Abstract
Hibernating myocardium is an important clinical syndrome protecting the heart with chronic myocardial ischemia, named for its assumed resemblance to hibernating mammals in winter. However, the effects of myocardial ischemic protection have never been studied in true mammalian hibernation, which is a unique strategy for surviving extreme winter environmental stress. The goal of this investigation was to test the hypothesis that ischemic stress may also be protected in woodchucks as they hibernate in winter. Myocardial infarction was induced by coronary occlusion followed by reperfusion in naturally hibernating woodchucks in winter with and without hibernation and in summer, when not hibernating. The ischemic area at risk was similar among groups. Myocardial infarction was significantly less in woodchucks in winter, whether hibernating or not, compared with summer, and was similar to that resulting after ischemic preconditioning. Whereas several genes were up or downregulated in both hibernating woodchuck and with ischemic preconditioning, one mechanism was unique to hibernation, i.e., activation of cAMP-response element binding protein (CREB). When CREB was upregulated in summer, it induced protection similar to that observed in the woodchuck heart in winter. The cardioprotection in hibernation was also mediated by endothelial nitric oxide synthase, rather than inducible nitric oxide synthase. Thus, the hibernating woodchuck heart is a novel model to study cardioprotection for two major reasons: (1) powerful cardioprotection occurs naturally in winter months in the absence of any preconditioning stimuli, and (2) it resembles ischemic preconditioning, but with novel mechanisms, making this model potentially useful for clinical translation.
Keywords: Hibernating myocardium, Cardioprotection, Ischemic preconditioning, Nitric oxide synthase, cAMP-response element binding protein
Introduction
Clinical hibernating myocardium is a syndrome in patients with chronically ischemic myocardium, where myocardial function is severely and chronically depressed resembling infarcted myocardium, but where the tissue remains viable by “hibernating”, i.e., not contracting, until blood flow is restored [17, 18, 41]. The clinical syndrome of hibernating myocardium was coined to reflect the state in mammalian hibernation, where bodily functions are down regulated in winter, in order allow the animals to survive prolonged periods of high energy demand (cold exposure) and reduced energy (food) availability [3]. In actuality, the cardiac picture of clinical hibernation may or may not resemble true hibernating hearts, since these data were not available at the time. The term was more likely selected to give this clinical syndrome an easily remembered name based on a well recognized animal model, potentially how atrial flutter was named, an arrhythmia resembling the flutter of butterfly wings. The concept in patients was that the reduced regional myocardial function reduced myocardial oxygen demand and consequently the need for myocardial blood flow, which could not increase due to coronary artery disease, thereby eliciting a form of cardioprotection. Again, this concept was developed in the absence of data on myocardial blood flow and function and cardioprotection in true mammalian hibernation. It is important to point out that the reduced myocardial blood flow in patients with hibernating myocardium is radically different from that in true mammalian hibernation, where coronary blood flow is maintained, despite marked decreases in heart rate and contractility [23].
The goal of this investigation was to study for the first time whether the heart during true mammalian hibernation is cardioprotected. If so, this could be a novel model for understanding basic and clinically relevant mechanisms relating to myocardial ischemia. We tested this hypothesis in the North American woodchuck (Marmota monax), a true mammalian hibernator, by comparing responses to identical periods of myocardial ischemia in summer and in winter. Since in hibernating woodchucks in winter, heart rate and other determinants of myocardial oxygen consumption (MVO2) are reduced, which by itself, would protect ischemic myocardium, we also studied animals at room temperature, thereby ruling out the confounding effects of hypothermia, severe bradycardia, and reduced myocardial contractility.
Methods
Animal models
This investigation conforms to the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and the Institutional Animal Care and Use Committee at Rutgers-New Jersey Medical School.
The woodchucks were obtained from Northeastern Wildlife, Idaho. The woodchucks studied were usually caught during spring and maintained through summer and winter. Terminal, open-chest experiments were conducted in anesthetized adult woodchucks (1–2 years old) in summer (June–July) weighing 4.3 ± 0.4 kg and in winter (December–January) weighing 3.4 ± 0.4 kg. Anesthesia was induced with ketamine/xylazine (35/5 mg/kg, intramuscular injection), and an intravenous catheter and endotracheal tube were placed. Anesthesia was maintained with a continuous rate infusion of 3 % pentobarbital, to avoid the preconditioning effects of inhalant anesthetics [1], and the animals were ventilated with room air via a VMC Matrix Spartan mechanical ventilator (tidal volume of 10–20 ml/kg). Woodchucks were placed on heating pads during the surgery, intravenous crystalloids were administered for maintenance fluid support, and rectal temperature was continuously monitored. Using sterile surgical technique, a left thoracotomy was performed at the 5th intercostal space. Tygon catheters (Norton Plastics, Akron, OH) were implanted in the descending aorta and in the left atrium for measurement of pressures and radioactive microsphere injection. A solid-state miniature pressure gauge was implanted in the left ventricular (LV) cavity to obtain LV pressure and its first derivative, LV dP/dt, as previously described [21–23]. The regional myocardial blood flow was measured using the radioactive microsphere technique [21–23].
