Abstract
Due to the lack of precise markers indicative of its occurrence and progression, coronary artery disease (CAD), the most common type of heart diseases, is currently associated with high mortality in the United States. To systemically identify novel protein biomarkers associated with CAD progression for early diagnosis and possible therapeutic intervention, we employed an iTRAQ-based quantitative proteomic approach to analyze the proteome changes in the plasma collected from a pair of wild type versus apolipoprotein E knockout (APOE −/−) mice which were fed with a high fat diet. In a multiplex manner ITRAQ serves as the quantitative ‘in-spectra’ marker for ‘cross-sample’ comparisons to determine the differentially expressed/secreted proteins caused by APOE knock-out. To obtain the most comprehensive proteomic datasets from this CAD-associated mouse model we applied both MALDI and ESI-based mass spectrometric (MS) platforms coupled with two different schemes of multidimensional liquid chromatography (2D-LC) separation. We then comparatively analyzed a series of the plasma samples collected at six and twelve weeks after the mice were fed with fat diets, where the 6-week or 12-week time point represents the early or intermediate phase of the fat-induced CAD, respectively. We then categorized those proteins showing abundance changes in accordance with APOE depletion. Several proteins such as the gamma and beta chains of fibrinogen, apolipoprotein B, apolipoprotein C-I, and thrombospondin-4 were among the previously known CAD markers identified by other methods. Our results suggested that these unbiased proteomic methods are both feasible and a practical means of discovering potential biomarkers associated with CAD progression.
Introduction
Coronary artery disease (CAD) is a chronic progressive disease that impacts approximately 13 million people in the United States [1]. Each year, more than half a million Americans die from CAD and, according to present trends in the United States, half of healthy 40-year old men and one out of three 40-year old women will likely develop CAD in the future [2]. Despite the development of multiple clinical, electrographic and biochemical tools for the detection of CAD, there are patients who progress to severe CAD without many symptoms or signs [3]. Therefore, discovery of novel protein biomarker(s) for this disease is critical in order to improve early diagnosis and therapeutic intervention to prevent CAD and its morbid sequelae. The apolipoprotein E knock out (APOE −/−) mouse is an established model of atherosclerosis that has been shown to closely mimic human atherosclerosis both in the spontaneous appearance of lesions and the distribution of lesions within the vasculature [4–9]. We suggest that the phenotype-specific plasma proteome from this mouse model could contain the best protein representatives of CAD and could be the systemic indicator for atherosclerosis. We therefore fed the APOE −/− versus WT mice pairs with high-fat diets. For proteomic screening of differentially expressed/secreted proteins a series of plasma samples was collected from mouse pairs sacrificed at 6 and 12 weeks after the fat treatment.
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) is widely used to assist mass spectrometry for large-scale protein separation and quantitation [10], but it suffers from a limited resolution in separating those proteins of extreme molecular weights or isoelectric points, the exclusion of hydrophobic proteins, and a limited dynamic range of detection [11]. In this regard, on-line gel-free techniques based on two-dimensional liquid chromatography separate the peptides derived from proteolytic digestion of protein mixtures. Therefore, no discrimination against proteins with particular physical properties can be expected, thereby allowing for tandem MS/MS analysis of a broad range of proteins. However, in plasma, the concentration differences between various proteins can be as much as 10 to 12 orders of magnitude. Currenly, chromatography and mass spectrometry technique is limited to identifying proteins whose concentrations differ by at most 4 orders of magnitude.[12] In order to detect the low abundant proteins, the three major proteins, i.e., albumin, IgG, transferrin, need to be removed before the proteomics analysis of plasma samples.
Recently, a quantitative method of isobaric tags for relative and absolute quantification (iTRAQ) was introduced to assist MS-based and non-biased high throughput quantitative analysis. For example, Ross et. al. made use of a 4-fold (4-plex) multiplex strategy to simultaneously determine relative protein levels in three yeast strains and provided a demonstration of the ability to measure the absolute quantity of specific target proteins through the use of internal peptide standards. [13]. Hu et. al. used iTRAQ method to quantify cerebellar protein changes in mice that are deficient in plasma membrane calcium ATPase 2 (PMCA2), an essential neuronal pump that extrudes calcium from cells. They reported that iTRAQ-based quantitative MS analysis could reveal broader proteome coverage than that provided by a 2D-PAGE-based analysis [14]. In this study we have employed the iTRAQ-based scheme to perform multiplexed quantitative analysis on multiple plasma samples collected from different mouse groups. Because peptide sequences tend to have a different ionization efficiency through matrix assisted laser desorption ionization (MALDI) and electrospray ionization (ESI), for each set of the iTRAQ-tagged peptide mixtures, we used both ionization mechanisms. Further, to increase the proteome coverage and quantitative precision, we combined either the first-dimension separation of strong cation exchange (SCX) or hydrophilic interaction liquid chromatography (HILIC) with the second dimensional separation of reverse phase liquid chromatography (RPLC) to identify those plasma proteins differentially expressed on the basis of the absence of the APOE gene.
Experimental Procedures
Mouse Sample Collection
Wild-type (WT) and APOE−/− C57BL6J mice, purchased from the Jackson Laboratory, were used to breed the mice. Pups were weaned at 3 weeks of age and fed a Western high-fat diet (Teklad, Madison, WI). Animals were euthanized at 6 or 12 weeks of age. At the time of euthanasia, blood was collected in Becton-Dickinson P100 tubes that are pre-loaded with protease inhibitors and anticoagulants, as well as a self-contained system for removing red blood cells and platelets. From a single P100 tube, 90% of the storage solution was discarded. At most 1 mL of blood from a single mouse was collected. Immediately after collection, the tube was inverted 8–10 times to mix the protease inhibitors and anticoagulant with the blood sample. After mixing, the tubes were placed in ice and then centrifuged at 2000 RCF at 4 °C for 15 minutes (centrifugation was done within 30 minutes of collection). Afterwards, the plasma was divided into 25 microliter aliquots and stored at −80 °C. All mouse work was approved by the Duke University Institutional Animal Care and User Committee.
Protein Depletion and Purification
For each group (i.e., wild type at 6 weeks, APOE−/− at age of 6 weeks, wild type at 12 weeks, and APOE−/− at 12 weeks after fat treatment), an equal amount of sample from each of three male and three female mice were pooled (Table 1). The three most abundant proteins (albumin, IgG, and transferrin) were depleted by using Multiple Affinity Removal Spin (MARS) Cartridge-mouse 3 (Agilent Technologies, Wilmington, DE) following the manufacturer’s protocol (Agilent Technologies, Wilmington, DE). Briefly, 20 μL of sample from each group was diluted to 200 μL by Agilent buffer A and loaded onto a MARS spin cartridge, centrifuged at 100 × g until all the samples passed through. The spin cartridge was then washed twice with 400 μL of buffer A, centrifuged at 100 × g and the eluted solution was collected in the depleted sample collection tube. The collected sample was buffer exchanged with 50 mM triethylammonium bicarbonate using Zeba™ desalting spin columns (Pierce, Rockford, IL).
Table 1a.
Up-regulated proteins identified and quantified by 2D-LC-MALDI-TOF/TOF.
