Abstract
Background and Purpose
Circulating lipoprotein-associated phospholipase A2 (Lp-PLA2) has emerged as a novel biomarker for cardiovascular diseases. However, the correlation between the plaque expression of Lp-PLA2 and plaque oxidative stress, inflammation, and stability as well as the clinical presentation remains poorly defined, especially for cerebrovascular disease. Therefore, this study was performed to test the hypothesis that Lp-PLA2 expression is higher in symptomatic than in asymptomatic carotid plaques of patients undergoing carotid endarterectomy.
Methods
The expression of Lp-PLA2 in 167 carotid artery plaques was determined by immunoblotting and immunostaining. Plaque oxidative stress, inflammation, and stability were quantified by NAD(P)H oxidase p67phox and MMP-2 immunoblotting, oxidized LDL (oxLDL) immunoreactivity, macrophage and Sirius red collagen staining. Lysophosphatidylcholine 16:0 (lysoPC) concentration was measured in 55 plaques using liquid chromatography tandem mass spectrometry.
Results
Lp-PLA2 expression was significantly higher in plaques of symptomatic patients than asymptomatic patients (1.66±0.19 versus 1.14±0.10, P<0.05) and localized mainly to shoulder and necrotic lipid core areas in colocalization with oxLDL and macrophage content. Similarly, Lp-PLA2 expression was related to collagen content, which was lower in plaques from symptomatic patients than in plaques from asymptomatic patients (9.1±2.2 versus 18.5±1.7% of staining/field, P<0.001). LysoPC plaque concentration was significantly higher in plaques of symptomatic than asymptomatic patients (437.0±57.91 versus 228.84±37.00 mmol/L, P<0.05).
Conclusions
Symptomatic carotid artery plaques are characterized by increased levels of Lp-PLA2 and its product lysoPC in correlation with markers of tissue oxidative stress, inflammation, and instability. These findings strongly support a role for Lp-PLA2 in the pathophysiology and clinical presentation of cerebrovascular disease.
Keywords: Lp-PLA2, lysophosphatidylcholine, carotid, atherosclerosis, unstable plaque
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a calcium-independent serine lipase that specifically hydrolyzes the sn-2 fatty acids of oxidized phospholipids such as oxidized low density lipoprotein (LDL).1 Circulating Lp-PLA2 derives mainly from hematopoietic and inflammatory cells and associates primarily with apolipoprotein B-containing particles.2– 4 Hence, 70% to 80% of the circulating Lp-PLA2 level is found in the LDL lipid fraction and only a small percentage in the high-density lipoprotein (HDL) lipid fraction.5 Importantly, systemic Lp-PLA2 has emerged as an independent marker for cardiovascular disease and events in several large population-based studies.6 –9
The biomarker evidence for Lp-PLA2 is strongest for coronary artery disease (CAD) and less conclusive for cerebrovascular disease.10 –12 Specifically, systemic Lp-PLA2 levels seem to predict the risk of ischemic stroke but do not correlate with carotid artery intima-media thickening or the presence of carotid plaques.9,13,14 These epidemiological data relate to pathohistological studies, indicating an association between plaque expression of Lp-PLA2 and plaque progression and instability in CAD but are yet to be consolidated for carotid artery disease.4,15
Lysophosphatidylcholine (lysoPC) is one of the main products of Lp-PLA2 hydrolytic activity. Importantly, lysoPC has been shown to stimulate both proliferation and apoptosis of endothelial and smooth muscle cells at low and high concentrations, respectively.16 –19 LysoPC also contributes to the recruitment of monocytes to the arterial wall and to matrix-metalloproteinase (MMP) production.20,21 Even so, the relationship between the expression profile of lysoPC and that of Lp-PLA2 in atherosclerotic plaques, including carotid artery plaques, has remained unknown.
The present study was designed to test the hypothesis that the expression of Lp-PLA2 and its product lysoPC differs between symptomatic and asymptomatic carotid artery plaques. For this purpose, we studied the expression of Lp-PLA2 and lysoPC and markers of oxidative stress, inflammation, and plaque stability in carotid endarterectomy (CEA) samples from patients without or with preceding symptoms of cerebral ischemia.
Methods
Patients
The study was approved by the Mayo Foundation Institutional Review Board. Written informed consent was obtained before surgery from all participants.
