Abstract
Skeletal muscle injury and repair are complex processes, including well-coordinated steps of degeneration, inflammation, regeneration, and fibrosis. We have reviewed the recent literature including studies by our group that describe how to modulate the processes of skeletal muscle repair and regeneration. Antiinflammatory drugs that target cyclooxy-genase-2 were found to hamper the skeletal muscle repair process. Muscle regeneration phase can be aided by growth factors, including insulin-like growth factor-1 and nerve growth factor, but these factors are typically short-lived, and thus more effective methods of delivery are needed. Skeletal muscle damage caused by traumatic injury or genetic diseases can benefit from cell therapy; however, the majority of transplanted muscle cells (myoblasts) are unable to survive the immune response and hypoxic conditions. Our group has isolated neonatal skeletal muscle derived stem cells (MDSCs) that appear to repair muscle tissue in a more effective manner than myoblasts, most likely due to their better resistance to oxidative stress. Enhancing antioxidant levels of MDSCs led to improved regenerative potential. It is becoming increasingly clear that stem cells tissue repair by direct differentiation and paracrine effects leading to neovascularization of injured site and chemoattraction of host cells. The factors invoked in paracrine action are still under investigation. Our group has found that angiotensin II receptor blocker (losartan) significantly reduces fibrotic tissue formation and improves repair of murine injured muscle. Based on these data, we have conducted a case study on two hamstring injury patients and found that losartan treatment was well tolerated and possibly improved recovery time. We believe this medication holds great promise to optimize muscle repair in humans.
Keywords: muscle injury, muscle derived stem cells, fibrosis, losartan, angiotensin receptor blocker, paracrine effect
INTRODUCTION
Vertebrate skeletal muscle comprises bundles of contractile muscle fibers that are multinucleated cells formed by the fusion of muscle cells (myocytes) into syncytia. The myofibers are surrounded by a membrane (sarcolemma), and each group forms a fascicle. Fascicles are in turn surrounded by connective tissue sheath called the endomysium. Groups of fascicles are enveloped by a perimysium and make up whole muscle. The skeletal muscle also contains connective, vascular, adipose, nerve, and other cell populations.
The myogenic cells in the skeletal muscle are found at a variety of different stages of maturity. The fully differentiated myofibers are surrounded by satellite cells that are within the basal lamina but outside the sarcolemma (Mauro, 1961). These cells express paired box protein 7 (Pax7) and myogenic differentiation (MyoD) surface markers, which indicate partial differentiation down the muscle lineage (Seale et al., 2000). Satellite cells remain quiescent in the muscle until an external stimulus such as an injury occurs, at which point they re-enter the cell cycle and proliferate (Hill et al., 2003). Proliferating satellite cells differentiate into myoblasts, which can then fuse to form new myofibers (Collins and Partridge, 2005). The myoblast population, which is more differentiated than satellite cells, fuse to become mature myofibers, as previously mentioned. These cells have also been used extensively for transplantation.
Distinct from both the satellite cell and the myoblast populations are the muscle derived stem cells (MDSCs). Although MDSCs are similar to satellite cells in playing a role in skeletal muscle regeneration, they are a separate population of cells that express distinct markers and phenotypes (Deasy et al., 2001; Huard et al., 2003). MDSCs are believed to be an earlier progenitor than satellite cells, expressing stem cell markers such as cluster of differentiation 34 (CD34) and stem cell antigen 1 (Sca-1), and they have the ability to differentiate down nonmuscle lineages to contribute to repair (Qu-Petersen et al., 2002). Pax-7 and Sca-1 positive cells have not been colocalized in skeletal muscle, providing further evidence that satellite cells and MDSCs are likely distinct populations (Zammit and Beauchamp, 2001; Fig. 1).
Figure 1.
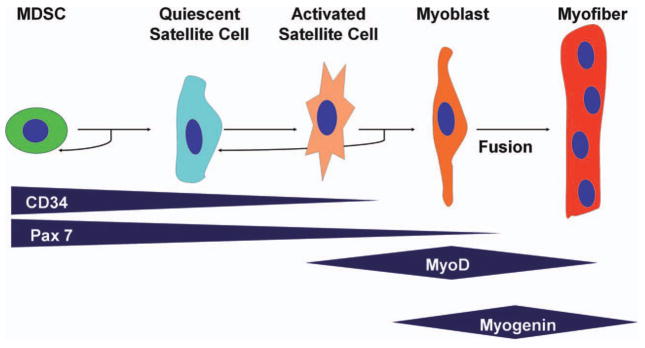
Generalized scheme of myogenic differentiation. Other markers are used by different investigators. (Adapted from Deasy et al., 2001, Blood Cells Mol Dis, 27, 924–933)
In the incidence of a traumatic injury or muscle damage due to a genetic disease (such as Duchenne muscular dystrophy [DMD]), the muscle goes through a complex and dynamic series of events resulting in an inflammatory phase, the activation of progenitor cells, regeneration of muscle tissue, formation of fibrosis, and varying degrees of restoration of function. These phases are detailed below.
Phases of Skeletal Muscle Injury and Repair
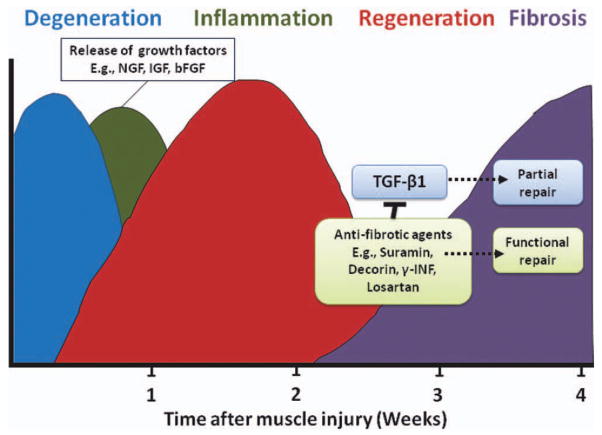
Acute skeletal muscle injuries are common injuries, which account for a large segment of the patients presenting to orthopaedic practitioners (Crisco et al., 1994; Woolf and Pfleger, 2003; Physicians, 2005; Carling et al., 2011). Research has shown that the natural progression of muscle injury proceeds through a highly interdependent sequence of steps, leading to the restoration of tissue architecture and function (Moyer and Wagner, 2011). However, the regenerative capacity of injured skeletal muscle is limited and very often, fibrotic tissue forms, delaying the muscle’s full functional recovery and predisposing it to recurrent injury (Li and Huard, 2002). Clinical findings reveal a high recurrence rate of skeletal muscle strain injuries, approaching 30% among some professional-level athletes (Woods et al., 2004). Numerous investigations have led to the identification of the molecular events and cellular transformations following muscle injury; however, the clinical treatment of this common condition still relies on conventional therapies of rest, ice, and antiinflammatory medications, which have a limited efficacy in preventing or treating the formation of posttraumatic muscle fibrosis (Almekinders, 1993; Jarvinen and Lehto, 1993; Worrell, 1994). Research conducted by our group and others showed that injured muscle undergoes a sequential process of healing phases, including muscle degeneration/inflammation, regeneration, and fibrosis (Huard et al., 2002; Li and Huard, 2002; Li et al., 2004). These phases of muscle healing can be modulated by different biological approaches that will be detailed in the sections later (Fig. 2).
Figure 2.
Healing process in the skeletal muscle. Several overlapping phases are accompanied by the release of growth factors that modulate regeneration and formation of fibrotic tissue. Use of antifibrotic agents minimizes muscle scarring and leads to better functional outcome.
