Abstract
We have previously reported the high regenerative potential of murine muscle-derived stem cells (mMDSCs) that are capable of differentiating into multiple mesodermal cell lineages, including myogenic, endothelial, chondrocytic, and osteoblastic cells. Recently, we described a putative human counterpart of mMDSCs, the myogenic endothelial cells (MECs), in adult human skeletal muscle, which efficiently repair/regenerate the injured and dystrophic skeletal muscle as well as the ischemic heart in animal disease models. Nevertheless it remained unclear whether human MECs, at the clonal level, preserve mMDSC-like chondrogenic and osteogenic potentials and classic stem cell characteristics including high proliferation and resistance to stress. Herein, we demonstrated that MECs, sorted from fresh postnatal human skeletal muscle biopsies, can be grown clonally and exhibit robust resistance to oxidative stress with no tumorigeneity. MEC clones were capable of differentiating into chondrocytes and osteoblasts under inductive conditions in vitro and participated in cartilage and bone formation in vivo. Additionally, adipogenic and angiogenic potentials of clonal MECs (cMECs) were observed. Overall, our study showed that cMECs not only display typical properties of adult stem cells but also exhibit chondrogenic and osteogenic capacities in vitro and in vivo, suggesting their potential applications in articular cartilage and bone repair/regeneration.
Keywords: myogenic endothelial cells, muscle stem cells, clonal analysis, osteogenesis, chondrogenesis
Recent studies suggest that stem/progenitor cell populations other than satellite cells repair/regenerate skeletal muscle.1–7 Our group previously reported that murine muscle-derived stem cells (mMDSCs), a unique population of myogenic stem cells isolated from the slowly adhering fraction of primary muscle cells by the “preplate” technique, proliferate long-term, self-renew, and differentiate into diverse cell lineages.8–13 mMDSCs express markers typically associated with stem cells, including CD34 and Sca-1. However, expression of these markers in mMDSCs is highly influenced by extended cell culture, leading to the difficulties of finding a valid marker profile for prospective identification and purification of native mMDSCs.
Recently, we have prospectively purified by cell sorting a unique stem cell population associated with the vasculature in the human skeletal muscle.14 These myogenic endothelial cells (MECs) (CD34+/CD56+/CD144+/CD45−), which presumably represent the human counterpart of mMDSCs, can undergo long-term proliferation, multi-lineage differentiation, and repair skeletal and cardiac muscles with high efficiency, similar to mMDSCs.14,15 Although MECs have been characterized in our prior studies,14,15 the true capacity of these cells to function as multi-lineage regenerative units has not yet been fully disclosed, especially in chondrogenesis and osteogenesis. One major limitation in characterizing their multipotent potential is the likely heterogeneous nature in stemness. In the present study, we investigated whether MECs, freshly sorted from adult human skeletal muscle based on their unique cell surface marker profile, preserve chondrogenic, and osteogenic potentials at the clonal level.
Our results showed that MEC clones possess stem cell characteristics equivalent to mMDSCs, including long-term proliferation with no karyotypic abnormalities and high resistance to stress. The robust ability of clonal MECs (cMECs) to differentiate into chondrogenic and osteogenic cell lineages in vitro and in vivo was demonstrated. This clonal study of human MECs highlights their regenerative potential for integrated musculoskeletal repair and regeneration.
METHODS
Human Muscle Biopsy Procurement and Animal Research
The procurement of adult human skeletal muscle biopsies from the National Disease Research Interchange (Philadelphia, PA) was approved by the Institutional Review Board at the University of Pittsburgh Medical Center (UPMC). After procurement, biopsies were immediately transported to our laboratory in Hanks’ balanced salt solution (HBSS; Invitrogen, Grand Island, NY) on wet ice. Animal experiments were approved by the Animal Research and Care Committee of the Children’s Hospital of Pittsburgh of UPMC (Protocol #42-04).
Cell Isolation and Cloning
Muscle biopsies were finely minced and digested with collagenases and dispase to obtain single cell suspension as previously described.14 Cells were immunofluorescently labeled and subject to cell sorting.14 Details are documented in Supplementary Material.