| ID | Name | Mice at 6 weeks | Mice at 12 weeks | ||
|---|---|---|---|---|---|
| APOE −/− vs WT | a EF | APOE −/− vs WT | a EF | ||
| Q60590 | Alpha-1-acid glycoprotein 1 precursor | c1.7 | 1.2 | ||
| P07361 | Alpha-1-acid glycoprotein 2 precursor | e2.1 | 1.5 | ||
| Q00898 | Alpha-1-antitrypsin 1–5 precursor | c2.6 | 1.4 | ||
| P29699 | b Alpha-2-HS-glycoprotein precursor | c1.3 | 1.2 | e1.3 | 1.2 |
| Q61838 | Alpha-2-macroglobulin precursor | c1.4 | 1.1 | ||
| P06728 | b Apolipoprotein A-IV precursor | c1.9 | 1.1 | c1.7 | 1.1 |
| P34928 | Apolipoprotein C-I precursor | c2.2 | 1.6 | ||
| Q01339 | b Beta-2-glycoprotein 1 precursor | e1.3 | 1.3 | e1.5 | 1.4 |
| Q61147 | Ceruloplasmin precursor | c1.4 | 1.2 | ||
| P16294 | Coagulation factor IX precursor | e1.4 | 1.3 | ||
| O88947 | Coagulation factor X precursor | e1.3 | 1.3 | ||
| Q02105 | Complement C1q subcomponent subunit C precursor | e1.5 | 1.5 | ||
| P06684 | Complement C5 precursor | e1.2 | 1.1 | ||
| Q8K182 | Complement component C8 alpha chain precursor | d1.7 | 1.4 | ||
| Q8BH35 | Complement component C8 beta chain precursor | e1.3 | 1.3 | ||
| Q8VCG4 | Complement component C8 gamma chain precursor | d1.5 | 1.3 | ||
| P03953 | Complement factor D precursor | e2.0 | 1.8 | ||
| Q06770 | Corticosteroid-binding globulin precursor | d1.7 | 1.4 | ||
| Q9ERU9 | E3 SUMO-protein ligase RanBP2 | e1.4 | 1.3 | ||
| Q01279 | Epidermal growth factor receptor precursor | c1.5 | 1.1 | ||
| Q8K0E8 | b Fibrinogen beta chain precursor | c1.3 | 1.1 | c1.6 | 1.1 |
| Q8VCM7 | b Fibrinogen gamma chain precursor | c1.3 | 1.1 | c1.8 | 1.1 |
| Q08879 | Fibulin-1 precursor | e1.8 | 1.6 | ||
| P13020 | Gelsolin precursor | e1.2 | 1.2 | ||
| Q61646 | Haptoglobin precursor | c3.3 | 1.3 | ||
| Q91X72 | b Hemopexin precursor | c1.3 | 1.1 | c1.5 | 1.1 |
| P01873 | Ig mu chain C region membrane-bound form | c3.5 | 1.5 | ||
| P08071 | Lactotransferrin precursor | d2.2 | 1.3 | ||
| P51885 | Lumican precursor | e1.3 | 1.2 | ||
| P11588 | Major urinary protein 1 precursor | c2.0 | 1.4 | ||
| P41317 | Mannose-binding protein C precursor | e1.2 | 1.1 | ||
| Q6KCD5 | Nipped-B-like protein | e2.7 | 1.4 | ||
| P11680 | Properdin precursor | e1.5 | 1.4 | ||
| P19221 | b Prothrombin precursor | c1.4 | 1.1 | c1.3 | 1.1 |
| Q9JJT9 | RNA U small nuclear RNA export adapter protein | e1.6 | 1.6 | ||
| P70274 | Selenoprotein P precursor | e1.8 | 1.8 | ||
| Q91WP6 | Serine protease inhibitor A3N precursor | c1.6 | 1.2 | ||
| Q921I1 | Serotransferrin precursor | d2.7 | 1.9 | ||
| P12246 | Serum amyloid P-component precursor | c2.8 | 1.4 | ||
| Q9Z1T2 | Thrombospondin-4 precursor | e2.3 | 1.6 | ||
The error factor (EF) is an asymmetrical confidence interval, so the true ratio for the protein is expected to be found between the listed ratio multiplied by the EF and the listed ratio divided by the EF at 95% of the time.
Proteins are up-regulated at both time points.
Probability (p) value is less than 0.001.
Probability (p) value is greater than 0.001 but less than 0.01.
Probability (p) value is greater than 0.01 but less than 0.05.
Protein Quantitation
The working bovine serum albumin (BSA) standard solution (1 mg/mL) was prepared by diluting the BSA stock solution (2 mg/mL) to 1 mg/mL with 50 mM triethylammonium bicarbonate, which was further diluted to a series of standard solutions of 0, 0.025, 0.05, 0.125, 0.25 and 0.5 mg/mL BSA with 50 mM triethylammonium bicarbonate buffer. Twenty microliters of each standard was added to cuvettes for standard solutions. Plasma samples and 50 mM triethylammonium bicarbonate buffer were added to the cuvettes. The concentrated dye reagent of Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA) was diluted 1:4 with HPLC water. One mL of diluted Bio-Rad protein assay solution was added to the standard and sample cuvettes. The reaction was allowed to take place for 5 minutes. The absorbance was read at a wavelength of 595 nm.
Protein Reduction, Alkylation, Digestion and iTRAQ Labeling
An equal amount of proteins from each group was lyophilized and prepared with iTRAQ reagents (Applied Biosystems, Foster City, CA) as described in the protocol from the company.[15] 50 μg depleted samples were dissolved in 25 μL 1 M triethylammonium biocarbonate sample buffer. The proteins were denatured by using 1 μL 2% SDS solution, reduced with 2 μL 50 mM Tris-(2-carboxy)ethylphosphine hydrochloride (TCEP), and alkylated with 1 μL of freshly prepared 84 mM iodoacetamide solution. Each sample was digested overnight at 37 °C with 10 μL of 1 μg/μL sequence-grade modified trypsin solution (Promega Corporation, Madison, WI). Applied Biosystems (AB)’s iTRAQ reagents 114, 115, 116, and 117 were resuspended in 70 μL of ethanol and added to the digested peptides from four pooled samples: wild type at 6 weeks, APOE−/− at 6 weeks, wild type at 12 weeks, and APOE−/− at 12 weeks. Samples were incubated at room temperature for one hour and the reactions were quenched by adding 100 μL HPLC grade water.
Off-line SCX Separation
The iTRAQ labeled peptides were separated by using an Agilent 1100 series HPLC system, which was fitted with a Polysulfoethyl A SCX column (100 × 2.1 mm, 5 μm, 300 Å, Poly LC, Columbia, MD). The composition of the mobile phase was 25% acetonitrile with 10 mM KH2PO4 (pH = 3) and the flow rate of mobile phase was 200 μL/min. The peptides retained on the column were eluted by sequential injection of 700 μL of a series of salt solutions: 0, 2.5, 5, 10, 25, 50, 75, 100, 125, 150, 175, 200, 225, 250, 275, 300, 325, 350, 400, 450, 500, and 1000 mM KCl in loading buffer. Each eluted fraction was desalted via a PepClean™ C - 18 spin column (Pierce, Rockford, IL, USA) and dried with a SpeedVac (Labconco, Kansas, MO).
Off-line HILIC Separation
HILIC separation of iTRAQ labeled peptides was performed by using an Agilent 1100 series HPLC system equipped with a HILIC column (150 mm × 2.1 mm, 5 μm, 200 Å; the Nest Group, Inc. MA). Buffer A is acetonitrile/water (v/v = 80%/20%) with 5 mM ammonium formate. Buffer B is acetonitrile/water (v/v = 30%/70%) with 10 mM ammonium formate. The peptides were separated with a gradient from 0% buffer B to 100% buffer B in 60 min at a flow rate of 200μL/min. Based on the intensity of the UV trace, 10 fractions were collected.
Reverse Phase LC-Separation and MALDI-Spotting
SCX fractions were separated and spotted on a MALDI plate via Applied Biosystems’ Tempo LC-MALDI equipped with Chromolith™ CapRod™ capillary column (150 mm × 0.1 mm, monolithic silica RP 18 end capped). Samples were separated in channel one, a two-buffer system using mobile phase A (2% acetonitrile, 98% water and 0.1% trifluoroacetic acid (TFA)) and mobile phase B (98% acetonitrile, 2% water and 0.1% TFA). The flow rate was set at 1 μL/min. The gradient was programmed: 0 – 5 min, 2% B; 5 – 95 min, 2% – 45% B; 95 –100 min, 45% – 80% B; 100 – 110 min, 80% B; 110 – 115 min, 80% – 2% B; 115 – 120 min, 2% B. Matrix spotting was accomplished using flow from channel 2 at a flow rate of 2 μL/min of matrix (5 mg/mL α-cyano-4-hydroxycinnamic acid in a solution of acetonitrile and water (75/25, v/v) with 2% ammonium citrate). The spotting was performed from 0 to120 min at a speed of 7 s/spot.
Each HILIC fraction was separated and spotted at a speed of 7.5 s per spot via Agilent LC-Probot. The reversed phase liquid chromatography separation was performed in a C18 LC Packings column (Dionex, CA, 150 mm × 75 mm, 5 μm, 100 Å). The components of buffer A were 5% acetonitrile, 95% water and 0.1% TFA. Buffer B was 5% water/95% acetonitrile with 0.1% TFA. The gradient for mobile phases was 0–150 min, 0% – 60% mobile phase B; 150–180 min, 60% – 90% mobile phase B; 180–210 min, 90% mobile phase B; 210 – 240 min, 90% - 0% mobile phase B. The matrix was 5 mg/mL α-cyano-4-hydroxycinnamic acid in 2% ammonium citrate, 50% acetonitrile and 0.1% TFA.
