Abstract
Purpose
Surgical repairs of tears in the vascular region of the meniscus usually heal better than repairs performed in the avascular region; thus, we hypothesized that this region might possess a richer supply of vascular-derived stem cells than the avascular region.
Methods
In this study, we analyzed 6 menisci extracted from aborted human fetuses and 12 human lateral menisci extracted from adult human subjects undergoing total knee arthroplasty. Menisci were immunostained for CD34 (a stem cell marker) and CD146 (a pericyte marker) in situ, whereas other menisci were dissected into two regions (peripheral and inner) and used to isolate meniscus-derived cells by flow cytometry. Cell populations expressing CD34 and CD146 were tested for their multi-lineage differentiation potentials, including chondrogenic, osteogenic, and adipogenic lineages. Fetal peripheral meniscus cells were transplanted by intracapsular injection into the knee joints of an athymic rat meniscal tear model. Rat menisci were extracted and histologically evaluated after 4 wk posttransplantation.
Results
Immunohistochemistry and flow cytometric analyses demonstrated that a higher number of CD34- and CD146-positive cells were found in the peripheral region compared with the inner region. The CD34- and CD146-positive cells isolated from the vascular region of both fetal and adult menisci demonstrated multilineage differentiation capacities and were more potent than cells isolated from the inner (avascular) region. Fetal CD34- and CD146-positive cells transplanted into the athymic rat knee joint were recruited into the meniscal tear sites and contributed to meniscus repair.
Conclusions
The vascularized region of the meniscus contains more stem cells than the avascular region. These meniscal-derived stem cells were multi-potent and contributed to meniscal regeneration.
Keywords: MENISCUS, MENISCAL TEAR, VASCULAR, AVASCULAR, MULTILINEAGE DIFFERENTIATION
The meniscus has an important role in the knee joint, including load transmission, shock absorption, stability, and lubrication (2,6,19,23). Injury or removal of the meniscus alters the loading environment in the knee joint, resulting in increased contact pressures and stresses on the articular cartilage, which is associated with the accelerated onset of osteoarthritis (11,18,28). Treatment options include partial meniscectomy, meniscus repair with fibrin clot, meniscus repair, and meniscal allograft transplantation (12,29); however, these present treatments do not always have good long-term outcomes.
Meniscal repairs performed in the vascular region have a much higher healing success rate than repairs performed in the avascular region (15,30). Unfortunately, most meniscal injuries occur in the inner two-thirds region, or avascular zone, and although repairs are possible, they have poor clinical outcomes in older patients (13,24–26).
A population of cells isolated from human skeletal muscle characterized by the expression of markers for both the myogenic and vascular endothelial compartments have a remarkable regenerative capacity (33). It has been demonstrated that the origin of muscle stem cells is associated with the walls of the blood vessels in skeletal muscle and have been characterized to express CD34 and CD146 surface cell markers (9,14). Other stem cell populations from vascular and perivascular origin have been found in several fetal and adult tissues (8).
Recently, meniscus regeneration using therapeutic strategies involving tissue engineering with mesenchymal stem cells, growth factors, and scaffolds have been attempted with promising results (3,7,22). On the basis of these data, we hypothesized that the vascularity differences between the inner (avascular) and the peripheral (vascular) parts of the meniscus accounted for their differential healing abilities. We have also tested whether these stem cells after transplantation into the joint fluid could contribute to meniscal repair after injury.
MATERIALS AND METHODS
Samples
Six menisci were extracted from spontaneously or therapeutically aborted human fetuses (21.3 ± 2.5 wk gestational age). The specimens were deidentified, coded, and provided by a certified Honest Broker. The institutional review board of the University of Pittsburgh approved the protocol. Twelve complete visually intact human lateral menisci were extracted from subjects undergoing total knee arthroplasty (61.1 ± 7.7 yr old, six men and six women). After proper de-identification, the specimens were placed into cold Hank’s Balanced Salt Solution and transported in the laboratory 6–8 h after surgery.
Immunohistochemical Staining
The excised menisci were frozen in 2-methylebutane precooled in liquid nitrogen then stored at −80°C until they were cryosectioned. Sections (8 μm thick) were fixed in 4% paraformaldehyde for 5 min and incubated in 10% sheep serum in 2% triton–phosphate-buffered saline (PBS) for 30 min at room temperature. Menisci were immunostained for CD34 (stem cell marker) and CD146 (pericyte marker), coupled with α-smooth muscle actin (α-SMA) to detect smooth muscle cells surrounding the vasculature. Primary antibodies were purified mouse antihuman CD34 (1:50; BD Pharmingen, Franklin Lakes, NJ) and mouse antihuman CD146 (1:1000; Calbiochem, San Diego, CA). These were incubated for 3 h at room temperature, followed by PBS washes and incubation with secondary antibodies, anti-mouse Cy-3 (Sigma-Aldrich, St. Louis, MO; 1: 500) for 1 h at room temperature. Fluorescein isothiocyanate (FITC)-conjugated anti-α-SMA (1:500; Sigma) was added for 1 h at room temperature to detect various cells around the arterioles. Control sections were stained without the addition of the primary antibodies. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI, 100 ng·mL; Sigma) for 5 min at room temperature.
Cell Isolation
The menisci were transported in sterile saline solution on ice. The excised menisci were dissected into two regions: the peripheral one third (peripheral) and the inner two thirds (inner). The tissues were minced into small pieces approximately 1–2 mm3, washed three times in PBS, and were then digested with 0.4% collagenase type I (0.4% w/v) (Invitrogen, Carlsbad, CA) in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/ streptomycin (standard medium) for 4 to 6 h, as previously described (1,20). Cells were spun down, resuspended in medium, passed through a 70-μm pore size nylon filter (BD, Bedford, MA), and washed twice with the same medium.