Gene Expression Profiling
Total RNA was extracted from the 1 × 106 cells using Nucleospin RNA kit (Clontech, Mountain View, CA). Details are listed in Supplementary Material. Primer sequences used for PCR are listed in Supplemental Table 1.
Cell Proliferation Analysis and Cell Survival under Oxidative Stress
For the single cell proliferation assay, cMECs were transduced with lentiviral eGFP reporter and further sorted to homogeny by FACS as previously reported.6 To test the capacity of cMECs against oxidative stress, MEC clones were plated onto collagen-coated plates and cultured with proliferation medium containing 400 μM hydrogen peroxide (H2O2) and 2 μl propidium iodide (1:500, PI, Sigma–Aldrich, St. Louis, MO). Bright-field and fluorescent images were taken in a time-lapsed microscopic imaging system. Details are summarized in Supplementary Material.
Tumorigenesis Assay and Karyotype Analysis
To examine the tumorigenic property of cMECs in vitro, we monitored the growth of cMECs plated at different densities on 1% agar in proliferation medium. For karyotype analysis, cMEC clones were cultured for 8 weeks and harvested. The cell pellets were processed for chromosome analysis. Detail procedures are listed in Supplementary Material.
Chondrogenic and Osteogenic Differentiation In Vitro
For in vitro chondrogenesis, 3.0 × 105 cMECs or unsorted human primary skeletal muscle cells (hPSMCs) were pelleted in 15-mL conical polypropylene tubes and cultured in chondrogenic medium (Cambrex, East Rutherford, NJ) supplemented with BMP4 (100 ng/ml; R&D system, Inc., Minneapolis, MN) and TGFβ3 (10 ng/mL; R&D system, Inc.). Pellets were harvested on Days 7 and 21, and then embedded in paraffin. Chondrogenesis was determined by Alcian blue staining, which stains the highly sulfated proteoglycans that are characteristic of the cartilaginous matrix as previously described.16,17 Pellets were counterstained with nuclear fast red. The diameter of each pellet was measured, and the volume of each pellet was estimated by the equation: V = 4/3πr3.
For in vitro osteogenesis, pellets of 3.0 × 105 cMECs were cultured and harvested on Days 7 and 21 in osteogenic medium: DMEM supplemented with dexamethasone [0.1 μM], ascorbate-2-phosphate [50 μM], β-glycerophosphate [10 mM] (all from Sigma–Aldrich), and BMP4 (200 ng/ml). Specimens were scanned with μCT (vivaCT40; Scanco USA, Inc., Wayne, PA) as formerly described.17 Pellets were subsequently sectioned and stained by von Kossa method.16,17 Unsorted hPSMCs were cultured under the same conditions as control.
Chondrogenic and Osteogenic Differentiation In Vivo
To track donor cells after implantation in vivo, cMECs and unsorted cells were genetically engineered to express nuclear LacZ (nLacZ) reporter gene with retroviral transduction as previously reported.16–18 The nLacZ gene transduction efficiency was around 80%. We subsequently co-transduced nLacZ-expressing cMECs with retroviral BMP4 gene as previously described.16,17 After expansion, 5 × 106 co-transduced cMECs or unsorted hPSMCs re-suspended in 100 μl HBSS were seeded onto the surface of a 6 × 6-mm piece of Gelfoam. After Gelfoam absorbed the cell suspension, 3 ml of DMEM supplemented with 10% FBS were added to each well and incubated overnight. On the following day, the cellseeded Gelfoam pieces were implanted into the gluteofemoral muscle pockets of SCID mice (8-week-old male; The Jackson Laboratory, Bar Harbor, ME). A total of 14 mice were used. Mice were sacrificed and scanned by μCT at 2, 4, 8, and 16 weeks after implantation. Tissue samples were harvested and treated with CRYO-GEL Embedding Medium (Cancer Diagnostics, Inc., Morriszille, NC), flash frozen in liquid nitrogen pre-cooled 2-methylbutane (Sigma–Aldrich), cryosectioned at 8 μm thickness, and stored at −80°C. X-gal staining revealed nLacZ-expressing cells based on their β-gal expression (blue nuclei). Briefly, frozen sections were fixed in 1% glutaraldehyde for 1 min, washed, and stained in X-gal solution with counterstain of eosin or immunostain with goat anti-osteocalcin (1:200; Santa Cruz Biotech, Santa Cruz, CA), following the manufacturer’s protocol (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA). Sections were also co-immunostained for goat anti-collagen type II (1:200; Santa Cruz Biotech) or goat anti-osteocalcin, with rabbit anti-β-galactosidase (β-gal) (1:200; Abcam, Cambridge, MA).