Protein Identification and Quantitation with AB 4800 MALDI TOF/TOF
An AB 4800 plus MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Foster City, CA) was used to analyze spotted target plates from SCX and HILIC fractions. This instrument was controlled by a 4000 series Explorer version 3.0. For MS analysis, the laser intensity was set at 3500, the number of laser shots was 3000, the MS range (m/z) was 800 to 4000, and the precursor mass tolerance was 200 ppm. The top 20 peaks in each MS spectrum with a signal-to-noise ratio (S/N) of 30 or greater were chosen for MS/MS analysis. For MS/MS analysis, the laser intensity was set at 4300 and the total number of shots was 5500. AB’s ProteinPilot software 2.0.1 was used to perform the data analysis with the Paragon Algorithm. All MS/MS data for all SCX and HILIC fractions from LC-MALDI TOF/TOF analysis were processed together and searched against the UniProtKB/Swiss-Prot database (released on 01/23/2007, about 260K entries).. The search parameters were 4-plex iTRAQ peptide labeling, alkylation with iodoacetamide, and tryptic digestion. The detected protein threshold (unused ProtScore (conf)) was set at 1.3 (95.0%). The protein abundance ratio, probability value (p), and error factor (EF) were calculated by using ProteinPilot 2.0.1. Only the unique peptides to a protein were used in its quantification.
Protein Identification and Quantitation with NanoLC-LTQ-Orbitrap
Another aliquot of peptides in each SCX and HILIC fraction was separated with on-line Eksigent nano LC system and analyzed by a LTQ-Orbitrap tandem mass spectrometer (Thermo Electron, San Jose, CA) equipped with a nano electrospray source (New Objective, Inc., Woburn, MA). The peptides were loaded onto an IntegraFrit™ sample trap ( ProteoPep™ II C18, 300 Å, 5 μm, 75 μm × 25 mm, New Objective, Inc., Woburn, MA) by using a mobile phase of water premixed with 0.1% formic acid, of which 50% was pumped with channel 1A and 50% with channel 1B. The retained peptides were washed isocratically by water premixed with 0.1% formic acid to remove any excess reagents. The cleaned peptides were resolved on a PicoFrit® analytical column (ProteoPep™ II C18, 300 Å, 5 μm, 50 μm × 100 mm, tip ID = 10 μm, New Objective Inc., Woburn, MA) with a multistep gradient in channel 2 of solvent 2A (water premixed with 0.1% formic acid) and solvent 2B (acetonitrile premixed with 0.1% formic acid) at a flow rate of 200 nL/min. The gradient started at 5% 2B and was held for 15 min, with linear increases to 60% 2B at 145 min and 90% 2B at 180 min. The gradient was held at 90% 2B for 15 min before going to 5% 2B at 197 min. The re-equilibration took about 33 min. The LTQ-Orbitrap tandem mass spectrometer was operated in the data-dependent mode. The full MS spectra were acquired in positive mode in Orbitrap (m/z = 300–2000, resolution = 60,000 ( at m/z 400) and the automatic gain control was set to 500,000 ions. 1 microscan was record.The three most abundant precursor ions in each full MS spectrum were chosen for Pulsed Q Dissociation (PQD) in LTQ. The normalized collision energy is 30%, Q activation setting was 0.7 with activation time of 0.1. The dynamic exclusion (repeat count 1, exclusion list size 500, exclusion duration 15s, exclusion mass width low 0.5, and exclusion mass width low 1.5) was enabled for LC-MS/MS experiments.
The LC-MS/MS raw data from nanoLC-LTQ-Orbitrap were converted to DTA files using Thermo Electron Bioworks 3.3.1 and correlated to theoretical fragmentation patterns of tryptic peptide sequences from the Fasta databases using SEQUEST™ (Thermo Fisher). The search parameters include: 1) fixed cysteine modifications of +57 for carbamidomethyl-cysteines, +144 for lysine-iTRAQ labeling and N-terminal peptides; 2) variable modifications allowing +16 with methionines for methionine sulphoxide and +144 for Y-iTRAQ labeling; 3) restricted to trypsin digested peptides and allowed for 2 missed cleavages; 4) peptide mass search tolerance was 50 ppm and fragment mass tolerance was set at ± 1 dalton. The criteria for peptide identification were 1) top 5 rankings as the hit for a MS/MS spectra and 2) peptide probability being lower than 0.01. The search results from all SCX and HILIC fractions were manually integrated. Proteins with a probability lower than 0.001 and matched with at least two peptides were considered as positive identifications. The intensities of iTRAQ reporter ions were manually extracted from Bioworks software. The protein abundance ratio was estimated by using the sum ratio of the reporter ion intensities across the spectra matched to its peptides [16] and were calculated by using self-developed script (Visual Basic for Applications in Microsoft Excel).
Results and Discussion
Comparative analysis of the plasma proteome changes in the paired APOE−/− versus wild-type mice collected at different time points following fat feeding
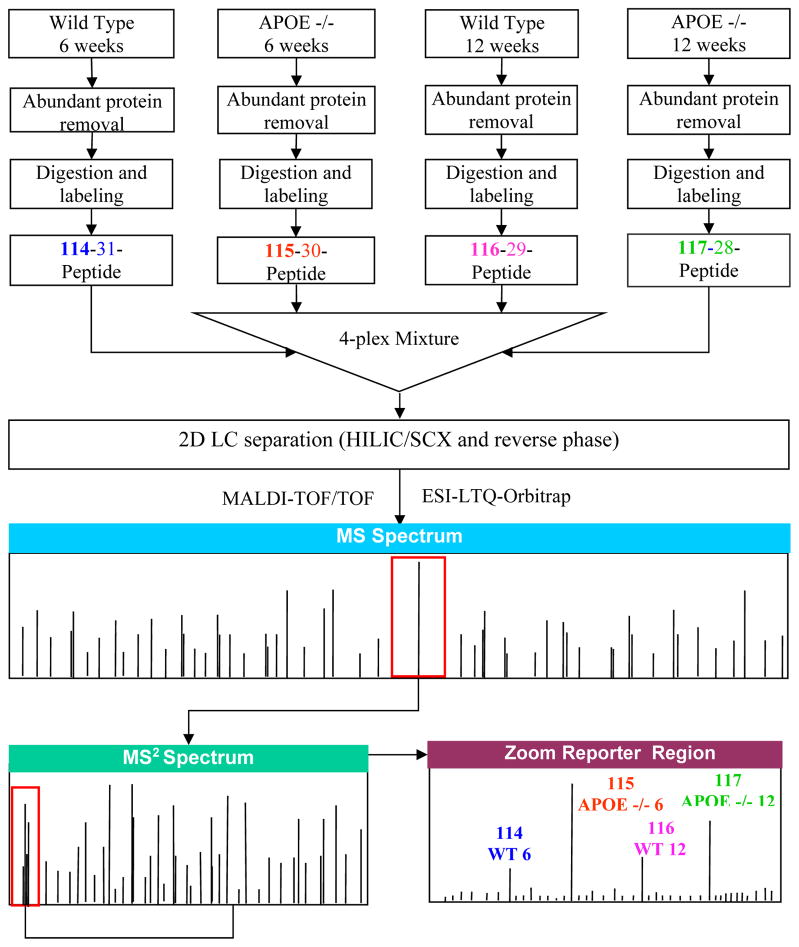
First, the most abundant proteins in the plasma such as albumin, IgG, transferrin, etc, were depleted. Following the buffer exchange, as shown in Figure 1, each of four samples was denatured, reduced, alkylated, and digested. 4-plex iTRAQ reagents were used to label the peptide digests obtained from both APOE−/− mice and their wild-type (WT) counterpart fed with the high fat diet for 6 and 12 weeks (wks), i.e., APOE−/− at 6 wks, APOE−/− at 12 wks, WT at 6 wks, WT at 12 wks, respectively. The iTRAQ-labeled peptides from each of the four samples were mixed at an equal mass ratio. We used both SCX and a more MS-compatible HILIC schemes to separate the iTRAQ-labeled peptide mixture respectively in the first LC dimension and then RP as the second dimension. Each of the separated peptide fractions from either 2D SCX-RP LC or 2D HILIC-RP LC was analyzed by both MALDI-4800-TOF/TOF and LTQ-Orbitap MS.
Figure 1.