Experimental group protocols
Five groups of woodchucks were used: summer (n = 5), summer with first window preconditioning consisting of two episodes of coronary artery occlusion (CAO) for 10 min followed by 10 min of coronary artery reperfusion (CAR) (n = 5), winter at room temperature (n = 4), winter in the hibernaculum prior to operation (n = 8), winter with first window preconditioning (n = 5), and winter with N-nitro-L-arginine (L-NA) (n = 3). For the group involving winter in the hibernaculum, the woodchucks were placed in a dark, quiet hibernaculum maintained at a constant temperature of 10 °C (50 °F) for 7–10 days [23]. While in the hibernaculum, deep bedding (Aspen woodchips) and fresh water were continuously available, and the woodchucks were carefully checked every other day. In all animals, while under anesthesia prior to initiating an ischemia–reperfusion protocol, the core body temperature was elevated to approximately 34 °C (93.2 °F) using warm sterile saline lavage, and a heating pad and lamp.
The ischemia/reperfusion protocol involved 60 min left anterior descending CAO followed by 2 h CAR. For winter + L-NA group, the woodchucks were removed from the hibernaculum, a systemic infusion (intravenous) of L-NA 35 mg/kg was given at room temperature for 10 min before 60 min of CAO to inhibit nitric oxide synthase (NOS).
In order to look at just the effects of ischemic preconditioning on cellular mechanisms, without the complicating influences of myocardial infarction, tissue samples from the heart of woodchucks in summer with ischemic preconditioning were collected from a separate group of animals after 2 episodes of CAO for 10 min followed by 10 min of CAR, but were not subjected to 60 min of lethal ischemia. All samples were taken from both the ischemic and remote zone and immediately frozen in liquid nitrogen.
Measurement of cardiac function
Measurements of global and regional myocardial function were recorded with a multiple-channel oscillograph and a computer-based data acquisition system (Notocord, France). Aortic and left atrial pressures were measured with strain manometers that were calibrated with a mercury manometer connected to the fluid-filled catheters. The solid-state LV pressure gauge was cross-calibrated with aortic and left atrial pressure measurements. LV dP/dt was obtained by electronically differentiating the LV pressure signal (Triton Technology Inc., San Diego, CA).
Measurement of infarct size
At the end of the experiments, the animals were anesthetized with sodium pentobarbital (120 mg/kg, iv to effect), and heparin (400 USP units/kg) was administered. The heart was excised and placed on a perfusion apparatus. The left anterior descending coronary artery was ligated at the level of the occluder. The ascending aorta was cannulated (distal to the sinus of Valsalva) and perfused retrogradely with Alcian blue dye. The driving pressure was maintained at approximately 120–140 mmHg. Full cross-sectional myocardial samples were then incubated in 1 % triphenyl tetrazolium chloride (TTC), which stains normal myocardium a deep red color and leaves infarcted myocardium unstained, and maintained at 37 °C for 10–12 min. Following TTC staining, the slices were photographed and the normal zone, ischemic zone, and infarcted area were analyzed using ImageJ software. The infarct size (INF) was expressed as a percent of the area at risk (AAR), while the AAR was expressed as a percent of the left ventricle and septum.
Intramyocardial injection of CREB adenovirus
Adenovirus vector harboring CREB (Ad-CREB) was generated using AdMax (Microbix). After titrating using Adeno-X-Rapid Titer Kit (Clontech), ~1011 PFU/ml of Ad-CREB was obtained. A total of 5 × 1010 PFU of Ad-CREB or adenovirus harboring β-Galactosidase. (LacZ) (Ad-LacZ) which was used as control was delivered via direct myocardial injection using a 31G needle attached to an insulin syringe at six sites within the estimated risk zone of the left ventricle from woodchucks in summer. Following adenovirus administration, the chest was closed and the animals recovered for 96 h. At that time, the animals underwent 60 min of CAO and 2 h of CAR followed by TTC staining, and infarct size was examined.
Immunoblotting
The samples for Western blotting were taken from the left ventricle in summer and winter woodchucks. For experiments with ischemic preconditioning in summer woodchucks, the samples were taken from the zone of the left ventricle where ischemia was induced. Protein extracts were prepared from the left ventricle of hearts with extraction buffer (20 mmol/L Tris–HCl [pH 7.4], 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 % Triton, 1 mmol/L sodium orthovanadate, 2.5 mmol/L sodium pyrophosphate, 1 mmol/ L β-glycerophosphate, and 5 μg/mL protease inhibitor cocktail). Protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA). Proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected with specific antibodies. The blots were incubated with horseradish peroxidase-labeled secondary antibodies. Immunoreactive bands were detected with chemiluminescence (ECL, PerkinElmer Life Sciences, Waltham, MA). The intensities of the resulting bands were quantified by Quantity One software on a GS-800 densitometer (Bio-Rad). Primary antibodies were used according to the manufacturer’s instructions. Western blotting for GAPDH was used to verify equal protein loading of the blots.
NOS activity
NOS activity was measured with the Ultrasensitive Colorimetric NOS assay kit (Oxford Biomedical Research, Rochester Hills, MI).
Statistical analysis
Results are shown as the mean ± SEM for the number of samples indicated in figure legends. Analysis of variance (ANOVA) test was used for analysis of >2 groups. A value of p <0.05 was considered significant.
Results
Hemodynamics in woodchucks in winter and summer
Table 1 shows the hemodynamic values during baseline, CAO, and 2 h CAR. Body temperature, LV systolic pressure, mean aortic pressure, heart rate, LV dP/dt max, LV dP/dt min, and the major determinant of myocardial oxygen consumption, triple product (heart rate × LV systolic pressure × myocardial contractility (LV dP/dt max) were similar in all groups throughout the entire duration of the protocol. These groups all had similar body temperature. During hibernation, without warming, our prior paper indicated that, compared with data in Table 1, marked reductions in body temperature (56 ± 1 °F), heart rate (27 ± 8 bpm), mean arterial pressure (47 ± 6 mmHg), and LV dP/dt (689 ± 129 mmHg/min) were observed, but coronary blood flow was maintained [23].
