ENaC mRNA was extracted for RT-qPCR analysis of the expression. The study demonstrated the existence of α-ENaC in the mouse StV. Tissues treated with ANP (10−6 mol/1) showed a significant reduction in α-ENaC mRNA expression (n=3, P<0.05). A maximum effect was reached at 2 h after treatment. The present results indicate that ANP may regulate cochlear ion transport and endolymph fluid balance in the inner ear via reducing expression of the α-ENaC mRNA in the mouse StV.
Keywords: atrial natriuretic peptide, α-subunit of the epithelial sodium channel, strial vascularis, reverse transcription-quantitative polymerase chain reaction
Introduction
Atrial natriuretic peptide (ANP), which was first extracted from rat atrial tissue by Baines et al, is now known to be a member of the natriuretic peptide family (1). The natriuretic peptide family consists of a family of 5 natriuretic peptides that include A-, B-, C-type natriuretic peptide, urodilatin and Dendroaspis natriuretic peptide (2). The pathophysiological actions of various natriuretic peptides are mediated by three different natriuretic peptide receptors; atrial natriuretic peptide receptor-A-type (NPR-A), NPR-B- and NPR-C (3). ANP, an A-type natriuretic peptide, has been reported to have a wide variety of biological functions, not only in the circulatory system but also in the kidney and other tissues (3). ANP acts to reduce the water, sodium and adipose loads on the circulatory system, thereby reducing blood pressure.
ANP receptors are distributed extensively in the cochlea, including the strial vascularis (StV). Lamprecht and Meyer zum Gottesberge (4) first reported that ANP receptors are expressed in the inner ear of guinea pigs. Previous studies have also identified ANP receptors in the inner ear of guinea pigs and rats, and demonstrated their presence in the StV, spiral ligament in the cochlea and the secretory epithelium of the vestibular organ (4–10).
Studies suggest that ANP may be locally synthesized and activates NPR-A in a paracrine/autocrine manner (4–8). Pronounced immunoreactivity for ANP was observed in the spiral ligament of the cochlear lateral wall. ANP has been demonstrated to be expressed mainly in the cytoplasm of the StV marginal cells of normal guinea pigs (11).
Epithelial sodium channel (ENaC) consists of three different subunits: α, β and γ, with the α-subunit (α-ENaC) being the most critical for channel functionality (12). Studies suggest that ENaC is important for regulating sodium transport across epithelia. ENaC has been shown to participate in transepithelial Na+ transport in epithelia of various organs, such as kidney, colon, lung, sweat glands and salivary glands (13). ENaC is located in the apical membrane of epithelia facing the endolymph, StV, spiral prominence and spiral limbus in the inner ear. The endolymph in the cochlea is characterized by a high K+ concentration of ~150 mM and low Na+ concentration of ~1 mM. ENaC may be involved in transepithelial Na+ transport through the apical membrane and maintenance of a K-rich and Na-poor composition in the endolymph (14).
ANP may participate in the regulation of the water-electrolyte balance (15). However, little is known regarding the exact mechanisms of ANP in the regulation of the water-electrolyte balance in the cochlea. Whether ANP has a regulatory effect on the ENaC in the StV remains unknown. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is a highly sensitive technology that allows high throughput quantification of gene expression (16). In the present study, RT-qPCR was used to evaluate whether ANP affects α-ENaC mRNA expression in the mouse StV.
Materials and methods
Animals
Healthy adult Kunming mice were provided by the Animal Care and Use committee at the Huaxi Medical School of Sichuan University. The study was approved by the Animal Care and Use Committee at the West China Medical School of Sichuan University (Sichuan, China). The temporal bone was removed and StV tissues were dissected at 4˚C, as previously described (17).
Tissues culture
StV tissues were dissected and rinsed in saline solution prior to being placed in culture plates. All the experimental specimens were cultured in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (1:1) containing 10% fetal bovine serum, 50 mM glucose, 100 U/ml penicillin and 2 µ1/ml insulin-transferrin selenium sodium mixture. StV tissues were co-cultured with ANP (10−6 mol/1) in a standard cell culture incubator at 37°C, 5% CO2 and 80% humidity for different times (2, 4, 6, 12, 24 and 48 h).