Work flow for quantitative proteomics. The pooled samples were depleted of albumin, transferrin and IgG with Agilent’s Mouse (Mu3) Multiple Affinity Removal Spin Cartridge. Equal amounts of proteins from each group were then denatured (2% SDS), reduced (TCEP, Tris-(2-carboxy)ethylphosphine hydrochloride), alkylated (iodoacetamide), digested (trypsin), and labeled with AB’s iTRAQ reagents (114, 115, 116, and 117). The iTRAQ-labeled peptides were mixed, and were analyzed by 2D LC (HILIC/SCX and reserve phase) followed by mass spectrometric analysis with an AB 4800 plus MALDI-TOF/TOF, and a ThermoFisher LTQ(PQD)-Orbitrap equipped with New Objective nanoESI. Protein identification and quantification were accomplished with AB’s ProteinPilot™ software 2.0.1 and ThermoFisher’s Bioworks 3.3.1.
In the 2D-LC-MALDI-TOF/TOF analysis, 300 proteins were quantified, and the threshold with 95% confidence in distinguishing those differentially expressed proteins was determined statistically by ProteinPilot, based on their identified peptides, with bias correction for uneven mixing. As a result, in the cross-sample quantitative comparisons provided by 4-plex quantitative iTRAQ labeling, 40 proteins were found up-regulated (Table 1a), and 39 proteins down-regulated (Table 1b) when the plasma proteome was compared for APOE−/− vs. WT, collected at one or both time points after feeding the fat diets. Specifically, 14, 19, and 7 proteins were observed up-regulated at 6 weeks only, 12 weeks only, and at both time points, respectively (Table 1a). Meanwhile 10 proteins were down-regulated at 6 weeks only, 11 proteins down-regulated at 12 weeks only, and 18 proteins down-regulated at both time points (Table 1b).
Table 1b.
Down-regulated proteins identified and quantified by 2D-LC-MALDI-TOF/TOF
| ID | Name | Mice at 6 weeks | Mice at 12 weeks | ||
|---|---|---|---|---|---|
| APOE −/− vs WT | a EF | APOE −/− vs WT | a EF | ||
| Q8VDM4 | 26S proteasome non-ATPase regulatory subunit 2 | e0.53 | 1.9 | ||
| Q00896 | Alpha-1-antitrypsin 1–3 precursor | d0.74 | 1.2 | ||
| O88327 | Alpha-catulin | e0.55 | 1.5 | ||
| Q00623 | b Apolipoprotein A-I precursor | c0.37 | 1.1 | c0.31 | 1.1 |
| P09813 | b Apolipoprotein A-II precursor | c0.20 | 1.5 | c0.17 | 1.3 |
| Q05020 | b Apolipoprotein C-II precursor | e0.36 | 2.0 | e0.31 | EF > 2 |
| P33622 | Apolipoprotein C-III precursor | d0.44 | 1.5 | ||
| P51910 | b Apolipoprotein D precursor | c0.42 | 1.4 | c0.37 | 1.3 |
| P08226 | b Apolipoprotein E precursor | c0.12 | 1.6 | c0.06 | 1.7 |
| P01887 | Beta-2-microglobulin precursor | c0.54 | 1.2 | ||
| P00920 | b Carbonic anhydrase 2 | c0.61 | 1.3 | c0.35 | 1.2 |
| Q06890 | Clusterin precursor | e0.85 | 1.1 | ||
| P01027 | b Complement C3 precursor | c0.69 | 1.1 | c0.68 | 1.1 |
| P01029 | b Complement C4-B precursor | c0.70 | 1.1 | c0.64 | 1.2 |
| P06683 | Complement component C9 precursor | e0.85 | 1.2 | ||
| P04186 | Complement factor B precursor | e0.83 | 1.2 | ||
| Q9JHU4 | b Dynein heavy chain, cytosolic | c0.49 | 1.6 | d0.30 | 1.9 |
| Q8K284 | General transcription factor 3C polypeptide 1 | e0.50 | 1.7 | ||
| Q9JHK4 | b Geranylgeranyl transferase type-2 alpha subunit | e0.19 | EF > 2 | e0.16 | EF > 2 |
| Q91VW5 | Golgin subfamily A member 4 | e0.14 | EF > 2 | ||
| P01898 | b H-2 class I histocompatibility antigen, Q10 alpha chain precursor | c0.38 | 1.2 | c0.33 | 1.2 |
| P63017 | Heat shock cognate 71 kDa protein | d0.71 | 1.1 | ||
| P01942 | b Hemoglobin subunit alpha | c0.55 | 1.1 | c0.32 | 1.1 |
| P02088 | b Hemoglobin subunit beta-1 | c0.46 | 1.3 | c0.26 | 1.3 |
| Q6P2L6 | Histone-lysine N-methyltransferase NSD3 | c0.12 | EF > 2 | ||
| Q61702 | Inter-alpha-trypsin inhibitor heavy chain H1 precursor | e0.79 | 1.3 | ||
| Q61703 | b Inter-alpha-trypsin inhibitor heavy chain H2 precursor | c0.49 | 1.2 | c0.62 | 1.1 |
| Q61730 | Interleukin-1 receptor accessory protein precursor | d0.75 | 1.2 | ||
| P39039 | Mannose-binding protein A precursor | e0.73 | 1.3 | ||
| Q62504 | Msx2-interacting protein | e0.29 | EF > 2 | ||
| Q61171 | b Peroxiredoxin-2 | e0.55 | 1.8 | e0.34 | EF > 2 |
| O70362 | b Phosphatidylinositol-glycan-specific phospholipase D 1 precursor | c0.54 | 1.2 | c0.63 | 1.2 |
| P97290 | Plasma protease C1 inhibitor precursor | d0.70 | 1.2 | ||
| Q8CJ40 | Rootletin | e0.73 | 1.3 | ||
| Q8K1I3 | Secreted phosphoprotein 24 precursor | e0.67 | 1.3 | ||
| P07759 | Serine protease inhibitor A3K precursor | e0.84 | 1.2 | ||
| P05366 | Serum amyloid A-1 protein precursor | c0.27 | 1.5 | ||
| P31532 | b Serum amyloid A-4 protein precursor | c0.46 | 1.5 | c0.49 | 1.3 |
| P52430 | b Serum paraoxonase/arylesterase 1 | e0.57 | 1.7 | d0.51 | 1.5 |
The error factor (EF) is an asymmetrical confidence interval, so the true ratio for the protein is expected to be found between the listed ratio multiplied by the EF and the listed ratio divided by the EF at 95% of the time.
Proteins are down-regulated at both time points.
Probability (p) value is less than 0.001.
Probability (p) value is greater than 0.001 but less than 0.01.
Probability (p) value is greater than 0.01 but less than 0.05.
In the quantitative proteomic dataset generated by nanoLC-ESI-LTQ(PQD)-Orbitrap MS, 397 proteins (P < 0.001) were quantified with at least two peptides for each individual protein. 21 plasma proteins were found up-regulated (Table 2a) with 13 proteins up-regulated in the APOE −/− mice fed with high fat at 6 weeks only, 6 proteins at 12-weeks only, and 2 proteins up-regulated at both time points. Meanwhile, 40 proteins were down-regulated when their levels in the APOE −/− mice compared to those of the WT mice (Table 2b). Among them, 5 proteins were down-regulated at 6-weeks only, 21 proteins at 12 weeks only, and 14 proteins at both time points.
Table 2a.
