Abstract
Background
Nosocomial infections caused by multidrug resistant organisms are commonly associated with increased length of hospital stays up to 12-18 days, and cost an estimated $6.7 billion per year. One common mode of transmission is cross-contamination between patients and providers via surface contaminants on devices such as telemetry systems.
Objectives
The purpose of this study was to determine the effect of a cleaning protocol on colonization of surface contaminants on telemetry systems in four cardiovascular step-down units. A secondary aim was to compare colonization in medical versus surgical units.
Methods
A prospective, cross-sectional, case-controlled intervention study was designed to evaluate organism colonization on telemetry systems cultured before and after cleaning with sodium hypochlorite wipes. Each randomly selected telemetry system served as its own control. Nurses used a standardized culture technique recommended by infection control. Colonization count pre- and post-intervention was analyzed using McNemar's Test and frequency tables. A standard cost-comparison analysis was conducted.
Results
Fifty-nine telemetry systems were tested, 30 in medical units and 29 in surgical units. Forty-one (69%) telemetry systems were colonized pre-intervention, and eighteen (24%) post-intervention (p < 0.001). In surgical units surface organisms were present in 14 (35%) cases as compared to 27 (66%) cases in medical units (p < 0.001). The cleaning strategy was cost-effective.
Conclusions
The number of organisms present on telemetry systems following a standardized cleaning intervention was significantly decreased, and cost comparison analysis supported use of a cleaning strategy for reusable leads as compared to investing in disposable leads.
Keywords: Telemetry, Surface contaminants, Nosocomial infection, Patient safety, Quality indicators
Nosocomial infections, especially those due to Gram-negative rod bacteria, are associated with increased hospital stays of up to 12-18 days longer than usual hospital stays in which a nosocomial infection does not occur (1). These infections result in an estimated $5.7 to 6.8 billion dollars per year in unnecessary healthcare costs in the United States (2, 3). Overall, the annual direct medical cost of nosocomial infections for hospitals is estimated to be $28.4 to 33.8 billion (2, 4-7). These rising costs and concerns for patient safety in the acute care setting have given rise to numerous questions concerning the factors associated with risk for infection, mechanisms of transmission of nosocomial infections and strategies to better protect patients through prevention of nosocomial infection.
A wide variety of risk factors for nosocomial infections have been identified. These factors range from patient-linked factors such as severity of illness, prolonged hospitalization, antecedent antibiotic therapy (8, 9), and older age with its associated increased susceptibility to infection (10-12), to equipment-linked factors such as pacemakers, implantable defibrillators and ventricular assist devices (13-15). In addition, multiple studies have shown provider and patient behaviors that result in cross-contamination to be a risk factor in nosocomial infections. Hospital personnel, surfaces and equipment have all been identified as common sources of nosocomial infections through cross-contamination (8, 13, 16-19).
Among these risk factors and identified mechanisms for transmission of hospital acquired infection, reusable EKG leads and telemetry boxes are particularly high risk as they represent healthcare equipment that is transferred between patients, touched by many hospital personnel during the patient stay, not traditionally cleaned on a daily basis, and often used by particularly high risk patients, such as those with open thoracic incisions who are elderly and possibly immune-compromised.
The transmission of high risk surface contaminants that is difficult to combat due to the life span of many common and potentially dangerous microbes. A literature review (20) found survival rates of nosocomial microbes ranging from hours to months for common pathogens such as Staphylococcus aureus, Escherichia coli and Enterococcus subspecies. Of these, the organisms of concern include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), certain gram-negative bacilli, and coagulase-negative staphylococci (21, 22). In particular, staphylococcus has been shown to pose an infectious risk to the patients on cardiac wards as a leading cause of major infection following cardiac surgery (23). Studies have shown both direct and indirect correlations between environmental contamination with these long-lived organisms and patient acquisition of both methicillin-resistant and vancomycin-resistant organisms (24, 25).