RNA isolation and reverse transcription
Total RNA from the tissues of the cultured StV in the mouse cochlea was isolated using the TRIzol reagent (MRC, Cincinnati, OH, USA). RNA was reverse transcribed into cDNA using the RevertAid™ First Strand cDNA Synthesis kit (MBI Fermentas, Inc., Burlington, ON, Canada) with the addition of random hexamer primers. Briefly, 5 µ1 purified RNA was used as template for cDNA synthesis in the presence of 1 µ1 Moloney murine leukemia virus reverse transcriptase (200 units), 1 µ1 random hexamer primer, 4 µ1 5X reaction buffer, 2 µ1 hexamer 1X (Roche, Indianapolis, IN, USA), 2 µ1 dNTP mix (10 mM each) and 6 µ1 RNase-free water. After incubation for 60 min at 42˚C, the reverse transcriptase was inactivated at 70˚C for 10 min and the cDNAs were stored at −20˚C until further analysis.
Primers
SCNN1A is a gene encoding the α-ENaC protein. Primers were designed according to the NCBI GenBank sequences of mice and were synthesized by the Sangon Biotech (Shanghai, China). The primer sequences for RT-qPCR are as follows. Outer primers: SCNN1A forward, CACAGCAGGTGT GCATT and reverse, CAGCCTGCAGTTTATAGT. Inner primers for the second-round PCR reaction: SCNN1A forward, CATTCACTCCTGCTTCCAG and reverse, GTAGCAGTA GCCCCAGGAG; ACTB forward, GAAGATCAAGATCAT TGCTCCT and reverse, TACTCCTGCTTGCTGATCCA.
First-round PCR reaction
The reaction mixture for the first-round PCR contains 3 µ1 10X PCR buffer, 1.8 µ1 25 mM MgCl2, 11 µmol each primer (SCNN1A forward and reverse) (Qiagen-Operon, Cologne, Germany), 0.36 µ1 deoxynucleoside triphosphate mix, 0.3 µ1 Taq DNA polymerase, 5 µ1 DNA template and water to a final volume of 30 µl. The first-round amplification was carried out in a thermocycler under the following conditions: 45 cycles at 94˚C for 2 min, 94˚C for 20 sec and 54˚C for 30 sec, with a final extension at 70˚C for 40 sec and at 70˚C for 5 min 4˚C.
Second-round PCR reaction
qPCR was used to determine the gene expression profiles of α-ENaC. cDNA was amplified by RT-qPCR using an FTC-2000 (Funglyn, Toronto, ON, Canada). Each analysis was performed in a total volume of 30 µ1 reaction mixture containing 5 µ1 cDNA sample, 1 µ1 SYBR-Green I and 2 µ1 gene-specific forward and reverse primers (10 µM each). Reference genes were included to normalize the data. Amplifications were performed as follows: 45 cycles at 94˚C for 2 min, 94˚C for 20 sec and 54˚C for 20 sec, with a final extension at 70˚C for 30 sec and at 80˚C for 20 sec.
The cycle threshold (Ct) number, defined as the number of PCR amplification cycles required to reach fluorescent intensity above the threshold, was determined for each gene and each developmental time point was analyzed. Using serial dilutions of the test sample cDNA, the standard curve was generated on the basis of the linear relationship of existing Ct and the logarithm of the copy number. The SCNN1A slope of the standard curve was shown to be −3.32 and a strong linear association was demonstrated (R2=1.00; Fig. 1A). The ACTB standard slope of the curve was also shown to be −3.32 and a strong linear association was demonstrated (R2=1.00; Fig. 1B).
Figure 1.