MUSCLE INFLAMMATION
Muscle degeneration and concomitant inflammation begins in the first few days postinjury. Resident macrophages are activated, releasing chemoattractants, leading to the recruitment of neutrophils and monocytes. Subsequently, inflammatory mediators such as tumor necrosis factor alpha (TNFα) are released, and immune, myogenic, and fibroblastic cell interactions are coordinated. This reaction can persist for several days depending on the severity of the injury (Moyer and Wagner, 2011).
Antiinflammatory drugs are often prescribed to relieve pain after muscle injury. However, the effect of these drugs, especially nonsteroidal antiinflammatory drugs (NSAIDs) on the skeletal muscle healing remains controversial. To examine the role NSAIDs play in the process of muscle healing, our group had performed two studies to determine the effect that cyclo-oxygenase-2 (Cox-2) has in modulating muscle recovery (Shen et al., 2005, 2006). In vitro experiments showed that a Cox-2-specific inhibitor (NS-398) slows the proliferation and maturation of differentiated myogenic precursor cells and thus delays the regenerative myogenesis process. Other investigators have found similar results using the Cox-2 selective inhibitor SC-236 (Bondesen et al., 2004, 2006). Our results thus indicate that NS-398 may hamper skeletal muscle healing. We examined the in vivo effect of NS-398 on skeletal muscle healing in a muscle laceration mouse model at different time points up to 4 weeks postinjury. The in vivo data were in agreement with in vitro results and showed delayed muscle regeneration at early time points after injury in mice treated with NS-398. Treating lacerated muscles with NS-398 led to the expression of higher levels of transforming growth factor-β1 (TGF-β1) than the untreated control muscles, and the lacerated muscles treated with NS-398 showed higher fibrosis deposition than the controls. As expected, we found fewer neutrophils and less macrophage infiltration in the muscles treated with NS-398. These results indicate that the inhibitory effect of NS-398 on the inflammatory responses delays skeletal muscle healing after laceration. Furthermore, we analyzed muscle healing following laceration injury on the tibialis anterior (TA) muscles of COX-2−/− mice and control wild type (Shen et al., 2005) by examining the histology and function of TA muscles at 5 and 14 days after injury. COX-2−/− mice TA muscles showed decreased regeneration relative to that observed in wild-type mice. These results demonstrate that the COX-2 pathway plays an important role in muscle healing, and consequently, the decision to use NSAIDs to treat muscle injuries warrants critical examination of evidence available. NSAIDs seem to impair the healing even in the heavily vascularized skeletal muscle tissue and probably affect the recovery of other soft tissues.
MUSCLE REGENERATION
In the first week postinjury, skeletal muscle promptly begins the regeneration process that peaks at 2 weeks and then decreases gradually at 3 to 4 weeks postinjury. Several studies have shown that growth factors play a variety of roles during muscle regeneration (Gospodarowicz et al., 1976; Inselburg and Applebaum, 1978; Linkhart et al., 1981; Florini et al., 1986; Olson et al., 1986; Allen and Boxhorn, 1989; Jennische, 1989; Jin et al., 1990; Yablonka-Reuveni et al., 1990; Anderson et al., 1991; Grounds, 1991; Harrington et al., 1992; Doumit et al., 1993; McFarland et al., 1993; Barnard et al., 1994; Coleman et al., 1995; Johnson and Allen, 1995; Jones and Clemmons, 1995; Lefaucheur and Sebille, 1995; Zdanowicz et al., 1995; Chambers and McDermott, 1996; Engert et al., 1996; Florini et al., 1996; Papadakis et al., 1996; Quinn and Haugk, 1996; Floss et al., 1997; Kurek et al., 1997; Lamberts et al., 1997; Barton-Davis et al., 1998; Damon et al., 1998; Springer et al., 1998; Tatsumi et al., 1998; Keller et al., 1999; Gowdak et al., 2000; Sheehan et al., 2000; De Deyne et al., 2002; Musaro et al., 2004; Wieteska-Skrzeczynska et al., 2011a,b). Using a mouse model, Menetrey et al. (2000) found that direct injections of insulin-like growth factor-1 (IGF-1), basic fibroblastic growth factor (bFGF), and, to a lesser extent, nerve growth factor (NGF), led to enhanced muscle healing in lacerated, contused, and strain-injured muscle at 2, 5, and 7 days after injury. Using human recombinant growth factors to treat muscle injuries has the advantage of feasibility and safety of the injection; however, the efficacy of direct injection of growth factors, in the form of recombinant proteins, is limited by the high concentrations typically required to elicit a measurable effect. Growth factors clearly have a dose-dependent effect on myoblast proliferation and differentiation in vitro. However, a series of three consecutive injections of a relatively high concentration (100 ng for each growth factor) of NGF, IGF-1, or bFGF is usually required to achieve significant enhancement of skeletal muscle healing in mice (Barton-Davis et al., 1998; Menetrey et al., 2000; Fukushima et al., 2001; Li and Huard, 2002; Chan et al., 2003; Foster et al., 2003; Sato et al., 2003; Li et al., 2004; Negishi et al., 2005). Rapid clearance of these molecules in the blood circulation and their relatively short biological half-lives necessitate large amounts to be administered. Further studies will be needed to investigate the potential synergetic effects of the association of two or more growth factors and the potential use of controlled release particles containing the factors.
Another method that can be effective in delivering high, maintainable concentrations of growth factors to injured muscle is gene therapy. Data obtained from previous studies had demonstrated that IGF-1 is a potent growth factor for stimulating muscle regeneration and improving muscle healing in vivo after injury (Barton-Davis et al., 1998; Menetrey et al., 2000; Fukushima et al., 2001; Li and Huard, 2002; Chan et al., 2003; Foster et al., 2003; Sato et al., 2003; Li et al., 2004; Negishi et al., 2005). Based on this information, we genetically engineered an adenoviral vector to encode the gene for IGF-1 and evaluated its ability to improve muscle healing after injury (Lee et al., 2000). Myoblasts ex vivo transduced by IGF-1 adenovirus and then injected into lacerated muscles of immunocompetent mice led to improved muscle repair (Lee et al., 2000). Although we have seen improvement in muscle strength in skeletal muscle treated with myoblasts adenovirally treated to express IGF-1, fibrosis or scar tissue was detected by histology. This suggested that the enhancement of muscle regeneration by either cell or gene therapy improves muscle regeneration but may not completely eliminate fibrosis (Lee et al., 2000). These results suggest that high levels of IGF-1 secretion can be achieved, and delivery is feasible, but the functional recovery of the injured muscle remained incomplete. It is likely that IGF-1 modulates several actions including a stimulatory effect on myofibroblast proliferation, deposition of extracellular matrix (ECM), which may interfere with the ability of this growth factor, even at high concentrations, to improve muscle healing after injury (Jones and Clemmons, 1995; De Deyne et al., 2002). Overall, these results indicate that reducing muscle fibrosis is a complicated process that likely involves several cell populations and growth factors.