Characterization of Meniscus-Derived Cells
Meniscus-derived cells from each region were characterized using fluorescence-activated cell sorting (FACS) for CD34 and CD146 expression. Cells were first incubated with mouse serum (1:10; Sigma) in FACS buffer for 10 min on ice and then incubated with CD34-APC, CD146-PE, and CD45-APC-Cy7 (BD Pharmingen) for 30 min. To exclude dead cells, 7-aminoactinomycin D (Via-probes; BD Pharmingen) was added to each tube. Live cells were analyzed using FACS using a FACSAria Cell-Sorting System (BD) and CellQuest software (BD). After examining the cell marker profiles, the hematopoietic (CD45-positive cells) cells were eliminated, and CD34+ and CD146 + cells were collected. Following a protocol described by Zheng et al. (33), the positive fractions were tested for their multilineage differentiation capacity. In addition, the adult and fetal CD34(+)CD146(−) cells from the peripheral (AP34 and FP34) and inner (AI34 and FI34) and the CD34(−)CD146(+) cells from the peripheral (AP146 and FP146) and inner (AI146 and FI146) meniscal segments were cultured in Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, 10% horse serum, 1% penicillin/streptomycin, and 0.5% chick embryo extract for 6 wk. Cultures were characterized by FACS for CD34 and CD146 expression again.
Assessment of Potential Multilineage Differentiation
Chondrogenic assay
To assess the ability of the meniscus-derived cells to undergo chondrogenesis, pellet cultures were performed as described previously (5,17,20). Pellets were extracted at day 21, and their sizes were measured using Image J software (National Institutes of Health, Bethesda, MD). Paraffin-embedded pellets were assessed for chondrogenesis by Alcian blue staining at a low pH to stain the highly sulfated proteoglycans that are characteristic of cartilaginous matrix and were counterstained with nuclear fast red, which stains cell nuclei. Total RNA was extracted from other pellets on day 21 for reverse transcription–polymerase chain reaction (RT-PCR) analysis. The expression of the chondrogenic genes, collagen type IIA2 (COL II) and aggrecan, was analyzed by RT-PCR.
Osteogenic assay
The osteogenic assay was performed as previously described (34,36). In brief, monolayer cultures of sorted cells from meniscal tissues were treated with standard medium supplemented with dexamethasone (0.1 mM) (Sigma), ascorbate-2-phosphate (50 μM) (Sigma), and β-glycerophosphate (10 mM) (Sigma), which also contained different concentrations of BMP4 (50, 100, and 250 ng·mL−1) (Sigma) and were incubated at 37°C in 5% CO2 for 6 d. Alkaline phosphatase (ALP) staining showed that meniscus-derived cells have more osteogenic potential in the 100- and 250-ng·mL−1 concentrations of BMP-4 than that at 50 ng·mL−1 of BMP-4 (data not shown). After this, 100 ng·mL−1 of BMP-4 was added to the media to make osteogenic medium. To assess their ability to undergo osteogenesis, cells (1.0 × 105) were cultured in six-well plates in osteogenic medium. The medium was changed every 3 d. Osteogenesis was assessed using ALP staining at day 6. Cells (2.5 × 105) were also placed in 15 mL conical polypropylene tubes and were centrifuged at 600g for 5 min to allow for pellet assays. Pellets were cultured for 21 d in osteogenic medium and evaluated for their bone volume using a micro computed tomography (CT) scan (SCANCO Medical, Wangen-Brüttisellen Switzerland). After scanning, pellets were embedded in paraffin, sectioned, and stained with von Kossa solution for the assessment of mineralization. Total RNA was extracted on day 21 from the cells in monolayer culture with osteogenic medium. The expression of the osteogenic genes, collagen type IA2 (COL I) and osteocalcin, was analyzed by RT-PCR.
Adipogenic assay
The adipogenesis assay was performed as described previously (34,36). Cells (1.0 × 105 per well) were cultured in six-well plates for 14 d in adipogenic medium made of standard medium supplemented with insulin (10 μM), dexamethasone (1 μM) (Sigma), isobutyl-methylxanthine (0.5 mM) (Sigma), and indomethacin (200 μM) (Sigma). Media were changed every 2 d. Adipogenesis was assessed using Oil Red O stain, which serves as an indicator of intracellular lipid accumulation. The cells were fixed for 10 min at room temperature in 10% neutral-buffered formalin and were washed with PBS. They were then incubated in Oil Red O reagent for 30 min and washed with 60% isopropanol one time and with PBS two times. Total RNA was extracted for RT-PCR on day 14 from the cells in monolayer culture maintained adipogenic medium. The expression of the adipogenic genes, peroxisome proliferator-activated receptor gamma (PPARγ) and lipoprotein lipase (LPL), was analyzed by RT-PCR.
RNA Isolation and RT-PCR
Total RNA was extracted from the cells or pellets using RNeasy plus Mini Kit (Qiagen; Hilden, Germany) following the manufacturer’s instructions. One microgram of total RNA was used for random hexamer-primed complementary DNA synthesis using reverse transcription of the SuperScript II preamplification system (Invitrogen). Equal amounts of complementary DNA synthesis were used as templates for RT-PCR amplification per 25-μL reaction volume using Taq DNA polymerase (Invitrogen) and 50 pmol of gene-specific primers. RT-PCR amplifications were performed by preheating the mixture to 95°C for 5 min followed by 35 cycles of 1 s at 95°C, 45 s at 58°C, and 1 s at 72°C. A final extension of 10 min was performed at 72°C. The PCR products were resolved by electrophoresis on 1.5% agarose gels and visualized by ethidium bromide staining. The messenger RNA (mRNA) expression of β-actin was used to normalize gene expression. Total RNA extracted from fetal cartilage, bone, and fat tissues were used as positive controls for chondrogenic, osteogenic, and adipogenic gene expression.
Animal Model of Meniscus Tear
A reproducible model of a meniscus tear was created in immunodeficient rats according to a previous report (10). The animal experiments conducted were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Twelve 10-wk-old female nude rats (National Institutes of Health–Whn NIHRNU-M; Taconic, Germantown, NY) were used for these studies. The animals were anesthetized with 2% isoflurane and O2 gas (1.5 L·min−1) delivered through an inhalation mask. A longitudinal incision was made over the knee, and a lateral parapatellar arthrotomy was performed. The medial meniscus was then incised sharply in an oblique direction starting from the free margin and extending peripherally for two-thirds of its width. The incision was located at the junction of the anterior one-third and posterior two-thirds. The wounds were closed in routine fashion. Ketorolac, used to control postoperative pain, was administered once immediately after surgery and then daily for 3–5 d postsurgery. Antibiotics were not used, and the animals were allowed food and water ad libitum.