Adipogenesis in Culture and Angiogenesis In Vitro and In Vivo
The details of in vitro adipogenesis and angiogenesis as well as in vivo angiogenesis are summarized in Supplementary Material.
RESULTS
Isolation and Characterization of Myogenic Endothelial Cell Clones
MECs (CD34+CD56+CD144+CD45−) were isolated by fluorescence activated cell sorting (FACS) from dissociated muscle biopsies as previously reported.14 Single sorted MEC was then automatically seeded by the autoclone system of the FACSAria sorter into each well of a collagen-coated 96-well plate (seeding density: 1 cell/well). Wells that did not contain exactly 1 cell/well were excluded from the study. A total of six MEC clones from two distinct muscle biopsies were obtained from 576 single-cell seeded wells. The average cloning efficiency was 1.04%, with MECs of donor #1 and #2 having the cloning efficiency of 0.69% and 1.39%, respectively. Clonal MECs (cMECs) at passage 6–15 were analyzed for their phenotypes, single cell proliferation, and multi-lineage differentiation capacity and subsequently used for transplantation experiments. Six MEC clones were individually analyzed for gene expression by RT-PCR. The results showed that genes of the lineage-specific markers were expressed in all clones at similar levels (Fig. 1A). Notably, in addition to the late myogenic markers: desmin, m-cadherin, and CD56, we also detected expression of the early myogenic transcription factors, Pax3, Pax7, and Myf5 in all six clones (Fig. 1A).
Figure 1.

Characterization of clonal myogenic endothelial cells (cMECs) in culture. (A) RT-PCR analysis was performed on all six FACS-sorted MEC clones and compared with HUVECs, cultured unsorted hPSMCs (Unsorted), and fresh skeletal muscle cell lysate (Fresh total cells). All MEC clones consistently expressed myogenic (desmin, CD56, Pax7, m-cadherin, Pax3 Myf5), endothelial (CD34, VE-cadherin, von Willebrand Factor (vWF)), smooth muscle/vascular mural (α-smooth muscle actin, PDGFR-β, NG2, CD146), and mesenchymal stem/stromal (CD90 and CD105) cell markers. (B) Analysis of cMEC clonogenic proliferation capacity by sub-cloning single cells from GFP-transduced MEC clones. A total of 80 sub-cloned single GFP-positive cMEC cells (31% of 258 seeded wells) were tracked. Among them, 1% died, 12% did not divide, 14% divided into two to four cells, and 73% were able to form colonies (>8 cells). (C) Colony growth rate (n = 4) after 17 days in culture showed that the population doubling time was 28.1 ± 5.5 h, and the cell division time was 16.8 ± 2.1 h. (D) After 10 passages in culture, the majority of cMEC metaphases analyzed possess an euploid number (46) of chromosomes.
Single Cells Derived from MEC Clones Undergo Clonogenic Proliferation
To precisely quantitate the clonogenic potential and long-term proliferation of cMECs in culture, we performed sub-cloning analysis. MEC clones were lentivirally transduced at passage 15 to express enhanced green fluorescence protein (eGFP). Sub-cloning analysis was performed with eGFP+ cMECs individually sorted into each well of a 384-well plate by the FACSAria autoclone system (Supplementary Fig. 1). Approximately 1/3 of all wells received exactly one cell per well; the remaining 2/3 received none. Proliferation of sub-cloned cMECs was monitored by a time-lapsed microscopic imaging system. The results showed that 73% of sub-cloned cMECs expanded to more than eight cells, 14% divided into two to four cells, 12% did not divide, and 1% eventually died (Fig. 1B). Cell doubling time and averaged cell division time were 28.1 ± 5.5 h and 16.8 ± 2.1 h, respectively (mean ± SD, n = 4; Fig. 1C). A video of single sub-cloned cMEC proliferation was presented (Supplemental Video).