Table 1.
Temperature, hemodynamics, and myocardial function at baseline, during 60 min coronary occlusion (CAO) and 2 h reperfusion (CAR) in summer (n = 5), summer ischemic preconditioning (IPC, n = 5), in winter (n = 5), and winter IPC (n = 5) woodchucks
| Baseline | CAO | CAR | |
|---|---|---|---|
| Body temperature (°F) | |||
| Summer | 93.5 ± 0.3 | 93.9 ± 0.3 | 94.7 ± 0.2 |
| Summer IPC | 93.9 ± 0.3 | 94.1 ± 0.6 | 94.0 ± 0.4 |
| Winter | 92.6 ± 0.5 | 93.5 ± 0.8 | 93.1 ± 0.8 |
| Winter IPC | 93.6 ± 0.5 | 93.7 ± 0.3 | 93.0 ± 0.5 |
| LV end-diastolic pressure (mmHg) | |||
| Summer | 4.2 ± 1.2 | 8.2 ± 1.3† | 8.4 ± 1.1† |
| Summer IPC | 4.0 ± 0.6 | 10.7 ± 0.7† | 9.8 ± 1.3† |
| Winter | 6.8 ± 1.1 | 12.7 ± 1.6† | 8.8 ± 0.5 |
| Winter IPC | 5.2 ± 1.0 | 11.5 ± 1.1† | 8.6 ± 0.7 |
| LV systolic pressure (mmHg) | |||
| Summer | 91.6 ± 8.7 | 79.6 ± 4.5 | 78.8 ± 6.5† |
| Summer IPC | 92.4 ± 7.9 | 83.6 ± 4.1 | 88.4 ± 7.2 |
| Winter | 97.6 ± 13.7 | 88.8 ± 8.2 | 87.2 ± 6.7 |
| Winter IPC | 92.8 ± 6.6 | 83.6 ± 7.0 | 87.2 ± 6.7 |
| Mean aortic pressure (mmHg) | |||
| Summer | 86.4 ± 8.0 | 76.0 ± 4.6 | 75.4 ± 6.3 |
| Summer IPC | 88.6 ± 7.9 | 80.6 ± 3.7 | 84.4 ± 6.6 |
| Winter | 91.8 ± 13.4 | 84.9 ± 7.8 | 84.4 ± 6.5 |
| Winter IPC | 88.8 ± 6.6 | 80.5 ± 6.7 | 80.0 ± 9.5 |
| Heart rate (beats/min) | |||
| Summer | 157.8 ± 10.8 | 152.6 ± 9.2 | 142.2 ± 3.9 |
| Summer IPC | 144.0 ± 11.0 | 150.0 ± 4.1 | 129.4 ± 12.0 |
| Winter | 143.4 ± 7.2 | 137.9 ± 1.1 | 112.0 ± 13.4† |
| Winter IPC | 151.0 ± 15.2 | 143.4 ± 6.3 | 123.6 ± 9.7 |
| LV dP/dt max (mmHg/s) | |||
| Summer | 2,200 ± 123 | 2,140 ± 27 | 1,980 ± 175† |
| Summer IPC | 2,380 ± 188 | 2,290 ± 123 | 2,160 ± 104 |
| Winter | 2,360 ± 268 | 2,220 ± 258 | 2,380 ± 325 |
| Winter IPC | 2,230 ± 167 | 2,100 ± 160† | 2,130 ± 164 |
| LV dP/dt min (mmHg/s) | |||
| Summer | 2,360 ± 259 | 2,110 ± 72 | 1,780 ± 219 |
| Summer IPC | 2,440 ± 192 | 2,200 ± 136 | 2,120 ± 129 |
| Winter | 2,300 ± 296 | 2,130 ± 286 | 2,120 ± 249 |
| Winter IPC | 2,070 ± 218 | 1,925 ± 145 | 1,990 ± 156 |
| Triple product (beats mmHg2/min s; ×106) | |||
| Summer | 32.1 ± 4.3 | 25.9 ± 1.7 | 22.5 ± 3.6† |
| Summer IPC | 32.8 ± 7.3 | 28.7 ± 2.8 | 24.6 ± 3.0 |
| Winter | 35.9 ± 10.9 | 28.0 ± 4.9 | 25.2 ± 6.9 |
| Winter IPC | 30.7 ± 3.1 | 24.7 ± 1.4 | 23.1 ± 4.2 |
p < 0.05 different from baseline using ANOVA and student’s t test
Woodchucks do not demonstrate increased collateral blood flow during CAO
Preexisting collateral blood vessels, as in dogs, are a major cause for variability in infarct size measurements. Woodchucks do not have preformed collaterals. Note that blood flow measured with microspheres in woodchucks during CAO both in summer and winter falls by over 90 % both in the subendocardium and subepicardium (compared to blood flow in the non-ischemic zone), indicating the absence of collateral flow (Table 2).
Table 2.