Up-regulated proteins identified and quantified by 2D-LC-ESI-LTQ(PQD)-Orbitrap
| Accession # | Name | Mice at 6 weeks APOE −/− vs WT |
Mice at 12 weeks APOE −/− vs WT |
|---|---|---|---|
| IPI00132542.1 | 10 days embryo whole body cDNA | 2.0 | |
| IPI00132134.2 | 12 days pregnant adult female placenta cDNA | 2.0 | |
| IPI00225477.4 | 2195 kDa protein | 2.9 | |
| IPI00463639.4 | 26 kDa protein | 2.1 | |
| IPI00123927.1 | Alpha-1-antitrypsin 1–5 precursor | 2.2 | |
| IPI00119676.1 | Apolipoprotein C-I precursor | 2.6 | |
| IPI00474450.1 | Dystrophin | 2.2 | |
| IPI00409148.2 | Haptoglobin precursor | 2.6 | |
| IPI00227857.4 | Hepatocyte growth factor activator precursor | 2.8 | |
| IPI00131111.1 | Histone-lysine N-methyltransferase, H3 lysine-36 and H4 lysine-20 specific | 7.8 | |
| IPI00177214.1 | Igh-6 protein | 2.4 | |
| IPI00119913.1 | Isoform 1 of Adenomatous polyposis coli protein | 2.1 | |
| IPI00115243.1 | Major urinary protein 5 precursor | 2.4 | |
| IPI00350772.5 | * PREDICTED: apolipoprotein B isoform 1 | 2.7 | 2.1 |
| IPI00461878.2 | PREDICTED: similar to limkain b1 isoform 1 | 4.0 | |
| IPI00553366.4 | PREDICTED: similar to tankyrase | 2.2 | |
| IPI00226216.5 | * Rho guanine nucleotide exchange factor (GEF) 19 | 2.3 | 2.0 |
| IPI00330896.1 | Spermatogenesis associated 20 | 2.7 | |
| IPI00230008.1 | Synaptotagmin-like homologue lacking C2 domains-b | 2.7 | |
| IPI00123111.2 | Transcription termination factor 1 | 2.8 | |
| IPI00314041.5 | Villin-1 | 2.5 |
Proteins are up-regulated at both time points.
Table 2b.
Down-regulated proteins identified and quantified by 2D-LC-ESI-LTQ(PQD)-Orbitrap
| Accession # | Name | Mice at 6 weeks APOE −/− vs WT |
Mice at 12 weeks APOE −/− vs WT |
|---|---|---|---|
| IPI00226680.1 | 10 days neonate cerebellum cDNA | 0.34 | |
| IPI00126208.1 | 11 days embryo whole body cDNA | 0.29 | |
| IPI00468603.4 | 12 days embryo embryonic body between diaphragm region and neck cDNA | 0.28 | |
| IPI00110658.1 | 13 days embryo liver cDNA, RIKEN full-length enriched library, clone:2510040B16 product:hemoglobin, beta adult major chain, full insert sequence | 0.30 | |
| IPI00755200.1 | 221 kDa protein | 0.28 | |
| IPI00649712.1 | *24 kDa protein | 0.38 | 0.32 |
| IPI00349896.3 | *47 kDa protein | 0.50 | 0.21 |
| IPI00350399.6 | *57 kDa protein | 0.49 | 0.29 |
| IPI00380781.2 | Adult male thymus cDNA, RIKEN full-length enriched library, clone:5830476A12 product:complement component 1, r subcomponent-like, full insert sequence | 0.50 | |
| IPI00121209.1 | * Apolipoprotein A-I precursor | 0.39 | 0.32 |
| IPI00136266.1 | * Apolipoprotein C-II precursor | 0.28 | 0.25 |
| IPI00323571.1 | * Apolipoprotein E precursor | 0.09 | 0.07 |
| IPI00130382.3 | * Apolipoprotein M | 0.35 | 0.42 |
| IPI00380509.5 | BC053994 protein | 0.29 | |
| IPI00109966.1 | Beta-2-microglobulin precursor | 0.50 | |
| IPI00121534.8 | Carbonic anhydrase 2 | 0.43 | |
| IPI00121319.1 | Cysteine-rich protein 2 | 0.20 | |
| IPI00128288.2 | * Dimethylaniline monooxygenase [N-oxide-forming] 4 | 0.08 | 0.06 |
| IPI00379245.2 | Glucosamine-6-phosphate isomerase | 0.27 | |
| IPI00319652.1 | Glutathione peroxidase 1 | 0.31 | |
| IPI00109996.1 | * H-2 class I histocompatibility antigen, L-D alpha chain precursor | 0.35 | 0.36 |
| IPI00469114.4 | * Hemoglobin subunit | 0.40 | 0.21 |
| IPI00124725.1 | Inter-alpha-trypsin inhibitor heavy chain H3 precursor | 0.49 | |
| IPI00329872.1 | Isoform 1 of Collagen alpha-1(I) chain precursor | 0.42 | |
| IPI00453688.1 | Isoform 1 of Signal-induced proliferation-associated 1-like protein 1 | 0.20 | |
| IPI00321666.1 | * MHC | 0.40 | 0.41 |
| IPI00117910.2 | Peroxiredoxin-2 | 0.44 | |
| IPI00109437.1 | * Pirin | 0.45 | 0.47 |
| IPI00129965.3 | PREDICTED: alpha-1-B glycoprotein isoform 1 | 0.27 | |
| IPI00355031.5 | PREDICTED: cortactin binding protein 2 isoform 1 | 0.47 | |
| IPI00462565.1 | PREDICTED: similar to 5T4 oncofetal trophoblast glycoprotein | 0.42 | |
| IPI00670418.2 | PREDICTED: similar to jumonji domain containing 1C isoform 3 | 0.21 | |
| IPI00620959.2 | PREDICTED: similar to MAP/microtubule affinity-regulating kinase 3 | 0.15 | |
| IPI00406030.2 | Rho interacting protein 3 | 0.42 | |
| IPI00605003.2 | RING finger protein 17 long transcript | 0.34 | |
| IPI00128040.1 | Serine protease HTRA1 precursor | 0.47 | |
| IPI00135547.1 | Serum amyloid A-4 protein precursor | 0.40 | |
| IPI00317356.9 | * Serum paraoxonase/arylesterase 1 | 0.41 | 0.45 |
| IPI00380247.2 | * SUMO-1-specific protease | 0.45 | 0.39 |
| IPI00116923.1 | Thyroid hormone receptor interactor 10 | 0.25 |
Proteins are down-regulated at both time points.
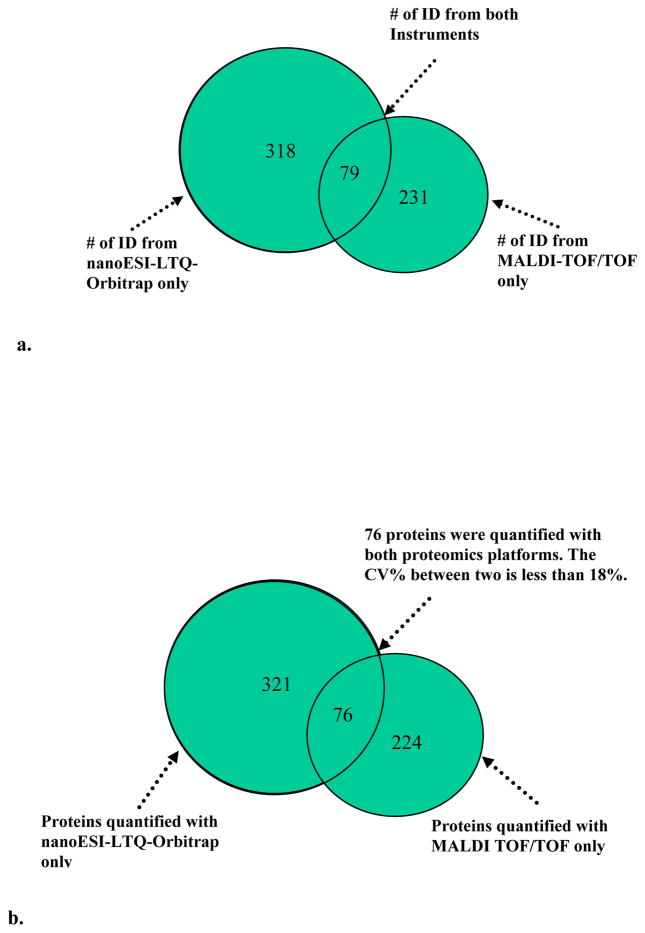
In combining the proteomic datasets obtained by both 2D-LC MALDI-TOF/TOF and ESI-LTQ(PQD)-Orbitrap MS approaches, we have identified a total of 628 proteins (Figure 2), of which 79 proteins were identified by both approaches while 231 were identified only by 2D-LC-MALDI-TOF/TOF and 318 only by the 2D-LC-ESI-LTQ(PQD)-Orbitrap MS approach. Among a total of 621 proteins quantified, 545 proteins were quantified only by either 2D-LC-MALDI-TOF/TOF or 2D-LC-ESI-LTQ(PQD)-Orbitrap approach, and 76 proteins were comparably quantified by using both approaches. The relative standard deviations for the quantitative data generated by both approaches were less than 15% and 18% for the paired analysis of APOE−/− vs. WT mice at week 6 and week 12, respectively (Figure 2), which provided the cross-method validation for the iTRAQ-based quantification accuracy.