Muscle Regeneration after Stem Cell Transplantation
A variety of muscle cell populations have been used for cell transplantation in studies treating patients who are afflicted with DMD, a muscle disease characterized by the lack of dystrophin expression at the sarcolemma of muscle fibers (Hoffman et al., 1987). Transplantation of committed myoblasts into dystrophin-deficient muscle delivers normal myoblasts that fuse with host muscle fibers and/or among themselves and consequently restores dystrophin expression; however, this approach is hindered by numerous limitations, including limited cell spreading, immune responses, and poor survival of the transplanted cells (Karpati et al., 1989; Morgan et al., 1990; Huard et al., 1991, 1994; Mendell et al., 1995; Fan et al., 1996; Gussoni et al., 1997). Although the immune response and the low spreading capacity of the cells have been overcome, at least in part (Kinoshita et al., 1994; Vilquin et al., 1995), the low survival of the transplanted cells is still a major limitation. Indeed, numerous studies report that only a small percentage of the transplanted cells (less than 1–5%) survive, therefore, approaches to increase cell survival postimplantation need to be optimized for myoblast transplantation therapies for DMD (Fan et al., 1996; Beauchamp et al., 1999; Hodgetts et al., 2000).
Efforts to promote donor myoblast survival initially focused on overcoming the inflammatory response (Guerette et al., 1997; Qu et al., 1998; Hodgetts et al., 2000). Myoblasts that are genetically engineered to express an inhibitor of the inflammatory cytokine, IL-1, showed an improved survival rate compared to nonengineered cells (Qu et al., 1998). By treating the host with CD4+/CD8+ to deplete antibodies, donor myoblast survival was enhanced in dystrophic animals (Hodgetts et al., 2000), and by treating the host animals with antibodies against Leukocyte function-associated molecule 1, death of the transplanted myoblasts was reduced (Guerette et al., 1997). This has led some investigators to focus their efforts on the isolation, identification, and characterization of the small subset of donor cells capable of surviving after transplantation (Baroffio et al., 1996; Beauchamp et al., 1999; Collins et al., 2007). Our efforts to isolate such a population are detailed in the following section.
MDSCs
Our group and others have isolated from mouse skeletal muscle a group of cells based on their adhesion characteristics to collagen-coated flasks. We used a modification of a method called the pre-plate technique (Qu-Petersen et al., 2002; Gharaibeh et al., 2008) to purify these cells from postnatal mouse skeletal muscles. In the preplating process, a skeletal muscle biopsy is mechanically broken and then enzymatically digested by a series of enzymes including dispase, collagenase, and trypsin. A single cell suspension is filtered and plated onto a series of collagen-coated flasks. The cells that are slowest to adhere (slowly adhering cells [SACs]) seem to proliferate very slowly at first and have a different morphology from the rapidly adhering cell fraction. After further passaging to remove other cells, including fibroblasts and myoblasts, SAC becomes highly enriched with MDSCs. Technical details of the protocol followed to isolate MDSCs are beyond the scope of this review (Gharaibeh et al., 2008). MDSCs have the potential for long-term proliferation without any significant changes to their cell characteristics, and they maintain a stable karyotype that has no significant numerical or structural abnormalities (Deasy et al., 2005). The end result of the preplate technique results is an enriched population of MDSCs, even though, it is a heterogeneous population. A marker profile for MDSCs is not very high for a single marker as one would obtain from isolation done by flow activated cell sorting (FACS), but typically MDSCs express high levels of Sca-1, as previously mentioned, very low levels of vimentin (a fibroblastic marker), low expression of desmin, and other differentiated muscle markers (Qu-Petersen et al., 2002).
We have shown that SAC repairs skeletal muscle in a more effective manner than myoblasts that tend to more rapidly adhere to collagen-coated flasks. Other investigators have shown that a subpopulation of muscle cells are slowly dividing (as determined by thymidine uptake), but when injected into dystrophic mice muscles, they undergo rapid proliferation and become major contributors to muscle repair (Beauchamp et al., 1999). Similarly, Collins et al. (2007) showed that among aged muscle satellite cells, there is also a subset of cells that survive the effects of aging, and this minority population is responsible for muscle regeneration while the majority of cells progress to apoptosis. Recent studies have used FACS to separate cells based on normally expressed cell-surface markers and showed that skeletal muscle precursor cells (SMPs) show heterogeneity (Cerletti et al., 2008; Biressi and Rando, 2010). The subfraction characterized by markers CD45− Sca-1− Mac-1− CXCR4+ β1-integrin+ showed a high level of muscle cell repair, while non-SMPs (CXCR4−/β1-integrin−) were rarely identified in the muscles after transplantation (Cerletti et al., 2008). One can speculate that these subpopulations secrete different factors and have autocrine and paracrine effects on the host skeletal muscle and other tissues. These signals probably support the survival of these progenitor cells and enhance their participation in skeletal muscle repair. Indeed, we have found in previous studies that the transplantation of MDSCs that were transduced with a retroviral vector to express NGF or directly stimulated with NGF protein, into the skeletal muscle of dystrophic mdx mice, resulted in a significantly larger engraftment with a higher number of dystrophin-positive myofibers than the transplantation of nontransduced MDSCs (Lavasani et al., 2006). Our observations of newly regenerated myofibers by the transplanted MDSCs, particularly the genetically engineered MDSCs, suggest that NGF that was released had an autocrine as well as paracrine effect on neighboring cells.
One important factor that may be involved in cell survival and tissue regeneration is the expression of antioxidants by the cells. A reduction of antioxidant levels negatively affects the regeneration index of myoblasts and satellite cells (Fulle et al., 2005; Lee et al., 2006; Urish et al., 2009), and hematopoietic stem cells (Ito et al., 2006), likely through the increased ability of the cells to survive after implantation. Our group has recently shown that the MDSCs express high levels of the antioxidants glutathione (GSH) and superoxide dismutase. These molecules likely play a critical role in the cells’ ability to survive the harsh transplantation microenvironment better than myoblasts and hence increase their ability to more efficiently regenerate the tissue (Urish et al., 2009; Drowley et al., 2010). Our group has recently shown that reduction of the antioxidant level of MDSCs by diethyl maleate decreased their regeneration potential, whereas an improvement in their regenerative potential was observed after enhancing their antioxidant levels with N-acetylcysteine. These results show a therapeutic potential for boosting antioxidant levels of stem cells before transplantation in skeletal and heart muscles (Urish et al., 2009; Drowley et al., 2010).
Donor Cell-Mediated Skeletal Tissue Repair Paracrine Action
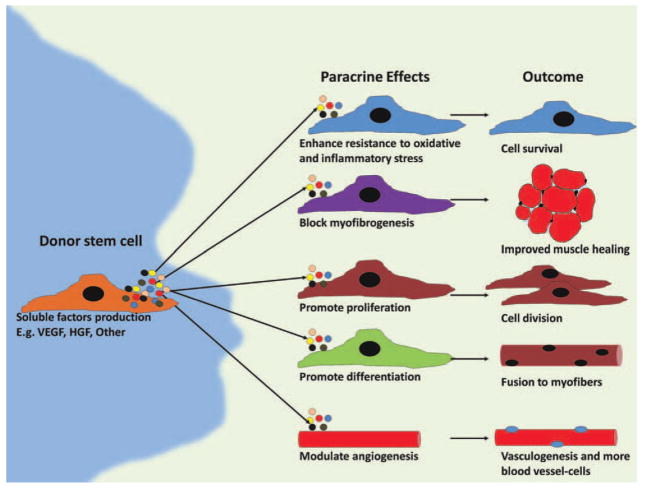
It is clear from numerous reports using cell therapy in animal models that cell survival, differentiation, and engraftment in host tissue are important factors in assessing the efficacy of cell therapy. At the same time, it has been demonstrated that the number of cells directly involved in tissue repair are not necessarily correlated with the improvement in function exhibited by the host tissue. This is especially true in the cardiac muscle (Payne et al., 2005). Recently, our group has found that intramuscular injection of MDSCs at 4 days postinjury greatly enhanced injured skeletal muscle healing by increasing angiogenesis and reducing scar tissue formation (Ota et al., 2011). High levels of expression of vascular endothelial growth factor (VEGF) 1 week after cell injection was correlated with increased vascularity, improved muscle regeneration, and strength at week 4 (Ota et al., 2011). Thus, it is believed that the abilities of MDSCs to secrete different molecules and engage in autocrine and paracrine activities are determining factors of the success of these stem cells in the repair process (Gharaibeh et al., 2011). Exciting results now show that stem cell-mediated repair may occur with little, if not in the absence of, donor cell differentiation. Results indicate that repair may be due to a largely overlooked characteristic of stem cells, paracrine signaling by surviving stem cells (Gnecchi et al., 2005; Murry et al., 2006; Gharaibeh et al., 2011; Fig. 3).