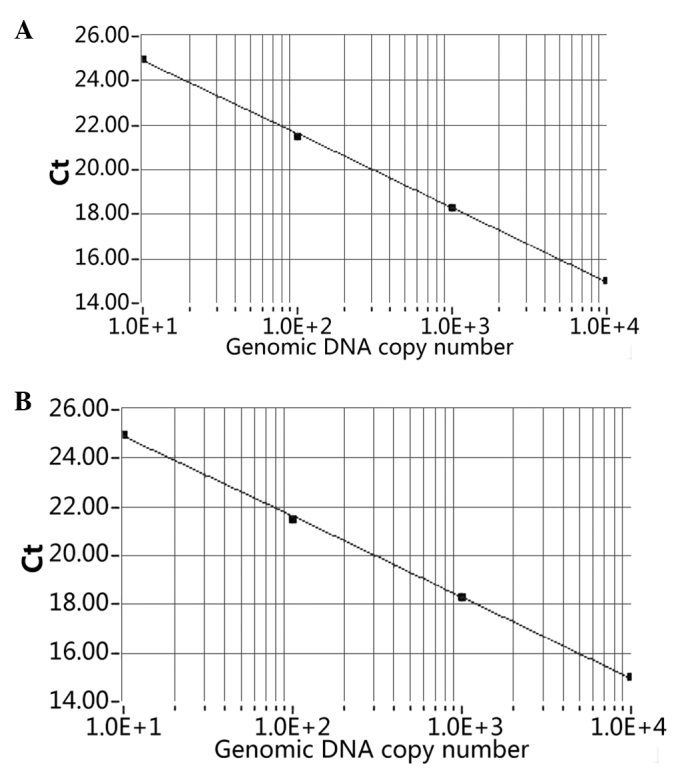
Standard curves for the (A) SCNN1A and (B) ACTB set generated from cycle threshold (Ct) values and plotted against the input standard genomic DNA molecules.
Analysis
Samples were normalized internally using the Ct number of the reference gene ACTB as follows: Δ Ct (sample) = Ct (sample)-Ct (ACTB). The mean Ct of α-ENaC in 0 h (control) was set to a relative quantity (RQ) value of 1 using the ΔΔ Ct, calculated as follows: ΔΔ Ct (sample) = Δ Ct (sample)-Δ Ct (control) and RQ=2−ΔΔCt. The data is presented as mean ± standard deviation. The one-way analysis of variance was performed using SPSS 11.0 statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Using RT-qPCR, α-ENaC mRNA was demonstrated to be expressed in the StV (Fig. 2). ACTB was examined as a reference gene. α-ENaC cDNA amplified from homogenates of the amplified fragments were of the expected sizes [124 basepairs (bp) for the α-ENaC gene (Fig. 2A) and 111 bp for the reference, ACTB cDNA (Fig. 2B)]. Tissues treated with ANP (10−6 mol/1) for different times (2, 6, 12, 24 and 48 h) showed that ANP decreased α-ENaC mRNA expression, and at the time of 2 h a maximum effect was reached (n=3, P<0.05) (Fig. 3). This effect was slightly reversed after 6 h. When α-ENaC mRNA level was evaluated chronologically in a series of cultured explants, a stability along with culture time was noticed, after 12, 24 and 48 h respectively (Fig. 3).
Figure 2.
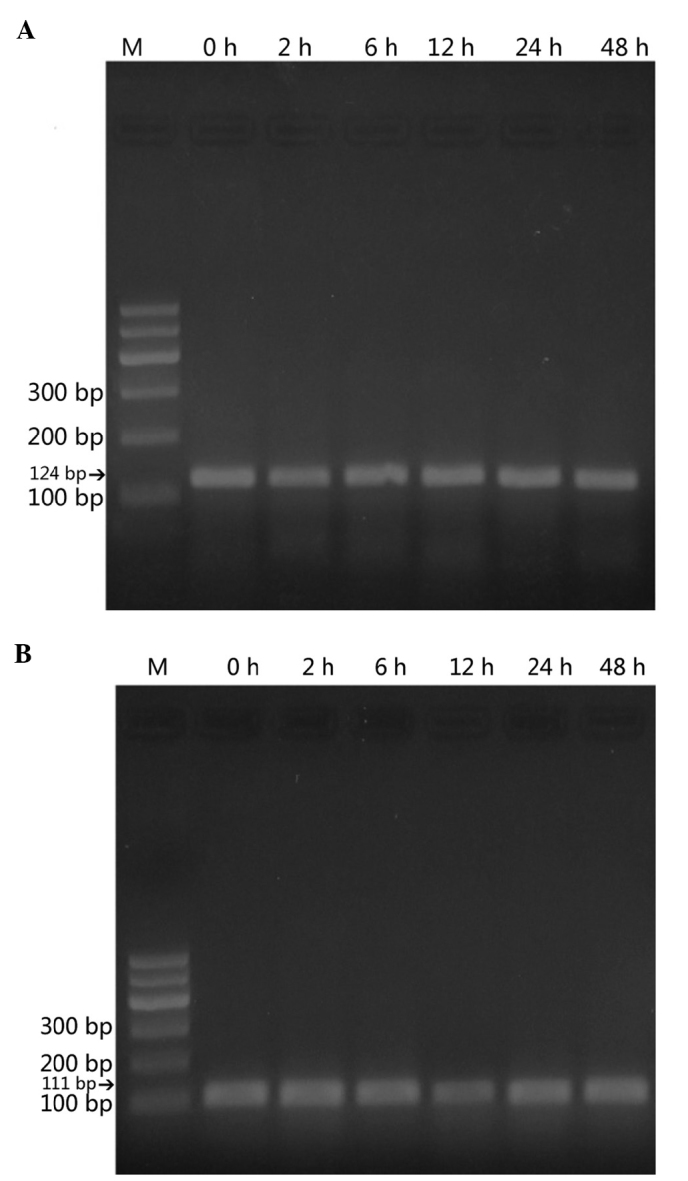
(A) α-subunit of the epithelial sodium channel (α-ENaC) cDNA amplified from the strial marginal cells. The amplified fragments were of the expected sizes of 124 bp for the α-ENaC gene. (B) The amplification of a 111 bp fragment of ACTB cDNA as a reference control is also shown.