Figure 2.
a) the comparison of results from 2D-LC-MALDI-TOF/TOF vs. 2D-LC-ESI-LTQ(PQD)-Orbitrap showed that a total of 628 proteins were identified. 79 proteins were identified from both techniques while 549 proteins with only one method. b) 621 proteins were quantified, among which 545 proteins were quantified with one method and 76 were comparably quantified with both methods.
For CAD biomarker discovery, by employing two platforms, we identified 58 proteins up-regulated and 70 proteins down-regulated in plasma samples from APOE−/− mice vs. WT mice at either 6 weeks or 12 weeks, or at both time points (Table 3). There were only 3 up-regulated and 9 down-regulated proteins detected by using both methods (Table 4a and Table 4b).
Table 3.
Differentially Expressed Proteins Associated with Response to Stimulus
| RefSeq_Protein | DAVID Gene Name | * WK 6 | * WK 12 |
|---|---|---|---|
| NP_038513 | Complement component 9 | − | |
| NP_033865 | Beta-2 Macroglobulin | − | |
| NP_035693 | Peroxiredoxin 2 | − | − |
| NP_059066 | Haptoglobin | + | |
| NP_598864 | Interleukin 1 Receptor Accessory Protein | − | |
| NP_034510 | Histocompatibility 2, D Region Locus 1 | − | − |
| NP_035543 | Complement Component 4b (Childo Blood Group) | − | − |
| NP_112442 | Heat Shock Protein 8 | − | |
| NP_862897 | Fibrinogen, B Beta Polypeptide | + | + |
| NP_038493 | Alpha-2-Hs-Glycoprotein | + | + |
| NP_032005 | Coagulation Factor IX | + | |
| NP_666260 | Complement Component 8, Alpha Polypeptide | + | |
| NP_031488 | Adenomatosis Polyposis Coli | + | |
| NP_033271 | Serine (or cysteine) peptidase inhibitor, clade a, member 1a | − | |
| NP_081338 | Riken cdna 1700013l23 gene | + | |
| NP_031601 | Class ii transactivator | − | − |
| NP_034521 | Histocompatibility 2, q region locus 10 | − | − |
| NP_033143 | Serum amyloid a 1 | − | |
| XP_909452 | Immunoglobulin heavy chain 6 (heavy chain of igm) | + | |
| NP_034298 | Coagulation factor ii | + | + |
| NP_033181 | Selenoprotein P, Plasma, 1 | + | |
| NP_032849 | Properdin factor, complement | + | |
| NP_033822 | Apolipoprotein a-i | − | − |
| NP_598643 | Riken cdna 4930439b20 gene | + | |
| NP_038487 | Complement factor d (adipsin) | + | |
| NP_033826 | Apolipoprotein e | − | − |
| NP_032186 | Glutathione peroxidase 1 | − | |
| NP_598623 | Fibrinogen, gamma polypeptide | + | + |
| NP_031600 | Complement component 1, q subcomponent, c chain | + | |
| NP_035446 | Serum amyloid a 4 | − | − |
| NP_033908 | Complement component 3 | − | − |
| NP_034536 | Hemolytic complement | + | |
| NP_035264 | Paraoxonase 1 | − | − |
| NP_038502 | Apolipoprotein a-ii | − | − |
| NP_038503 | Apolipoprotein h | + | + |
| NP_032794 | Orosomucoid 1 | + | |
| NP_033906 | Serine (or cysteine) peptidase inhibitor, clade g, member 1 | − | |
| NP_034905 | Mannose binding lectin (a) | − | |
| NP_033278 | Serine (or cysteine) peptidase inhibitor, clade a, member 3n | + | |
| NP_031998 | Coagulation factor X | + | |
| NP_032224 | Complement factor b | − | |
| NP_034906 | Mannose binding lectin (c) | + | |
| NP_035146 | Orosomucoid 2 | + |
Proteins up-regulated are represented by “+” and down-regulated by “−”.
Table 4a.
List of up-regulated proteins identified by both methods.
| Protein Name | Mice at 6 weeks APOE −/− vs WT |
Mice at 12 weeks APOE −/− vs WT |
|---|---|---|
| Alpha-1-antitrypsin 1–5 precursor | a2.6 | |
| b2.2 | ||
| Apolipoprotein C-I precursor | a2.2 | |
| b2.6 | ||
| Haptoglobin precursor | a3.3 | |
| b2.6 |
Protein identified and quantified by using 2D-LC-MALDI-TOF/TOF (P<0.001).
Protein identified and quantified by using 2D-LC-ESI-LTQ(PQD)-Orbitrap (two fold cut-off).
Table 4b.
List of down-regulated proteins identified by both methods.
| Protein Name | Mice at 6 weeks APOE −/− vs WT |
Mice at 12 weeks APOE −/− vs WT |
|---|---|---|
| Apolipoprotein A-I precursor | a0.37 | a0.31 |
| d0.39 | d0.32 | |
| Apolipoprotein C-II precursor | c0.36 | c0.31 |
| d0.28 | d0.25 | |
| Apolipoprotein E precursor | a0.12 | a0.059 |
| d0.09 | d0.07 | |
| Beta-2-microglobulin precursor | a0.54 | |
| d0.50 | ||
| Carbonic anhydrase 2 | a0.61 | a0.35 |
| d0.43 | ||
| Hemoglobin subunit alpha | a0.55 | a0.32 |
| d0.40 | d0.21 | |
| Peroxiredoxin-2 | c0.55 | c0.34 |
| d0.44 | ||
| Serum amyloid A-4 protein precursor | a0.46 | a0.49 |
| d0.40 | ||
| Serum paraoxonase/arylesterase1 | c0.57 | b0.51 |
| d0.41 | d0.45 |
Protein identified and quantified by using 2D-LC-MALDI-TOF/TOF (P<0.001).
Protein identified and quantified by using 2D-LC-MALDI-TOF/TOF (0.001P<0.01).
Protein identified and quantified by using 2D-LC-MALDI-TOF/TOF (0.01<P<0.05).
Protein quantified by using 2D-LC-ESI-LTQ(PQD)-Orbitrap (0.5 cut-off).
Clinical implications of the APOE-dependent differentially expressed plasma proteins identified by iTRAQ-based quantitative proteomics approaches
Consistent with previous findings [9, 17–27], certain dys-regulated proteins were identified at two time points along with the progression of CAD in the APOE−/− mice compared to the WT control mice. For example, the increased levels of the gamma and beta chains of fibrinogen (precursors) were observed in the plasma of the APOE−/− mice at both 6 and 12 weeks, and their abundances increased further with longer fat feeding from 6 to 12 weeks (Table 4a). This is in accordance with the fact that fibrinogen plays a significant role for the development of CAD and is generally elevated in patients diagnosed with CAD[24]. Apolipoprotein A-I (precursor) was found down-regulated in APOE−/− mice at both time points, which is also consistent with the established negative correlation between apolipoprotein A-I and the extent of CAD [9, 17–23]. Thrombospondin-4 (precursor) was also found up-regulated in APOE−/− mice in our study, and its elevated levels have been associated with atherosclerosis in human [25–27]. The identification of these differentially expressed proteins in this murine model that are known to play active roles during CAD development in humans has supported the utilization of these iTRAQ-based proteomic approaches for the discovery of novel protein biomarkers. Of note, the average abundance ratios of APOE, i.e., listed as ‘APOE precursor’ in protein databases, for APOE−/− mice vs. WT at 6 weeks and 12 weeks were 0.11 and 0.07 (average of the measured results from MALD-TOF/TOF (0.12 and 0.06) and LTQ-Orbitrap (0.09, 0.07), respectively, which were essentially close to the background level. This is consistent with the expectation that APOE was absent in APOE−/− mice, another proof for the validity of using these methods.