Cell Transplantation
Three days after the meniscal injury, 20 rats divided into four groups received intracapsular administration of 5 × 105 fetal peripheral meniscus-derived cells sorted as follows: 1) CD34+ cells, 2) CD146+ cell, 3) CD34−CD146− cell, and 4) PBS but no cells. All the cells were suspended in 50 μL PBS for delivery.
Tissue Extraction
Rats were euthanized with CO2 overdose at 4 wk post-transplantation. The knee joints were extracted and quickly embedded in optimal cutting temperature (OCT) compound, snap frozen in liquid nitrogen, and stored at −80°C for histochemical and immunohistochemical staining as described in the following sections.
Histological Examination
The knee joints in OCT blocks were sectioned, and 6-μm serial sections were mounted on silane-coated glass slides (Sigma) and air dried for 1 h before being fixed with 4% paraformaldehyde at 4°C for 5 min and stained immediately. Hematoxylin and eosin (H&E) staining was performed at week 4 for morphological analysis according to standard procedures (n = 6 in each group). The number of cells recruited into the tear site were quantified from H&E micrographs in equivalent size regions using Image J software (National Institutes of Health, Bethesda, MD).
Immunofluorescent Staining
To follow the fate of the transplanted cells in the rat knee joint, the cells were stained with 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (DiI; Sigma) following the manufacture’s protocol. Also, to assess the healing of meniscus, immunohistochemistry (n = 6, in each group) was performed at week 4 with antihuman type II collagen (hCol2) antigen (Sigma). The first antibodies for immunostaining were Col2 antigen used at 1:100 dilution at room temperature for 1 h. Alexa Fluor 488-conjugated donkey antirabbit IgG (Molecular Probes) were used at 1:200 dilution at room temperature for 2 h as the secondary antibody for hCol2 staining. DAPI solution was applied for 5 min for nuclear staining. After staining, we also evaluated the number of each of the stained cells in five randomly selected fields (250 × 250 μm) of the tissue at the meniscal tear sites using Northern Eclipse software (Empix Imaging Inc., Cheektowaga, NY).
Statistical Methods
Statistical differences were tested using Student’s t-test between the groups. Independent variables consisted of meniscus cells from different regions (peripheral vs inner cells) and sorted cells (CD34 vs CD146 cells). All data were reported as the mean ± SE of 5–10 samples from a minimum of three replicate studies, depending on the assay. Animal experiments included a group size of five rats and histological replicates of at least three analyses. The threshold for statistical significance was set at P < 0.05.
RESULTS
Stem cells from fetal meniscus
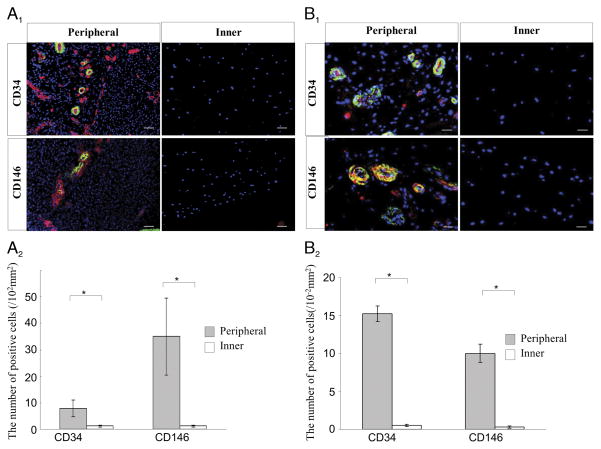
Fetal meniscus tissues showed more CD34, CD146, and α-SMA-expressing cells in the periphery of the meniscus than that in the inner meniscus (Fig. 1A1). CD34-positive cells in the peripheral region were located in α-SMA-positive arterioles, and CD146-positive cells in the peripheral region were located surrounding α-SMA-positive arterioles (Fig. 1A1) (P = 0.046 for CD34 and P = 0.013 for CD146) (Fig. 1A2).
FIGURE 1.
A, Immunohistochemical staining for CD34 and CD146 in fetal meniscal tissues in vivo. A1, The left panels are representative images of the peripheral vascular region, and the right panels are the inner avascular region. CD34, CD146 (red), and α-SMA (green) (scale bars = 25 μm). A2, The number of positive cells for CD34 and CD146 in the fetal meniscus is shown in the graph (*P < 0.05). B, Immunohistochemical staining for the CD34 and CD146 in adult meniscal tissues. B1, The left panels are the peripheral vascular region, and the right panels are the inner avascular region. CD34, CD146 (red), α-SMA (green) (scale bars = 50 μm). B2, The number of positive cells for CD34 and CD146 in the adult menisci are shown in the graph (*P < 0.05). A full color version of this figure can be viewed in the online version of the article (http://journals.lww.com/acsm-msse).
Stem cells from adult meniscus
Adult meniscal tissues also showed more CD34-positive cells in the peripheral region, which were located in α-SMA-positive arterioles, and CD146-positive cells in the peripheral region were located surrounding α-SMA-positive arterioles (Fig. 1B1). Adult meniscal tissues showed more positive staining for CD34, CD146, and α-SMA in the periphery of the meniscus than that in the inner meniscus (Fig. 1B1) (P = 0.012 for CD34 and P = 0.013 for CD146) (Fig. 1B2).
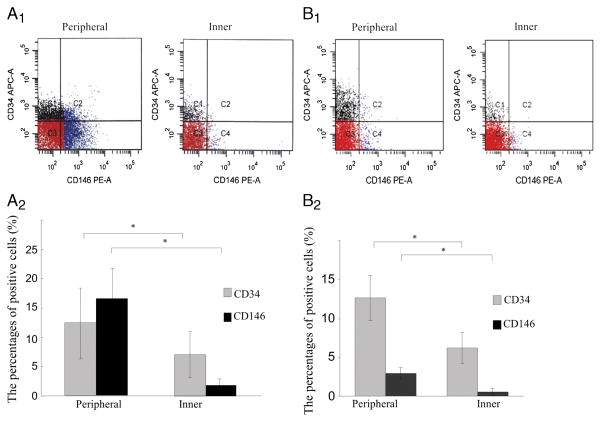
For FACS analysis, CD34-positive cells/CD45-negative cells and CD146-positive/CD45-negative cells were obtained from the peripheral and inner regions of the fetal meniscus (Fig. 2A1). There were significant differences in the percentage of CD34- and CD146-positive cells between the cells isolated from the peripheral and inner regions of the meniscus (P = 0.041 for CD34 and P = 0.03 for CD146) (Fig. 2A2).
