Abstract
Exocrine pancreas is a source of several enzymes that are essential for the digestive process. The exocrine pancreatic secretion is tightly regulated by the neuroendocrine system. The endocrine pancreas is tightly integrated anatomically and physiologically with the exocrine pancreas and modulates its function. Compound-induced pancreatitis is not a common event in toxicology or drug development but it becomes a significant liability when encountered. Understanding the species-specific differences in physiology is essential to understand the underlying pathobiology of pancreatic disease in animal models and its relevance to human disease. This review will mainly focus on understanding the morphology and physiology of the pancreas, unique islet-exocrine interactions, and pancreatitis.
Keywords: Animal Models, Digestive system, Endocrine system, OTHER
Introduction
Pancreas is not the most common target organ in toxicological studies. However, pancreatitis can be a serious liability in drug development. Understanding the normal morphology and physiology is essential in order to appreciate the pathology of any organ system. In this paper, I will focus on the normal morphology and physiology of exocrine pancreas and the pathogenesis of pancreatitis.
Morphology
The pancreas develops from a common multipotent cell population within the foregut (dorsal and ventral buds) endoderm. The exocrine and endocrine pancreas arise from a common progenitor cell population expressing Pdx1, Ptf1a and Sox9. These cells in the presence of other factors like Ngn3, NeuroD, Hnf6 and Pax4 contribute to the proliferation and differentiation of the endocrine pancreas. The absence of proendocrine factors and transcription factors like Ptf1a and Mist1 leads to the development of exocrine pancreas (Reichert and Rustgi, 2011; Benitez et al., 2012). Overall, pathways associated with Hedgehog, Notch and Wnt play critical roles in the development, differentiation and proliferation of pancreas. The exocrine pancreas is morphologically mature at birth but attain functional maturity at weaning in most animals (Walthall et al., 2005).
There is species-specific variation in the organization of pancreatic tissue. In rabbits, the pancreatic tissue is diffusely distributed through out the mesentery and in primates, dogs and hamsters it is compact. While, in rats and mice it is intermediate since the tail (splenic portion) is relatively compact but the head is dispersed within the duodenal loop’s mesentery. The pancreas is the only organ in the body composed of exocrine and endocrine components intermixed within the parenchyma. In rats and mice, the pancreas may be anatomically divided into gastric lobe, duodenal head and tail. The numbers of islets of Langerhans distributed within each of these regions appears to vary by strains. For example, within a 10-week old CD1 mouse on average there are 110 islets in the gastric lobe, 453 islets in the duodenal head and 686 islets in the tail, whereas, in a 10-week old NOD mouse on average there were 120 islets in the gastric lobe, 549 islets in the duodenal head and 489 islets in the tail (El-Gohary et al., 2012). The exocrine pancreatic component comprises ~90% of the pancreatic mass and is comprised of acinar, centroacinar and ductal cells; the endocrine component islets of Langerhans comprise about 1-2% and the interstitium with the blood vessels, lymphatics, nerves and fibrous connective tissue stroma comprise the remainder. The pancreas is covered by a thin layer of loose connective tissue that forms septa resulting in the division of the gland into lobules that contain numerous acini. In order to explain the organization of the exocrine pancreatic acini, two models have been proposed. One is the acinar model supported by Takahashi (1984) where the lobules are arranged in grape-like clusters interconnected by ducts and the other is the reticular model supported by Bockman (1976) where the lobules bud off a network of anastomosing tubules (Bockman, 1976; Takahashi, 1984). There are proponents for both the models since they are backed up by data from morphological studies involving retrograde injection of dyes and latex, scanning electron microscopy and 3D reconstruction of serial semithin sections (Motta et al., 1997). These models seem to support the morphology across several species and possibly the pathogenesis of some lesions within the exocrine pancreas. For example, the ductular morphology of the tubular complexes as well as the ductular phenotype seen in pancreatic adenocarcinoma seem to support the anastomosing model since in the dimethylbenzanthracene treated rat model, the acinar cells lose their zymogen granules and attain a ductular phenotype and proliferate progressively to form ductular complexes and subsequently an adenocarcinoma with ductular phenotype (Bockman et al., 2003). The pancreatic acinus consists of a single layer of pyramidal acinar cells arranged concentrically around a lumen. The narrow apices of the pyramidal cells facing the lumen contain eosinophilic zymogen granules and the broader base of the pyramidal cells is basophilic due to abundant rough endoplasmic reticulum and also contains the nucleus. Each pancreatic acinus is surrounded by a thin basal lamina, scant reticular stroma, and pancreatic stellate cells (similar to the hepatic stellate cells). The quiescent pancreatic stellate cells stain positive for vimentin, desmin, GFAP and Nestin. When activated, these stellate cells express α-smooth muscle actin (Omary et al., 2007). The pancreatic stellate cells play important roles in the pathogenesis of chronic pancreatitis and pancreatic cancer. Located centrally within the acinus, the centroacinar cells form an interface between the acinus and the intercalated duct. The intercalated duct continues into intralobular ducts formed by the ductular cells. The centroacinar cells and the duct cells have carbonic anhydrase and secrete bicarbonate and water that results in the flushing of acinar secretions into the pancreatic ducts. The intralobular ducts fuse to form the interlobular ducts that mainly open into the pancreato-hepatic (biliary) duct or to a lesser extent directly into the duodenal lumen.
The exocrine pancreas is physiologically and morphologically compartmentalized into peri- and tele-insular regions. The peri-insular acinar cells are in the immediate proximity of the islets of Langerhans and are bigger with abundant cytoplasm, larger zymogen granules and larger nuclei than the tele-insular acinar cells that are farther from the islets (Aughsteen and Kataoka, 1993). Due to differences in sizes of the peri- and tele-insular acinar cells, at lower magnification, the islets of Langerhans appear to be surrounded by “halos”. These physiologically normal size differences in the peri- and tele-insular exocrine acinar cells form the so-called “peri-insular halos” (Cosnier, 1955). The difference in the size of peri- and tele-insular acinar cells is greater in mice than in rats, resulting in more prominent peri-insular halos in mice. The peri-insular acinar cells are more resistant (3 hours) to pilocarpine-induced degranulation than the tele-insular acinar cells (1 hour) (Putzke and Said, 1975). The size of peri-insular halos is markedly reduced in alloxan-induced diabetic pancreata compared to control non-diabetic rats. These features of the peri-insular halos are due to the hormones (mainly insulin and possibly other factors) secreted by islets of Langerhans that are locally enriched due to the islet-acinar capillary anastomoses or due to diffusion via the fenestrated capillaries (Wayland, 1997). The peri-insular halos are not recorded in routine toxicity studies but recording any alterations within these halos may provide important information about chemicals affecting islet cells such as alloxan or about chemicals directly affecting the secretions from exocrine pancreas like pilocarpine. Thus, it is important to be cognizant of the heterogeneity of exocrine pancreatic acini during routine toxicologic pathology studies since it may be useful in identifying chemicals that may target the islets of Langerhans (like alloxan) or the exocrine pancreas (like pilocarpine).