We studied 167 carotid plaques specimens from 164 consecutive patients undergoing CEA as previously described.22,23 The decision for the surgical intervention followed present clinical guidelines and included symptomatic and asymptomatic patients as defined by carotid artery imaging (ultrasound duplex defining stenosis as 70% to 99% and MRA defining 80% to 90% and 90% to 99% as severe and critical, respectively). Demographic data and a detailed clinical history were obtained for each patient by chart review with attention to cerebral ischemic events, CAD risk factors, and medications. CAD was defined as past history of angina pectoris, MI, CABG, or PTCA. In accordance with previously published studies such as the EVA-3 S trial, carotid artery plaques were defined as symptomatic if they were associated with a cerebral ischemic event ipsilateral to the collected plaque within 120 days of surgery.24 Symptomatic patients included patients with prior ischemic stroke (neurological event lasting more then 24 hours) and transient ischemic attack (TIA, reversible neurological event lasting less then 24 hours). Patients with multiple events were categorized by the most severe event and those with bilateral carotid disease were defined in accordance with the side of the symptoms.
Plaque Specimens
After surgery, plaques were dissected transversely at the site of the maximum plaque diameter which was mostly located at the bifurcation and mainly involving the intracerebral carotid artery. One half was fixed in formalin and embedded in paraffin, the other half was immediately frozen and stored at −80°C.
Western Blotting for Lp-PLA2, MMP-2, and NAD(P)H Oxidase p67phox
Western blotting was performed as previously described, using the following primary antibodies: anti-Lp-PLA2 (monoclonal 4B4, 1:1000, gift from DiaDexus, San Francisco, Calif), anti-MMP-2 (1:7500, Chemicon International), anti-NAD(P)H oxidase p67phox (1:500; Santa Cruz), and anti–β-actin (Sigma, 1:1000).4,22,23,25,26 Densitometric signals were analyzed using ImageJ software (National Institutes of Health).
Immunostaining for Lp-PLA2, oxLDL, and Macrophages
Fifty randomly selected samples were immunostained, as previously detailed, with the following primary antibodies: anti-LpPLA2 (4B4 1:500, gift from DiaDexus, San Francisco, CA), anti-CD68 (1:500, Dako), anti-Cu+2-oxLDL (1:200, Calbiochem, San Diego, CA).23 Diaminobenzidine (DAB) was used as chromogen. Primary antibody isotype immunoglobulins were used as negative controls.
Digital images of the stained specimens were taken (SPOT Advanced 3.3, Diagnostic Instruments Inc) and the percentage of stained area within the entire specimen was quantified by MetaMorph Meta Imaging Series 4.6.
Immunofluorescence Staining for Lp-PLA2, lox1, and Macrophages
Twenty representative slides were costained by immunofluorescence for Lp-PLA2, oxLDL (Calbiochem), lox1 (H-140: sc-20753, Santa-Cruz Biotechnology Inc), and macrophages. Fluorescence was observed using Zeiss LSM-510 confocal laser scanning microscope (Carl Zeiss Inc).
Liquid Chromatography Tandem Mass Spectrometry for lysoPC
Plaque content of lysoPC was measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) in 55 randomly selected samples. Tissue homogenate was extracted with butanol after the addition of 17:0 Lyso-PC as internal standard. The dried down extract was reconstituted in methanol for analysis using a triple quadruple MS (SCIEX API 3000) with a Turbo Ion Spray Source. Chromatographic separation was achieved using a Phenomenex C8 column. Mobile phase consisted of a mixture of methanol (90%), 100 mmol/L ammonium acetate (10%), and formic acid (0.05%). 16:0 lysoPC was determined by selective reaction monitoring. Interassay coefficients of variation were 2.0% at 131 μmol/L and 12.0% at 45 μmol/L.
Sirius Red Staining for Collagen
The interstitial collagen content of carotid plaques was evaluated by Sirius red in fifty randomly selected samples as outlined before.23 Slides were visualized under both bright-field and polarized light microscope, and pictures were taken with identical exposure settings for all sections. The content of collagen, identified by birefringence under polarized light, was quantified as percent of plaque area.27
TUNEL Staining for Apoptosis
Cells undergoing apoptosis were identified by the Terminal deoxynucleotidyl transferase (TdT) end labeling (TUNEL) technique in 41 carotid artery samples applying the ApopTag In Situ Apoptosis Detection Kit (Intergen Company).23 Quantification was made by manual count of the number of TUNEL+cells relative to the total number of cells in the plaque.