Gene Expression, Tumorigenesis, Karyotype Analysis, and Resistance to Oxidative Stress
Gene expression analyses revealed that cMECs express genes associated with undifferentiated cells (GABRB3 and DNMT3B), and the stemness gene, IL6ST when compared to the unsorted human primary skeletal muscle cells (hPSMCs; Supplemental Table 2). In contrast, hPSMCs expressed genes associated with myogenic differentiation including: Runx-2, Noggin, MYF5, MyoD1, Des, and Actc (Supplementary Table 2). To assess their tumorigenesis, cMECs cultured for 2 months were analyzed for anchorage independent growth, a hallmark of transformed tumor cells. Cells were plated on a layer of 1% agar at different densities, and colony growth was scored at 2 and 3 weeks post-seeding. Non-adherent cell growth was only observed when cMECs were plated at a very high density of 2.5 × 104 cells/cm2. Cells plated at low densities (25 and 250 cells/cm2) and regular culture density (2,500 cells/cm2) did not grow in an anchorage-independent manner and eventually died (Supplementary Fig. 2). Furthermore, tumorigenesis in vivo was examined by implanting expanded cMECs into skeletal muscle pockets in the hind limbs of SCID mice. No evidence of tumor growth at 12 weeks post-transplantation was observed physically and histologically (data not shown). Finally, karyotype analyses revealed little-to-no structural (data not shown) and numerical (Fig. 1D) abnormalities in the chromosome of all long-term cultured MEC clones.
Non-clonal MECs displayed a superior regenerative capacity in both skeletal and cardiac muscles when compared to myoblasts and/or endothelial cells, a behavior hypothesized to be associated with MECs’ ability to withstand oxidative stress.14,15 Whether cMECs retain the resistance to oxidative stress was examined by culturing the cells in 400 μM H2O2 and analyzing their survival every 12 h over a 72-h period.19 Among five MEC clones tested, four withstood the oxidative stress better than non-clonal MECs (p > 0.05), which had a survival rate of 30.6% at 72 h (Supplementary Table 3). This result suggested that most of the cMECs (80%) have high resistance to extended exposure of oxidative stress.
cMECs Undergo Chondrogenic Differentiation In Vitro and In Vivo
To induce chondrogenic differentiation, cMECs and hPSMCs were pellet-cultured in chondrogenic induction medium supplemented with BMP4 and TGF-β3 for up to 3 weeks. Gross morphology of pellets was compared (Fig. 2A). The average volume of the pellets formed by cMECs was significantly larger than those formed by hPSMCs at Days 7 and 21 (n = 3 per group, p < 0.01, two-tailed unpaired t-test) (Fig. 2B). Pellets were then sectioned and stained specifically for the cartilaginous matrix with Alcian blue (pH = 1). Clonal MEC pellets displayed a stronger chondrogenic potential, based on the intensity of Alcian blue staining, when compared to the unsorted cells, at all three time points (Fig. 2C, inset: whole pellet; main: higher magnification). This assay has been used in the past to determine the extent of chondrogenic potential of myogenic cells.14,16,17 To investigate their chondrogenic potential in vivo, we co-transduced cMECs with retroviruses encoding BMP4 and nuclear LacZ (nLacZ) genes. Co-transduced hPSMCs were used as a control. The transduction efficiency was near 80%, revealed by the positive β-gal staining (blue) localized to the nuclei of cMECs (Fig. 2D.a). The presence of round chondrocytes with LacZ+ nuclei (Fig. 2D.b) and positive immunostaining for collagen type II (Fig. 2D.c) were observed within the implanted scaffold. A few collagen type II-positive cells co-expressed β-galactosidase, confirming the presence of functional chondrogenic cells originated from donor cMECs (Fig. 2D.d). A lack of chondrogenesis was observed in hPSMCs-seeded scaffolds (data not shown). Together these data suggest that cMECs were able to differentiate into chondrocytes in vitro and in vivo, albeit to a different extent.