Strategies to combat nosocomial infections have been established by multicenter trials (26), endorsed by the Centers for Disease Control (CDC), and are followed by hospital infection control departments. These strategies include improving the time to identification and isolation of infected patients, increasing the use of disposable patient care items and equipment, and improving the effective identification and disinfection of non-disposables. In 2008 the CDC published guidelines for cleaning and sterilization of hospital and patient care items using the Spaulding classification scheme, which categorizes instruments and equipment to be cleaned and reprocessed according to the level of risk associated with their intended use (27). Items and equipment for patient care are identified as belonging to one of three categories: 1) critical, 2) semi-critical and 3) non-critical, based on the degree of risk of infection involved in the use of that particular item. With these classifications, it is now easier to determine what type of disinfectant to use to clean patient care surfaces (28). Critical items are those associated with a high risk of infection if the item is contaminated. These items include surgical equipment, cardiac and urinary catheters, implants, and ultrasound probes used in sterile body cavities. Semi-critical items are those that come in contact with mucous membranes or non-intact skin. Respiratory therapy, anesthesia equipment, and esophageal probes are examples of these. Non-critical items are those that come in contact with intact skin but not mucous membranes. Examples of non-critical items are blood pressure cuffs, bed rails, linens, patient furniture, and floors (29).
Cardiac telemetry systems are items that fall between the classifications of semi-critical and noncritical items for disinfection. Skin integrity and implantable hardware determine the level of risk and susceptibility. For this reason telemetry systems on surgical and medical patient care areas may require different cleaning protocols; however evidence to substantiate this hypothesis is lacking. While non-critical surfaces are not commonly associated with transmission of infections to patients (24), two studies found electrocardiography wires do have potential to be a source of colonization for organisms and nosocomial infections. An outbreak of vancomycin-resistant enterocci on a burn unit was traced to a lead wire as a potential source of cross-contamination (30). In addition, Maki & Brookmeyer presented an abstract of a study at the Interscience Conference of Antimicrobial Agents Chemotheraputics in 2003, revealing that one third of cultured lead wires were reservoirs for resistant organisms. These two studies have been cited as the basis and the rationale for using disposable cardiac telemetry lead wires (31, 32), however the complete results of the preliminary data from these studies were not published. More research is needed to build on the body of evidence related to the role of telemetry systems as a source of nosocomial infections. Although the Spalding classification scheme is helpful for prioritizing cleaning and sterilization of hospital and patient care items, little independent research has been done to demonstrate the most effective strategy for minimizing colonization of microbial flora on EKG electrodes and telemetry boxes.
The purpose of this study was to evaluate the effectiveness of a new cleaning protocol on telemetry systems in four cardiovascular step-down units, two surgical and two medical units. The study aims were: to determine the effect of current cleaning practice on numbers and types of surface contaminants on telemetry systems, to determine if a difference exists in pathogen growth between two medical and two surgical units, and to evaluate the cost-comparison for a cleaning strategy as compared to use of disposable EKG electrodes.
Methods
The study used a prospective, cross-sectional, randomized case-controlled intervention design to evaluate colonization of surface contaminants on telemetry systems in four cardiovascular progressive care units.
Setting and Sample
The study subject was the telemetry systems used by both medical and surgical cardiology progressive care units in a tertiary care academic medical center. Sixteen randomly selected telemetry systems were obtained from each of 4 clinical units, 2 medical and 2 surgical, each with 31 possible rooms. The telemetry systems came from occupied and unoccupied clean rooms. Those telemetry systems currently in use in isolation rooms were excluded to decrease the possible incidence of the study team introducing bacteria. Samples were collected on two randomly selected days in June 2010 between 8:00 a.m. and 12 p.m. Research team members were trained by an infection control team in standardized sampling methods to obtain uniform sterile culture samples.
Procedure
In each of the four progressive care units, cases were randomly selected for inclusion in the cross sectional study. On each clinical unit, each telemetry box and lead wire served as its own case and control, pre- and post-intervention. Single sterile culture swabs and individually wrapped 0.52% sodium hypochlorite wipes (Dispatch©) were used. A designated team of staff nurses served as the investigators. The team was instructed by infection control on the uniform technique of swabbing pre-intervention, cleaning, and swabbing post-intervention. Hand washing and gloving was maintained pre- and post-swabbing. Telemetry systems were cultured before and after cleaning using “S” pattern from front to back of unit then swabbing entire length of lead wires from insertion plug to lead snaps. A five-minute waiting period after cleaning with the Dispatch© wipe was observed per manufacturer's recommendation prior to re-swabbing. Each swab was labeled accordingly. All cultures obtained were refrigerated within two hours and shipped within 48 hours of collection to an independent laboratory for analyses.