Figure 3.
Schematic of potential scenario of events taking place during cell therapy. Donor stem cells proliferate, differentiate, apoptose, senesce, or more importantly produce trophic factors that would have autocrine and paracrine effects on other donor and host cells. Furthermore, certain factors (e.g., VEGF) promote agniogenesis, which can positively affect other processes.
Trophic signaling or release of cytokines or other signaling molecules may be a key for recruiting host cells to participate in the repair, perhaps by having an effect in the local microenvironment, and/or by inducing a systemic effect, or by mediating an inflammatory response. Several reports have shown that transplanted muscle cells may induce angiogenesis in the host tissue by expressing VEGF, but it is not fully understood whether other bioactive factors may be secreted by the cells or whether they have any immunomodulatory activity (Springer et al., 1998, 2003; Deasy et al., 2009). On a similar note, evidence indicating that antiinflammatory drugs delay muscle repair implicates inflammatory cells in the repair process as mentioned before, but the role that particular host cells play in the repair process is still unclear (Almekinders and Gilbert, 1986; Obremsky et al., 1994; Mishra et al., 1995; Shen et al., 2005).
Based on these findings, further analysis of the identity of specific molecules expressed in this paracrine activity will likely affect therapeutic strategies and indeed the need for stem cell transplantation.
FIBROSIS
Excessive formation of connective tissue between skeletal muscle fibers forms muscle scar. This usually begins between the second and third week after muscle injury and continues to increase in size over time (Li et al., 2001; Huard et al., 2002). Our findings strongly indicate that scar tissue formation leads to incomplete regeneration of injured muscle tissue. Various reports have implicated TGF-β1 in the onset of fibrosis in various tissues (Czaja et al., 1989; Okuda et al., 1990; Brandes et al., 1991; Coimbra et al., 1991; Barnes and Abboud, 1993; Khalil et al., 1993; Sporn and Roberts, 1993; Westergren-Thorsson et al., 1993; Yamamoto et al., 1993; Barcellos-Hoff et al., 1994; Kagami et al., 1994; Logan et al., 1994; Wolf et al., 1994; Wynn, 2007). However, very few studies have examined the direct role of TGF-β1 in skeletal muscle fibrosis (Fanbin et al., 2011). It was shown that TGF-β1 is expressed at high levels and is associated with fibrosis in the skeletal muscle of DMD patients (Yamazaki et al., 1994; Bernasconi et al., 1995; Zanotti et al., 2005; Cohn et al., 2007). Research has shown significantly higher levels of TGF-β1 mRNA levels in muscle biopsy specimens of patients with dermatomyositis (Confalonieri et al., 1997; Amemiya et al., 2000). The studies concluded that excessive TGF-β1 is correlated with chronic inflammation, the accumulation of ECM, and fibrosis (Confalonieri et al., 1997; Amemiya et al., 2000). Members of the TGF-β superfamily have been shown to be involved in Marfan syndrome and other inherited or acquired myopathies (Cohn et al., 2007; Burks and Cohn, 2011). We have used immunohistochemistry to study the expression of TGF-β1 and found strong expression of this cytokine in injured skeletal muscles in mice (Li et al., 2004). Taken together, these results clearly implicate TGF-β1 in initiating a cascade of events that occur after muscle trauma or with the onset of muscle disease and suggest that neutralizing TGF-β1 or down regulating its expression could eliminate or reduce scar formation.
Modulating Fibrosis with Antifibrotic Agents
Our group recently showed that inhibiting the expression of TGF-β1 using several antifibrotic agents led to reduced muscle fibrosis. Muscle fibrosis is reduced resulting in nearly complete recovery of injured by using decorin, suramin, relaxin, gamma interferon (IFN-γ) and α-IFN (Fukushima et al., 2001; Chan et al., 2003; Foster et al., 2003; Sato et al., 2003; Chan et al., 2005; Li et al., 2005; Negishi et al., 2005; Cohn et al., 2007; Habashi et al., 2011). However, clinical use is hampered by lack of oral dosing formulations, serious side-effect profiles of some of these antifibrotic agents, and lack of U.S. Food and Drug Administration (FDA) approval for use in humans.
Research results had linked pathologic fibrosis in various tissues to an end-product of the blood pressure-regulating renin–angiotensin system, angiotensin II. Modulation of angiotensin II levels with angiotensin II receptor blockers or angiotensin converting enzyme (ACE) inhibitors has shown decreased fibrosis and improved function in kidney, liver, lung tissue, and the aortic wall (Lim et al., 2001; Paizis et al., 2001; Suga et al., 2002; Otsuka et al., 2004; Habashi et al., 2011). In diseases such as congestive heart failure, injured cardiac muscle is dysfunctional due to increased amounts of fibrosis. Use of ACE inhibitors or angiotensin receptor blockers to decrease the levels of angiotensin II in myocardium has demonstrated measurable improvement in cardiac output (Swedberg and Kjekshus, 1988; Gremmler et al., 2000; Habashi et al., 2011). Interestingly, investigators have observed a relationship between treatment for hypertension by the use of medications containing ACE and skeletal muscle health in the elderly. Patients treated with antihypertensive drugs had the unexpected positive side effect of decreased rates of muscle wasting and a reduction in the relative amount of adipose tissue within their musculature (Onder et al., 2006). In the other studies that utilized ACE inhibitors and studies of persons carrying a deletion of ACE gene, a direct effect of the renin–angiotensin system on the skeletal muscle was demonstrated (Folland et al., 2000; Onder et al., 2006). Muscle hypertrophic growth in response to overloading is believed to be modulated by the renin–angiotensin system (Folland et al., 2000; Onder et al., 2006). Other evidence has elucidated the mechanism by which the angiotensin II receptor blockade modulates TGF-β1 and showed that it is implicated in the prevention of muscle regeneration in murine models of Marfan syndrome (Cohn et al., 2007).
Angiotensin II receptor blocker, Losartan potassium (Cozaar; Merck & Co, West Point, PA), is an FDA approved drug that has minimal side effects, and has been in clinical use for over 20 years (Burks et al., 2011; Fakhfakh et al., 2011). Losartan use has been associated with reduction in fibrosis in several tissues (Lim et al., 2001; Paizis et al., 2001; Otsuka et al., 2004; Burks et al., 2011). Burks et al. (2011) have found that blocking angiotensin II Type I receptor by Losartan improved muscle remodeling in an aging mouse model, protected against sarcopenia by regulating TGF-β1 signaling pathways, and helped against muscle loss in immobilized hindlimbs by regulating the activity of the IGF-1/Akt/mammalian target of rapamycin. Our group had investigated the effect of losartan on improving muscle healing after injury in a murine model and have found that losartan-treated mice exhibited a histological, dose-dependent improvement in muscle regeneration (Bedair et al., 2008). Furthermore, gastrocnemius muscles in mice given losartan solubilized in their drinking water and sacrificed 3 or 5 weeks after injury showed a significant reduction in fibrous tissue formation within the area of injury compared with control animals (Bedair et al., 2008). We are performing additional studies to understand the mechanism of action of losartan, as well as potential effects of dosing and timing of application to improve muscle healing in animal models (Kobayashi et al., in press).