Figure 2.
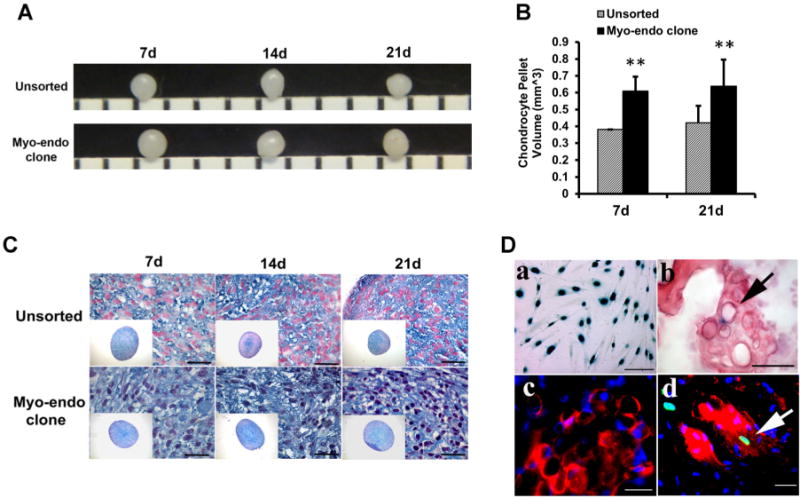
Chondrogenic differentiation in vitro and in vivo. (A) Gross morphology of cartilage-like pellets formed by cMECs and unsorted muscle cells after being pellet-cultured in chondrogenic medium for 3 weeks. (B) cMEC pellets had significantly larger volume than unsorted hPSMC pellets at Days 7 and 21 (**p < 0.01, Student’s t-test). Data were shown as mean ± SD (n = 3). (C) Chondrogenic differentiation is revealed by Alcian blue/nuclear fast red staining of pellets cultured in chondrogenic medium supplemented with BMP4 and TGFβ3 at different time points (Days 7, 14, and 21) (10× magnification, scale bar represents 25 μm). (D.a) cMECs were genetically engineered to express nLacZ reporter gene. Positive staining (blue) is localized to the nuclei of the cells (scale bar represents 25 μm). (D.b) Three weeks after transplantation, chondrogenesis in vivo by cMECs was demonstrated by co-localization of round chondrocytes with blue nuclei (arrow) after staining for β-galactosidase/eosin (scale bar represents 50 μm) (D.c) Chondrogenesis of cMECs was also revealed by positive immunostaining for collagen type-II (red) (scale bar represents 25 μm), and (D.d) Co-localization of collagen type-II (red) and β-galactosidase (green, arrow) signals was also detected (scale bar represents 50 μm).
cMECs Undergo Osteogenic Differentiation in Vitro and In Vivo
To assay the production of mineralized extracellular matrix, cMECs were pellet-cultured in osteogenic induction medium supplemented with BMP4. Osteogenic differentiation was revealed by von Kossa staining after 7 and 21 days in culture. Compared with hPSMCs, pellets formed by cMECs exhibited more intense mineralization (Fig. 3A). cMEC pellets maintained in control proliferation medium with no BMP4 remained negative for von Kossa staining, suggesting no spontaneous osteogenic differentiation of cMECs without a proper inductive signal (Fig. 3A). Mineralization within the pellet was detected by micro-computerized tomography (μCT) at 7 and 21 days (Fig. 3B). μCT images showed that cMECs produced a significantly higher volume (Fig. 3C) and density (Fig. 3D) of mineralized matrix when compared to hPSMCs at both time points (n = 3 per group, both p < 0.01, two-tailed unpaired t-test).
Figure 3.