Transmural myocardial blood flows (ml/min/g) in ischemic and non-ischemic zones after 60 min CAO for summer (n = 3), summer ischemic preconditioning (IPC, n = 3), winter (n = 3), and winter ischemic preconditioning (n = 3) woodchucks
| Summer | Summer IPC | Winter | Winter IPC | |
|---|---|---|---|---|
| Ischemic zone | ||||
| Endo | 0.047 ± 0.007 | 0.040 ± 0.022 | 0.034 ± 0.025 | 0.027 ± 0.006 |
| Epi | 0.044 ± 0.009 | 0.030 ± 0.008 | 0.033 ± 0.027 | 0.029 ± 0.007 |
| Trans | 0.045 ± 0.008 | 0.035 ± 0.015 | 0.034 ± 0.026 | 0.028 ± 0.006 |
| Non-ischemic zone | ||||
| Endo | 1.033 ± 0.184 | 1.152 ± 0.173 | 1.176 ± 0.349 | 1.204 ± 0.185 |
| Epi | 0.874 ± 0.197 | 1.020 ± 0.141 | 1.006 ± 0.158 | 1.154 ± 0.099 |
| Trans | 0.953 ± 0.189 | 1.086 ± 0.152 | 1.091 ± 0.247 | 1.179 ± 0.142 |
The woodchuck heart in winter exhibits intrinsic cardioprotection
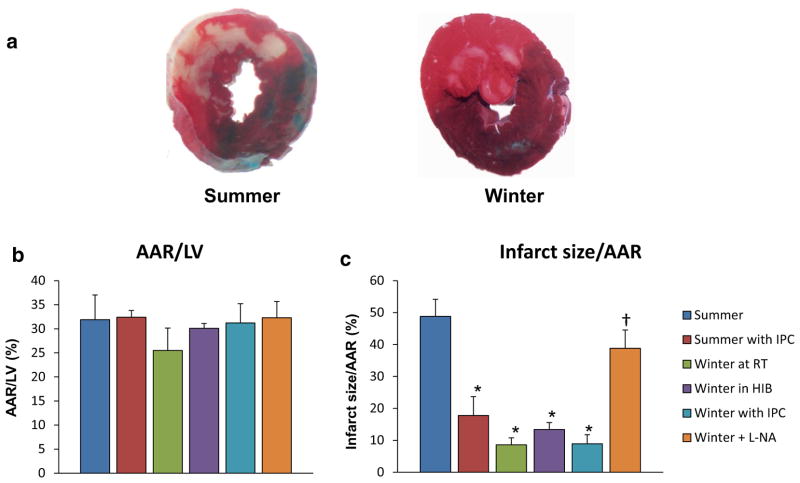
The extent of myocardial ischemia induced by 60 min left anterior descending CAO, as reflected by the area at risk for infarction following CAO was similar among the summer groups (32 ± 5.1 %), summer with first window preconditioning (32 ± 1.4 %), winter at room temperature (28 ± 1.6 %), winter in the hibernaculum prior to operation (33 ± 3.9 %), and winter with first window preconditioning (31 ± 4.0 %) (Fig. 1a), but the resultant myocardial infarction, expressed as a fraction of the area at risk, was markedly smaller in both winter at room temperature (8.6 ± 2.2 %) and winter in the hibernaculum prior to operation (13 ± 2.2 %) compared with summer (49 ± 5.4 %), at least as powerful, or even more so, than summer with ischemic preconditioning (19 ± 5.9 %). The woodchucks in winter did not show significant further protection by first window preconditioning (8.9 ± 2.9 %) (Fig. 1b). These data indicate that woodchucks exhibit powerful myocardial ischemic protection during winter compared to woodchucks in summer, similar to that elicited by ischemic preconditioning.
Fig. 1.
a Representative cross-sectional slices following the control protocol, 0.1 % Alcian blue dye perfusion and TTC staining in summer (left) and winter (right). The winter animals displayed significantly less infarction when compared with their summer cohorts. b Area at risk (AAR), expressed as a percent of the left ventricle and septum (LV), was similar in all groups. c Infarct size (INF), expressed as a percent of the AAR, was significantly decreased in the winter animals at room temperature (n = 4), winter in the hibernaculum prior to operation (n = 8), and winter with first window preconditioning (n = 5) when compared to summer animals (n = 5). With addition of L-NA, the cardioprotection was lost in the winter animals (n = 3). Furthermore, infarct size was reduced following ischemic preconditioning in the summer (n = 5) when compared to summer animals (n = 5). *p <0.05 vs. summer. †p <0.05 vs. each winter group. Values are presented as mean ± SEM
Endothelial nitric oxide synthase (eNOS) is involved in ischemic protection in woodchucks in winter
The extent of myocardial protection conferred in winter in these animals is at least as powerful, or even more so, than ischemic preconditioning, the most potent mechanism of cardioprotection studied to date. Accordingly, we examined a major mechanism mediating second window preconditioning, occurring 1–2 days after a myocardial ischemic challenge, i.e., inducible nitric oxide synthase (iNOS). We did not find upregulation of iNOS, a key mechanism mediating the second window [8], but did demonstrate increased NOS activity and levels of eNOS and phosphorylation at Ser1177 of eNOS in the woodchuck heart in winter compared with summer (Fig. 2), and importantly, L-NA, which blocks NO activity, eliminated the cardioprotection in the winter woodchuck heart (Fig. 1b), indicating that eNOS mediates the protection developed by the woodchuck heart in winter. Although the numbers of animals was low, the statistical difference between the group with L-NA, where cardioprotection was blocked, and the other three winter groups where cardioprotection was observed, was highly significant, p <0.00002.