Statistics
Data were presented as percentage or mean±SEM. Two group comparisons were made by t test or U-test for continuous variables or the χ2 test for categorical variables. Multiple group comparisons were performed by ANOVA followed by Tukey-Kramer posthoc analysis. Regression coefficients were calculated based on Pearson or Spearman product moment. Statistical significance was assumed for P<0.05.
Results
One hundred and sixty-seven carotid artery plaques were collected from 164 patients, including 107 asymptomatic and 60 symptomatic patients. Except for smoking and CAD, there were no major intergroup differences in baseline characteristics (Table). Ten patients suffered multiple events, of which 3 patients had additional TIAs before their stroke.
Lp-PLA2 Expression
Lp-PLA2 concentration was significantly higher in plaques of symptomatic patients than in plaques of asymptomatic patients (Lp-PLA2/β-actin density ratio: 1.66±0.19 versus 1.14±0.10, P<0.01). This difference was attributable to increased Lp-PLA2 plaque expression mainly in patients presenting with TIA (Figure 1).
Figure 1.
Expression of Lp-PLA2, NAD(P)H oxidase p67phox, and MMP-2 in carotid artery plaque of asymptomatic and symptomatic patients with a differential expression pattern in TIA and stroke patients (top panel: representative blots; bottom panel: quantitative results; *P<0.05 vs asymptomatic, †P<0.05 vs TIA).
Plaque expression of Lp-PLA2 negatively correlated withbplasma HDL (r=−0.29, P=0.001) but no other cardiovascular risk factor or particular cardiovascular drug. As for concomitant CAD, expression of Lp-PLA2 in the carotidbplaque was significantly higher in 24 patients with a history of CAD and percutaneous transluminal coronary angioplasty (Lp-PLA2/β-actin density ratio: 2.00±0.27 versus 1.28±0.11, P<0.05).
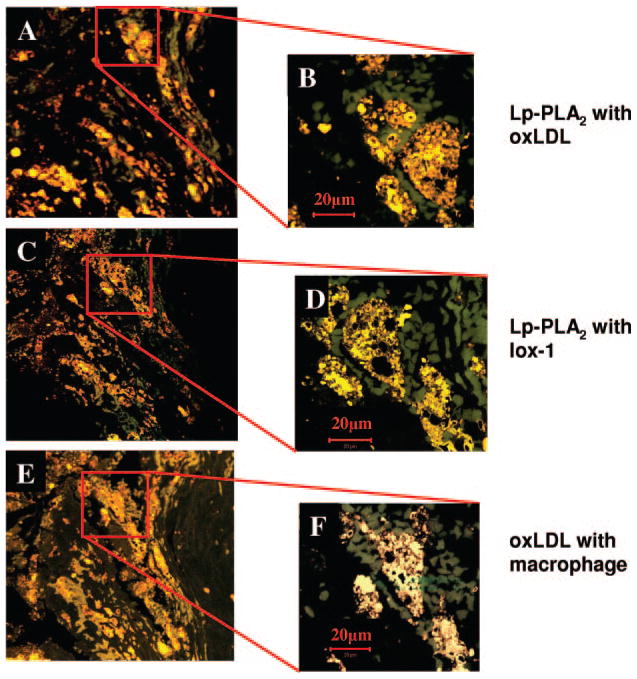
Lp-PLA2 immunoreactivity was mainly seen in the lipid necrotic core and plaque shoulders (Figure 2) in colocalization with oxLDL, lox1, and macrophages (Figure 3).
Figure 2.
Representative immunostaining for Lp-PLA2 (positive brown staining) showing expression in the core and shoulder area; insets show high magnification of the necrotic core and shoulder. Original magnification × 10, inset ×25.
Figure 3.
Immunofluorescence of a carotid artery plaque shoulder region showing colocalization (yellow) of Lp-PLA2 (red) and ox-LDL (green; panel A&B) and with lox-1 (green; panel C&D). The same area is characterized by colocalization (yellow) of macrophages (red) and oxLDL (green; panel E&F).