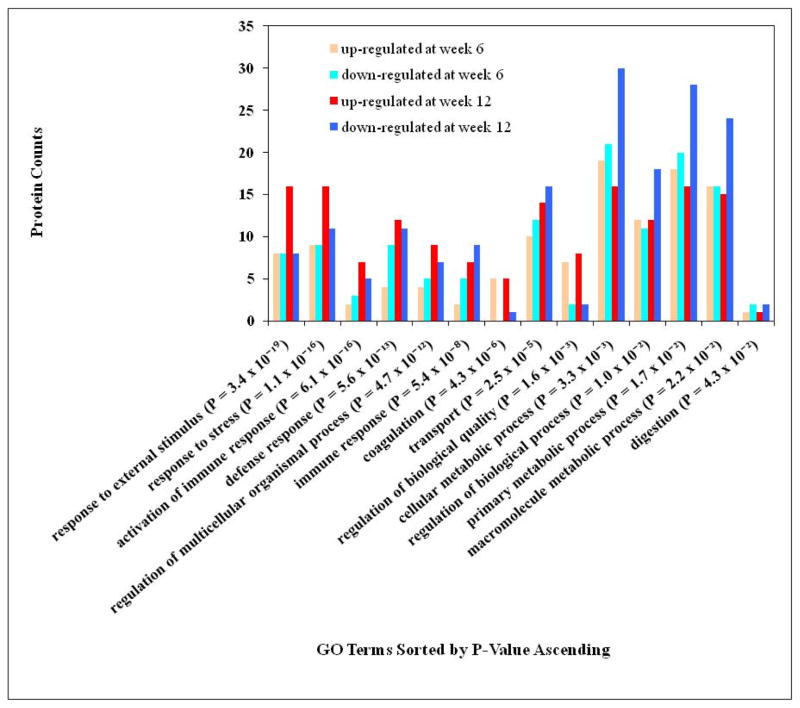
The putative biological functions associated with the differentially expressed proteins found here were investigated to determine if they are involved in the known process related to CAD. To do this, the differentially expressed proteins were converted to RefSeq protein identifiers with the DAVID gene ID conversion tool and submitted for functional annotation analysis by using DAVID Bioinformatics Resources 2008[28, 29]. The largely enriched annotation categories associated with the differentially expressed proteins at week 6 and 12 are listed in Figure 3. A p-value less than 0.05 (p < 0.05) represents the high enrichment of particular categories. The enrichment p-value in the functional annotation chart is the probability calculated based on EASE Score, a modified Fisher exact test. The protein count at each category (or the number of unique DAVID IDs) and p-value corresponding to the list of differentially expressed proteins identified by this study are shown in Figure 3. The functional annotations that are related to CAD were clustered into specific functional groups. In general, there were more regulated proteins associating with these enriched functional categories in the week 12 plasma than in that of week 6, as expected if these proteins are associated with the progression of CAD.
Figure 3.
Functional annotation chart for up- and down-regulated proteins at week 6 and week 12. Protein count is the number of unique DAVID IDs corresponding to the differential protein list from this study. P-value is the probability calculated based on EASE Score, a modified Fisher Exact Test.
The known APOE-associating proteins, such as apolipoproteins (A-I, A-II, A-IV, B, C-I, C-III, D and H), serum paraoxonase/arylesterase 1, serum amyloid P-component, serum amyloid A1, inter-alpha trypsin inhibitor, heavy chain 3, selenoprotein P, plasma 1, and lactotransferrin [30] were found either up- or down-regulated at either or both time points in the plasma isolated from APOE−/− mice. For certain proteins previously known to be associated with human CAD including those involved in the complement system, proteolysis, and coagulation, etc [27], we observed a good cross-species correlation between what were detected in the APOE−/− mice and in the developed phase of human CAD patients. Thus, our proteomic findings in the mouse model with fat-dependent progression of CAD could be indicative of novel CAD markers for the diagnosis of the human disease. We therefore categorized those APOE-dependent differentially expressed proteins according to their previously known functions.
43 proteins or 34% of all differentially expressed proteins identified in this study were found to be associated with stimulus response (Table 3). The detailed distribution of these proteins includes those related to the response to external stimulus (p = 3.4 × 10−19), response to stress (p = 1.1 × 10−16), activation of immune response (p = 6.1 × 10−16), defense response (p = 5.6 × 10−13), and immune response (p = 5.4 × 10−8). Certain proteins in the category of host defense (28 proteins, p = 5.6 × 10−13) or related to immune response (19 proteins, p = 5.4 × 10−8) were previously known to be associated with CAD and its development [4, 27]. The number of proteins associated with these functional categories was increased in the plasma of APOE−/− mice with prolonged fat feeding from 6 to 12 weeks (Figure 3). Similar genes associated with defense response were previously found to be significantly over-presented in the progression of atherosclerosis [4, 31]. At week 12, the number of defense response-associated proteins (23) was significantly over-expressed, with a p value of 2.2 × 10−12, of which 18 proteins were associated with acute inflammatory response. There were 12 proteins (i.e., riken cdna 1700013l23 gene, complement component 1, q subcomponent, c chain, haptoglobin, orosomucoid 1, coagulation factor ii, serine (or cysteine) peptidase inhibitor, clade a, member 3n, properdin factor, complement, hemolytic complement, alpha-2-Hs-glycoprotein, riken cdna 4930439b20 gene, complement component 8, alpha polypeptide, and orosomucoid 2) up-regulated at week 12 compared to only 4 proteins (i.e., coagulation factor ii, alpha-2-Hs-glycoprotein, mannose binding lectin c, and complement factor d or adipsin) up-regulated at week 6. Furthermore, 14 proteins in the category of defense response were involved in the complementary activation, which supports a report that most of the differentially displayed proteins in CAD patients were members of the complement system[27].
The second significant enriched category associated with the differentially expressed proteins was blood coagulation (7 proteins, p = 4.3 × 10−6). This observation is consistent with the results from previous human proteomics studies which suggested that some coagulation-associated proteins could be candidates for CAD biomarkers [27]. 6 out of 7 proteins detected in this category, including coagulation factor II, IX and X, fibrinogen gamma, and B-beta polypeptide and apolipoprotein H, were significantly up-regulated in APOE−/− mice compared to wild-type mice. Among them, coagulation factor II, fibrinogen gamma and B-beta polypeptide were known to be involved in the process of platelet activation.
We also found that 37 proteins previously known to be associated with transport processes[29] were differentially expressed when the plasma proteome of APOE−/− was compared to that of wild type mice. Among those over-presenting proteins associated with lipid transport (9 proteins, p = 2.9 × 10−7), 4 proteins are associated with cholesterol transport (p = 2.8 × 10−4) including apolipoprotein A-I, apolipoprotein E and M, which were down-regulated at both time points, and apolipoprotein B which was up-regulated at both points. Apolipoprotein A-I and M are the proteins in high density lipoprotein (HDL), which can remove cholesterol from atheroma within arteries and transport it back to the liver for excretion or re-utilization. The lower HDL increases the risk for developing CAD and therefore the abundance of apolipoprotein A-I and M were lower in the APOE −/− mice. On the other hand, apolipoprotein B is the primary protein of low-density lipoproteins, which is responsible for carrying cholesterol to tissues. The high level of apolipoprotein B observed in our study is consistent with a previous report that suggested it as a potential CAD biomarker [32].
As expected with the knowledge of CAD pathology, many proteins associated with metabolism were over-presented, including cellular metabolism (66 proteins, p = 0.0033), primary metabolism (63 proteins, p = 0.017), and macromolecule metabolism (56 proteins, p = 0.022) (Figure 3). Interestingly, we observed that the number of down-regulated proteins in this category increased from week 6 to week 12 while there was not much change in the number of up-regulated proteins. There were 9 proteins related to lipid metabolism, including 6 proteins involved in cholesterol metabolism, 3 proteins in lipid catabolism, and 8 proteins related to lipoprotein metabolism (p = 9.9 × 10−7). At week 6, 8 differently expressed proteins (i.e., apoplipoprotein A-I, A-II, B, C-II, and E, serum amyloid A1, glycosylphosphatidylinositol specific phospholipase d1, and paraoxonase 1) were associated with lipid metabolic process (p = 0.021) and 7 proteins (including apolipoprotein A-I, A-II, A-IV, C-I, E, M, and rab geranylgeranyl transferase, a subunit ) were associated with lipoprotein metabolic process (p = 6.2 × 10−7 ). This observation is consistent with a report from a mouse genomics study which reported that lipid/lipoprotein metabolism was significantly over-represented at 6 weeks. Also, there were 46 proteins associated with protein metabolism, including 23 proteins associated with proteolysis.
Limitations and future work
Considering still limited sensitivity and sequence coverage of currently available mass spectrometry-based technology, qantitative proteomics analysis of pooled plasma is required to generate sufficient proteins for MS-characterization. By doing so, the biological variations could be diminished in the pooled samples and might result in genetic errors in biomarker candidates. However, previous work has studied biological variation in human plasma characterized by two-dimensional difference gel electrophoresis (2-D DIGE) using plasma samples from eleven healthy subjects collected three times over a two week period. Their results have shown that for 70% of the high-quality protein spots, the coefficient of variation of the standardized abundance was less than 30% across all subjects. In our case, since the experimental mice were grown in the identical condition, the variation between them is expected to be much smaller than 30%. Taking a consideration of individual genetic variations and their impact on the accuracy of biomarker identification, we propose to first analyze pooled samples by using MS-based quantitative proteomics in the discovery phase and then further characterize/validate individual biomarkers of clinical significance on individual samples using biological assays such as immunoblotting.