FIGURE 2.
Marker profile of meniscal cells by FACS analysis. A1, CD34-positive/CD45-negative cells and CD146-positive/CD45-negative cells from the peripheral (left panel) and inner regions (right panel) of the fetal meniscus. A2, The percentage of positive cells for CD34 and CD146 of the fetal meniscus is shown in the graph (*P < 0.05). B1, CD34-positive/CD45-negative cells and CD146-positive/CD45-negative cells from the peripheral (left panel) and inner regions (right panel) of the adult meniscus. B2, The percentage of positive cells for CD34 and CD146 of the adult meniscus are shown in the graph(*P < 0.05).
The characterization of the meniscal cells from adult menisci by FACS analysis revealed that the percentage of cells positive for CD34 and CD146 were, respectively, 12.62% ± 2.90% and 2.90% ± 0.80% in the peripheral cells and 6.20% ± 2.00% and 0.53% ± 0.43% in the inner cells (Fig. 2B1). Adult meniscus has significantly more CD34-and CD146-positive cells in the peripheral than that in the inner region (P = 0.013 for CD34 and P = 0.047 for CD146) (Fig. 2B2).
Multilineage potential
Because the CD146-positive fraction of cells from the adult meniscus do not proliferate well, only two populations of adult-derived meniscal cells were tested for their multilineage potentials: the CD34-positive cells in the peripheral meniscal region (AP34) and the CD34-positive cells in the inner region of the meniscus (AI34).
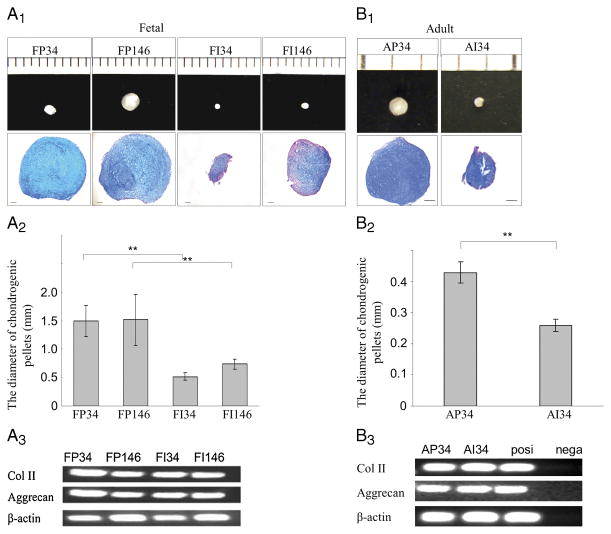
In addition, fetal meniscus cells were sorted for their expression of CD34 and CD146 after eliminating the hematopoietic (CD45-positive) cells. Four populations were tested for their multilineage potential; CD34-positive cells in the peripheral region of the meniscus (FP34), CD146-positive cells in the peripheral region of the meniscus (FP146), CD34-positive cells in the inner meniscal region (FI34), and CD146-positive cells in the inner meniscal region (FI146). All sorted cells from the fetal meniscal cells formed chondrogenic pellets. FP146- and FP34-positive cells showed the largest chondrogenic pellet sizes among all the populations tested (P = 0.001 for PF34 vs PI34, P = 0.002 for PF146 vs PI146) (Fig. 3A2). Pellets formed by the FP34 and FP146 cells appeared to stain more intensely with Alcian blue than FI34 and FI146 pellets (Fig. 3A1). The mRNA expression of COLII and aggrecan was detected in all the pellets (Fig. 3A3).
FIGURE 3.
Chondrogenic potential of meniscus-derived cells. Human meniscus-derived vascular cells were cultured for 21 d in chondrogenic medium. A1, Fetal meniscus-derived vascular cells made chondrogenic pellets (scale shows 1 mm gradation) and were stained with Alcian blue (scale bars = 100 μm). A2, Quantification of chondrogenic pellets sizes. CD146- and CD34-positive cells from the peripheral meniscus region produced significantly larger pellets compared with inner cells (*P < 0.05). A3, RT-PCR analysis for mRNA expression of COLII, aggrecan, and β-actin in the pellets of FP34, FP146, FI34, and FI146 with chondrogenic induction for 21 d. B1, Adult meniscus-derived vascular cells (CD34-positive cells) produced chondrogenic pellets (scale shows 1 mm gradation) and were stained with Alcian blue (scale bars = 100 μm). B2, Quantification of chondrogenic pellets sizes. CD34-positive cells from the peripheral meniscus region produced significantly larger pellets compared with the inner cells (*P < 0.05). B3, RT-PCR analysis for mRNA expression of COLII, aggrecan, and β-actin by the pellets of AP34 and AI34 with chondrogenic induction for 21 d.
All cell populations also produced chondrogenic pellets from adult-derived menisci. AP34 cells showed significantly larger sizes than AI34 cells (P = 0.005 for peripheral vs inner) (Figs. 3B1 and 3B2). Both pellets of AP34 and AI34 cells appeared to be stained well with Alcian blue (Fig. 3B1). The mRNA expression of COLII and aggrecan was detected from both pellets (Fig. 3B3).