Fig. 2.
a Upregulated NOS activity was found in woodchuck myocardium in winter, *p <0.05. b Western blot analysis of iNOS. There is no change in the level of iNOS in woodchucks in winter vs. summer. c–d Western blot analysis of eNOS and its phosphorylated form (Ser1177). Both are significantly elevated in woodchucks in winter (n = 5). *p <0.05 vs. summer (n = 5). GAPDH was used as a loading control. Values are presented as mean ± SEM
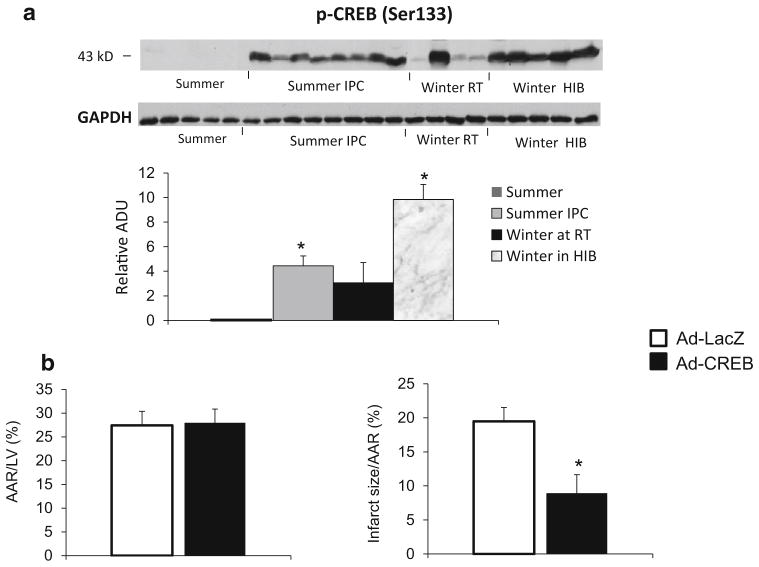
Activation of CREB, a mechanism mediating ischemic protection in woodchucks in winter
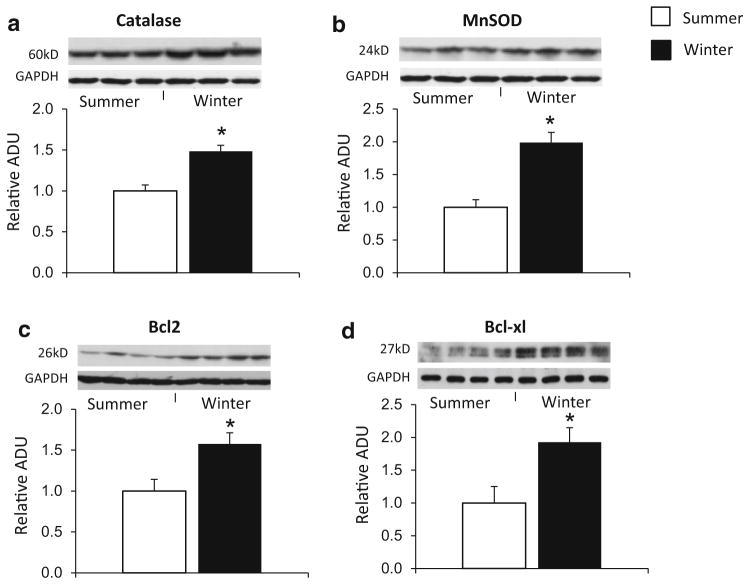
CREB was activated, as reflected by a dramatic increase in the levels of its phosphorylated form (Ser133), in woodchuck myocardium in the winter at room temperature and even higher in hibernating woodchucks compared to that in the summer, but also activated in woodchuck myocardium in summer following first window of ischemic preconditioning (Fig. 4a). Activation of CREB activity via adenovirus-mediated overexpression of CREB in woodchuck myocardium in the summer significantly reduced the infarct size (Fig. 4b), resulting in improved cardioprotection in woodchucks in summer. These results support the concept that CREB is involved in myocardial ischemic protection in winter woodchucks. Furthermore, anti-apoptotic factors B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xl) are also elevated in the hearts of woodchucks in winter (Fig. 3c, d), suggesting that the cardioprotection generated by woodchucks in winter is associated with the reduction of apoptosis by promoting Bcl-2 and Bcl-xl.
Fig. 4.
a Western blot analysis for p-CREB (Ser133) in woodchuck myocardium. Compared to levels in summer without ischemic preconditioning, p-CREB was increased with ischemic preconditioning in summer and more in winter with and without hibernating. GAPDH was used as a loading control. *p <0.05 vs. summer without ischemic preconditioning (IPC). b The infarct size was smaller after CREB virus injection woodchucks in summer, *p <0.05, compared with control LacZ virus. Values are presented as mean ± SEM. These results suggest that the activation of CREB is a novel mechanism involved in the cardioprotection in woodchucks in winter and the cardioprotection induced by IPC in woodchucks in summer
Fig. 3.
Western blot analysis for catalase (a), MnSOD (b), Bcl2 (c), and Bcl-xl (d) in woodchuck myocardium. The levels of these proteins are all elevated in woodchucks in winter. *p <0.05 vs. summer. GAPDH was used as a loading control
Involvement of mechanisms mediating traditional ischemic preconditioning
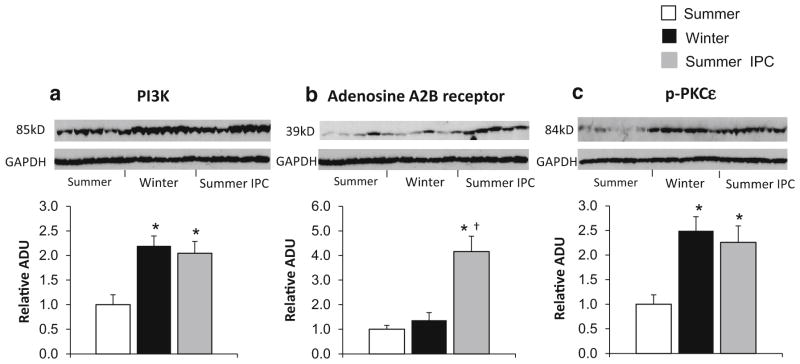
Except for iNOS and eNOS, several other key proteins mediating the protection of traditional ischemic preconditioning were examined, including Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), adenosine A2B receptor, and Protein kinase C-epsilon (PKCε) [10, 30, 44], known mediators of first window of ischemic preconditioning which regulates cell survival, were significantly upregulated in both summer first window of ischemic preconditioning group and winter group (Fig. 5a), indicating that the animals in winter displayed an inherent change in the myocardium independent of an ischemic stimulus. In addition, PKCε phosphorylation, another known mediator of classical preconditioning, was significantly increased in both winter and the summer first window of ischemic preconditioning group (Fig. 5c). Resistance to oxidative stress is involved in ischemic preconditioning and was also observed in the hearts of woodchucks in winter, as reflected by increased levels of antioxidants, catalase (Fig. 3a) and magnesium superoxide dismutase (MnSOD) (Fig. 3b).