Islet-Acinar axis
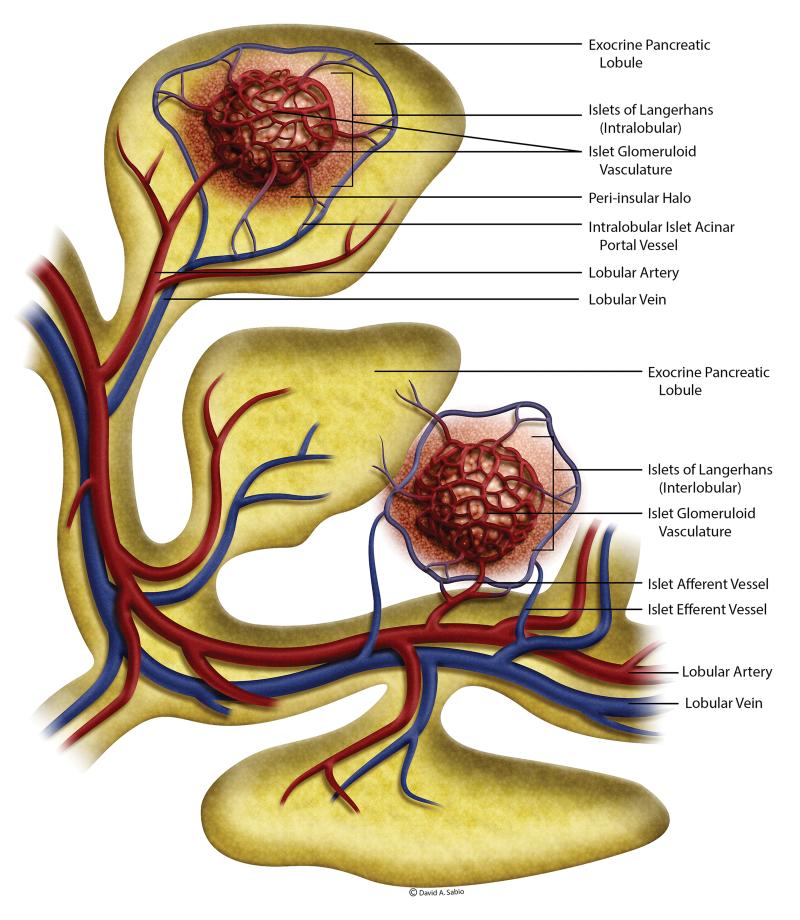
The rat pancreas is supplied by the splenic, pancreato-duodenal and other minor arteries arising from the celiac or superior mesenteric arteries and drain into the splenic, pancreato-duodenal and other veins into the portal vein. There are about 5000 or more lobules within the rat exocrine pancreas (Murakami and Fujita, 1992). The lobular artery and vein enter the lobules near their base and form lobular capillary plexus and then drain into the lobular veins (Figure 1). The islets of Langerhans comprise ~2% of the pancreas but about 20% of the arterial blood entering the pancreas is supplied to the islets. In a typical rat pancreas, there are about 400 islets that may be located in almost equal numbers either within the exocrine lobules (intralobular) or between tissue spaces along the secretory ducts (interlobular) (Murakami and Fujita, 1992). The intralobular and interlobular afferents arise from lobular and interlobular arteries or their branches, respectively.
Figure 1.
An artist’s rendering of the vasculature within the rat pancreas. The islets and acini have a tightly integrated blood supply. The islets may be located intralobular or interlobular and have corresponding blood supply. The blood draining from the intralobular islets pass through the exocrine acini before joining the venous system. The peptides from the islets contributes to the regulation of peri-islet acini (please see text for more details). (Adapted from Murakami and Fujita, 1992)
Based on their size, the islets receive one or more afferent vessels and give out 3 or more efferent vessels (Figure 1). The intralobular efferents drain either into lobular capillaries or into venous branches in the lobules and the interlobular efferents drain into venous branches in the interlobular spaces or along the secretory ducts. Thus, the islet-acinar axis forms a direct connection between the islet and lobular capillaries. This islet-acinar portal system has been demonstrated in man, monkey, horse, rabbit, dog, cat, mouse, and rat. However, there are some species differences in this regard. In man (and monkey, pig, cattle, rabbit, dog, cat), most of the islets are intralobular in location and emit only islet-acinar portal vessels and very few islets within the interlobular spaces issue the islet-venous efferent vessels that are continuous with the interlobular veins. In contrast, in rat and mouse, the intralobular vessels drain into both islet-acinar portal vessels and intralobular veins and the interlobular arteries drain into the interlobular veins (Murakami et al., 1993).
In the rat, the afferent arteries supplying the islets are first divided in the peripheral zone of the islet and then break into several capillaries resulting in a glomerular meshwork that penetrates the islets and converge to form the efferent supply that passes through the surrounding exocrine acini before joining the venous drainage. The vascular plexus within the islets is about 5 times denser than the acinar lobular plexus (Henderson and Moss, 1985). The capillary network in islets has about ten times more fenestrae than capillaries of the exocrine tissue (Henderson and Moss, 1985). This unique anatomic arrangement of the islet-acinar portal system facilitates the interaction of several proteins secreted by the islets with the surrounding exocrine tissue.
Influence of the islet-acinar axis on exocrine function
The islets of Langerhans are distributed within the exocrine pancreas and this arrangement serves an important physiological function since several islet-derived hormones and peptides directly influence the exocrine function. In addition to these peptides and islet-derived hormones, neurotransmitters like acetylcholine and norepinephrine, play an important role in the exocrine pancreatic homeostasis. Several unique cell types within the islets secrete corresponding hormones and peptides for influencing exocrine function. The alpha (α) cells secrete glucagon, the beta (β) cells produce insulin and amylin, the delta (δ) cells produce somatostatin (SST), the epsilon (ε) cells produce ghrelin, and the PP or F cells produce pancreatic polypeptide and adrenomedullin. In addition, islets also contain several neuropeptides/cotransmitters that modulate exocrine pancreatic function. The stimulatory neuropeptides/cotransmitters include pituitary adenylate cyclase-activating peptide (PACAP), and nitric oxide (NO), vasoactive intestinal peptide (VIP), and Angiotensin II. The inhibitory factors for exocrine pancreatic secretion include SST, pancreatic polypeptide, ghrelin, pancreastatin, adrenomedullin, galanin, calcitonin gene-related peptide (CGRP), neuropeptide Y (NPY), and peptide YY (PYY) (Barreto et al., 2010).
Insulin is one of the major islet hormones and the most well characterized regulator of exocrine pancreatic function. Insulin positively influences pancreatic growth and exocrine function. Insulin binds to its own receptor on the acinar cells to stimulate and potentiate amylase secretion (Mossner et al., 1985). In addition, it also potentiates secretagogue-stimulated secretion of amylase. The role of glucagon on exocrine function is not clear due to contradictory data (Barreto et al., 2010). Pituitary adenylate cyclase-activating peptide (PACAP) has a direct stimulatory effect on the exocrine pancreatic secretion as well as flow rate without affecting the secretin and VIP levels (Alonso et al., 1994). This stimulatory effect of PACAP is mediated by a cholinergic mechanism and it has a synergistic effect with CCK (Naruse et al., 1992; Kitagawa et al., 1995). NO exerts a stimulatory effect of exocrine pancreatic secretion probably via the generation of cyclic guanosine monophosphate and the release of endogenous neurotransmitter in the pancreas (Ember et al., 2001; Yago et al., 2001). Vasoactive intestinal peptide (VIP) shares structural similarity with secretin as well as glucagon. It has a stimulatory action on exocrine pancreatic secretion, especially with the increasing pancreatic secretion flow rate and secretin levels, suggesting that these effects are likely secondary to its effect on secretin levels (Alonso et al., 1994). The pancreas contains a local renin-angiotensin system and the angiotensin II receptors AT1 and AT2 are expressed in pancreatic acinar cells, ducts, islets and blood vessels (Leung and Carlsson, 2001; Tsang et al., 2004). Angiotensin II stimulates exocrine pancreatic secretion (Tsang et al., 2004) but inhibits the synthesis and release of insulin from the islets (Lau et al., 2004).