Conclusion
Two iTRAQ quantitative proteomics platforms (2D-LC-MALDI-TOF/TOF and 2D-LC-ESI-LTQ(PQD)-Orbitrap) for CAD plasma biomarker discovery were established via a CAD mouse model. This study demonstrated that these two platforms provide complementary results in protein identification and quantitation. Only about 12% of total reported proteins were identified and comparably quantified by using both platforms. This may be due to the two different ionization mechanisms of MALDI (which produces and fragments single charged peptides) vs. ESI (which produces mostly multiple charged peptides for fragmentation). Based on the results from this study, it may be worthwhile to use both approaches to significantly increase the number of identified and quantified protein biomarkers in biological applications of proteomics techniques. The 128 differentially expressed proteins (58 up-regulated and 70 down-regulate proteins) and their associated biological mechanisms, such as immune modulation and inflammation, are completely analogous to mouse transcriptome findings at early and intermediate CAD disease stages [4] and suggest that the differential proteomic approaches used here are a feasible and productive approach to biomarker discovery for CAD. The identity of the biological functions associated with the differentially expressed proteins in CAD, as described above, may assist in defining both novel biomarkers as well as novel mechanism contributing to CAD.
Our study demonstrated that there was a panel of plasma proteins instead of a single protein associated with the CAD pathogenesis. Validation of this protein pattern as the “signature” of CAD is of particular interest. However, the effectiveness of conventional biomarker validation methods such as Western blotting depends on the availability and quality of antibodies and then the cost and time involvement for developing immunoassays for each new target will be substantial[33]. The iTRAQ approach described above was particularly designed for target discovery, For the purpose of high throughput validation a mTRAQ methodology is recently developed, which relies on multiple reaction monitoring (MRM) to analyze tryptic peptides from the proteins of interest. We therefore propose to use mTRAQ reagent, to quantify the list of target proteins identified in the discovery phase across all samples. The protein panel will be validated first with plasma samples from individual APOE −/− mice and the correspodning controls, and then be tested with individual plasma samples from CAD patients.
Acknowledgments
This work was partially supported by NIH 1R01AI064806, a gift to the UNC-Duke Proteomics Center from an anonymous donor in honor of Michael Hooker, and a collaboration grant from Duke Institute for Genome Sciences and policy.
References
- 1.You SA, Archacki SR, Angheloiu G, Moravee CS, et al. Proteomics approach to coronary artherosclerosis shows ferritin light chain as a significant marker: evidence consistent wit iron hypothesis in atherosclerosis. Physiol Genomics. 2003;13:25–30. doi: 10.1152/physiolgenomics.00124.2002. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, Furie K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Fazzini PF, Prati PL, Rovelli F, Antoniucci D, et al. Epidemiology of silent-myocardial-ischemia in asymptomatic middle-aged men. American Journal of Cardiology. 1993;72:1383–1388. doi: 10.1016/0002-9149(93)90184-e. [DOI] [PubMed] [Google Scholar]
- 4.Karra R, Vemullapalli S, Dong C, Herderick EE, et al. Molecular evidence for arterial repair in atherosclerosis. Proceedings National Academy of Sciences. 2005;102:16789–16794. doi: 10.1073/pnas.0507718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein-E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 6.Daugherty A. Mouse models of atherosclerosis. The American Journal of the Medical Sciences. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima Y, Plump AS, Raine EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of athersclerosis throughout the arterial tree. Arteriosclerosis and Thrombosis. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, et al. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 9.Plump AS, Smitt JD, Hayek T, Aalto-Setälä K, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 10.Srinivas PR, Verma M, Zhao Y, Srivastava S. Proteomics for cancer biomarker discovery. Clinical Chemistry. 2002;48:1160–1169. [PubMed] [Google Scholar]
- 11.Gygi SP, Corthals GL, Zhang Y, Rochon Y, Aebersold R. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proceedings National Academy of Sciences. 2000;97:9390–9395. doi: 10.1073/pnas.160270797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung KE, Kho AT, Sarracino D, Richard LG, et al. Mass spectrometry-based study of the plasma proteome in a mouse intestinal tumor model. Journal of Proteome Research. 2006;5:1866–1878. doi: 10.1021/pr060120r. [DOI] [PubMed] [Google Scholar]
- 13.Ross P, Huang Y, Marchese J, Williamson B, et al. Multiplexed protein quantitation in saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molecular and Cellular Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Qian J, Borisov O, Pan S, et al. Optimized proteomic analysis of a mouse model of cerebellar dysfunction using amine-specific isobaric tags. Proteomics. 2006;6:4321–4334. doi: 10.1002/pmic.200600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AB Applied Biosystems. 2005. Applied BIosystems iTRAQ Reagents Application Kit - Plasma, Amine-Modifying Labeling Reagents for Plasma Sample Applications. [Google Scholar]
- 16.Griffin TJ, Xie H, Bandhakavi S, Popko J, et al. iTRAQ reagent-based quantitative proteomic analysis on a linear ion trap mass spectrometer. Journal of Proteome Research. 2007;6:4200–4209. doi: 10.1021/pr070291b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rong JX, Li J, Reis ED, et al. Elevating high-density lipoprotein cholesterol in apoliprotein E-efficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 18.Major AS, Dove DE, Ishiguro H, SU yR, et al. Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI((−/−)) mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:1790–1795. doi: 10.1161/hq1101.097798. [DOI] [PubMed] [Google Scholar]
- 19.Miller NE. In: Atherosclerosis: Mechanism and Approaches to Therapy. Miller NE, editor. Raven Press; New York, NY: 1983. [Google Scholar]
- 20.Genest JJ, Bard J, Fruchart J, Ordovas J, Schaefer E. Familial hypoalphalipoproteinemia in premature caronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 1993;13:1728–1737. doi: 10.1161/01.atv.13.12.1728. [DOI] [PubMed] [Google Scholar]
- 21.Rubin EM, Ishida BY, Clift SM, Krauss RM. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and appearance of two new high density lipoprotein size subclasses. Proceedings National Academy of Sciences. 1991;88:434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin EM, Krauss RM, Spangler EA, GVJ, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 23.Paszty C, Maeda N, Verstuyt J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atheroslcerosis in mice. J CLin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: A meta analysis and review of the literature. Annuals of Internal Medicine. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 25.McCarty JJ, Parker A, Salem R, et al. Large scale association analysis for identification of genes underlying premature coronary heart disease cumulative perspective from analysis of 111 candidate genes. Journal of Medical Genetics. 2004;41:334–341. doi: 10.1136/jmg.2003.016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenina OI, Desai SY, Krukovets I, et al. Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation. 2003;108:1514–1519. doi: 10.1161/01.CIR.0000089085.76320.4E. [DOI] [PubMed] [Google Scholar]
- 27.Donahue MP, Rose K, Hochstrasser D, Vonderscher J, et al. Discovery of Proteins Related to Coronary Artery Disease Usig Industrial-Scale Proteomics Analysis of Pooled Plasma. American Heart Journal. 2006;152:478–485. doi: 10.1016/j.ahj.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Dennis GJ, Sherman BT, Hosack DA, Yang J, et al. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biology. 2003;4:3. [PubMed] [Google Scholar]
- 30.Jensen LJ, Kuhn M, Stark M, Chaffron S, et al. Nucleic Acids Research. 2009;37:D412–416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wingrove JA, Daniels SE, Sehnert AJ, Tingley W, et al. Correlation of peripheral-blood gene expression with the extent of caronary artery stenosis. Circulation: Cardiovascular Genetics. 2009;1:31–38. doi: 10.1161/CIRCGENETICS.108.782730. [DOI] [PubMed] [Google Scholar]
- 32.Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:661–670. doi: 10.1161/01.ATV.0000255580.73689.8e. [DOI] [PubMed] [Google Scholar]
- 33.Anderson L. Candidate-based proteomics in the search for biomarkers of cardiovascular disease. The Journal of Physiology. 2005;563:23–60. doi: 10.1113/jphysiol.2004.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]