Figure 3.
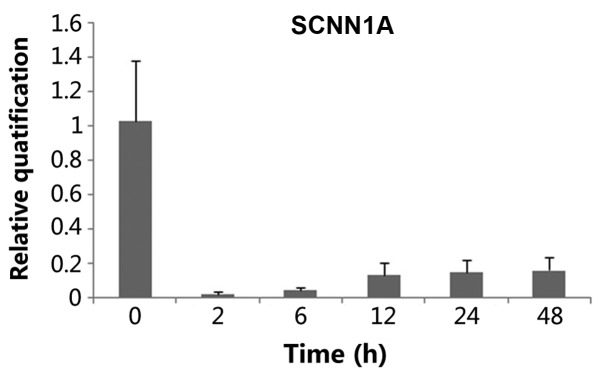
mRNA expression of α-subunit of the epithelial sodium channel (α-ENaC) by reverse transcription-quantitative polymerase chain reaction. α-ENaC mRNA expression was normalized to the expression of α-ENaC in 0 h (control), which was arbitrarily set to a value of 1. P<0.05 is considered to indicate a significantly different expression and data are expressed as the average ± standard deviation.
Discussion
The StV is mainly responsible for generating the high K+ and low Na+ levels in the endolymph, which plays an important role in normal auditory function (17). The StV is bound by several types of parasensory epithelium. Previously is has been suggested that several different ion channels are present in these parasensory epithelium cells, which include the IsK/KvLQT1, Ca2+-activated nonselective cation, Cl− and ENaC channels (12, 18). The IsK/KvLQT1 channel has been shown to be the main pathway across the apical membrane of strial marginal cells for secreting K+ into the lumen of the inner ear. ENaC has been shown to participate in reabsorbing Na+ in the apical membrane of epithelia, which is located in the apical membrane of epithelia facing endolymph.
ENaC is critical in maintaining the sodium balance and water-electrolyte balance. Together with basolateral Na+/K+-ATPase, ENaC regulates sodium reabsorption and plays a major role in the control of total body salt and water homeostasis (12). In numerous types of cells, it has been shown that ENaC is regulated by a variety of extrinsic factors, such as proteolytic cleavage, mechanical and cytoskeletal activity. ENaC has also been shown to be under the control of different hormones, such as aldosterone, arginine vasopressin, endothelin, insulin and ANP (19).