Culture Method
Swabs were submitted for bacterial and fungal environmental viable cultures. Upon receipt, each swab was diluted in one milliliter of sterile water and plated using 0.1, 0.01 and 0.001 dilutions on the appropriate laboratory media used for target bacteria or fungi isolation. This method is referenced in the American Industrial Hygiene Association book, “Field Guide for the Determination of Biological Contamination in the Environmental Samples” (33).
Bacterial cultures were incubated at 35°C for 48 hours and 25°C for five days for fungal cultures. After incubation, colonies were counted and identified using gram stains, biochemical colonial morphology identification methods, and organism specific identification methods. The cultures were performed by an independent laboratory accredited by the American Industrial Hygiene Association in the Environmental Microbiology Laboratory Accreditation Program (EMLAP) for environmental bacteriology and mycology.
In addition to the cleaning intervention, we performed a cost-comparison to evaluate the cost difference associated with use of disposable as compared to non-disposable EKG leads. Total cost was calculated as unit price multiplied by patient volume (total cost = price of unit × cost of cleaning × volume of units used). To calculate the per patient costs for non-disposable lead use we summed the following cost variables: The annualized depreciation on the cost of single-unit, non-disposable leads; the cleanser cloths and gloves; and the average time for nurses to acquire materials and complete the cleaning and drying process as recommended by the lead manufacturer. By comparison, for disposable leads we summed the following cost variables: the time for acquisition of disposable leads, “product creep” or the incremental increase in the cost of disposable leads, and the cost of multiple lead packs used in patients with longer length of stay (length of >5 days requires replacing disposable leads).
Analysis Method
The primary aim, to identify a relationship between cleaning practices and microbial growth on EKG telemetry boxes and wires, was evaluated by organism counts of growth and specificity. To determine if pathogens growing on telemetry boxes and lead wires were a source of cross-contamination between patients, a Pearson product moment correlation was used. To determine whether differences existed in nosocomial organisms identified from rooms on the surgical floors of the Heart Center as compared to rooms on the medical floors of the hospital, descriptive parametric and non-parametric statistics were used and reported as means and standard deviations and medians and range, as appropriate. In addition, differences in organism counts were evaluated and compared on the surgical versus medical floors using a chi square test of difference (χ2).
Results
Of the 62 possible patient rooms, 59 rooms had samples eligible for analysis. Two sets of samples were lost after the swabbing, and three sets of samples were inadvertently not collected. A total of 118 swabs were collected and available for analysis, 59 prior to cleaning and 59 after cleaning. Types of organisms found on telemetry systems included: coagulation-negative staphylococcus, bacillus, fermentative gram-negative rods, non-fermentative gram-negative rods, and micrococcus (Figure 1). Though many organisms were represented in the sample, only five telemetry units had more than one type of organism that grew concomitantly during this study.
Figure 1. Microbial Growth by Unit Before and After Cleaning Intervention.