Somatostatin (SST) acts as a hormone and as a neurotransmitter. It acts as a hormone by inhibiting CCK or cerulein stimulated amylase secretion and inhibits insulin secretion. SST binds to its own receptor on the acinar cells and reduces intracellular cyclic AMP and subsequent Ca2+ signaling (Ohnishi et al., 1994). SST acts as a neurotransmitter by modulating vagal and sympathetic pathways or via the SST receptor 2 in the dorsal vagal complex (DVC). In addition, it can indirectly inhibit pancreatic secretion via the intrapancreatic cholinergic mechanism by inhibiting acetylcholine release in the peripheral nerve terminals (Heintges et al., 1994). Pancreatic polypeptide inhibits exocrine pancreatic secretion during both the interdigestive and postprandial states. It has been demonstrated to inhibit CCK-stimulated release of amylase and by inhibiting the stimulatory effect of insulin on amylase secretion (Louie et al., 1985). Ghrelin inhibits exocrine pancreatic secretion but its precise mechanism of action is not known but is thought to be via intrapancreatic neurons (Zhang et al., 2001) or by inhibiting insulin secretion (Tong et al., 2010). Pancreastatin inhibits glucose induced insulin secretion (Tatemoto et al., 1986). In addition, it also inhibits exocrine secretion irrespective of the stimulus used and this is probably mediated by modulation of presynaptic acetylcholine release (Herzig et al., 1992) and/or reduction of local pancreatic blood flow (Migita et al., 1999). Adrenomedullin colocalizes with pancreatic polypeptide in the PP or F cells and inhibits insulin secretion (Martinez et al., 1996). Galanin inhibits the secretion of insulin (McDonald et al., 1985) and somatostatin (Amiranoff et al., 1990). Its effect on amylase secretion is dependent on the stimulus, i.e. it inhibited amylase secretion stimulated by cerulein at physiological concentrations but it had no effect on carbachol-stimulated amylase secretion (Barreto et al., 2009). The cerulein-induced stimulation of amylase secretion is secondary to inhibition of insulin secretion and inhibition of postganglionic cholinergic nerves (Barreto et al., 2010). This indicates the close influence of endocrine pancreas on exocrine pancreatic secretion. Calcitonin gene-related peptide (CGRP)–immunoreactive neurons within the pancreas may play a role in influencing exocrine secretion. CGRP inhibits exocrine secretions indirectly by stimulation and release of SST into systemic circulation (Mulholland et al., 1989) and by sympathetic noradrenergic efferent nerves via α-adrenergic receptor (Messmer et al., 1993). Neuropeptide Y (NPY) dose dependently inhibits CCK-stimulated exocrine pancreatic secretion but does not alter bicarbonate concentration in secretin-stimulated pancreatic secretions (Mulholland et al., 1991). The action of NPY on pancreatic exocrine secretions is likely indirect via alteration of intrapancreatic neurotransmission (Mulholland et al., 1991) or via its splanchnic vasoconstrictive effect (Sumi et al., 1991). Peptide YY (PYY) is structurally similar to pancreatic polypeptide and significantly inhibits secretin- and CCK- stimulated pancreatic exocrine secretion (Tatemoto, 1982). PYY acts via intrapancreatic cholinergic nerves and is independent of adrenergic nerves or extrapancreatic nerves (DeMar et al., 1991; Brodish et al., 1993; Brodish et al., 1995). Peptide YY acts via the Y1 receptor in rats and Y2 receptor in dogs (Grandt et al., 1995; Teyssen et al., 1996). Substance P binds to neurokinin receptors on acinar cells and modulates pancreatic neural signaling, and blood flow that subsequently influence exocrine pancreatic secretion (Barreto et al., 2010).
Physiology of exocrine pancreatic secretion
The function of the exocrine pancreas is tightly regulated by the neuroendocrine system. Please refer to the excellent reviews on the topic for more details (Konturek et al., 2003a; Owyang and Logsdon, 2004; Wang and Cui, 2007; Owyang, 2009; Singer and Niebergall-Roth, 2009)
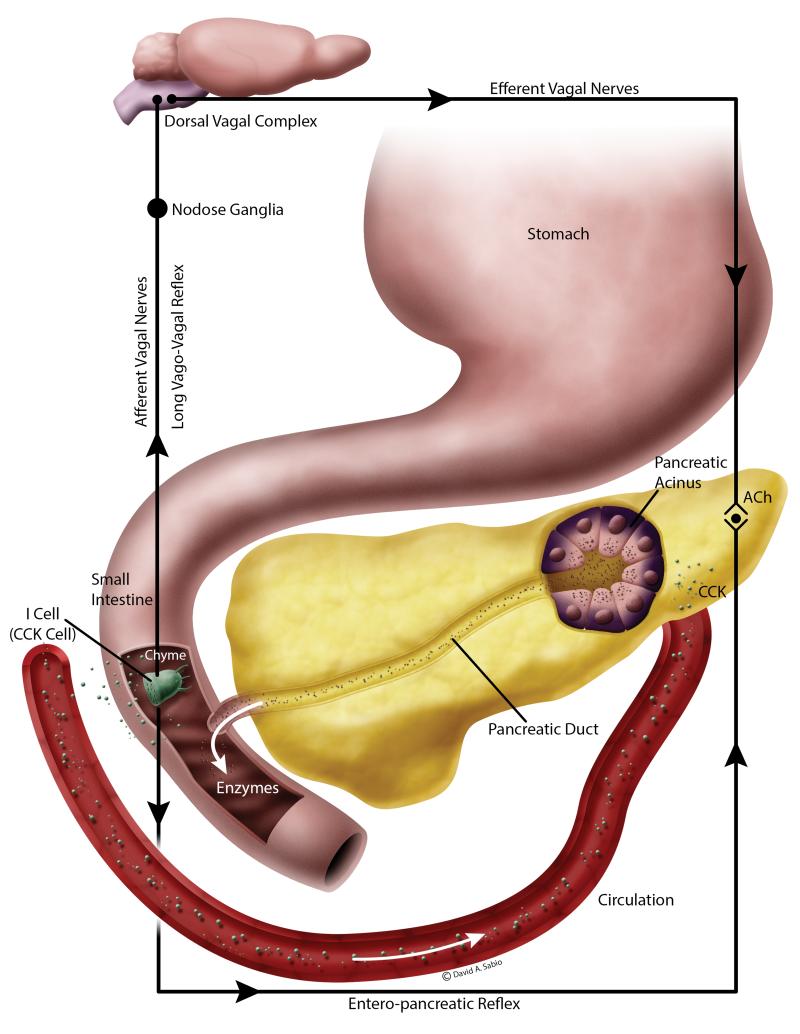
The exocrine pancreatic secretions are elicited via a complex interplay of neural, humoral and paracrine mediators (Figure 2). The islets and the exocrine tissue are richly innervated with central and autonomic nervous system with afferent and efferent signaling. The vagus nerve serves a major role in the regulatory pathway. In addition, enteropancreatic neurons between the pancreas and the gastrointestinal tract mediate the vago-vagal enteropancreatic reflexes that are important in the intestinal phase of the exocrine pancreatic secretion. The vago-vagal enteropancreatic reflex consists of the afferent and efferent fibers of the vagus nerve that coordinate responses to the gut stimuli via the dorsal vagal complex. The intrapancreatic postganglionic neurons are activated by efferents arising from the duodenal mucosa that is in contact with chyme (intestinal phase). Acetylcholine released by these neurons act on the M1 and M3 muscarinic receptors on the acinar cells to elicit exocrine secretions (Singer and Niebergall-Roth, 2009).