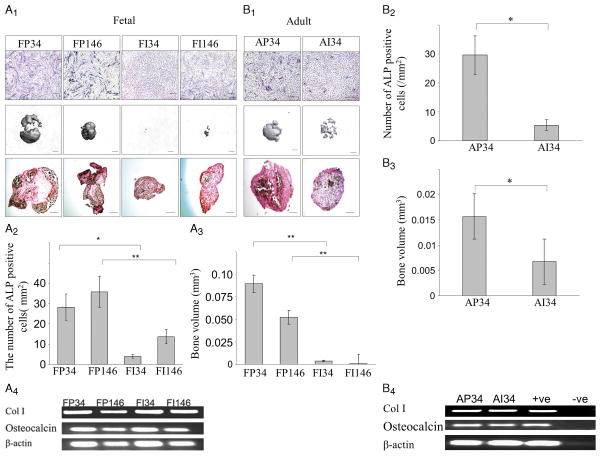
ALP staining of the cells cultured in a monolayer revealed a larger number of positive cells in the periphery (Fig. 4A1) than the inner part of the fetal meniscus. The number of ALP-positive cells of P34, P146, I34, and I146 were 28.20 ± 6.50, 35.80 ± 7.69, 3.80 ± 0.84, and 13.60 ± 3.51, respectively (Fig. 4A2). Peripheral cells displayed significantly more ALP-positive cells than the cells isolated from inner regions of the meniscus (P = 0.012 for PF34 vs PI34, P = 0.008 for PF146 vs PI146) (Fig. 4A2). Micro-CT analysis showed greater mineralization with peripheral cells compared with inner cells (Fig. 4A1). P34 pellets were the largest bone volume among all populations tested (P = 0.005 for PF34 vs PI34, P = 0.005 for PF146 vs PI146) (Fig. 4A3). Preliminary analysis of the von Kossa–positive cells in three fields/cell type showed a greater number of positive cells in the peripheral meniscal cell pellets then in the inner meniscal cell pellets (Fig. 4A1). The mRNA expression of COLI and osteocalcin was detected in all pellets (Fig. 4A3)
FIGURE 4.
Osteogenic potential of meniscus-derived cells. A, Human fetal meniscus-derived vascular cells were cultured in osteogenic medium. A1, Monolayer culture of fetal meniscus-derived vascular cells from each region of the meniscus were also maintained and treated with osteogenic medium for 21 d (scale bars = 50 μm). Pellet cultures of the meniscus-derived vascular cells from each region of the fetal meniscus were also maintained and treated with osteogenic medium for 21 d. Bone volumes were assessed by micro-CT (scale bars = 250 μm). Pellets were von Kossa stained (scale bars = 250 μm). A2, Quantification of the number of ALP-positive cells is shown in the graph. CD146-positive cells in the peripheral region of the meniscus showed more ALP-positive cells (*P < 0.05). A3, Quantification of bone volume in pellets is shown (*P < 0.05). A4, RT-PCR analysis for mRNA expression of COLI, osteocalcin, and β-actin in the cells of FP34, FP146, FI34, and FI146 after osteogenic induction for 21 d. B. Human adult meniscus-derived vascular cells (CD34-positive cells) were cultured in osteogenic medium. B1, Monolayer culture of meniscus-derived vascular cells from each region of the meniscus were also maintained and treated with osteogenic medium for 21 d (scale bars = 50 μm). Pellet culture of meniscus-derived vascular cells from each region of the meniscus were also maintained and treated with osteogenic medium for 21 d. Bone volumes were assessed by micro-CT (scale bars = 250 μm). Pellets of the adult meniscus-derived cells were von Kossa stained (scale bars = 250 μm). B2, Quantification of the number of ALP-positive cells is shown. CD146-positive cells in the peripheral region of the meniscus showed more ALP-positive cells (*P < 0.05). B3, Quantification of the bone volume of the pellets is shown. (*P < 0.05). (e) Pellets were von Kossa stained (scale bars = 250 μm). B4, RT-PCR analysis for mRNA expression of COLI, osteocalcin, and β-actin in the cells of AP34 and AI34 after osteogenic induction for 21 d.
ALP staining also revealed a larger number of positive cells in the periphery than the inner region of the adult meniscus (Fig. 4B1). The number of ALP-positive cells detected in the AP34 and AI34 populations was 29.60 ± 6.60 and 5.40 ± 1.82·mm−2, respectively. Peripheral cells displayed significantly more ALP-positive cells than the cells isolated from inner regions of the meniscus (P = 0.012 for peripheral vs inner) (Fig. 4B2). Micro-CT analysis showed greater mineralization with the peripherally derived cells compared with cells isolated from the inner region of the meniscus (Fig. 4B1). Peripheral cell pellets had significantly more bone volume than inner cell pellets (P = 0.045 for peripheral vs inner; Fig. 4B3). Moreover, von Kossa staining showed a greater number of positive cells in the peripheral cell pellets then the inner cell pellets (Fig. 4B1). The mRNA expression of COLI and osteocalcin was detected in both pellets (Fig. 4B4).
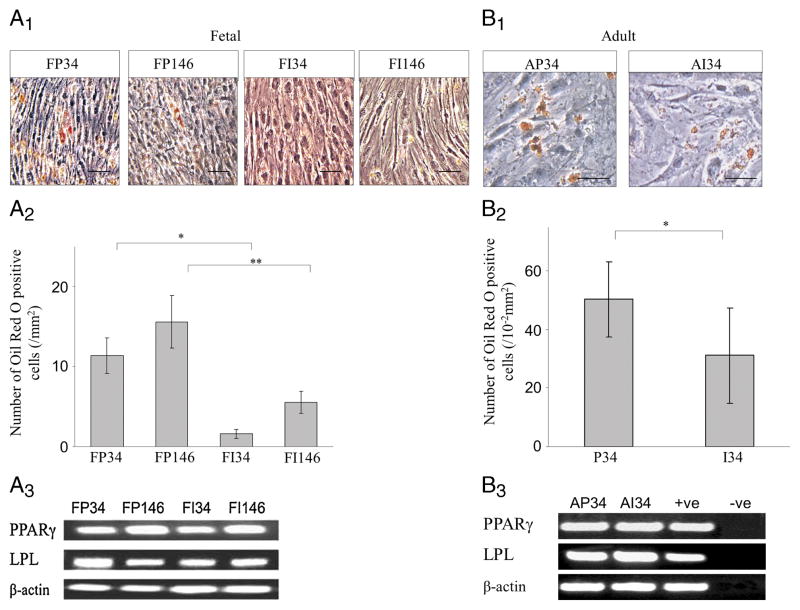
The differentiation of fetal meniscal cells into adipogenic cells was assessed by staining with Oil Red O. All fetal cell populations (Fig. 5A1) were positive for Oil Red O. Oil Red O staining demonstrated that the peripherally derived cells showed significantly more adipose cells (lipid droplets) compared with the inner cells (P = 0.015 for PF34 vs PI34, P = 0.004 for PF146 vs PI146) (Fig. 5A2). The mRNA expression of PPARγ and LPL was detected in all the cells (Fig. 5A3).