LysoPC
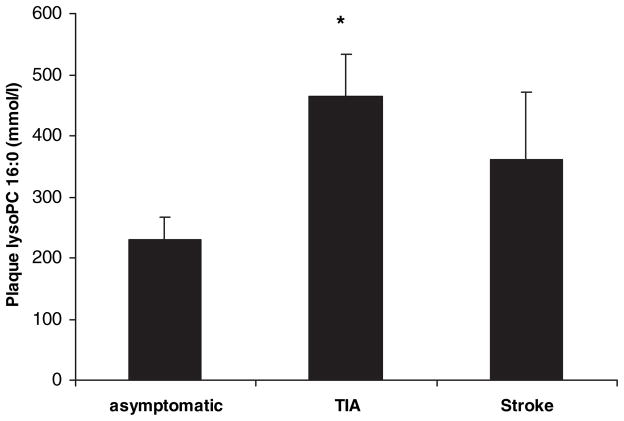
LysoPC concentration, was significantly higher in plaques of symptomatic than asymptomatic patients (437±58 versus 229±37 mmol/L, P<0.005). Among symptomatic patients, those with preceding TIA had the highest plaque lysoPC content (Figure 4). The expression of lysoPC correlated with Lp-PLA2 (r=0.36, P<0.01) and was inversely correlated with the elapsed time from the last ischemic event (r=−0.52, P<0.01).
Figure 4.
Concentration of lysoPC in carotid plaques of asymptomatic, TIA, and stroke patients showing an increased lysoPC content in plaques of symptomatic patients with a differential expression pattern in TIA and stroke patients. *P<0.05 vs asymptomatic.
MMP-2
Plaque MMP-2 expression was significantly higher in symptomatic than in asymptomatic patients (MMP-2/β-actin density ratio: 2.37±0.30 versus 1.47±0.22, P<0.05). Like Lp-PLA2 and lysoPC, this difference was attributable to a significantly higher MMP-2 expression in carotid plaques from TIA patients (Figure 1). MMP-2 correlated with Lp-PLA2, (r=0.33, P<0.01) but no other variables.
NAD(P)H Oxidase Subunit p67phox
Expression of p67phox was also higher in plaques from symptomatic compared with asymptomatic patients (p67phox/β-actin density ratio: 2.38±0.25 versus 1.34±0.18, P<0.005). Again, this difference was related to a high expression of p67 in TIA patients (Figure 1), and p67phox expression correlated only with Lp-PLA2 (r=0.30, P<0.01).
OxLDL
Expression of oxLDL was also higher in plaques from symptomatic compared with asymptomatic patients (27.7±2.7 versus 17.3±2.6% area, P<0.01), which was attributable to high expression of oxLDL in TIA patients (29.1±3.6% area).
Plaque Stability
Macrophage content was significantly higher in symptomatic than in asymptomatic plaques (10.8±1.7 versus 4.5±1.3% area, P<0.01). This was attributable to significantly higher macrophage content in carotid plaques from TIA patients compared with asymptomatic patients (Figure 5).
Figure 5.
Concentration of macrophages and collagen in carotid plaques of asymptomatic, TIA, and stroke patients showing an increased macrophage and a decreased collagen content in symptomatic patients, again with a differential pattern in TIA and stroke patients. *P<0.05 vs asymptomatic.
Collagen content was lower in plaques from symptomatic patients (without a significant difference between TIA and stroke patients) than in plaques from asymptomatic patients (9.1±2.2 versus 18.5±1.7% of staining/field, P<0.001, Figure 5).
The number of TUNEL+cells, indicating apoptosis, was significantly lower in asymptomatic than in symptomatic plaques (10.2±8.2 versus 17.8±12.9%, P<0.05). Overall, plaque collagen content was inversely correlated to the percentage of TUNEL+cells in the carotid plaque (r=−0.45, P<0.01) and plaque macrophage content (r=−0.51, P<0.01). Also, there was a significant positive correlation between plaque macrophage content and percentage of TUNEL+cells in the carotid plaque (r=0.42, P<0.05). There was no quantitative correlation of Lp-PLA2 expression with plaque collagen content, macrophage count, or percentage of TUNEL+cells. However, plaque lysoPC content correlated strongly with the percentage of TUNEL+cells in the carotid plaques (r=−0.76, P<0.05).
Discussion
The present study demonstrates increased Lp-PLA2 and lysoPC levels in carotid artery plaques of patients with symptomatic cerebrovascular disease. Plaque content of Lp-PLA2 correlated with markers of tissue oxidative stress, inflammation, and instability. These observations suggest a clinically relevant role for Lp-PLA2 in atherosclerotic cerebrovascular disease.