Figure 2.
Cholecystokinin (CCK) stimulation of the exocrine pancreas is species dependent. In rodents, CCK released from the I cells within the intestine enter the circulation and directly stimulate the CCK1 receptors on the exocrine acini to stimulate secretions. In addition, CCK can also activate the sensory nerve fibers resulting in the activation of long vago-vagal and short enteropancreatic cholinergic reflexes. However, in humans, the latter process is the most accepted physiological process of exocrine stimulation. (Adapted from Wang and Cui, 2007)
Besides cholinergic nerves, the peptide cholecystokinin (CCK) is a very important mediator of exocrine pancreatic secretory activity and also has a trophic effect on pancreas (Yamamoto et al., 2003). CCK is a heterogenous protein secreted by I cells in the small intestine and by neurons in the brain. The plasma CCK that originates from the small intestine is a mixture of several types of CCKs (CCK-58, 33, 22, 8) but the prominent molecular forms in the blood are different in different species, i.e. CCK-58 in rats and dogs; CCK-33 in humans, and CCK-22 in pigs (Eysselein et al., 1987; Cantor and Rehfeld, 1989; Rehfeld et al., 2001; Reeve et al., 2003; Wang and Cui, 2007). CCK, in addition, also inhibits gastric emptying, gastric acid secretion, and gall bladder contraction. Since physiological levels of CCK stimulate exocrine pancreatic secretion in humans and rodents, it was assumed that CCK stimulates CCK-A receptor. However, exocrine pancreatic secretion by physiological levels of CCK (but not supraphysiological levels) is inhibited completely by atropine suggests the importance of cholinergic stimulation in eliciting exocrine secretion (Konturek et al., 2003b). In addition, human pancreatic acini do not have any functional CCK receptors and are not responsive to physiological concentrations of CCK in vitro (Ji et al., 2001; Miyasaka et al., 2002). In contrast, rodent pancreatic acini are responsive to physiological concentrations of CCK in vitro owing to the presence of CCK-A receptors on the pancreatic acini. Thus, it is generally accepted that in humans exocrine pancreatic secretion is almost exclusively mediated through the cholinergic stimulation of the acini where as in rodents, there is a dual mechanism of stimulation, both direct CCK-A receptor stimulation as well as indirectly via the cholinergic stimulation (Owyang, 2009).
Independent of the action of CCK, the intestinal serotonin also mediates exocrine pancreatic secretion. Luminal factors such as osmolarity and disaccharides activate 5-HT3 receptors, whereas mechanical stimulation activates both 5-HT3 and 5-HT2 receptors on mucosal vagal afferent fibers in the intestine (Li et al., 2001). Activation of these serotonin receptors stimulates exocrine pancreatic secretion. The enterochromaffin cells within the intestinal mucosa are the richest source of serotonin in the gut. Serotonin may act as a paracrine substance and mediate exocrine pancreatic secretion via a cholinergic pathway similar to that of CCK since acute vagotomy, and perivagal or luminal application of capsaicin abolishes serotonin-induced pancreatic secretion (Zhu et al., 2001). CCK and serotonin may act at the level of the nodose ganglia to synergistically increase exocrine pancreatic secretion and this is exemplified by the robust postprandial exocrine pancreatic secretion mediated by modest increases in plasma CCK levels (Owyang and Logsdon, 2004).
Secretin is another major hormone that influences exocrine pancreatic secretion. It plays an important role in the secretion of bicarbonate rich fluid that helps in flushing the pancreatic zymogens from the acinar lumen into the pancreatic ducts. In addition, secretin plays inhibitory roles in gastric acid secretion, and gastric motility. Luminal acid influenced release of secretin is mediated by a secretin releasing peptide that in turn is dependent on vagal afferent pathways. Secretin’s action at physiological doses is abolished by atropine. Thus, similar to CCK, secretin’s actions are also mediated by vagal afferent cholinergic pathways (Li et al., 1990). Similar to serotonin, CCK and secretin also acts at the level of the nodose ganglia to synergistically increase exocrine pancreatic secretion (Owyang, 2009).
Drug-induced pancreatitis
Pancreatitis in humans may be manifested as a pain in the upper abdomen radiating to the back, and nausea that aggravates with eating. It can be mild or fulminant with severe systemic effects. In general human population, the incidence of pancreatitis is about 2% but in certain cohorts like the HIV patients, the incidence can be up to 40% (Trivedi and Pitchumoni, 2005). The principal causes of pancreatitis in humans include alcoholism and biliary obstruction. However the etiology of pancreatitis may be divided into several categories, such as, toxic (alcoholism, pesticides, and drugs); obstructive (gallstones, tumors, worms and congenital defects); metabolic (hyperlipidemia, hypercalcemia and acidosis); infectious (parasitic, viral and bacterial) and genetic (Cystic fibrosis transmembrane conductance regulator (CFTR), Pancreatic secretory trypsin inhibitor (PSTI1), Serine protease inhibitor Kazal-type 1 (SPINK1), Cationic trypsinogen1 (PRSS1)). The index of suspicion of drug-induced pancreatitis is very low especially when compared to drug-induced liver injury due to the subclinical nature of early pancreatitis and the absence of pancreatic enzyme evaluation in routine clinical pathology. As a result, it is under-diagnosed and under-reported. Even when it is suspected, it is difficult to establish a link between pancreatitis and the suspect drug due to the long latency of onset of the disease. In addition, even if pancreatitis is diagnosed, usually alcohol or biliary diseases are implicated and the drug remains a silent actor and is never a suspect (Trivedi and Pitchumoni, 2005). There have been several surveys aimed at identifying drugs associated with pancreatitis and several algorithms have been proposed (Mallory and Kern, 1980; Trivedi and Pitchumoni, 2005; Badalov et al., 2007). In these surveys, several categories of drugs have been implicated to be associated with pancreatitis, such as antimicrobials (pentavalent antimonials, tetracycline, pentamidine, trimethoprim/sulfamethoxazole, sulfasalazine); HIV drugs (didanosine, nefilnavir); Diuretics (furosemide); GI and pancreatobiliary (azathioprine, 6-mercaptopurine, mesalamine); Immunosuppressive (L-asparaginase, dexamethasone) and Nervous system (valproic acid, opiates). The mechanism of action of these drug-induced pancreatitis is not really well addressed since most of these drugs are not direct pancreatic toxicants but are “associated” with other etiological factors or idiosyncratic factors that result in pancreatitis in a temporal and context dependent manner. Compared to the liver, the pancreas has a respectable complement of xenobiotic metabolizing enzymes and the distribution of these enzymes within the pancreatic parenchyma varies greatly depending on the cell type. In all the species examined, the endocrine pancreas had the greatest amount of the phase I and II metabolizing enzymes compared to the exocrine pancreas and it is not surprising that the endocrine pancreas is more often a target of pancreatic toxicity than exocrine pancreas (Ulrich et al., 2002). There are very few studies that examined metabolism of drugs and chemicals within the pancreatic tissue and as a result only a few xenobiotics (Cyanohydroxybutene, 2,3,7,8-tetrachlorodibenzo-p-dioxin, L-arginine, tacrolimus, organophosphates) can be definitely identified as pancreatic toxicants (Nalesnik et al., 1987; Tani et al., 1990; Longnecker, 2002; Yoshizawa et al., 2005; Walgren et al., 2007; Singh et al., 2007).