We have previously shown the presence of NPR-A transcripts in the mouse StV, as well as in the nonstrial tissue of the cochlear lateral wall and vestibular organ by PCR (9, 10). Gene expression of NPR-A in the StV was higher compared to the nonstrial and vestibula (9, 10). In the present study, the effect of ANP on the expression of the α-ENaC mRNA was investigated in the mouse StV by the RT-qPCR technique. The study showed that ANP significantly decreases α-ENaC mRNA expression in the mouse StV and at the time of 2 h a maximum effect was reached. ANP may exert its inhibitory activity on Na+ reabsorption through suppression of ENaC gene expression in the mouse StV.
The inhibitory effect by ANP on the α-ENaC mRNA may reduce the clearance of Na+. Inhibition of ENaC is expected to lead to an increase in endolymphatic Na+ concentration, subsequently resulting in an elevation in endolymph volume. An increase of the endolymph volume may aggravate the endolymphatic hydrops. ANP is believed to influence the ionic homeostasis of the endolymph by regulating the ENaC in StV. This effect may be more evident in pathological conditions, such as severe congestive heart failure when endogenous ANP is significantly increased.
The precise mechanisms of ANP in strial marginal cells remain unclear. ANP was shown to mediate the majority of its biological functions through interaction with NPR-A via cyclic guanosine monophosphate (cGMP), a second messenger (20). ANP is a classical inhibitor of Na+ reabsorption induced by aldosterone at renal distal tubule and collecting duct segments, possibly via cGMP and protein kinase G intracellular second messengers (21–23). Previously, it has been shown that cGMP is involved in the control of gene expression via its action on different gene-promoter regions or by controlling the activities of transcription factors (24, 25).
The finding of the present study that α-ENaC is expressed in the StV supports a functional role for ENaC in the regulation of Na+ concentration. Although the pathophysiological significance of the inhibition of the α-ENaC mRNA expression by ANP in the mouse StV remains unknown in the present study, it may take part in the modulation of homeostasis of the endolymph. Further investigation is required to explore the detailed molecular and cellular mechanisms by which ANP modulates the expression of the α-ENaC mRNA in the mouse StV.
In conclusion, to the best of our knowledge, the present study is the first to directly access the effect of ANP on α-ENaC mRNA expression in the mouse StV. These results indicate that ANP has a physiological importance in the regulation of ion transport and endolymph fluid balance in the inner ear.
Acknowledgements
The authors would like to thank Ms Yanfang Chen for her technical assistance. The present study was supported by the National Natural Science Foundation of China (grant no. NSFC 81271079 to Dr Yuedi Tang).
Glossary
Abbreviations
- ANP
atrial natriuretic peptide
- ENaC
epithelial sodium channel
- α-ENaC
α-subunit of the epithelial sodium channel
- StV
strial vascularies
- RT-PCR
reverse transcription-polymerase chain reaction
- ACTB
β-actin
References
- 1.Baines AD, De Bold AJ, Sonnenberg H. Natriuretic effect of atrial extract on isolated perfused rat kidney. Can J Physiol Pharmacol. 1983;61:1462–1466. doi: 10.1139/y83-208. [DOI] [PubMed] [Google Scholar]
- 2.Cea LB. Natriuretic peptide family: new aspects. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:87–98. doi: 10.2174/1568016053544309. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 4.Lamprecht J, Meyer zum Gottesberge AM. The presence and localization of receptors for atrial natriuretic peptide in the inner ear of the guinea pig. Arch Otorhinolaryngol. 1988;245:300–301. doi: 10.1007/BF00464636. [DOI] [PubMed] [Google Scholar]