FIGURE 5.
Adipogenic potential of the meniscus-derived cells. Human meniscus-derived vascular stem cells accumulated lipid-filled droplets when cultured for 2 wk in adipogenic medium. A, Human fetal meniscus-derived vascular cells were cultured in adipogenic medium. A1, Oil Red O staining revealed lipid-filled intracellular vacuoles (scale bar = 100 μm). A2, Quantification of Oil Red O–stained cells (*P < 0.05). A3, RT-PCR analysis for mRNA expression of PPARγ, LPL, and β-actin in the cells of FP34, FP146, FI34, and FI146 after adipogenic induction for 21 d. B, Human adult meniscus-derived vascular cells (CD34-positive cells) were cultured in adipogenic medium. B1, Oil Red O staining revealed lipid-filled intracellular vacuoles (scale bars = 100 μm). B2, Quantification of Oil Red O–stained cells (*P < 0.05). B3, RT-PCR analysis for mRNA expression of PPARγ, LPL, and β-actin in the cells of AP34 and AI34 after adipogenic induction for 21 d.
CD34-positive cells isolated from the both the peripheral and inner part of the adult menisci also positively stained for Oil Red O (Fig. 5B1). Oil Red O staining demonstrated that peripheral cell population had significantly more adipose cells (lipid droplet–expressing cells) compared with the inner cells (P = 0.011 for peripheral vs inner) (Fig. 5B2). The mRNA expression of PPARγ and LPL was detected in both cell populations (Fig. 5B3).
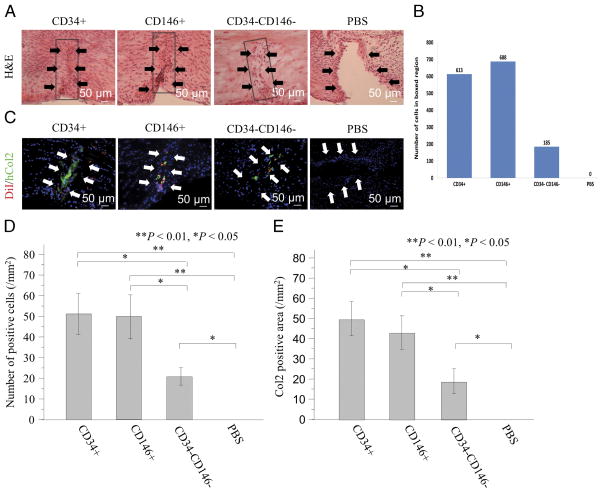
Histological evaluation was performed using H&E staining to assess meniscal healing at week 4. In almost all cases, the location of the defect was marked by a line formed by several layers of spindle-like cells. The CD34+ and CD146+ groups showed an increased cellularity with signs of regeneration at the meniscal tear sites. These repopulated cells resembled fibrochondrocyte-like cells (Fig. 6A). The CD34− CD146− group displayed an incomplete healing of the meniscus showing a central nonhealing region with a defect lined by a layer of spindle-shaped cells and a peripheral area showing healing with collagen and fibrochondrocyte-like and fibroblast-like cells. In the PBS groups, the lack of healing was obvious, and the cut edges of the menisci were populated with flattened spindle-shaped cells with an obvious gap within the defect area. The total number of cells recruited into the injured area was quantified by image analysis and was found to be higher for CD34+ and CD146+ than CD 34−CD146− (Fig. 6B).
FIGURE 6.
Histological and immunohistological evidence of meniscus healing after fetal cell transplantation. A, The peripheral, fetal CD34+, and CD146+ transplanted groups showed an increased cellularity with signs of regeneration at the tear sites of the meniscus, and these repopulated cells resembled fibrochondrocyte-like cells. The CD34−CD146− group displayed an incomplete healing of the meniscus showing a lack of healing in the central region. A layer of spindle-shaped cells and fibrochondrocyte-like and fibroblast-like cells was present within the defect area. B, The total number of cells recruited into the repair of the tear site were higher in CD34+ and CD 146+ cells than cells negative for both markers. In the PBS groups, the lack of healing was obvious. C, The immunostaining for DiI and hCol2 demonstrated the recruitment of transplanted cells to the meniscal healing sites and an enhancement of meniscus healing in the CD34+ and the CD146+ groups when compared with the other groups. D, The number of stained human cells at the meniscal healing sites was significantly higher in the CD34+ and CD146+ groups when compared with the other groups (**P < 0.01, *P < 0.05). E, The number of hCol2-positive cells was significantly greater in the CD34+ and the CD146+ groups than the other groups (**P < 0.01, *P < 0.05).
Immunohistological assessment
The immunostaining for DiI demonstrated the recruitment of transplanted cells at the meniscal healing sites especially in the CD34+ and the CD146+ groups (Fig. 6B). The number of stained human cells at the healing sites was significantly higher in the CD34+ and the CD146+ groups compared with the other groups (CD34+ = 51.0 ± 9.9, CD146+ = 49.5 ± 10.7, CD34−CD146− = 20.3 ± 4.3, PBS = 0.0 ± 0.0/mm2, respectively; P = 0.002 for CD34+ vs PBS, P = 0.029 for CD34+ vs CD34−CD146−, P = 0.004 for CD146+ vs PBS, P = 0.045 for CD146+ vs CD34−CD146−, and P = 0.030 for CD34−CD146− vs PBS) (Fig. 6C). Immunofluorescent staining showed higher levels of expression of hCol2 in the CD34+ and the CD146+ groups when compared with the other groups (Fig. 6B). The number of hCol2-positive cells was significantly greater in the CD34+ and the CD146+ groups than the other groups (P = 0.005 for CD34+ vs PBS, P = 0.029 for CD34+ vs CD34−CD146−, P = 0.008 for CD146+ vs PBS, P = 0.049 for CD146+ vs CD34−CD146−, P = 0.033 for CD34−CD146− vs PBS) (Fig. 6D).