Lp-PLA2 and CVD–Epidemiological Link
The West of Scotland Coronary Prevention Study (WOSCOPS), the Atherosclerosis Risk in Communities study (ARIC), and the Monitoring of Trends and Determinants in Cardiovascular Disease study (MONICA) identified higher circulating Lp-PLA2 mass concentration as an independent predictor for first-time acute myocardial infarction (AMI) or cardiac death in patients at risk but without angiographically confirmed CAD (adjusted attributable risk 1.17 to 1.21).6 – 8 Likewise, the Rotterdam study showed a 40% to 100% higher risk of cardiac death and AMI in patients with systemic Lp-PLA2 activity above the lowest quartile.9 This study also highlighted a similarly elevated risk for ischemic stroke in the subgroup of patients with elevated systemic Lp-PLA2 activity.9 These findings are reminiscent of the 2-fold elevated risk of ischemic stroke identified for elevated circulating Lp-PLA2 mass concentrations in the ARIC study.7 In patients with angiographically confirmed CAD, Brilakis et al found a correlation of Lp-PLA2 plasma mass concentrations with the extent of CAD on univariate but not on multivariable analysis even though they confirmed a prognostic merit.28 Similarly, Lp-PLA2 plasma activity did not correlate with the extent of carotid artery disease by ultrasound evaluation.14 Taken together, circulating Lp-PLA2 levels seem to correlate with the prognosis but not necessarily with the atherosclerotic burden of cardiovascular disease. In a pursuit to further define a pathomechanistic biomarker role for Lp-PLA2, pathoanatomical studies are a valuable approach.
Lp-PLA2 and CVD–Pathoanatomic Link
The present study demonstrates increased Lp-PLA2 expression in carotid artery plaques from patients with symptoms within 3 months before CEA. Intriguingly, the increase in Lp-PLA2 expression was particularly strong in the necrotic core and shoulder regions in colocalization with macrophages and oxLDL. As macrophages are a major source of Lp-PLA2 and oxLDL is a major substrate for Lp-PLA2, these findings are very plausible from a pathophysiological standpoint. They also resemble the findings obtained by Kolodgie et al, who showed strong Lp-PLA2 expression in the necrotic core and surrounding macrophages as well as in the fibrous cap of vulnerable and ruptured coronary artery plaques. Likewise, Hakkinen et al were able to show increasing levels of Lp-PLA2 mRNA expression in aortic samples with advancing stages of atherosclerosis and mainly confined to inflammatory cells.4 Hence, the present study on carotid plaques adds to previous studies by identifying Lp-PLA2 not only as a biomarker but potentially even as one important pathophysiological mediator of atherosclerosis.
Lp-PLA2 and CVD–Pathomechanistic Link
The increased content of lysoPC in the carotid plaques of symptomatic patients in the present study corresponds to an increase in Lp-PLA2 enzymatic activity. LysoPC is also of greater biological significance. Its can recruit monocyte/macrophages to the atherosclerotic plaque where these inflammatory cells further produce Lp-PLA2 and lysoPC, hence creating a vicious cycle.29 The colocalization of Lp-PLA2 with oxLDL and lox1 in macrophages and the correlation of Lp-PLA2 with the increased expression of p67phox furthermore supports the concept that LDL undergoes oxidative modifications, thereby becoming a substrate for Lp-PLA2, which finally generates potent biomolecules such as lysoPC.30 Native LDL could contribute to this process not only as a substrate but also by carrying Lp-PLA2 into the subintimal space. However, the present study did not show a correlation between LDL serum concentrations and Lp-PLA2 plaque content. On the contrary, there was an inverse correlation between carotid plaque Lp-PLA2 levels and HDL serum concentrations. These findings may suggest that LDL-bound transport of Lp-PLA2 within the plaque is less important than production of Lp-PLA2 within the plaque, which is still captured by HDL in the context of reverse cholesterol transport and may contribute to the circulating level of Lp-PLA2.