Even though supramaximal stimulation of pancreas is not a common cause of pancreatitis in humans, secretagogue hyperstimulation models such as cerulein-induced hyperstimulation with or without sensitizers like ethanol and LPS are used to understand the pathophysiology of pancreatitis (Lerch and Gorelick, 2013). The cerulein hyperstimulation model is most widely used because of its ease of induction, non-invasiveness, and reproducibility. The pathogenesis of secretagogue-induced pancreatitis has been reviewed in several recent papers (Saluja et al., 2007; Van Acker et al., 2007; Lerch and Gorelick, 2013). Several factors have been implicated in the pathogenesis of secretagogue-induced pancreatitis. A detailed review is beyond the scope of this paper. For more information on the pathogenesis of secretagogue-induced pancreatitis, please refer to the following reviews (Saluja et al., 2007; Van Acker et al., 2007; Lerch and Gorelick, 2013). Briefly, at supramaximal doses the pancreatic enzyme secretion is blocked resulting in colocalization of zymogens and lysosomes, activation of trypsinogen by cathepsin B and subsequent activation of other zymogens by trypsin, the resulting acinar cell injury and release of chemokines and cyokines causes inflammation and infiltration of leukocytes. The leukocytes secrete more cytokines and potentiate a self-propagating inflammatory reaction and systemic disease (Saluja et al., 2007).
The CCK-A receptors in the rat pancreas exist in both high- and low-affinity states and these contribute to some understanding of the pathogenesis of secretagogue-induced pancreatitis. CCK and its agonists cerulein or carbachol have a biphasic or bell shaped dose response curves, i.e. at physiological dose stimulation there is an increase in amylase secretion, but at high (supramaximal) doses, the secretion of amylase is inhibited. In addition at supramaximal stimulation, pancreatitis is observed. A novel CCK agonist CCK-JMV-180 has a unique monophasic dose response curve compared to CCK and its agonists in that, its stimulation curve is more or less a plateau at both low and high doses (Saluja et al., 1989). This indicates that exocrine pancreatic secretion at physiological doses of CCK and its agonists as well as CCK-JMV-180 is mediated by high-affinity receptors. In contrast, the inhibition of exocrine secretion and induction of pancreatitis at high doses of CCK and its agonists is mediated by low-affinity receptors. CCK-JMV-180 acts as an antagonist of the low affinity receptors and prevents the inhibition of pancreatic exocrine secretion and also pancreatitis. Thus, the inhibition of enzyme secretion at high doses via the low affinity receptors plays an important role in secretagogue-induced pancreatitis (Saluja et al., 2007).
Calcium signaling plays an important role in the molecular pathways of several metabolic cells. CCK and its agonists bind to G-protein coupled transmembrane receptors on the basolateral surface of the pancreatic acinar cells and activate phospholipase C resulting in the cleavage of phosphatidylinositol-4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 causes the release of Ca2+ from the endoplasmic reticulum (Thorn et al., 1993a; Thorn et al., 1993b). Based on the type of stimulation, the duration of the intracellular Ca2+ oscillations or spikes can vary. These Ca2+ spikes result in a burst of exocytic activity causing the release of zymogens. With acetylcholine, these spikes are restricted to the secretary pole of the cell where as, with CCK, the spikes are local followed by distribution to the entire cell. With physiological stimulation, the zymogens are released only from the secretory pole where as, with supramaximal stimulation, there is a sustained increase in Ca2+ spikes resulting in a significant increase in zymogen release followed by sustained increase at lower level (Matozaki et al., 1990; Thorn and Petersen, 1993). Hypercalcemia has also been associated with pancreatitis and increased serum calcium sensitizes the exocrine pancreas to secretogogue-induced pancreatitis (Mithofer et al., 1995a; Mithofer et al., 1995b; Frick et al., 1995). Hypocalcemia observed during pancreatitis is usually a sequela of pancreatitis and is likely related to precipitation and sequestration of calcium in soft tissues, and alterations in calcitonin and parathyroid hormone (Edmondson, 1952; Norberg et al., 1975; Bhattacharya et al., 1985; Izquierdo et al., 1985).
The subapical actin cytoskeleton is important for the secretion of zymogens after appropriate stimulation of the acinar cells. During supramaximal stimulation, the actin cytoskeleton and the associated intermediate filaments are ablated resulting in the prevention of apical exocytosis. However, treatment with CCK-JMV-180 prevents this process suggesting that the low affinity receptors may play a role in this process (O’Konski and Pandol, 1993). Once the zymogen granules are not secreted, they accumulate within the acinar cell. Upon excess stimulation, these accumulated zymogen granules colocalize with lysosomal enzymes like cathepsin B (Watanabe et al., 1984; Saito et al., 1987; Saluja et al., 1987) and convert the inactive trypsinogen into trypsin, which in turn activates a host of other peptidases, nucleases, lipases and hydrolases. The activation of these enzymes results in hyperamylasemia, hyperlipasemia, pancreatic edema, acinar cell degeneration and necrosis.
Supramaximal stimulation by CCK results in a powerful, biphasic activation of NF-κB (Gukovsky et al., 1998). This activation of NF-κB was not noted during pancreatic stimulation with physiological levels of CCK or with JMV-CCK-180. In addition, JMV-CCK-180 is able to abolish cerulein-induced NF-κB activation (Gukovsky et al., 1998). NF-κB transcriptionally regulates the expression of IL6 and IL8 that recruit inflammatory cells to the site of hyperstimulation (Zaninovic et al., 2000). The recruited inflammatory cells secrete more inflammatory cytokine mediators such as TNF-α, IL1, IL2, IL6, IL8 and platelet activating factor (PAF) as well as anti-inflammatory cytokines such as IL10 and IL1R antagonist resulting in systemic inflammation and sepsis (Makhija and Kingsnorth, 2002).