The clinical implications for this application of angiotensin receptor blockers are potentially far-reaching and include not only sports and military-related injuries but also diseases like the muscular dystrophies. However, thus far there are no clinical reports on the use of this drug to optimize healing after muscle injury. We hypothesized that treatment of patients with doses of losartan that do not affect their blood pressure would antagonize the effects of TGF-β1, as we have shown in murine muscles (Bedair et al., 2008), and thus result in a clinically significant reduction of fibrosis formation after a common muscle injury. We have conducted a limited clinical case study on two college athletes to document the effect of losartan on muscle fibrosis and followed their tolerability and time needed to reach strength and flexibility levels that would correlate to ability to return to play. Furthermore, we followed up on any future recurrences of injury. Below we summarize the preliminary results obtained in this case study.
Case Study
At the initial evaluation in the clinic, a history and physical examination were performed on each of the two patient–athletes. The two patients had normal neurovascular examinations. They underwent a work up that included measurements of isometric hamstring muscle force and flexibility, plain radiographs of the pelvis, to rule out fracture of the ischial tuberosity, and magnetic resonance imaging (MRI; 1.5 T; GE-Sigma, Waukesha, WI) of the involved muscles was performed to better characterize the injury. The MRIs confirmed in both a partial thickness tear of the biceps femoris with surrounding edema. No fracture or significant hematoma was present. After obtaining the patients’ consent for treatment, they were started on a 30-day course of losartan (Cozaar, Merck and Co.) at the oral daily dose of 50 mg. In addition to the medication, the subjects underwent routine standard of care rehabilitation supervised by a physical therapist. Return to sports activities progressed from jogging to running and sprinting. The patients were evaluated every 7 days by a study physician (Y.C.) that included measurement of blood pressure and hamstring flexibility and strength as described earlier. At the conclusion of treatment, both patients underwent testing on an isokinetic dynamometer (Biodex II; Shirley, New York) at 60° and 180° per second to assess the torque generating capacity of the hamstring and quadriceps muscles.
Serial isometric strength and flexibility measurements, performed at different time points, are summarized in Table 1. In addition to the isometric tests, iso-kinetic tests were performed. Both patients reported no side effects, while they were taking the losartan and remained normotensive throughout the 30-day course of the medication.
TABLE 1.
Results of Strength and Flexibility Measurements
| 10 days postinjury | 3 weeks postinjury | 4 weeks postinjury | 7 weeks postinjury | 9 weeks postinjury | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Strength (%) | Pop angle (°) | Strength (%) | Pop angle (°) | Strength (%) | Pop angle (°) | Strength (%) | Pop angle (°) | Strength (%) | Pop angle (°) | |
| Subject 1 | 47.2/54.1 | 30 (5) | 71.0/79.6 | 5 (5) | 85.5/92.6 | 5 (5) | 91.5/83.5 | 5 (5) | 83.3/107.1 | 5 (5) |
| Subject 2 | 59.6/60.1 | 13 (10) | 101.4/85.5 | 24 (15) | 103.8/92.5 | 15 (12) | 110.5/99.7 | 26 (18) | 132.5/110.8 | 14 (12) |
In both patients, we initiated treatment with losartan after the acute period of necrosis/degeneration and inflammation (10 days after injury for the first patient and 5 days after injury for the second patient). Patient 2 has demonstrated normal hamstring flexibility and strength by 3 weeks after injury and was without activity-limiting pain at that time (Fig. 4). Patient 1 was back to normal strength after 9 weeks. While meeting this criteria would normally correlate with appropriate time to return to athletics, both athletes suffered injury at the end of their season and thus a return to sport was not a primary end point. After 1 year, neither individual has suffered any recurrence of injury.
Figure 4.
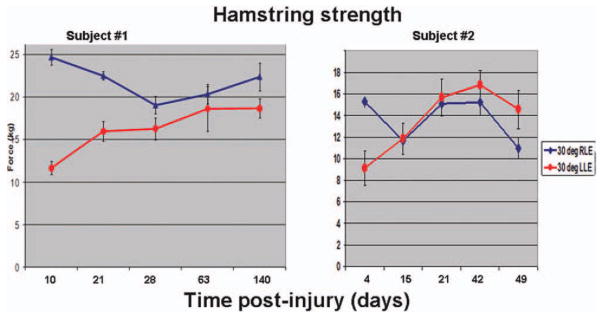
Hamstring strength in two hamstring injury patients treated with Losartan (affected leg readings are charted in red).
Extrapolating the time to return to play for hamstring injuries from the previous studies is a difficult and an inexact task, as evidenced by the many variables involved and a wide range of time reported in the studies ranging from mean of 14 days to 62 weeks (Pomeranz and Heidt, 1993; Slavotinek et al., 2002; Connell et al., 2004; Brooks et al., 2006; Askling et al., 2007, 2008). For our patients, both have been able to return to normal levels of activity after 9 weeks or even shorter for Patient 2. Despite the difficulty of extrapolating from this limited data, in our clinical experience this may be a shorter period of recovery than expected for patients with a similar degree of recurrent injury.
We are encouraged by the results of this case study that angiotensin receptor blockers may provide a clinically safe and effective option for a nonsurgical treatment of common skeletal muscle injuries. This may also be combined with growth factors and stem cell therapies. However, we do recognize the limitations of making treatment decisions based on case reports. We believe that a prospective, blinded, placebo controlled randomized clinical trial is necessary to determine the benefits of losartan use in comparison to conventional approaches, to the treatment of hamstring muscle rupture. Future research should consider the effects of the losartan on recovery of hamstring flexibility and strength, degree of fibrosis, time to return to full participation in sports, and the frequency of recurrence, as well as any adverse effects encountered by otherwise healthy individuals.
CONCLUSIONS
Review of recent literature indicates that muscle injury includes well-coordinated and interdependent phases, including degeneration, inflammation, regeneration, and fibrosis. Stem cells isolated from different tissues have great therapeutic potential, especially when combined with growth factors to modulate their growth and differentiation into certain lineages. From the skeletal muscle, our group has isolated a population termed MDSCs that has been effective in skeletal muscle repair and has great potential for future therapies for musculoskeletal diseases.
Biologically active signals produced by donor stem cells are believed to elicit response from donor cells and chemoattract host cells to the injury site. It is still unclear, though, which host cells are involved in the repair processes after stem cell transplantation. Blood vessel cells, immune and inflammatory cells, and resident cells at the injury site all appear to play a role in the regeneration process.
Recent investigations had shown that few donor stem cells are found within the regenerated tissues, but this should not detract from the potential of stem cells. Current findings indicate that the improvement in function is probably a result of paracrine signaling by donor stem cells (Yoon et al., 2005; Santhanam et al., 2007; Togel et al., 2007; Chen et al., 2008; Gharaibeh et al., 2011).
It is clear that the multipotentiality of the stem cells may not represent a major determinant for the success of stem cell therapy. This idea invites a revisit to the notion that using embryonic stem cells in therapeutic strategies is favored over adult derived stem cells because the latter are less able to differentiate into multiple lineages. We speculate that it is the stem cells’ resistance to oxidative stress and paracrine signaling capacity that will lead to their increased ability to attract host cells, and it is this feature that is a key to successful stem cell therapy.