DISCUSSION
Multipotent CD34- and CD146-positive cells were isolated from adult and fetal human meniscus biopsies using FACS sorting. Sorting the cells eliminated the hematopoietic cells. A higher number of CD34- and CD146-positive cells were found in the peripheral (vascular) compared with the inner region. Fetal meniscal cells were transplanted into meniscal tear sites in the rat knee and potentially contributed to meniscus repair.
Multipotent vascular endothelial cells (CD34+), vascular pericytes (CD146+), and myoendothelial cells (CD56+/ CD34+) were isolated by our group from human skeletal muscle samples (9,33). It is very likely that a better outcome of repair in meniscal tears in the vascular region is attributed to more abundant populations of meniscus-derived stem cells or other blood vessel wall-derived perivascular, progenitor cells.
We obtained three major findings from this study. First, immunohistochemical staining showed that CD34- and CD146-positive cells were present in greater numbers in the peripheral region of both fetal and adult menisci than the inner region. Flow cytometry data confirmed our immunohistochemistry results. These results suggested that the vascularized region of the meniscus contains cell populations that are probably vessel-derived stem cells. Verdonk et al. (31) also reported the presence of CD34-positive cells in the outer vascular regions and the superficial region of the meniscus. It was reported that meniscal tears in the peripheral region can heal, probably because they receive a richer blood supply (4). Superior repair outcomes in the vascular meniscus show that these two factors are probably at play. Our data indicated that CD34- and CD146-positive cells, also found in blood vessel walls (9,33), were more abundant in the peripheral (vascular) region of the meniscus than the inner region.
Second, FACS sorted CD34-positive cells from the peripheral and inner regions showed a loss of CD34 expression during their 6 wk in culture. In contrast, the expression of CD146 increased in expression. These results indicate that the cells’ phenotype is dynamic, and thus cell population purity after culture must be carefully considered when using tissue engineering or other therapeutic strategies for meniscus repair. We have observed similar loss of surface marker expression in CD34 and CD144 myogenic cells after long-term expansion (33,35).
Third, it has been reported that CD34-positive cells are committed to an endothelial and perivascular cell lineage (i.e., pericytes and smooth muscle cells) (16,32). In addition, it has been reported that vascular pericytes may arise from CD34-positive cells (14). Matsumoto et al. (21) has suggested that CD34-positive cells might be considered vascular wall progenitor cells that differentiate into other lineages in response to certain microenvironmental signals. CD34-positive cells from the peripheral vascular region and the inner avascular region of human menisci underwent multilineage differentiation: chondrogenic, osteogenic, and adipogenic differentiation. CD34-positive cells from the peripheral-vascularized region displayed a higher multi-lineage differentiation potential compared with the inner-avascular region, but the reason for the difference is not fully understood.
Pellets made from adult peripheral and inner meniscus had the potential to form cartilage in vitro; however, pellets from the peripheral region were more effective at chondrogenesis. Similar results were found in the fetal menisci. Mesenchymal stem cells obtained from bone marrow have been used in meniscus repair (27). Following these findings, we believe that meniscal cells (alone or with other cells) could be used in cell therapy studies to treat meniscal tears.
Furthermore, we examined whether these vascular populations had differentiation potentials for other lineages. It is of note that osteogenic and adipogenic differentiation was confirmed and that their ability to differentiate was distinct to cells derived from the peripheral region. These findings suggest that meniscal vascular cells have characteristics of stem cells and can be found in greater numbers in the peripheral region of the meniscus than the inner region, which we posit leads to the low success rate of healing in the inner region. The similarity of the adult and fetal meniscal cell populations’ phenotypes indicate that there is probably a set of genes that identify populations of cells that have been conserved over the developmental history of the meniscal tissue. Perhaps these cells play an important role in the homeostasis and healing process when the correct signals activate them in the postnatal meniscus.
Finally, results of our in vivo studies showed that meniscus-derived cell populations delivered into the injured rat meniscus contributed to the repair process of the tissue. These results are encouraging because they show novel evidence that meniscal cells with stem cell markers (CD34+ and CD146+) can be successfully isolated, grown in sufficient numbers, transplanted, and incorporated into meniscal tissue.
Study limitations
There are some limitations to the current study. The distinction between the vascular and the avascular regions of the meniscus is difficult to make, especially in fetal menisci. Fetal menisci are different from adult menisci and should be histologically and physiologically assessed in future studies. Further, the adult menisci studied were obtained from older patients and may not be directly comparable with those from younger individuals. We should, therefore, investigate the potential differences between cell populations obtained from menisci from individuals of different ages and gender. In addition, histological studies need to be used to make standardized assessments of meniscal healing but represent rather descriptive studies.
Despite the significance of the in vivo findings of this study, we should emphasize that it was performed in a small animal model of meniscus tear. Therefore, applications to patients still require additional studies on large animal models to demonstrate biomechanical improvement after stem cell therapy and to determine proper delivery method, dosing, timing, and whether there is a need for additional series of injections.
SUMMARY
Our study showed that fetal and adult meniscus-derived CD34-positive and CD146-positive cells not expressing CD45 hematopoietic markers, which were found more prevalent in the peripheral vascular region than that in the inner avascular region, exhibited potential for multilineage differentiation. These results suggest that these populations exhibit stem cell characteristics and may contribute to meniscal regeneration. Consequently, the ability of the vascularized region of the meniscus to heal after injury may not be related exclusively to blood cells, but may also rely on blood vessel–derived cells. To further confirm the cells’ role in the meniscal healing process, we performed in vivo transplantation studies using meniscal injury/defect animal model. Our results suggest that meniscus-derived vascular cells exhibit stem cell characteristics and likely play an important role in meniscal healing after injury. Results of the in vivo experiments have demonstrated that they are incorporated into the injured meniscus tissue in rats. Although the present findings provide important clinical insight for cell-based therapies aimed at enhancing meniscal repair and regeneration after injury, our present study has some limitations. Nevertheless, the present findings may provide important clinical insight for novel regenerative medicine therapies aimed at enhancing meniscal repair and regeneration after injury.