The finding that plaque Lp-PLA2 expression was correlated with plaque MMP-2 expression links plaque stability with markers of oxidative stress and inflammation. This consideration is furthermore underscored by the observations that plaques from patients with symptoms before CEA were characterized by a lower collagen content and plaque collagen content was inversely correlated with the number of macrophages and TUNEL+cells. Moreover, plaque lysoPC content strongly correlated with the number of TUNEL+cells. These results agree with previous reports on the proapoptotic effect of high concentrations of lysoPC.18 –19 As the enzymatic activity of Lp-PLA2 is the source of lysoPC in the atherosclerotic plaque, these findings imply a destabilizing role for Lp-PLA2 in carotid artery disease as recently pointed out for coronary artery disease.15
Thus, the present study confirms characteristics of the symptomatic carotid plaque.35 Furthermore, atherosclerotic plaque Lp-PLA2 activity, resulting in the generation of lysoPC, may provide a mechanistic link between the biological activity and the clinical consequences of the atherosclerotic plaque.
Lp-PLA2 and CVD–Clinical Presentation and Implication
It was previously suggested that the mechanisms leading to TIA might be different from those leading to stroke. Indeed, patients presenting with TIA have a tendency for multiple cerebral ischemic events, including a higher risk of stroke, unlike stroke patients who tend to have only a single major event.33,34 This could be attributable to a persistent level of tissue instability in plaques manifesting with TIA whereas plaques collected after stroke show a gradual decline in instability in the setting of plaque remodeling over time.34 In our study we demonstrated for the first time a differential expression of several markers of plaque bioactivity and instability between patients with preceding TIA compared with those with preceding stroke. Specifically, the expression of Lp-PLA2, lysoPC, NAD(P)H oxidase p67phox, and MMP-2 was higher in carotid plaques after TIA than after stroke. Each of these markers could by itself contribute to plaque instability and together they might create a cycle enhancing plaque instability with plaque macrophages as a center point. Along these lines, Lp-PLA2 was correlated with each of the other marker, suggesting a more unique role for Lp-PLA2 in differential plaque instability.
If an important fraction of the clinically relevant Lp-PLA2 activity was derived from inflammatory cells within the plaque and not from outside the plaque, simple reduction of circulating LDL levels might not be clinically sufficient. Indeed, statins have been shown to decrease circulating Lp-PLA2 levels in conjunction with a decrease in LDL serum concentrations, but they do not reduce de novo synthesis and secretion by macrophages.31 This may explain why the Lp-PLA2-attributable risk of cardiovascular events was not reduced by statins in the WOSCOPS study.8 These considerations may furthermore explain why even high-dose statin therapy is still associated with a 20% CV event rate in a population at risk in general.31 Lp-PLA2 inhibitors may therefore fill an important therapeutic gap, especially in patients with high circulating Lp-PLA2 levels.32
Study Limitations
Even though suggestive, the present study findings do not ascertain a causal relationship between Lp-PLA2 and the clinical consequences of carotid artery disease. Future outcome-directed prospective studies with Lp-PLA2 inhibitors would be ideally suited to provide an answer. They would furthermore give insight into the significance of Lp-PLA2 activity versus expression in atherosclerotic plaques and the relative contribution of and correlation with circulating Lp-PLA2 levels. Lp-PLA2 plasma levels or activity were not available in this study but would provide a valuable perspective.
Unfortunately the endarterectomy samples received for the current study contained atherosclerotic plaques not necessarily in conjunction with the entire vascular circumference. For this reason, established valuable pathoanatomic staging system of atherosclerotic disease could not be applied to the present findings. However, the distinction between clinically relevant symptomatic and asymptomatic plaques was made on the background of the previously outlined histological differences between these 2 clinical presentations of carotid artery disease.35
Finally, even though intriguing, results obtained in subset analyses such as the distinction between TIA and stroke will need to be confirmed in larger series of patients.
Conclusions
Symptomatic carotid artery plaques are characterized by increased levels of Lp-PLA2 and its product lysoPC in correlation with markers of tissue oxidative stress, inflammation, and instability. These findings strongly support a role for Lp-PLA2 in the pathophysiology and clinical presentation of cerebrovascular disease.
Table.