Choosing the most robust stem cell population(s) with the greatest potential for differentiation toward other cell lineages will continue to be of valuable interest to restore the structure and function of damaged tissues. Furthermore, additional efforts to isolate factors involved in the paracrine action of different stem cell populations under different conditions will very likely be utilized by future therapeutic applications.
The TGF-β1 is a cytokine that plays a significant role in the formation of fibrotic tissue in skeletal muscle and other tissues. Antifibrotic agents modulating TGF-B1 effects, including angiotensin receptor II blocker (such as losartan), are very effective in reducing muscle fibrosis and are likely to be a strategy that can be used in clinical therapies in the future. We believe that more rigorous clinical trials using losartan are necessary to determine the benefits of losartan use, in comparison to conventional approaches alone for the treatment of skeletal muscle injuries. This research should also consider the effects of the losartan on recovery of muscle flexibility and strength, and amount of fibrosis, as well as any adverse effects encountered by otherwise healthy individuals. Furthermore, future experiments on the use of losartan should compare the effects of using losartan on muscle healing when used with or without antiinflammatory drugs that seem to delay muscle regeneration.
Acknowledgments
Indirectly supported by Department of Defense Contract (W81XWH-08-0076), the National Institutes of Health (R01 DE013420-09), the William F. and Jean W. Donaldson Chair at the Children’s Hospital of Pittsburgh, the Henry J. Mankin Endowed Chair in Orthopaedic Surgery at the University of Pittsburgh, and the Orris C. Hirtzel and Beatrice Dewey Hirtzel Memorial Foundation.
Contributor Information
Burhan Gharaibeh, Stem Cell Research Center, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania. Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Yuri Chun-Lansinger, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Tanya Hagen, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Sheila Jean McNeill Ingham, Stem Cell Research Center, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania. Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Vonda Wright, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Freddie Fu, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Johnny Huard, Stem Cell Research Center, Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania. Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Almekinders LC. Anti-inflammatory treatment of muscular injuries in sports. Sports Med. 1993;15:139–145. doi: 10.2165/00007256-199315030-00001. [DOI] [PubMed] [Google Scholar]
- Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med. 1986;14:303–308. doi: 10.1177/036354658601400411. [DOI] [PubMed] [Google Scholar]
- Amemiya K, Semino-Mora C, Granger RP, Dalakas MC. Downregulation of TGF-beta1 mRNA and protein in the muscles of patients with inflammatory myopathies after treatment with high-dose intravenous immunoglobulin. Clin Immunol. 2000;94:99–104. doi: 10.1006/clim.1999.4823. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Liu L, Kardami E. Distinctive patterns of basic fibroblast growth factor (bFGF) distribution in degenerating and regenerating areas of dystrophic (mdx) striated muscles. Dev Biol. 1991;147:96–109. doi: 10.1016/s0012-1606(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during slow-speed stretching: clinical, magnetic resonance imaging, and recovery characteristics. Am J Sports Med. 2007;35:1716–1724. doi: 10.1177/0363546507303563. [DOI] [PubMed] [Google Scholar]
- Askling CM, Tengvar M, Saartok T, Thorstensson A. Proximal hamstring strains of stretching type in different sports: injury situations, clinical and magnetic resonance imaging characteristics, and return to sport. Am J Sports Med. 2008;36:1799–1804. doi: 10.1177/0363546508315892. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard W, Bower J, Brown MA, et al. Leukemia inhibitory factor (LIF) infusion stimulates skeletal muscle regeneration after injury: injured muscle expresses lif mRNA. J Neurol Sci. 1994;123:108–113. doi: 10.1016/0022-510x(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Barnes JL, Abboud HE. Temporal expression of autocrine growth factors corresponds to morphological features of mesangial proliferation in Habu snake venom-induced glomerulonephritis. Am J Pathol. 1993;143:1366–1376. [PMC free article] [PubMed] [Google Scholar]
- Baroffio A, Hamann M, Bernheim L, et al. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. doi: 10.1046/j.1432-0436.1996.6010047.x. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, et al. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedair HS, Karthikeyan T, Quintero A, et al. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36:1548–1554. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, et al. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S, Rando TA. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21:845–854. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol. 2006;290:C1651–C1659. doi: 10.1152/ajpcell.00518.2005. [DOI] [PubMed] [Google Scholar]
- Brandes ME, Allen JB, Ogawa Y, Wahl SM. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J Clin Invest. 1991;87:1108–1113. doi: 10.1172/JCI115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34:1297–1306. doi: 10.1177/0363546505286022. [DOI] [PubMed] [Google Scholar]
- Burks TN, Cohn RD. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet Muscle. 2011;1:19–32. doi: 10.1186/2044-5040-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling C, Le Gall F, Orhant E. A four-season prospective study of muscle strain reoccurrences in a professional football club. Res Sports Med. 2011;19:92–102. doi: 10.1080/15438627.2011.556494. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RL, McDermott JC. Molecular basis of skeletal muscle regeneration. Can J Appl Physiol. 1996;21:155–184. doi: 10.1139/h96-014. [DOI] [PubMed] [Google Scholar]
- Chan YS, Li Y, Foster W, et al. Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol. 2003;95:771–780. doi: 10.1152/japplphysiol.00915.2002. [DOI] [PubMed] [Google Scholar]
- Chan YS, Li Y, Foster W, et al. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- Chen J, Park HC, Addabbo F, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra T, Wiggins R, Noh JW, et al. Transforming growth factor-beta production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol. 1991;138:223–234. [PMC free article] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Collins C, Partridge T. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4:1338–1341. doi: 10.4161/cc.4.10.2114. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, et al. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Confalonieri P, Bernasconi P, Cornelio F, Mantegazza R. Transforming growth factor-beta 1 in polymyositis and dermatomyositis correlates with fibrosis but not with mononuclear cell infiltrate. J Neuropathol Exp Neurol. 1997;56:479–484. doi: 10.1097/00005072-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Connell DA, Schneider-Kolsky ME, Hoving JL, et al. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR Am J Roentgenol. 2004;183:975–984. doi: 10.2214/ajr.183.4.1830975. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Jokl P, Heinen GT, et al. A muscle contusion injury model. Biomechanics, physiology, and histology. Am J Sports Med. 1994;22:702–710. doi: 10.1177/036354659402200521. [DOI] [PubMed] [Google Scholar]
- Czaja MJ, Weiner FR, Flanders KC, et al. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108:2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon SE, Haugk KL, Birnbaum RS, Quinn LS. Retrovirally mediated overexpression of insulin-like growth factor binding protein 4: evidence that insulin-like growth factor is required for skeletal muscle differentiation. J Cell Physiol. 1998;175:109–120. doi: 10.1002/(SICI)1097-4652(199804)175:1<109::AID-JCP12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- De Deyne PG, Kinsey S, Yoshino S, Jensen-Vick K. The adaptation of soleus and edl in a rat model of distraction osteogenesis: IGF-1 and fibrosis. J Orthop Res. 2002;20:1225–1231. doi: 10.1016/S0736-0266(02)00047-5. [DOI] [PubMed] [Google Scholar]
- Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27:924–933. doi: 10.1006/bcmd.2001.0463. [DOI] [PubMed] [Google Scholar]