Fig. 5.
Western blot analysis of traditional IPC regulators, PI3K, adenosine A2B receptor and PKCε. a PI3K was significantly upregulated in woodchucks in both winter and summer with IPC, *p <0.05 vs. summer. n = 5 per group. b Adenosine A2B receptor was only increased in woodchucks in summer with IPC, *p <0.05 vs. summer and †p <0.05 vs. winter, but did not change in woodchucks in winter or summer without IPC (n = 5 per group). c PKCε phosphorylation was significantly increased in both the summer IPC and winter control animals when compared to the summer control, p <0.05, n = 5 per group. Values are presented as mean ± SEM
However, there are also key first window ischemic preconditioning mechanisms, such as adenosine A2B receptors, which were upregulated in the summer woodchucks after ischemic preconditioning, but were not upregulated in woodchucks in winter (Fig. 5b), indicating that the protective mechanisms regulating woodchucks in winter share some but not all mechanisms mediating traditional ischemic preconditioning.
Discussion
The current investigation demonstrated that naturally hibernating mammals exhibit powerful myocardial protection in the winter against ischemia/reperfusion injury, but do not exhibit this cardioprotection in summer, when not hibernating. What triggers the cardioprotection in winter is not known, but is likely related to what is thought to trigger other components of adaptation to winter stress in naturally hibernating mammals, e.g., dynamic maintenance of conduction and repolarization patterns through improved gap junction function [12]; maintenance of myocardial blood flow involved in NO-dependent vasodilation [23]; maintenance of Na+/K+ ion homeostasis [20], improved calcium handling [46]; and enhanced ATP preservation [14]. It is clear, however, that the trigger for natural hibernation does not involve myocardial ischemia, juxtaposing this model against the clinical model and models of hibernating myocardium in pigs [9]. The cardioprotection is at least as powerful as that induced by ischemic preconditioning, which to date has been thought to be the most potent mediator of cardioprotection [4, 24, 27, 31, 47]. In contrast to ischemic preconditioning, the protection in the woodchuck heart is not induced by myocardial ischemia and also unlike ischemic preconditioning is not transient, but rather appears to be present for months, as we have found ischemic protection throughout the winter months, from December until early February, coincident with Groundhog Day.
When mammals hibernate naturally, they do so in a cold environment and their body temperature falls dramatically [37], and our prior data, summarized in the Results, show marked decreases in heart rate and other determinants of MVO2, which would lead to cardioprotection. Since hypothermia is also known to induce cardioprotection [16, 36, 45], it was important to rule out this possibility, which we did by studying woodchucks at normal body temperature, which also normalized hemodynamic factors leading to cardioprotection, i.e., the triple product (Table 1). In fact, both woodchucks winter housed at room temperature and in the cold in the hibernaculum prior to experiments demonstrated similar protection (Fig. 1).
In addition, we eliminated other variables which may have influenced the response to ischemia–reperfusion with this model; specifically, our experiments were performed in vivo, and our anesthetic protocol did not involve the use of opioids [15, 35] or volatile anesthetics [1, 25, 32], each of which are known to protect the heart against ischemia and reduce infarct size. Our results differ from a prior study by McKean et al. [29] which had demonstrated the lack of cardioprotection in the marmot heart in the face of ischemia and hypoxia. However, that prior study was performed ex vivo using a Langendorff model and used only summer animals. It was also important to confirm that differences seen in infarct size in our study were not due to increased collateral blood flow and differences in myocardial tissue blood flow during coronary artery occlusion. Our blood flow data confirmed the lack of flow at 55 min of coronary occlusion and the lack of innate collateral vessels in this model and species (Table 2).
Examination of genes regulated in the woodchuck heart and classical first and second window preconditioning found many commonly regulated genes [2, 10, 30, 44]. However, some key genes regulating first and second window preconditioning, e.g., the adenosine A2B receptor, iNOS and COX2 were not upregulated in woodchucks in winter, indicating that the protective mechanisms regulating woodchucks in winter without ischemic preconditioning are not identical to those mediating traditional ischemic preconditioning. In woodchucks in summer with first window preconditioning, we did observe upregulation of PI3K, PKCε, MnSOD, and eNOS which were also found in woodchucks in winter. The increases in eNOS, which have been reported to be involved in signal transduction in first window ischemic preconditioning [5, 33, 43], and also in the hibernating woodchucks, could also be responsible for the maintenance of myocardial blood flow during hibernation in the woodchuck, which occurs in the face of markedly reduced metabolic demand [23]. Furthermore, by linking the mechanisms between intrinsic cardioprotection from hibernating woodchucks to hypothermia-induced cardioprotection in non-hibernating species [36, 38, 39], the current study would help us to understand the protective mechanisms elicited during hypothermia. Some similarities were observed, e.g., PI3K, which was upregulated in hibernating woodchucks was also found to mediate hypothermic cardioprotection [39], and adenosine receptors were not found to be involved in the cardioprotection in both mild hypothermia [7] and woodchucks in winter.