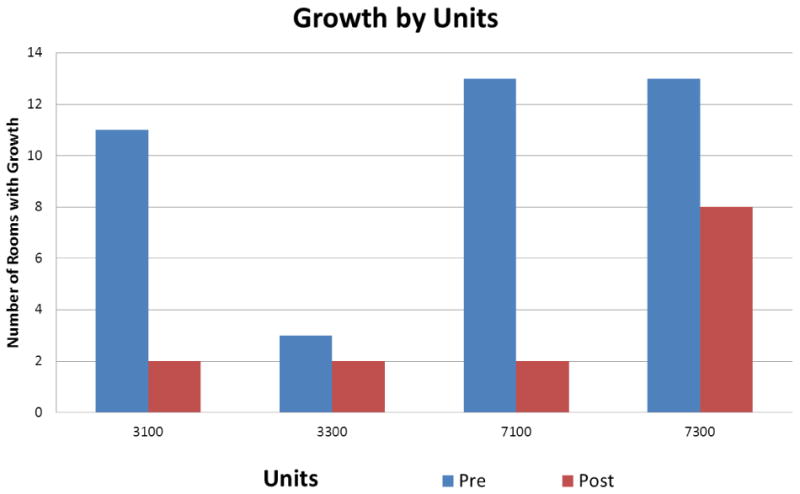
Key: Surgical Telemetry Units – 3100/3300; Medical Telemetry Units – 7100-7300
Overall Organism Growth
There was a significant reduction in organism count pre- and post-intervention (p<0.0001). Prior to the intervention organisms were present on 70% (n=41) of all randomly selected telemetry systems. Following the cleaning intervention organisms were present on 24% of telemetry systems (n=14). In three cases the telemetry systems were organism free at baseline and were subsequently found to have had organisms introduced, either during cleaning or in the culturing process itself (Table 1).
Table 1. Survival Time of Common Surface Contaminants.
| Source | Organism/Route of Transmission | Length of Survival |
|---|---|---|
| Multi-drug resistant pathogens | Vancomycin Resistant Enterococcus (VRE), Methylocillin Resistant Staph Aureus (MRSA), Acinetobecter, Klebsiella, Pseudomonas, Shigella | Several months |
| Respiratory pathogens | Pertussis, Hemophylus Influenza, Vibrio cholera | 2-6 days |
| Respiratory tract viruses | Coxsackie, Severe Acute Respiratory Syndrome (SARS), Influenza | 2-6 days |
| Gastrointestinal viruses | Rota virus | 2 months |
| Gastrointestinal bacteria | Clostridium Difficile | Several months |
| Fungal pathogens | Candida albicans | 4 months |
| Blood borne viruses | Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV) | Greater than one week |
Medical versus surgical units
The types of organisms found pre- and post-intervention did not differ between medical and surgical units. Of the fifty-nine telemetry systems tested, 30 were in medical units and 29 were in surgical units. Forty one (n=41) telemetry systems were positive for organism growth before cleaning, 27 (66%) were in medical rooms and 14 (34%) were in surgical rooms. Following the intervention, the medical units had growth in 10 (30.6%) cases and surgical units had organism growth in four (21.4%) cases. A chi-square test was used to evaluate the difference in organism growth in medical versus surgical units, and no statistically significant difference was found (p= 0.5788), however the baseline difference of 27 versus 14 systems with growth was noted. Further post-hoc analyses were conducted to validate the findings and to determine possible reasons for the difference at baseline.
In the one surgical unit with much lower rates of organism growth (19%, as compared to 85-93 % in the other units), a practice difference related to storage of unused telemetry boxes was identified as the likely cause of the difference. This difference in storage practices was described in field notes of clinical practice observations, which were conducted by the research team on each of the participating units.
Discussion
Findings from this study, conducted in four cardiovascular progressive care units in a university-based hospital, indicate that a standard cleaning protocol significantly reduces surface pathogens on EKG telemetry systems. These findings suggest that though telemetry lead wires are a source of potentially harmful surface contaminants, representing an increased concern for patient safety, a standard cleaning strategy can reduce organism growth to safe and acceptable colony counts. A review of the literature revealed only two studies testing telemetry lead wires as a source of surface contamination (34, 35). Of these, the most recently reported study observed 320 telemetry units across four hospitals and various settings of care (34). In this study, Albert and colleagues tested telemetry units for surface contaminants in these areas and found them to harbor harmful pathogens, with the emergency department and telemetry units harboring more organisms than the operating rooms or critical care areas (34).
The increased prevalence of organisms found in telemetry units by Albert and colleagues was a foundational starting point for the current study. To build on Albert's findings, this study team developed a cleaning intervention for telemetry units and included two medical cardiology telemetry units and two surgical cardiovascular telemetry units. The current study adds to the existing literature by evaluating and demonstrating the benefit of a standard cleaning protocol in reducing the presence of harmful pathogens. The cleaning strategy, developed in collaboration with an infection control team for this study, was found to effectively remove organisms present on telemetry systems (Table 3).