Osteogenic differentiation in vitro and in vivo. (A) von Kossa staining of cell pellets cultured in the osteogenic inductive conditions at different time points. Compared to unsorted cell pellets, cMEC pellets cultured in the osteogenic inductive medium containing BMP4 appear to display more extensive mineralization at Days 7 and 21. Nevertheless, control cMEC pellets maintained in proliferation medium exhibited no mineralization (scale bar represents 250 μm). (B) MicroCT images showed that cMEC pellets have a significantly higher (C) mineralized matrix volume and (D) mineralized matrix density when compared with unsorted cell pellets at Days 7 and 21 (**p < 0.01, Student’s t-test). Data were shown as mean ± SD (n = 3). (E) cMECs or unsorted hPSMCs were retrovirally transduced to express BMP4, seeded onto Gelfoam, and implanted into the intramuscular pocket of SCID mice. MicroCT imaging demonstrated that cMEC implants appear to give rise to larger and more organized ectopic mineralized tissue than unsorted cells at all time points. (F) There is a significant difference of mineralized tissue volume between the two groups (p < 0.01 at all time points, Student’s t-test). Data were shown as mean ± SEM (n = 3). Osteogenesis in vivo by cMECs was also confirmed by co-localization of (G.a) β-galactosidase-positive nuclei (blue) within eosin-positive cells and (G.b) positive immunohistochemical staining for β-galactosidase (blue) and osteocalcin (brown) in the newly formed mineralized tissue as well as (G.c) co-localized immunofluorescent staining of osteocalcin (red) and β-galactosidase (green, arrow) (scale bar represents 50 μm).
To evaluate their osteogenic potential in vivo, cMECs or hPSMCs were co-transduced with retroviruses encoding nLacZ and BMP4 genes and seeded onto a gelatin sponge (5 × 106 cells), followed by implantation into an intramuscular pocket of SCID mice. μCT imaging revealed that transduced cMECs form dense ectopic bone consistently at 2, 4, 8, and 16 weeks post-implantation while transduced hPSMCs fail to form any organized structure (Fig. 3E). A significant difference in mineralized tissue volume between the two groups was observed at all time points (n = 3 per group, all p < 0.01, two-tailed unpaired t-test) (Fig. 3F). To track donor cMECs undergoing osteogenic differentiation, β-gal/eosin co-staining was performed on the sections of the cMEC-formed ectopic bone structure (Fig. 3G.a). Osteogenic differentiation of donor cMECs was also confirmed by co-localization of the positive immunohistochemical signals of nLacZ and osteocalcin (Fig. 3G.b) as well as the positive immunofluorescent signals of β-galactosidase and osteocalcin (Fig. 3G.c). Collectively, cMECs exhibited robust osteogenic differentiation capacity under appropriate inductive signals in vitro and in vivo.
cMECs Differentiate into Adipocytes and Remain Angiogenic In Vitro and In Vivo
To understand whether cMECs are able to differentiate into other mesodermal cell lineages, we examined their adipogenic potential in vitro. cMECs cultured in adipogenic induction medium were subsequently stained positive by Oil Red O, revealing the accumulated cytoplasmic lipid droplets (Supplementary Fig. 3A). cMECs maintained in control medium were not adipogenic (Supplementary Fig. 3B).
To investigate the angiogenic capacity of cMECs, Matrigel culture was used to observe the formation of capillary-like structures.20 After incubation for 16 h, cMECs cultured in Matrigel formed capillary-like network (Supplementary Fig. 3C) while hPSMCs failed to form similar structures under the same condition (Supplementary Fig. 3D). Next we subcutaneously implanted Matrigel plugs encapsulating 1.0 × 106 cMECs, hPSMCs, or no cells into the back of SCID mice (n = 4 per group). Capillary formation within the implanted plug was determined by anti-CD31 immunostaining (Supplementary Fig. 3E–G). The cMEC-plugs displayed significantly higher capillary density than the hPSMC-plugs (p < 0.01, two-tailed unpaired t-test) (Supplementary Fig. 3H). Implants with no cells exhibited no presence of CD31-positive structures. To confirm the human origin of the newly formed microvessels within the Matrigel plugs, Lamin A/C, a human nuclear specific antigen, was used to identify donor cMECs. A fraction of microvascular endothelial cells within the cMEC plugs indeed co-expressed CD31 and Lamin A/C, indicative of their human origin (Supplementary Fig. 3I–L). These results suggest that human cMECs are not only capable of differentiating into major mesenchymal cell lineages but also retain their angiogenic capacity and participate in neovascularization in vivo after long-term culture.