In summary, a thorough understanding of unique histological features and physiology of exocrine pancreas is essential in order to better understand the pathophysiology of pancreatitis. Inspite of the substantial strides made in understanding the pathophysiology of pancreatitis, it still remains a significant clinical problem mainly due to the myriad factors that are associated with the pathogenesis and diagnosis of pancreatitis.
Acknowledgements
I would to thank Drs. Robert Sills (NIEHS), Mark Cesta (NIEHS), Jerry Hardisty (EPL, Inc.) and Rodney Miller (EPL, Inc.) for their support and encouragement. In addition, I would like to acknowledge Mr. David Sabio (EPL, Inc.) for the fine illustrations presented in this manuscript.
Reference
- Alonso RM, Rodriguez AM, Garcia LJ, Lopez MA, Calvo JJ. Comparison between the effects of VIP and the novel peptide PACAP on the exocrine pancreatic secretion of the rat. Pancreas. 1994;9:123–128. doi: 10.1097/00006676-199401000-00018. [DOI] [PubMed] [Google Scholar]
- Amiranoff B, Lorinet AM, Laburthe M. Galanin inhibits somatostatin release by the rat islet cell tumor in culture, Rin-m. Eur J Pharmacol. 1990;191:401–405. doi: 10.1016/0014-2999(90)94174-v. [DOI] [PubMed] [Google Scholar]
- Aughsteen AA, Kataoka K. Morphometric studies on the juxta-insular and tele-insular acinar cells of the pancreas in normal and streptozotocin-induced diabetic rats. J Electron Microsc (Tokyo) 1993;42:79–87. [PubMed] [Google Scholar]
- Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648–661. doi: 10.1016/j.cgh.2006.11.023. quiz 644. [DOI] [PubMed] [Google Scholar]
- Barreto SG, Carati CJ, Toouli J, Saccone GT. The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol. 2010;299:G10–22. doi: 10.1152/ajpgi.00077.2010. [DOI] [PubMed] [Google Scholar]
- Barreto SG, Woods CM, Carati CJ, Schloithe AC, Jaya SR, Toouli J, Saccone GT. Galanin inhibits caerulein-stimulated pancreatic amylase secretion via cholinergic nerves and insulin. Am J Physiol Gastrointest Liver Physiol. 2009;297:G333–339. doi: 10.1152/ajpgi.00078.2009. [DOI] [PubMed] [Google Scholar]
- Benitez CM, Goodyer WR, Kim SK. Deconstructing pancreas developmental biology. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Luther RW, Pate JW, Crawford AJ, Moore OF, Pitcock JA, Palmieri GM, Britt LG. Soft tissue calcium and magnesium content in acute pancreatitis in the dog: calcium accumulation, a mechanism for hypocalcemia in acute pancreatitis. J Lab Clin Med. 1985;105:422–427. [PubMed] [Google Scholar]
- Bockman DE. Anastomosing tubular arrangement of the exocrine pancreas. Am J Anat. 1976;147:113–118. doi: 10.1002/aja.1001470111. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853–859. doi: 10.1097/01.lab.0000074918.31303.5a. [DOI] [PubMed] [Google Scholar]
- Brodish RJ, Kuvshinoff BW, Fink AS, McFadden DW. Peptide YY inhibits pancreatic secretion via cholinergic pathways. J Surg Res. 1993;55:103–108. doi: 10.1006/jsre.1993.1115. [DOI] [PubMed] [Google Scholar]
- Brodish RJ, Kuvshinoff BW, McFadden DW, Fink AS. Adrenergic pathways do not mediate peptide YY-induced inhibition of pancreatic exocrine secretion. Pancreas. 1995;10:187–193. doi: 10.1097/00006676-199503000-00013. [DOI] [PubMed] [Google Scholar]
- Cantor P, Rehfeld JF. Cholecystokinin in pig plasma: release of components devoid of a bioactive COOH-terminus. Am J Physiol. 1989;256:G53–61. doi: 10.1152/ajpgi.1989.256.1.G53. [DOI] [PubMed] [Google Scholar]
- Cosnier J. [Peculiar image of the exocrine peri-insular zones in the white rat, the peri-insular halo] C R Seances Soc Biol Fil. 1955;149:263–265. [PubMed] [Google Scholar]
- DeMar AR, Taylor IL, Fink AS. Pancreatic polypeptide and peptide YY inhibit the denervated canine pancreas. Pancreas. 1991;6:419–426. doi: 10.1097/00006676-199107000-00008. [DOI] [PubMed] [Google Scholar]
- Edmondson HA. Pancreatitis and carcinoma of the pancreas; some aspects of the pathologic physiology. Calif Med. 1952;77:176–179. [PMC free article] [PubMed] [Google Scholar]
- El-Gohary Y, Sims-Lucas S, Lath N, Tulachan S, Guo P, Xiao X, Welsh C, Paredes J, Wiersch J, Prasadan K, Shiota C, Gittes GK. Three-dimensional analysis of the islet vasculature. Anat Rec (Hoboken) 2012;295:1473–1481. doi: 10.1002/ar.22530. [DOI] [PubMed] [Google Scholar]
- Ember Z, Yago MD, Singh J. Distribution of nitric oxide synthase and secretory role of exogenous nitric oxide in the isolated rat pancreas. Int J Pancreatol. 2001;29:77–84. doi: 10.1385/IJGC:29:2:077. [DOI] [PubMed] [Google Scholar]
- Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR., Jr. Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem. 1987;262:214–217. [PubMed] [Google Scholar]
- Frick TW, Mithofer K, Fernandez-del Castillo C, Rattner DW, Warshaw AL. Hypercalcemia causes acute pancreatitis by pancreatic secretory block, intracellular zymogen accumulation, and acinar cell injury. Am J Surg. 1995;169:167–172. doi: 10.1016/s0002-9610(99)80127-5. [DOI] [PubMed] [Google Scholar]
- Grandt D, Siewert J, Sieburg B, al Tai O, Schimiczek M, Goebell H, Layer P, Eysselein VE, Reeve JR, Jr., Muller MK. Peptide YY inhibits exocrine pancreatic secretion in isolated perfused rat pancreas by Y1 receptors. Pancreas. 1995;10:180–186. doi: 10.1097/00006676-199503000-00012. [DOI] [PubMed] [Google Scholar]
- Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- Heintges T, Luthen R, Niederau C. Inhibition of exocrine pancreatic secretion by somatostatin and its analogues. Digestion. 1994;55(Suppl 1):1–9. [PubMed] [Google Scholar]
- Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol. 1985;70:347–356. doi: 10.1113/expphysiol.1985.sp002920. [DOI] [PubMed] [Google Scholar]
- Herzig KH, Louie DS, Tatemoto K, Chung OY. Pancreastatin inhibits pancreatic enzyme secretion by presynaptic modulation of acetylcholine release. Am J Physiol. 1992;262:G113–117. doi: 10.1152/ajpgi.1992.262.1.G113. [DOI] [PubMed] [Google Scholar]
- Izquierdo R, Bermes E, Jr., Sandberg L, Saxe A, Oslapas R, Prinz RA. Serum calcium metabolism in acute experimental pancreatitis. Surgery. 1985;98:1031–1037. [PubMed] [Google Scholar]
- Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380–1390. doi: 10.1053/gast.2001.29557. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Naruse S, Ishiguro H, Hayakawa T, Nokihara K. The effect of pituitary adenylate cyclase activating polypeptide (PACAP) on amylase secretion from guinea pig pancreatic acini. Biomed Pept Proteins Nucleic Acids. 1995;1:73–76. [PubMed] [Google Scholar]
- Konturek SJ, Pepera J, Zabielski K, Konturek PC, Pawlik T, Szlachcic A, Hahn EG. Brain-gut axis in pancreatic secretion and appetite control. J Physiol Pharmacol. 2003a;54:293–317. [PubMed] [Google Scholar]
- Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol. 2003b;481:1–14. doi: 10.1016/j.ejphar.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Lau T, Carlsson PO, Leung PS. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47:240–248. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Leung PS, Carlsson PO. Tissue renin-angiotensin system: its expression, localization, regulation and potential role in the pancreas. J Mol Endocrinol. 2001;26:155–164. doi: 10.1677/jme.0.0260155. [DOI] [PubMed] [Google Scholar]
- Li P, Lee KY, Chang TM, Chey WY. Mechanism of acid-induced release of secretin in rats. Presence of a secretin-releasing peptide. J Clin Invest. 1990;86:1474–1479. doi: 10.1172/JCI114864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G916–923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- Longnecker DS. Abnormal methyl metabolism in pancreatic toxicity and diabetes. J Nutr. 2002;132:2373S–2376S. doi: 10.1093/jn/132.8.2373S. [DOI] [PubMed] [Google Scholar]
- Louie DS, Williams JA, Owyang C. Action of pancreatic polypeptide on rat pancreatic secretion: in vivo and in vitro. Am J Physiol. 1985;249:G489–495. doi: 10.1152/ajpgi.1985.249.4.G489. [DOI] [PubMed] [Google Scholar]
- Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401–410. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- Mallory A, Kern F., Jr. Drug-induced pancreatitis: a critical review. Gastroenterology. 1980;78:813–820. [PubMed] [Google Scholar]
- Martinez A, Weaver C, Lopez J, Bhathena SJ, Elsasser TH, Miller MJ, Moody TW, Unsworth EJ, Cuttitta F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology. 1996;137:2626–2632. doi: 10.1210/endo.137.6.8641217. [DOI] [PubMed] [Google Scholar]
- Matozaki T, Goke B, Tsunoda Y, Rodriguez M, Martinez J, Williams JA. Two functionally distinct cholecystokinin receptors show different modes of action on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. Studies using a new cholecystokinin analog, JMV-180. J Biol Chem. 1990;265:6247–6254. [PubMed] [Google Scholar]
- McDonald TJ, Dupre J, Tatemoto K, Greenberg GR, Radziuk J, Mutt V. Galanin inhibits insulin secretion and induces hyperglycemia in dogs. Diabetes. 1985;34:192–196. doi: 10.2337/diab.34.2.192. [DOI] [PubMed] [Google Scholar]
- Messmer B, Zimmerman FG, Lenz HJ. Regulation of exocrine pancreatic secretion by cerebral TRH and CGRP: role of VIP, muscarinic, and adrenergic pathways. Am J Physiol. 1993;264:G237–242. doi: 10.1152/ajpgi.1993.264.2.G237. [DOI] [PubMed] [Google Scholar]
- Migita Y, Nakano I, Goto M, Ito T, Nawata H. Effect of pancreastatin on cerulein-stimulated pancreatic blood flow and exocrine secretion in anaesthetized rats. J Gastroenterol Hepatol. 1999;14:583–587. doi: 10.1046/j.1440-1746.1999.01918.x. [DOI] [PubMed] [Google Scholar]
- Mithofer K, Fernandez-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995a;109:239–246. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Mithofer K, Warshaw AL, Frick TW, Lewandrowski KB, Koski G, Rattner DW, Fernandez-del Castillo C. Calcium administration augments pancreatic injury and ectopic trypsinogen activation after temporary systemic hypotension in rats. Anesthesiology. 1995b;83:1266–1273. doi: 10.1097/00000542-199512000-00017. [DOI] [PubMed] [Google Scholar]
- Miyasaka K, Shinozaki H, Jimi A, Funakoshi A. Amylase secretion from dispersed human pancreatic acini: neither cholecystokinin a nor cholecystokinin B receptors mediate amylase secretion in vitro. Pancreas. 2002;25:161–165. doi: 10.1097/00006676-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Mossner J, Logsdon CD, Williams JA, Goldfine ID. Insulin, via its own receptor, regulates growth and amylase synthesis in pancreatic acinar AR42J cells. Diabetes. 1985;34:891–897. doi: 10.2337/diab.34.9.891. [DOI] [PubMed] [Google Scholar]
- Motta PM, Macchiarelli G, Nottola SA, Correr S. Histology of the exocrine pancreas. Microsc Res Tech. 1997;37:384–398. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<384::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mulholland MW, Garcia R, Garcia I, Taborsky GJ, Jr., Helton S. Inhibition of pancreatic exocrine secretion in the rat by calcitonin gene-related peptide: involvement of circulating somatostatin. Endocrinology. 1989;124:1849–1856. doi: 10.1210/endo-124-4-1849. [DOI] [PubMed] [Google Scholar]
- Mulholland MW, Lally K, Taborsky GJ., Jr. Inhibition of rat pancreatic exocrine secretion by neuropeptide Y: studies in vivo and in vitro. Pancreas. 1991;6:433–440. doi: 10.1097/00006676-199107000-00010. [DOI] [PubMed] [Google Scholar]
- Murakami T, Fujita T. Microcirculation of the rat pancreas, with special reference to the insulo-acinar portal and insulo-venous drainage systems: a further scanning electron microscope study of corrosion casts. Arch Histol Cytol. 1992;55:453–476. doi: 10.1679/aohc.55.453. [DOI] [PubMed] [Google Scholar]
- Murakami T, Fujita T, Miyake T, Ohtsuka A, Taguchi T, Kikuta A. The insulo-acinar portal and insulo-venous drainage systems in the pancreas of the mouse, dog, monkey and certain other animals: a scanning electron microscopic study of corrosion casts. Arch Histol Cytol. 1993;56:127–147. doi: 10.1679/aohc.56.127. [DOI] [PubMed] [Google Scholar]
- Nalesnik MA, Todo S, Murase N, Gryzan S, Lee PH, Makowka L, Starzl TE. Toxicology of FK-506 in the Lewis rat. Transplant Proc. 1987;19:89–92. [PMC free article] [PubMed] [Google Scholar]
- Naruse S, Suzuki T, Ozaki T. The effect of pituitary adenylate cyclase activating polypeptide (PACAP) on exocrine pancreatic secretion in dogs. Pancreas. 1992;7:543–547. doi: 10.1097/00006676-199209000-00006. [DOI] [PubMed] [Google Scholar]
- Norberg HP, DeRoos J, Kaplan EL. Increased parathyroid hormone secretion and hypocalcemia in experimental pancreatitis: necessity for an intact thyroid gland. Surgery. 1975;77:773–779. [PubMed] [Google Scholar]
- O’Konski MS, Pandol SJ. Cholecystokinin JMV-180 and caerulein effects on the pancreatic acinar cell cytoskeleton. Pancreas. 1993;8:638–646. doi: 10.1097/00006676-199309000-00018. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Mine T, Kojima I. Inhibition by somatostatin of amylase secretion induced by calcium and cyclic AMP in rat pancreatic acini. Biochem J. 1994;304(Pt 2):531–536. doi: 10.1042/bj3040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang C. Neurohormonal and Hormonal Control of Exocrine Pancreatic Secretion. In: Beger ALWHG, Büchler MW, Kozarek RA, Lerch MM, Neoptolemos JP, Shiratori K, Whitcomb DC, Rau BM, editors. The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery. Second Edition Blackwell Publishing Ltd; Oxford, UK: 2009. pp. 113–126. [Google Scholar]
- Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–969. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Putzke HP, Said F. Different secretory responses of periinsular and other acini in the rat pancreas after pilocarpine injection. Cell Tissue Res. 1975;161:133–143. doi: 10.1007/BF00222119. [DOI] [PubMed] [Google Scholar]
- Reeve JR, Jr., Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol. 2003;285:G255–265. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Sun G, Christensen T, Hillingso JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab. 2001;86:251–258. doi: 10.1210/jcem.86.1.7148. [DOI] [PubMed] [Google Scholar]
- Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I, Hashimoto S, Saluja A, Steer ML, Meldolesi J. Intracellular transport of pancreatic zymogens during caerulein supramaximal stimulation. Am J Physiol. 1987;253:G517–526. doi: 10.1152/ajpgi.1987.253.4.G517. [DOI] [PubMed] [Google Scholar]
- Saluja A, Hashimoto S, Saluja M, Powers RE, Meldolesi J, Steer ML. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987;253:G508–516. doi: 10.1152/ajpgi.1987.253.4.G508. [DOI] [PubMed] [Google Scholar]
- Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- Saluja AK, Saluja M, Printz H, Zavertnik A, Sengupta A, Steer ML. Experimental pancreatitis is mediated by low-affinity cholecystokinin receptors that inhibit digestive enzyme secretion. Proc Natl Acad Sci U S A. 1989;86:8968–8971. doi: 10.1073/pnas.86.22.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer MV, Niebergall-Roth E. Secretion from acinar cells of the exocrine pancreas: role of enteropancreatic reflexes and cholecystokinin. Cell Biol Int. 2009;33:1–9. doi: 10.1016/j.cellbi.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Singh S, Bhardwaj U, Verma SK, Bhalla A, Gill K. Hyperamylasemia and acute pancreatitis following anticholinesterase poisoning. Hum Exp Toxicol. 2007;26:467–471. doi: 10.1177/0960327107076814. [DOI] [PubMed] [Google Scholar]
- Sumi S, Inoue K, Kogire M, Doi R, Yun M, Kaji H, Hosotani R, Fujimura M, Uchida K, Kiyama S, Kitagawa K, Yajima H, Fujii N, Tobe T. Effect of synthetic porcine neuropeptide Y (NPY) on splanchnic blood flows and exocrine pancreatic secretion in dogs. Dig Dis Sci. 1991;36:1523–1528. doi: 10.1007/BF01296392. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Scanning electron microscopy of the rat exocrine pancreas. Arch Histol Jpn. 1984;47:387–404. doi: 10.1679/aohc.47.387. [DOI] [PubMed] [Google Scholar]
- Tani S, Itoh H, Okabayashi Y, Nakamura T, Fujii M, Fujisawa T, Koide M, Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. 1990;35:367–374. doi: 10.1007/BF01537416. [DOI] [PubMed] [Google Scholar]
- Tatemoto K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 1982;79:2514–2518. doi: 10.1073/pnas.79.8.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Teyssen S, Grandt D, Niebergall-Roth E, Schimiczek M, Goebell H, Eysselein VE, Reeve JR, Jr., Singer MV. Inhibition of canine exocrine pancreatic secretion by peptide YY is mediated by PYY-preferring Y2 receptors. Pancreas. 1996;13:80–88. doi: 10.1097/00006676-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993a;14:746–757. doi: 10.1016/0143-4160(93)90100-k. [DOI] [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993b;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Thorn P, Petersen OH. Calcium oscillations in pancreatic acinar cells, evoked by the cholecystokinin analogue JMV-180, depend on functional inositol 1,4,5-trisphosphate receptors. J Biol Chem. 1993;268:23219–23221. [PubMed] [Google Scholar]
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39:709–716. doi: 10.1097/01.mcg.0000173929.60115.b4. [DOI] [PubMed] [Google Scholar]
- Tsang SW, Cheng CH, Leung PS. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul Pept. 2004;119:213–219. doi: 10.1016/j.regpep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Ulrich AB, Standop J, Schmied BM, Schneider MB, Lawson TA, Pour PM. Species differences in the distribution of drug-metabolizing enzymes in the pancreas. Toxicol Pathol. 2002;30:247–253. doi: 10.1080/019262302753559588. [DOI] [PubMed] [Google Scholar]
- Van Acker GJ, Weiss E, Steer ML, Perides G. Cause-effect relationships between zymogen activation and other early events in secretagogue-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1738–1746. doi: 10.1152/ajpgi.00543.2006. [DOI] [PubMed] [Google Scholar]
- Walgren JL, Mitchell MD, Whiteley LO, Thompson DC. Identification of novel peptide safety markers for exocrine pancreatic toxicity induced by cyanohydroxybutene. Toxicol Sci. 2007;96:174–183. doi: 10.1093/toxsci/kfl189. [DOI] [PubMed] [Google Scholar]
- Walthall K, Cappon GD, Hurtt ME, Zoetis T. Postnatal development of the gastrointestinal system: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2005;74:132–156. doi: 10.1002/bdrb.20040. [DOI] [PubMed] [Google Scholar]
- Wang BJ, Cui ZJ. How does cholecystokinin stimulate exocrine pancreatic secretion? From birds, rodents, to humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R666–678. doi: 10.1152/ajpregu.00131.2006. [DOI] [PubMed] [Google Scholar]
- Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457–467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Wayland H. Microcirculation in pancreatic function. Microsc Res Tech. 1997;37:418–433. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<418::AID-JEMT6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Yago MD, Manas M, Ember Z, Singh J. Nitric oxide and the pancreas: morphological base and role in the control of the exocrine pancreatic secretion. Mol Cell Biochem. 2001;219:107–120. doi: 10.1023/a:1010834611480. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Otani M, Jia DM, Fukumitsu K, Yoshikawa H, Akiyama T, Otsuki M. Differential mechanism and site of action of CCK on the pancreatic secretion and growth in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G681–687. doi: 10.1152/ajpgi.00312.2002. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Marsh T, Foley JF, Cai B, Peddada S, Walker NJ, Nyska A. Mechanisms of exocrine pancreatic toxicity induced by oral treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Harlan Sprague-Dawley Rats. Toxicol Sci. 2005;85:594–606. doi: 10.1093/toxsci/kfi121. [DOI] [PubMed] [Google Scholar]
- Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G666–676. doi: 10.1152/ajpgi.2000.279.4.G666. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen M, Chen X, Segura BJ, Mulholland MW. Inhibition of pancreatic protein secretion by ghrelin in the rat. J Physiol. 2001;537:231–236. doi: 10.1111/j.1469-7793.2001.0231k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]