- Deasy B, Gharaibeh B, Pollett J, et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Cell Biol. 2005;16:3323–3333. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy BM, Feduska JM, Payne TR, et al. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther. 2009;17:1788–1798. doi: 10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumit ME, Cook DR, Merkel RA. Fibroblast growth factor, epidermal growth factor, insulin-like growth factors, and platelet-derived growth factor-BB stimulate proliferation of clonally derived porcine myogenic satellite cells. J Cell Physiol. 1993;157:326–332. doi: 10.1002/jcp.1041570216. [DOI] [PubMed] [Google Scholar]
- Drowley L, Okada M, Beckman S, et al. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther. 2010;18(10):1865–1873. doi: 10.1038/mt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhfakh R, Lamarre Y, Skuk D, Tremblay JP. Losartan enhances the success of myoblast transplantation. Cell Transplant. 2011 doi: 10.3727/096368911X576045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Fanbin M, Jianghai C, Juan L, et al. Role of transforming growth factor-beta1 in the process of fibrosis of denervated skeletal muscle. J Huazhong Univ Sci Technolog Med Sci. 2011;31:77–82. doi: 10.1007/s11596-011-0154-4. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Falen SL, Van Wyk JJ. Biphasic concentration dependency of stimulation of myoblast differentiation by somatomedins. Am J Physiol. 1986;250:C771–C778. doi: 10.1152/ajpcell.1986.250.5.C771. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11:2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folland J, Leach B, Little T, et al. Angiotensin-converting enzyme genotype affects the response of human skeletal muscle to functional overload. Exp Physiol. 2000;85:575–579. [PubMed] [Google Scholar]
- Foster W, Li Y, Usas A, et al. Gamma interferon as an antifibrosis agent in skeletal muscle. J Orthop Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Badlani N, Usas A, et al. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- Fulle S, Di Donna S, Puglielli C, et al. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40:189–197. doi: 10.1016/j.exger.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Gharaibeh B, Lu A, Tebbets J, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- Gharaibeh B, Lavasani M, Cummins JH, Huard J. Terminal differentiation is not a major determinant for the success of stem cell therapy–cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther. 2011;2:31–43. doi: 10.1186/scrt72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Weseman J, Moran JS, Lindstrom J. Effect of fibroblast growth factor on the division and fusion of bovine myoblasts. J Cell Biol. 1976;70:395–405. doi: 10.1083/jcb.70.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowdak LH, Poliakova L, Wang X, et al. Adenovirus-mediated VEGF(121) gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia. Circulation. 2000;102:565–571. doi: 10.1161/01.cir.102.5.565. [DOI] [PubMed] [Google Scholar]
- Gremmler B, Kunert M, Schleiting H, Ulbricht LJ. Improvement of cardiac output in patients with severe heart failure by use of ACE-inhibitors combined with the AT1-antagonist eprosartan. Eur J Heart Fail. 2000;2:183–187. doi: 10.1016/s1388-9842(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Towards understanding skeletal muscle regeneration. Pathol Res Pract. 1991;187:1–22. doi: 10.1016/S0344-0338(11)81039-3. [DOI] [PubMed] [Google Scholar]
- Guerette B, Asselin I, Skuk D, et al. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997;6:101–107. doi: 10.1177/096368979700600203. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington MA, Daub R, Song A, et al. Interleukin 1 alpha mediated inhibition of myogenic terminal differentiation: increased sensitivity of Ha-ras transformed cultures. Cell Growth Differ. 1992;3:241–248. [PubMed] [Google Scholar]
- Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat. 2003;203:89–99. doi: 10.1046/j.1469-7580.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Huard J, Bouchard JP, Roy R, et al. Myoblast transplantation produced dystrophin-positive muscle fibres in a 16-year-old patient with Duchenne muscular dystrophy. Clin Sci (Lond) 1991;81:287–288. doi: 10.1042/cs0810287. [DOI] [PubMed] [Google Scholar]
- Huard J, Roy R, Guerette B, et al. Human myoblast transplantation in immunodeficient and immunosuppressed mice: evidence of rejection. Muscle Nerve. 1994;17:224–234. doi: 10.1002/mus.880170214. [DOI] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A:822–832. [PubMed] [Google Scholar]
- Huard J, Cao B, Qu-Petersen Z. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res C Embryo Today. 2003;69:230–237. doi: 10.1002/bdrc.10020. [DOI] [PubMed] [Google Scholar]
- Inselburg J, Applebaum B. Proteins synthesized in minicells containing plasmid ColE1 and its mutants. J Bacteriol. 1978;133:1444–1451. doi: 10.1128/jb.133.3.1444-1451.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Jarvinen MJ, Lehto MU. The effects of early mobilisation and immobilisation on the healing process following muscle injuries. Sports Med. 1993;15:78–89. doi: 10.2165/00007256-199315020-00002. [DOI] [PubMed] [Google Scholar]
- Jennische E. Sequential immunohistochemical expression of IGF-I and the transferrin receptor in regenerating rat muscle in vivo. Acta Endocrinol (Copenh) 1989;121:733–738. doi: 10.1530/acta.0.1210733. [DOI] [PubMed] [Google Scholar]
- Jin P, Rahm M, Claesson-Welsh L, et al. Expression of PDGF A-chain and beta-receptor genes during rat myoblast differentiation. J Cell Biol. 1990;110:1665–1672. doi: 10.1083/jcb.110.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SE, Allen RE. Activation of skeletal muscle satellite cells and the role of fibroblast growth factor receptors. Exp Cell Res. 1995;219:449–453. doi: 10.1006/excr.1995.1251. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G, Pouliot Y, Zubrzycka-Gaarn E, et al. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989;135:27–32. [PMC free article] [PubMed] [Google Scholar]
- Keller HL, St Pierre Schneider B, Eppihimer LA, Cannon JG. Association of IGF-I and IGF-II with myofiber regeneration in vivo. Muscle Nerve. 1999;22:347–354. doi: 10.1002/(sici)1097-4598(199903)22:3<347::aid-mus7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Khalil N, Whitman C, Zuo L, et al. Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest. 1993;92:1812–1818. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Guerette B, et al. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle Nerve. 1994;17:1407–1415. doi: 10.1002/mus.880171210. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Uehara K, Ota S, et al. The timing of Losartan administration represents a major determinant for the improvement of skeletal muscle healing after injury. J Appl Physiol in press. [Google Scholar]
- Kurek JB, Bower JJ, Romanella M, et al. The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve. 1997;20:815–822. doi: 10.1002/(sici)1097-4598(199707)20:7<815::aid-mus5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- Lavasani M, Lu A, Peng H, et al. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum Gene Ther. 2006;17:180–192. doi: 10.1089/hum.2006.17.180. [DOI] [PubMed] [Google Scholar]
- Lee CW, Fukushima K, Usas A, et al. Biological intervention based on cell and gene therapy to improve muscle healing after laceration. J Musculoskel Res. 2000;4:265. [Google Scholar]
- Lee S, Shin HS, Shireman PK, et al. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic Biol Med. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Sebille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol. 1995;57:85–91. doi: 10.1016/0165-5728(94)00166-l. [DOI] [PubMed] [Google Scholar]
- Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cummins J, Huard J. Muscle injury and repair. Curr Opin Orthop. 2001;12:409–415. [Google Scholar]
- Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Negishi S, Sakamoto M, et al. The use of relaxin improves healing in injured muscle. Ann NY Acad Sci. 2005;1041:395–397. doi: 10.1196/annals.1282.060. [DOI] [PubMed] [Google Scholar]
- Lim DS, Lutucuta S, Bachireddy P, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart TA, Clegg CH, Hauschika SD. Myogenic differentiation in permanent clonal mouse myoblast cell lines: regulation by macromolecular growth factors in the culture medium. Dev Biol. 1981;86:19–30. doi: 10.1016/0012-1606(81)90311-0. [DOI] [PubMed] [Google Scholar]