Patient Characteristics
| Asymptomatic (n=107) | Symptomatic (n=60) | Stroke (n=21) | TIA (n=39) | P Value | |
|---|---|---|---|---|---|
| Age, y | 70±1 | 70±1 | 67±2 | 71±1 | n.s. |
| Sex M/F, n | 65/42 | 42/18 | 16/5 | 26/13 | n.s. |
| BMI, kg/m2 | 29.1±0.5 | 28.6±0.7 | 29.0±1.3 | 28.3±0.8 | n.s. |
| Medical history | |||||
| CAD, n (%) | 48 (44) | 20 (33) | 12 (57) | 8 (20)* | 0.008 |
| Hypertension, n (%) | 91 (85) | 48 (80) | 17 (81) | 31 (79) | n.s. |
| Diabetes, n (%) | 15 (14) | 15 (25) | 6 (29) | 9 (23) | n.s. |
| Smoking, n (%) | 12 (11) | 14 (23) | 9 (42)* | 5 (12) | 0.014 |
| Hypercholesterolemia, n (%) | 71 (66) | 38 (63) | 12 (57) | 26 (66) | n.s. |
| Lipid profile | |||||
| Total cholesterol, mmol/L | 4.9±0.1 | 4.9±0.2 | 4.8±0.3 | 4.9±0.2 | n.s. |
| LDL, mmol/L | 2.7±0.1 | 2.9±0.2 | 2.9±0.3 | 2.9±0.2 | n.s. |
| HDL, mmol/L | 1.2±0.1 | 1.1±0.1 | 1.2±0.1 | 1.1±0.1 | n.s. |
| Statins, n (%) | 71 (66) | 28 (46) | 12 (57) | 16 (41) | n.s. |
| Aspirin, n (%) | 82 (76) | 49 (81) | 16 (76) | 33 (84) | n.s. |
| Days since last event | 0 | 25±4 | 26±6 | 25±5 | n.s. |
BMI indicates body mass index; CAD, coronary artery disease; LDL, low density lipoprotein; HDL, high density lipoprotein. Values represent mean±SEM for continuous variables and No. (%) for categorical.
Acknowledgments
The authors are grateful to Shelly Gintz, Monica L. Olson for her help in collecting and processing endarterectomy specimens, and to Meagan Shultz her technical help in processing the lysophosphatidylcholine essay.
Sources of Funding
This study was supported by NIH grants: K24, HL-69840, and R01 HL-63911 and by and award from the Mayo Clinic Clinical Immunology and Immunotherapeutic program.
Footnotes
Disclosures
None.
References
- 1.MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, Leach CA, Leake DS, Milliner KJ, Patterson RA, Suckling KE, Tew DG, Hickey DM. Lipoprotein-associated phospholipase a2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: Use of a novel inhibitor. Biochem J. 1999;338:479– 487. [PMC free article] [PubMed] [Google Scholar]
- 2.Asano KOS, Fukunaga K, Shiomi T, Mori T, Iwata M, Ikeda Y, Yamaguchi K. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun. 1999;261:511–514. doi: 10.1006/bbrc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 3.Stafforini DM, Elstad MR, McIntyre TM, Zimmerman GA, Prescott SM. Human macrophages secret platelet-activating factor acetylhydrolase. J Biol Chem. 1990;265:9682–9687. [PubMed] [Google Scholar]
- 4.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, Rice SQ, Tew DG, Karkola K, Yla-Herttuala S. Lipoprotein-associated phospholipase a(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 5.Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase a(2), platelet-activating factor acetylhydrolase: A potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413– 419. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 6.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase a2 adds to risk prediction of incident coronary events by c-reactive protein in apparently healthy middle-aged men from the general population: Results from the 14-year follow-up of a large cohort from southern germany. Circulation. 2004;110:1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase a2, high-sensitivity c-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837– 842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 8.Packard CJ, O’Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase a2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 9.Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase a2 activity is associated with risk of coronary heart disease and ischemic stroke: The Rotterdam Study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 10.Yang EH, McConnell JP, Lennon RJ, Barsness GW, Pumper G, Hartman SJ, Rihal CS, Lerman LO, Lerman A. Lipoprotein-associated phospholipase a2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106–111. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- 11.Winkler K, Winkelmann BR, Scharnagl H, Hoffmann MM, Grawitz AB, Nauck M, Bohm BO, Marz W. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: The Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2005;111:980–987. doi: 10.1161/01.CIR.0000156457.35971.C8. [DOI] [PubMed] [Google Scholar]
- 12.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase a2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586–1593. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 13.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, Myerson M, Wu KK, Sharrett AR, Boerwinkle E. Lipoprotein-associated phospholipase a2, high-sensitivity c-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 14.Kardys I, Oei HH, van der Meer IM, Hofman A, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase a2 and measures of extracoronary atherosclerosis: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 2006;26:631– 636. doi: 10.1161/01.ATV.0000201289.83256.cf. [DOI] [PubMed] [Google Scholar]