- 5.Koch T, Gloddek B, Gutzke S. Binding sites of atrial natriuretic peptide (ANP) in the mammalian cochlea and stimulation of cyclic GMP synthesis. Hear Res. 1992;63:197–202. doi: 10.1016/0378-5955(92)90085-2. [DOI] [PubMed] [Google Scholar]
- 6.Seebacher T, Beitz E, Kumagami H, Wild K, Ruppersberg JP, Schultz JE. Expression of membrane-bound and cytosolic guanylyl cyclases in the rat inner ear. Hear Res. 1999;127:95–102. doi: 10.1016/S0378-5955(98)00176-2. [DOI] [PubMed] [Google Scholar]
- 7.Meyer zum Gottesberge A, Schleicher A, Drummer C, Gerzer R. The volume protective natriuretic peptide system in the inner ear. Comparison between vestibular and cochlear compartments. Acta Otolaryngol Suppl. 1995;520:170–173. doi: 10.3109/00016489509125219. [DOI] [PubMed] [Google Scholar]
- 8.Meyer zum Gottesberge A, Lamprecht J. Localization of the atrial natriuretic peptide binding sites in the inner ear tissue-possibly an additional regulating system. Acta Otolaryngol Suppl. 1989;468:53–57. doi: 10.3109/00016488909139021. [DOI] [PubMed] [Google Scholar]
- 9.Long L, Tang Y, Xia Q, Xia Z, Liu J. Detection of atrial natriuretic peptide receptor in the labyrinth of the mouse inner ear. Neuro Endocrinol Lett. 2008;29:577–580. [PubMed] [Google Scholar]
- 10.Long L, Tang Y, Xia Q, Xia Z, Liu J. The expression of atrial natriuretic peptide receptor in the mouse inner ear labyrinth. Neuro Endocrinol Lett. 2010;31:126–130. [PubMed] [Google Scholar]
- 11.Chen HX, Wang JL, Liu QC, Qiu JH. Distribution and location of immunoreactive atrial natriuretic peptides in cochlear stria vascularis of guinea pig. Chin Med J (Engl) 1994;107:53–56. [PubMed] [Google Scholar]
- 12.Loffing J, Schild L. Functional domains of the epithelial sodium channel. J Am Soc Nephrol. 2005;16:3175–3181. doi: 10.1681/ASN.2005050456. [DOI] [PubMed] [Google Scholar]
- 13.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 14.Couloigner V, Fay M, Djelidi S, et al. Location and function of the epithelial Na channel in the cochlea. Am J Physiol Renal Physiol. 2001;280:F214–F222. doi: 10.1152/ajprenal.2001.280.2.F214. [DOI] [PubMed] [Google Scholar]
- 15.Yoon YJ, Hellstrom S. Immunohistochemical localization of alpha-atrial natriuretic polypeptide in the rat cochlea. Acta Otolaryngol. 1992;112:604–610. doi: 10.3109/00016489209137448. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology (NY) 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- 17.Marcus DC, Chiba T. K+ and Na+ absorption by outer sulcus epithelial cells. Hear Res. 1999;134:48–56. doi: 10.1016/S0378-5955(99)00074-X. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi S, Ando M, Kozakura K, Saito H, Irimajiri A. Ion channels in basolateral membrane of marginal cells dissociated from gerbil stria vascularis. Hear Res. 1995;83:89–100. doi: 10.1016/0378-5955(94)00191-R. [DOI] [PubMed] [Google Scholar]
- 19.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 20.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 21.Fried TA, Osgood RW, Stein JH. Tubular site(s) of action of atrial natriuretic peptide in the rat. Am J Physiol. 1988;255:F313–F316. doi: 10.1152/ajprenal.1988.255.2.F313. [DOI] [PubMed] [Google Scholar]
- 22.Argenzio RA, Armstrong M. ANP inhibits NaCl absorption and elicits Cl secretion in porcine colon: evidence for cGMP and Ca mediation. Am J Physiol. 1993;265:R57–R65. doi: 10.1152/ajpregu.1993.265.1.R57. [DOI] [PubMed] [Google Scholar]
- 23.Waldman SA, Rapoport RM, Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984;259:14332–14334. [PubMed] [Google Scholar]
- 24.Kiemer AK, Bildner N, Weber NC, Vollmar AM. Characterization of heme oxygenase 1 (heat shock protein 32) induction by atrial natriuretic peptide in human endothelial cells. Endocrinology. 2003;144:802–812. doi: 10.1210/en.2002-220610. [DOI] [PubMed] [Google Scholar]
- 25.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93:1034–1046. doi: 10.1161/01.RES.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]