DISCUSSION
Our group has previously demonstrated that mMDSCs differentiate into diverse cell lineages including bone, cartilage, muscle, endothelial, and blood cells.8–13 mMDSCs repair skeletal and cardiac muscles more efficiently than myoblasts and vascular endothelial cells.9,11 Recently, we have purified myogenic endothelial cells (MECs) from adult human skeletal muscle that co-express cell surface markers of both myogenic and endothelial cell lineages and exhibit superior regenerative capacities in injured skeletal and cardiac muscle, similar to mMDSCs.14,15 Nevertheless, the osteogenic and chondrogenic potentials of MECs were not fully examined at the clonal level. The present study employed the clonogenic assay to evaluate the osteogenic and chondrogenic capacities of single MEC and further characterize their stem cell properties.
We herein established a protocol that enable us to prospectively purify MEC clones from fresh human muscle biopsies directly by FACS sorting, using the previously reported combination of cell surface markers for MEC isolation.14 RT-PCR analysis revealed that all of the MEC clones expressed genes of myogenic (desmin, CD56, Pax3, Pax7, m-cadherin, and MYf5), endothelial (CD34, CD144, and vWF), smooth muscle/vascular mural (α-smooth muscle actin, PDGFR-β, NG2, and CD146), and mesenchymal stem/stromal (CD90 and CD105) cell lineages, showing consistency between clones from the two donor sources. cMECs displayed robust multipotency in vitro and in vivo, including chondrogenesis, osteogenesis, adipogenesis, and angiogenesis/vasculogenesis, in addition to myogenesis reported previously.14 Ectopic implantation into the skeletal muscle pocket triggered the endochondral bone formation by BMP4-transduced cMECs, which can be divided into several phases, including cartilage formation, cartilage resorption, and bone formation.16 Though we have showed evidence of cartilage formation and chondrogenic differentiation from implanted cMECs in the current study, whether the cartilaginous tissue formed by cMECs remains permanent is unclear and requires further investigation. Furthermore, cMECs could not differentiate into hematopoietic cells in vitro under inductive conditions, even with the presence of OP9 stromal cells (data not shown).
Most MEC clones tested (80%) were shown to resist oxidative stress more efficiently than non-clonal MECs. However, one of the five MEC clones tested did not exhibit superior resistance to oxidative stress at 72 h (MEC clone 4, Supplementary Table 3), possibly due to the natural variability in oxidative-stress resistance among MEC clones. cMECs up-regulated genes associated with early progenitor cells, GABRB3 and DNMT3B, and a gene correlated to stemness, IL6ST. When compared to hPSMC, cMECs expressed much lower level of genes associated with advanced myogenic differentiation, including Runx-2, Noggin, MyoD1, MYF5, Desmin, and α-actin. The tumorigenic assay and karyotype analysis revealed no tumorigeneity in long-term expanded cMECs, suggesting the safety of this novel multi-lineage stem cell population in regenerative applications.