- Logan A, Berry M, Gonzalez AM, et al. Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci. 1994;6:355–363. doi: 10.1111/j.1460-9568.1994.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DC, Pesall JE, Gilkerson KK. The influence of growth factors on turkey embryonic myoblasts and satellite cells in vitro. Gen Comp Endocrinol. 1993;89:415–424. doi: 10.1006/gcen.1993.1049. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Menetrey J, Kasemkijwattana C, Day CS, et al. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br. 2000;82:131–137. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- Mishra DK, Friden J, Schmitz MC, Lieber RL. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am. 1995;77:1510–1519. doi: 10.2106/00004623-199510000-00005. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990;111:2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011;23:568–573. doi: 10.1097/BOR.0b013e32834bac92. [DOI] [PubMed] [Google Scholar]
- Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Musaro A, Giacinti C, Borsellino G, et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci USA. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi S, Li Y, Usas A, et al. The effect of relaxin treatment on skeletal muscle injuries. Am J Sports Med. 2005;33:1816–1824. doi: 10.1177/0363546505278701. [DOI] [PubMed] [Google Scholar]
- Obremsky WT, Seaber AV, Ribbeck BM, Garrett WE., Jr Biomechanical and histologic assessment of a controlled muscle strain injury treated with piroxicam. Am J Sports Med. 1994;22:558–561. doi: 10.1177/036354659402200420. [DOI] [PubMed] [Google Scholar]
- Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990;86:453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Sternberg E, Hu JS, et al. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G, Vedova CD, Pahor M. Effects of ACE inhibitors on skeletal muscle. Curr Pharm Des. 2006;12:2057–2064. doi: 10.2174/138161206777442137. [DOI] [PubMed] [Google Scholar]
- Ota S, Uehara K, Nozaki M, et al. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med. 2011;39:1912–1922. doi: 10.1177/0363546511415239. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Takahashi H, Shiratori M, et al. Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist. Thorax. 2004;59:31–38. doi: 10.1136/thx.2003.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paizis G, Gilbert RE, Cooper ME, et al. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol. 2001;35:376–385. doi: 10.1016/s0168-8278(01)00146-5. [DOI] [PubMed] [Google Scholar]
- Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- Payne TR, Oshima H, Sakai T, et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264–1274. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- Physicians AAoF. . ICD-9 Codes for Family Medicine 2005–2006. Family Practice Management. 2005;12(8):48A. [Google Scholar]
- Pomeranz SJ, Heidt RS., Jr MR imaging in the prognostication of hamstring injury. Work in progress. Radiology. 1993;189:897–900. doi: 10.1148/radiology.189.3.8234722. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Balkir L, van Deutekom JC, et al. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Haugk KL. Overexpression of the type-1 insulin-like growth factor receptor increases ligand-dependent proliferation and differentiation in bovine skeletal myogenic cultures. J Cell Physiol. 1996;168:34–41. doi: 10.1002/(SICI)1097-4652(199607)168:1<34::AID-JCP5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, Smith LA, He T, et al. Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase-2. Circ Res. 2007;100:1379–1388. doi: 10.1161/01.RES.0000265848.55035.5d. [DOI] [PubMed] [Google Scholar]
- Sato K, Li Y, Foster W, et al. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin L, Girgis-Garbado A, et al. Pax7 is required for the specification of the myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23:239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Shen W, Li Y, Tang Y, et al. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol. 2005;167:1105–1117. doi: 10.1016/S0002-9440(10)61199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Prisk V, Li Y, et al. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2al-pha. J Appl Physiol. 2006;101:1215–1221. doi: 10.1152/japplphysiol.01331.2005. [DOI] [PubMed] [Google Scholar]
- Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. Am J Roentgenol. 2002;179:1621–1628. doi: 10.2214/ajr.179.6.1791621. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. A major advance in the use of growth factors to enhance wound healing. J Clin Invest. 1993;92:2565–2566. doi: 10.1172/JCI116868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer ML, Chen AS, Kraft PE, et al. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- Springer ML, Ozawa CR, Banfi A, et al. Localized arteriole formation directly adjacent to the site of VEGF-induced angiogenesis in muscle. Mol Ther. 2003;7:441–449. doi: 10.1016/s1525-0016(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Suga S, Mazzali M, Ray PE, et al. Angiotensin II type 1 receptor blockade ameliorates tubulointerstitial injury induced by chronic potassium deficiency. Kidney Int. 2002;61:951–958. doi: 10.1046/j.1523-1755.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) Am J Cardiol. 1988;62:60A–66A. doi: 10.1016/s0002-9149(88)80087-0. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, et al. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Togel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- Urish KL, Vella JB, Okada M, et al. Antioxidant levels represent a major determinant in the regenerative capacity of muscle stem cells. Mol Biol Cell. 2009;20:509–520. doi: 10.1091/mbc.E08-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilquin JT, Guerette B, Kinoshita I, et al. FK506 immunosuppression to control the immune reactions triggered by first-generation adeno-virus-mediated gene transfer. Hum Gene Ther. 1995;6:1391–1401. doi: 10.1089/hum.1995.6.11-1391. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G, Hernnas J, Sarnstrand B, et al. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieteska-Skrzeczynska W, Grzelkowska-Kowalczyk K, Rejmak E. Growth factor and cytokine interactions in myogenesis. Part II. Expression of IGF binding proteins and protein kinases essential for myogenesis in mouse C2C12 myogenic cells exposed to TNF-alpha and IFN-gamma. Pol J Vet Sci. 2011a;14:425–431. doi: 10.2478/v10181-011-0063-2. [DOI] [PubMed] [Google Scholar]
- Wieteska-Skrzeczynska W, Grzelkowska-Kowalczyk K, Tokarska J, Grabiec K. Growth factor and cytokine interactions in myogenesis. Part I. The effect of TNF-alpha and IFN-gamma on IGF-I-dependent differentiation in mouse C2C12 myogenic cells. Pol J Vet Sci. 2011b;14:417–424. doi: 10.2478/v10181-011-0062-3. [DOI] [PubMed] [Google Scholar]
- Wolf YG, Rasmussen LM, Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J Clin Invest. 1994;93:1172–1178. doi: 10.1172/JCI117070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Hawkins RD, Maltby S, et al. The Football Association Medical Research Programme: an audit of injuries in professional football—analysis of hamstring injuries. Br J Sports Med. 2004;38:36–41. doi: 10.1136/bjsm.2002.002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17:338–345. doi: 10.2165/00007256-199417050-00006. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990;111:1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nakamura T, Noble NA, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Minota S, Sakurai H, et al. Expression of transforming growth factor-beta 1 and its relation to endomysial fibrosis in progressive muscular dystrophy. Am J Pathol. 1994;144:221–226. [PMC free article] [PubMed] [Google Scholar]
- Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- Zammit P, Beauchamp J. The skeletal muscle satellite cell: stem cell or son of stem cell? Differentiation. 2001;68:193–204. doi: 10.1046/j.1432-0436.2001.680407.x. [DOI] [PubMed] [Google Scholar]
- Zanotti S, Negri T, Cappelletti, et al. Decorin and biglycan expression is differentially altered in several muscular dystrophies. Brain. 2005;128(Pt 11):2546–2555. doi: 10.1093/brain/awh635. [DOI] [PubMed] [Google Scholar]
- Zdanowicz MM, Moyse J, Wingertzahn MA, et al. Effect of insulin-like growth factor I in murine muscular dystrophy. Endocrinology. 1995;136:4880–4886. doi: 10.1210/endo.136.11.7588220. [DOI] [PubMed] [Google Scholar]