Another question that arises relates to any molecular relationship between clinical hibernating myocardium induced by myocardial ischemia and natural hibernating myocardium in the woodchuck. As noted above, a critical feature involved in clinical hibernating myocardium, i.e., chronic low coronary flow, is not observed in the hibernating mammal [23]. Having noted that there are some genes related to downregulation of mitochondrial metabolism and even cardioprotection that overlap, but there are others, e.g., stress proteins that are upregulated in clinical models of hibernation [9], and do not change or are decreased in the woodchuck model.
We also searched for a novel mechanism of cardioprotection that is not generally thought to mediate first or second window preconditioning, or known to be involved in clinical hibernating myocardium. Using a proteomic approach, from the network analysis, we found that CREB protein network was activated in the winter woodchuck hibernation [26]. In the present investigation, we found that CREB was activated in the hearts of woodchucks in winter at both room temperature and in the hibernaculum. CREB is a ubiquitously expressed nuclear transcription factor, which is phosphorylated at residue Ser 133 by various kinases in response to extracellular stimuli including growth factors and stress, and plays an important role in cell proliferation, differentiation, adaptation, survival, and apoptosis [19, 28]. Furthermore, when CREB was upregulated via Ad-CREB administration in the summer woodchuck heart, it induced cardioprotection similar to that observed in the woodchuck heart in winter. It has been reported that the effects of CREB on apoptosis are associated with elevation of Bcl-2 [13, 34] and Bcl-xl [11], which are also elevated in the hearts of woodchucks in winter and woodchucks in summer with ischemic preconditioning. CREB is also an important component of the protective actions of brown fat [6, 40, 42]. Since hibernating animals utilize these brown fat mechanisms, it is interesting to speculate that this also is involved in ischemic protection in the woodchuck heart.
In summary, the woodchuck heart in winter exhibits intrinsic protection against myocardial ischemia. This cardioprotection is as powerful as ischemic preconditioning in attenuating the detrimental effects of ischemia–reperfusion; however, the mechanism is different. The cardioprotection observed in hibernating woodchucks does not use one single pathway entirely, but is mediated by eNOS and CREB mechanisms.
Acknowledgments
This study was supported by funding from National Institutes of Health (5R01HL091781, 5R21HL97264, 5R01HL093481, 1R01HL106511, 5P01AG027211, 1R01HL102472, 5R01HL033107, 5T32HL069752, 5R01HL095888, 5P01HL069020).
Footnotes
Conflict of interest: No conflicts of interest.
References
- 1.Bienengraeber MW, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics. Vascul Pharmacol. 2005;42:243–252. doi: 10.1016/j.vph.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.RES.87.11.972. [DOI] [PubMed] [Google Scholar]
- 3.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 4.Carroll R, Yellon DM. Myocardial adaptation to ischaemia—the preconditioning phenomenon. Int J Cardiol. 1999;68(Suppl 1):S93–S101. doi: 10.1016/s0167-5273(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 5.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM, Zhang H, Schulz TJ, Huang TL, Espinoza DO, Kristiansen K, Unterman TG, Tseng YH. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology. 2011;152:3680–3689. doi: 10.1210/en.2011-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbera L, Chenoune M, Lidouren F, Ghaleh B, Cohen MV, Downey JM, Berdeaux A, Tissier R. Adenosine and opioid receptors do not trigger the cardioprotective effect of mild hypothermia. J Cardiovasc Pharmacol Ther. 2012;17:173–180. doi: 10.1177/1074248411412969. [DOI] [PubMed] [Google Scholar]
- 8.Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann N Y Acad Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- 9.Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, Dreyfus GD, Gaussin V, Pennell DJ, Vatner DE, Camici PG, Vatner SF. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res. 2004;95:433–440. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 10.Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y. Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis. 2004;172:201–210. doi: 10.1016/S0021-9150(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 11.Eliseev RA, Vanwinkle B, Rosier RN, Gunter TE. Diazoxide-mediated preconditioning against apoptosis involves activation of cAMP-response element-binding protein (CREB) and NFkappaB. J Biol Chem. 2004;279:46748–46754. doi: 10.1074/jbc.M406217200. [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VV, Glukhov AV, Sudharshan S, Egorov Y, Rosenshtraukh LV, Efimov IR. Electrophysiological mechanisms of antiarrhythmic protection during hypothermia in winter hibernating versus nonhibernating mammals. Heart Rhythm. 2008;5:1587–1596. doi: 10.1016/j.hrthm.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeland K, Boxer LM, Latchman DS. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res Mol Brain Res. 2001;92:98–106. doi: 10.1016/S0169-328X(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 14.Grabek KR, Karimpour-Fard A, Epperson LE, Hindle A, Hunter LE, Martin SL. Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiol Genomics. 2011;43:1263–1275. doi: 10.1152/physiolgenomics.00125.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Hale SL, Kloner RA. Mild hypothermia as a cardioprotective approach for acute myocardial infarction: laboratory to clinical application. J Cardiovasc Pharmacol Ther. 2011;16:131–139. doi: 10.1177/1074248410387280. [DOI] [PubMed] [Google Scholar]