Table 3. Organism Counts Pre- And Post-Cleaning (n=59).
| Organism Count Pre-Intervention | Organism Count Post-Intervention | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Type of Clinical Area | Yes | No | Total | Yes | No | Total | |
| Medical Units | 27 | 3 | 30 | 10 | 20 | 30 | |
| Percentage “Yes” | 90% | 33% | |||||
| Surgical Units | 14 | 15 | 29 | 4 | 25 | 29 | |
| Percentage “Yes” | 48% | 7% | |||||
| Total | 41 | 18 | 59 | 14 | 45 | 59 | 0.0001 |
| Percentage “Yes” | 70% | 24% | |||||
The prevalence and type of organisms found to be present on the telemetry systems during the course of this study was similar between medical and surgical units. Interestingly, the prevalence of organisms was significantly lower in one surgical step-down unit as compared to the other three units (Figure 1). This unit had just three telemetry systems with organism presence pre-intervention and two post-intervention. The rationale for the difference was further investigated and was subsequently attributed to a different unit storage policy for the telemetry boxes. The majority of clinical areas store the telemetry boxes in a charging station in each of the patient care rooms when not in use. In this unit however, the boxes were cleaned and then stored in a central location at the nurse's station rather than in the patient room. In all other units telemetry boxes were stored in the individual patient rooms between use, not at the central station in view of the patients, families and care team. Staff conducted brief observations of practice patterns and concluded that the storage location may have contributed to greater visibility, increased public scrutiny and possibly a more thorough cleaning than was occurring in the other three units.
Attempts to reduce the risk for nosocomial infection by reducing surface contaminants have focused in recent years on the potential advantage of disposable EKG telemetry lead sets. Given the significant decrease in surface contaminants that were achieved using a short cleaning intervention in this study, and the similarities in actual rates of nosocomial infection across the four participating telemetry units, the cost associated with cleaning versus disposable leads was further evaluated.
Actual expenditures for disposable EKG lead sets include; the cost of a new set of EKG leads for each patient admitted to the ward and fees associated with maintaining a two-week supply of lead sets on hand. These leads remain on each patient for the duration of their hospital stay and although the telemetry box itself requires cleaning between patients, nursing staff are relieved of the extra task of cleaning individual leads between patients. The cost of disposable EKG leads changes in accordance with patient census, length of stay, and daily handling of the product. If the patient census and length of stay remain stable, then the cost of disposable EKG leads is predictable and stocking and storage costs are stable over time. However, changes in the clinical setting may affect the cost of disposable leads. Factors that result in an abrupt rise in patient census, a significant decrease in length of stay, or aggressive handling of the product resulting in damage, cause the cost of disposable EKG leads to rise dramatically.
By contrast, the cost of cleaning EKG leads between patients is minimal and includes the nurses' time, a pair of gloves and a simple cleaning product. The protocol used in this study demonstrated a statistically significant (p<0.0001) reduction in organism presence on the telemetry systems and EKG lead wires at a relatively low total cost ($0.21 per patient). The cost of nosocomial infection is primarily associated with prolonged length of hospital stay, during which patients incur preventable bed-days and require additional diagnostic and therapeutic interventions (7). Though the current study was not large enough in scope to demonstrate effect on rates of infection, the use of a standard cleaning protocol may translate into additional savings through lower rates of hospital-associated infections, reduced length of stay, and decreased cost of in-patient care. Cleaning non-disposable EKG leads presents an opportunity for measurable cost-savings at two levels; both the daily cost of care delivery as well as the larger costs of reduced nosocomial infection.