Acknowledgments
Funding for this project was provided to Johnny Huard by the Henry J. Mankin endowed chair at the University of Pittsburgh and the William F. and Jean W. Donaldson endowed chair at the Children’s Hospital of Pittsburgh.
The authors are grateful for the technical and scientific advice provided by Bruno Péault, Alison Logar, James Cummins, Jessica Tebbets, Bo Zheng, Guangheng Li, Karin Corsi, Laurie Meszaros, Lauren Drowley (SCRC), and James J Irrgang, Sarah Henry, and Kimberly Francis (Orthopaedic Surgery, University of Pittsburgh).
Johnny Huard received remuneration as a consultant and royalties from Cook MyoSite, Inc., Pittsburgh, PA. The results obtained in this investigation do not constitute endorsement by the American College of Sports Medicine.
Footnotes
None of the other authors have any conflict of interest to declare.
References
- 1.Adesida AB, Grady LM, Khan WS, Hardingham TE. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res Ther. 2006;8(3):R61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed AM, Burke DL, Yu A. In-vitro measurement of static pressure distribution in synovial joints–part II: retropatellar surface. J Biomech Eng. 1983;105(3):226–36. doi: 10.1115/1.3138410. [DOI] [PubMed] [Google Scholar]
- 3.Arnoczky SP. Building a meniscus. Biologic considerations. Clin Orthop Relat Res. 1999;(367 Suppl):S244–53. [PubMed] [Google Scholar]
- 4.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90–5. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 5.Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14(4):270–5. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 6.Bourne RB, Finlay JB, Papadopoulos P, Andreae P. The effect of medial meniscectomy on strain distribution in the proximal part of the tibia. J Bone Joint Surg Am. 1984;66(9):1431–7. [PubMed] [Google Scholar]
- 7.Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25(9):1523–32. doi: 10.1016/s0142-9612(03)00499-x. [DOI] [PubMed] [Google Scholar]
- 8.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(6):1104–9. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 9.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Dutton AQ, Choong PF, Goh JC, Lee EH, Hui JH. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J Bone Joint Surg Br. 2010;92(1):169–75. doi: 10.1302/0301-620X.92B1.22629. [DOI] [PubMed] [Google Scholar]
- 11.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178–87. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 12.Goble EM, Kohn D, Verdonk R, Kane SM. Meniscal substitutes—human experience. Scand J Med Sci Sports. 1999;9(3):146–57. doi: 10.1111/j.1600-0838.1999.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 13.Heckmann TP, Barber-Westin SD, Noyes FR. Meniscal repair and transplantation: indications, techniques, rehabilitation, and clinical outcome. J Orthop Sports Phys Ther. 2006;36(10):795–814. doi: 10.2519/jospt.2006.2177. [DOI] [PubMed] [Google Scholar]
- 14.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289(6):C1396–407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 15.Huang TL, Lin GT, O’Connor S, Chen DY, Barmada R. Healing potential of experimental meniscal tears in the rabbit. Preliminary results. Clin Orthop Relat Res. 1991;(267):299–305. [PubMed] [Google Scholar]
- 16.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113(10):1311–25. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 18.Lanzer WL, Komenda G. Changes in articular cartilage after meniscectomy. Clin Orthop Relat Res. 1990;(252):41–8. [PubMed] [Google Scholar]
- 19.Macconaill MA. The function of intra-articular fibrocartilages, with special reference to the knee and inferior radioulnar joints. J Anat. 1932;66(Pt 2):210–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto TKR, Mifune Y, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43(3):434–9. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Mauck RL, Martinez-Diaz GJ, Yuan X, Tuan RS. Regional multi-lineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec (Hoboken) 2007;290(1):48–58. doi: 10.1002/ar.20419. [DOI] [PubMed] [Google Scholar]
- 23.Medlar RC, Mandiberg JJ, Lyne ED. Meniscectomies in children. Report of long-term results (mean, 8.3 years) of 26 children. Am J Sports Med. 1980;8(2):87–92. doi: 10.1177/036354658000800205. [DOI] [PubMed] [Google Scholar]
- 24.Noyes FR, Barber-Westin SD. Arthroscopic repair of meniscal tears extending into the avascular zone in patients younger than twenty years of age. Am J Sports Med. 2002;30(4):589–600. doi: 10.1177/03635465020300042001. [DOI] [PubMed] [Google Scholar]
- 25.Noyes FR, Heckmann TP, Barber-Westin SD. Meniscus repair and transplantation: a comprehensive update. J Orthop Sports Phys Ther. 2012;42(3):274–90. doi: 10.2519/jospt.2012.3588. [DOI] [PubMed] [Google Scholar]
- 26.Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341–5. [PubMed] [Google Scholar]
- 27.Port J, Jackson DW, Lee TQ, Simon TM. Meniscal repair supplemented with exogenous fibrin clot and autogenous cultured marrow cells in the goat model. Am J Sports Med. 1996;24(4):547–55. doi: 10.1177/036354659602400422. [DOI] [PubMed] [Google Scholar]
- 28.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–7. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 29.Sekiya JK, West RV, Groff YJ, Irrgang JJ, Fu FH, Harner CD. Clinical outcomes following isolated lateral meniscal allograft transplantation. Arthroscopy. 2006;22(7):771–80. doi: 10.1016/j.arthro.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi N, Suzuki Y, Sagehashi Y, Yamaguchi T, Itoh H, Iwata H. Histologic examination of meniscal repair in rabbits. Clin Orthop Relat Res. 1997;(338):253–61. doi: 10.1097/00003086-199705000-00034. [DOI] [PubMed] [Google Scholar]
- 31.Verdonk PC, Forsyth RG, Wang J, et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13(7):548–60. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–3. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 33.Zheng B, Cao B, Crisan M, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nature Biotechnol. 2007;25(9):1025–34. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 34.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12(7):1891–901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, Chen CW, Li G, et al. Isolation of myogenic stem cells from cultures of cryopreserved human skeletal muscle. Cell Transplant. 2012;21(6):1087–93. doi: 10.3727/096368912X636876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]