- 17.Heusch G. Hibernating myocardium. Physiol Rev. 1998;78:1055–1085. doi: 10.1152/physrev.1998.78.4.1055. [DOI] [PubMed] [Google Scholar]
- 18.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288:H984–H999. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 19.Ichiki T. Role of cAMP response element binding protein in cardiovascular remodeling: good, bad, or both? Arterioscler Thromb Vasc Biol. 2006;26:449–455. doi: 10.1161/01.ATV.0000196747.79349.d1. [DOI] [PubMed] [Google Scholar]
- 20.Kamm KE, Zatzman ML, Jones AW, South FE. Maintenance of ion concentration gradients in the cold in aorta from rat and ground squirrel. Am J Physiol. 1979;237:C17–C22. doi: 10.1152/ajpcell.1979.237.1.C17. [DOI] [PubMed] [Google Scholar]
- 21.Kudej RK, Ghaleh B, Sato N, Shen YT, Bishop SP, Vatner SF. Ineffective perfusion-contraction matching in conscious, chronically instrumented pigs with an extended period of coronary stenosis. Circ Res. 1998;82:1199–1205. doi: 10.1161/01.RES.82.11.1199. [DOI] [PubMed] [Google Scholar]
- 22.Kudej RK, Kim SJ, Shen YT, Jackson JB, Kudej AB, Yang GP, Bishop SP, Vatner SF. Nitric oxide, an important regulator of perfusion-contraction matching in conscious pigs. Am J Physiol Heart Circ Physiol. 2000;279:H451–H456. doi: 10.1152/ajpheart.2000.279.1.H451. [DOI] [PubMed] [Google Scholar]
- 23.Kudej RK, Vatner SF. Nitric oxide-dependent vasodilation maintains blood flow in true hibernating myocardium. J Mol Cell Cardiol. 2003;35:931–935. doi: 10.1016/S0022-2828(03)00174-3. [DOI] [PubMed] [Google Scholar]
- 24.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–1299. doi: 10.1161/01.RES.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 25.Kwok WM, Aizawa K. Preconditioning of the myocardium by volatile anesthetics. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:249–255. doi: 10.2174/1568016043356318. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Liu T, Chen W, Jain MR, Vatner DE, Vatner SF, Kudej RK, Yan L. Proteomic mechanisms of cardioprotection during mammalian hibernation in woodchucks, Marmota monax. J Proteome Res. 2013;12:4221–4229. doi: 10.1021/pr400580f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.CIR.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 28.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 29.McKean T, Mendenhall W. Comparison of the responses to hypoxia, ischaemia and ischaemic preconditioning in wild marmot and laboratory rabbit hearts. J Exp Biol. 1996;199:693–697. doi: 10.1242/jeb.199.3.693. [DOI] [PubMed] [Google Scholar]
- 30.Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- 31.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 32.Pratt PF, Jr, Wang C, Weihrauch D, Bienengraeber MW, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics: new applications for old drugs? Curr Opin Anaesthesiol. 2006;19:397–403. doi: 10.1097/01.aco.0000236139.31099.b5. [DOI] [PubMed] [Google Scholar]
- 33.Redel A, Stumpner J, Smul TM, Lange M, Jazbutyte V, Ridyard DG, Roewer N, Kehl F. Endothelial nitric oxide synthase mediates the first and inducible nitric oxide synthase mediates the second window of desflurane-induced preconditioning. J Cardiothorac Vasc Anesth. 2013;27:494–501. doi: 10.1053/j.jvca.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 35.Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther. 2001;89:123–137. doi: 10.1016/S0163-7258(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz BG, Kloner RA, Thomas JL, Bui Q, Mayeda GS, Burstein S, Hale SL, Economides C, French WJ. Therapeutic hypothermia for acute myocardial infarction and cardiac arrest. Am J Cardiol. 2012;110:461–466. doi: 10.1016/j.amjcard.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 37.Storey KB, Storey JM. Metabolic rate depression: the biochemistry of mammalian hibernation. Adv Clin Chem. 2010;52:77–108. [PubMed] [Google Scholar]
- 38.Tissier R, Chenoune M, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. The small chill: mild hypothermia for cardioprotection? Cardiovasc Res. 2010;88:406–414. doi: 10.1093/cvr/cvq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tissier R, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. Myocardial protection with mild hypothermia. Cardiovasc Res. 2012;94:217–225. doi: 10.1093/cvr/cvr315. [DOI] [PubMed] [Google Scholar]
- 40.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, Lopez M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. N Engl J Med. 1998;339:173–181. doi: 10.1056/NEJM199807163390307. [DOI] [PubMed] [Google Scholar]
- 42.Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol. 2005;25:8311–8322. doi: 10.1128/MCB.25.18.8311-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C, Talukder MA, Varadharaj S, Velayutham M, Zweier JL. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc Res. 2013;97:33–43. doi: 10.1093/cvr/cvs287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Liu Y, Yang XM, Hu F, Cui L, Swingle MR, Honkanen RE, Soltani P, Tissier R, Cohen MV, Downey JM. Cardioprotection by mild hypothermia during ischemia involves preservation of ERK activity. Basic Res Cardiol. 2011;106:421–430. doi: 10.1007/s00395-011-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yatani A, Kim SJ, Kudej RK, Wang Q, Depre C, Irie K, Kranias EG, Vatner SF, Vatner DE. Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am J Physiol Heart Circ Physiol. 2004;286:H2219–H2228. doi: 10.1152/ajpheart.01096.2003. [DOI] [PubMed] [Google Scholar]
- 47.Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55:438–455. doi: 10.1016/S0008-6363(02)00442-X. [DOI] [PubMed] [Google Scholar]