In the current study, we have chosen hPSMCs as our donor-matched control cells due to their abundance and easy isolation while native MECs and other populations of early stem/progenitor cells are relatively scarce and difficult to obtain from the same biopsy. Interestingly, under inductive conditions, hPSMC pellet cultures exhibited certain levels of chondrogenic and osteogenic differentiation. This can be explained, at least in part, by the potential trans-differentiation of myoblasts within the hPSMC culture as well as the heterogeneous nature of hPSMCs.16,21 Recently, we have demonstrated that hPSMCs contain a small percentage of blood-vessel-derived stem/progenitor cells, including MECs and pericytes, which were shown to be chondrogenic in nature even after long-term cryopreservation.21
Our previous studies of mMDSCs indicated that these cells reside in areas that are normally occupied by capillaries running alongside myofibers.8,9 Similarly, MECs are associated with the vasculature in human skeletal muscle, specifically the capillaries located within the interstitial space between myofibers. The hypothesis that MECs represent a developmental intermediate between myogenic and endothelial cells was further supported by the evidence suggesting that muscle satellite cells and endothelial cells are close neighbors and privileged developmental partners.22 Despite the unclear developmental relationship between MECs and other blood-vessel-associated stem/progenitor cells such as mesoangioblasts23–25 and pericytes,5,6 our data suggest that cMECs are indeed one of the multi-lineage mesodermal stem cell populations residing in a vascular niche within the adult skeletal muscle1,26,27 and likely represent a human counterpart of mMDSCs.28 Overall, these cells not only represent a promising cell source for musculoskeletal repair but also provide further evidence to the involvement of vasculature in postnatal musculoskeletal regeneration.
Supplementary Material
Acknowledgments
The authors wish to thank Alison Logar for her expert assistance in flow cytometry and James H. Cummins for his editorial assistance. This work was supported in part by grants from the National Institutes of Health (R01-AR049684; RO1-DE13420-06; IU54AR050733-01) and the Department of Defense (AFIRM grant W81XWH-08-2-0032) and by the William F. and Jean W. Donaldson Endowed Chair at the Children’s Hospital of Pittsburgh, the Henry J. Mankin Endowed Chair at the University of Pittsburgh, the Orris C. Hirtzel and Beatrice Dewey Hirtzel Memorial Foundation, and the Lemieux Foundation at the University of Pittsburgh. Johnny Huard received remuneration as a consultant for Cook MyoSite, Inc. during the period of this investigation.
Footnotes
Additional supporting information may be found in the online version of this article.
Conflicts of interest: Johnny Huard received remuneration as a consultant and royalties from Cook MyoSite, Inc., Pittsburgh during the period of time the studies for this manuscript were performed. All the other authors declare no conflict of interest.
References
- 1.Peault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 2.De Angelis L, Berghella L, Coletta M, et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 4.Sampaolesi M, Torrente Y, Innocenzi A, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 5.Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 6.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao B, Zheng B, Jankowski RJ, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 11.Payne TR, Oshima H, Sakai T, et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264–1274. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 12.Gharaibeh B, Lu A, Tebbets J, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protocols. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda R, Usas A, Kubo S, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Cao B, Crisan M, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotech. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Payne TR, Zheng B, et al. Myogenic endothelial cells purified from human skeletal muscle improve cardiac function after transplantation into infarcted myocardium. J Am Coll Cardiol. 2008;52:1869–1880. doi: 10.1016/j.jacc.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Peng H, Corsi K, et al. Differential effect of BMP4 on NIH/3T3 and C2C12 cells: implications for endochondral bone formation. J Bone Miner Res. 2005;20:1611–1623. doi: 10.1359/JBMR.050513. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Corsi-Payne K, Zheng B, et al. The Dose of growth factors influences the synergistic effect of vascular endothelial growth factor on bone morphogenetic protein-4 induced ectopic bone formation. Tissue Eng A. 2009;15:2123–2133. doi: 10.1089/ten.tea.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng B, Cao B, Li G, et al. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 19.Drowley L, Okada M, Payne TR, et al. Sex of muscle stem cells does not influence potency for cardiac cell therapy. Cell Transplant. 2009;18:1137–1146. doi: 10.3727/096368909X471305. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, Chen C-W, Li G, et al. Isolation of myogenic stem cells from cultures of cryopreserved human skeletal muscle. Cell Transplant. 2012;21:1087–1093. doi: 10.3727/096368912X636876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christov C, Chretien F, Abou-Khalil R, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minasi MG, Riminucci M, De Angelis L, et al. The mesoangioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 24.Cossu G, Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Sampaolesi M, Blot S, D’Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 26.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C-W, Corselli M, Péault B, et al. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