- 15.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, Virmani R. Lipoprotein-associated phospholipase a2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 16.Wolfram Kuhlmann CR, Wiebke Ludders D, Schaefer CA, Kerstin Most A, Backenkohler U, Neumann T, Tillmanns H, Erdogan A. Lysophosphatidylcholine-induced modulation of ca(2+)-activated k(+)channels contributes to ros-dependent proliferation of cultured human endothelial cells. J Mol Cell Cardiol. 2004;36:675– 682. doi: 10.1016/j.yjmcc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Chai YC, Howe PH, DiCorleto PE, Chisolm GM. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem. 1996;271:17791–17797. doi: 10.1074/jbc.271.30.17791. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi M, Okazaki H, Ogata Y, Takeuchi K, Ikeda U, Shimada K. Lysophosphatidylcholine induces apoptosis in human endothelial cells through a p38-mitogen-activated protein kinase-dependent mechanism. Atherosclerosis. 2002;161:387–394. doi: 10.1016/s0021-9150(01)00674-8. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CC, Yen MH, Liu HW, Lau YT. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: In comparison with oxidized ldl. Atherosclerosis. 2000;151:481– 491. doi: 10.1016/s0021-9150(00)00453-6. [DOI] [PubMed] [Google Scholar]
- 20.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: A chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue N, Takeshita S, Gao D, Ishida T, Kawashima S, Akita H, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine increases the secretion of matrix metalloproteinase 2 through the activation of nadh/nadph oxidase in cultured aortic endothelial cells. Atherosclerosis. 2001;155:45–52. doi: 10.1016/s0021-9150(00)00530-x. [DOI] [PubMed] [Google Scholar]
- 22.Sattler KJ, Woodrum JE, Galili O, Olson M, Samee S, Meyer FB, Zhu XY, Lerman LO, Lerman A. Concurrent treatment with reninangiotensin system blockers and acetylsalicylic acid reduces nuclear factor kappab activation and c-reactive protein expression in human carotid artery plaques. Stroke. 2005;36:14–20. doi: 10.1161/01.STR.0000150643.08420.78. [DOI] [PubMed] [Google Scholar]
- 23.Versari D, Herrmann J, Gossl M, Mannheim D, Sattler K, Meyer FB, Lerman LO, Lerman A. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2132–2139. doi: 10.1161/01.ATV.0000232501.08576.73. [DOI] [PubMed] [Google Scholar]
- 24.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 25.Tew DG, Southan C, Rice SQ, Lawrence MP, Li H, Boyd HF, Moores K, Gloger IS, Macphee CH. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1996;16:591–599. doi: 10.1161/01.atv.16.4.591. [DOI] [PubMed] [Google Scholar]
- 26.Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 27.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: Implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 28.Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase a2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137–144. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 29.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S. Oxidized ldl in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 30.Kugiyama K, Sugiyama S, Ogata N, Oka H, Doi H, Ota Y, Yasue H. Burst production of superoxide anion in human endothelial cells by lysophosphatidylcholine. Atherosclerosis. 1999;143:201–204. doi: 10.1016/s0021-9150(98)00288-3. [DOI] [PubMed] [Google Scholar]
- 31.O’Donoghue M, Morrow DA, Sabatine MS, Murphy SA, McCabe CH, Cannon CP, Braunwald E. Lipoprotein-associated phospholipase a2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the prove it-timi 22 (pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction) trial. Circulation. 2006;113:1745–1752. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 32.Johnson A, Zalewski A, Janmohamed S, Sawyer J, Rolfe T, Staszkiewicz W, Alvarez S. Lipoprotein-associated phospholipase a2 (lp-pla2) activity, an emerging cv risk marker, can be inhibited in atherosclerotic lesions and plasma by novel pharmacologic intervention: The results of a multicenter clinical study. Circulation. 2004:III–590. [Google Scholar]
- 33.Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJ. Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. Cmaj. 2004;170:1105–1109. doi: 10.1503/cmaj.1030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The oxford plaque study. Circulation. 2006;113:2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 35.Golledge J, Greenhalgh RM, Davies AH. symptomatic carotid plaque. Stroke. 2000;31:774–781. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]