Cost estimates of one episode of nosocomial infection average $20,549 to $25,903 (2, 3). Surgical site infection (SSI) costs range from $11,874 to $34,670 and central line associated blood stream infections (CLABSI) cost $7,288 to $29,156 per patient (2, 3). With the rising cost of nosocomial infections, disposable EKG leads may in fact offer an opportunity to decrease risk of transmission and cross-contamination. Manufacturers of EKG leads suggest that the products may improve hospital system safety profiles by enhancing infection prevention strategies in patient care areas (34, 36). However, many claims for the advantages offered by disposable EKG leads have been reported and financially supported by the manufacturer, causing opportunity for bias that may inadvertently influence the recommendations.
While disposable EKG leads appear to be a responsible choice for preventing infection, the cost of these leads is often misrepresented by disregarding accumulated costs over time. When annualized, the cost of these leads significantly exceeds the costs associated with non-disposable leads. In addition, the extra investment for disposable leads has not been clearly linked with clinical benefit in terms of actual decreases in the incidence of infection a larger, randomized trial.
The current study showed that there was a statistically significant (p<0.0001) reduction in organism presence on EKG systems and wires when they were cleaned using the recommended cleaning product. Because the cleaning strategy was effective in significantly reducing surface contaminants, cleaning the non-disposable leads was determined to be more cost effective as compared to purchasing disposable leads.
Several limitations of this study are important to consider in interpretation of the results. One important consideration was the exclusion of telemetry units in isolations rooms. These units were not eligible for this study due to the different practice patterns in caring for EKG systems for patients with known resistant microbial infestation. Though no cases of methylocillin resistant staph aureus (MRSA), vancomycin resistant enterococcus (VRE) or Clostridium difficele were found, the hypochlorite wipes used in this cleaning protocol strategy have been tested and are approved for use against these organisms. Other limitations were the cost of the laboratory swab cultures and the use of a single facility to evaluate the cleaning strategy. As a result of the cost of culturing before and after cleaning, only one facility was studied and the number of telemetry systems used was small (n=59).
Implications for Practice
Though disposable EKG lead sets offer a strategy for significantly reducing patient exposure to surface contaminants at a minimal cost (36). Though costs associated with actual cases of nosocomial infection far outweigh the costs associated with disposable leads, careful cleaning of non-disposable leads may be even more cost-effective, as results from this study suggest.
The clinical implications of this study include the following recommendations: 1) to adopt a practice of cleaning telemetry systems using sodium hypochlorite wipes between patients and at intervals of 3 days in patients with prolonged length of stay; 2) to establish a hospital-wide cleaning protocol for cleaning telemetry systems and other semi-critical items, such as stethoscopes and bedside temperature monitors for example; and 3) to adopt a centralized telemetry storage system as was observed in the one surgical step-down unit in this study that had significantly fewer organisms present at baseline. The importance of implementing a process standard or practice policy to implement a proven telemetry cleaning strategy was also a key clinical implication of this project.
Conclusion
The results of this study indicate that the proper use of a bleach-based cleaning product can significantly reduce organism growth on EKG telemetry systems. The findings support the effectiveness of a standardized protocol for cleaning telemetry systems in cardiovascular patient care areas, and suggest that implementation of such a protocol is equally effective in both medical and surgical patient care areas. In a cost comparison analysis, the use of disposable EKG leads was not supported as being clinically indicated for patient safety.
Table 2. Organism Types Identified Prior to a Cleaning Intervention.
| Organisms | Total Number of Rooms |
|---|---|
| Coagulation-negative Staphylococcus | 36 |
| Bacillus | 1 |
| Fermentative gram-negative rod | 2 |
| Non-fermentative gram negative rod | 1 |
| Micrococcus | 1 |
| Total | 41 |
Acknowledgments
The authors would like to acknowledge Dr. Daniel Sexton, Chief of Infectious Diseases and Head of the Department of Infection Control for consultation on study design, and Minionette Wilson for administrative support.
This publication was made possible by Grant Number 1 UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- 1.Chen YY, Chou YC, Chou P. Impact of nosocomial infection on cost of illness and length of stay in intensive care units. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2005;26(3):281–7. doi: 10.1086/502540. Epub 2005/03/31. [DOI] [PubMed] [Google Scholar]
- 2.Evans HL, Lefrak SN, Lyman J, Smith RL, Chong TW, McElearney ST, et al. Cost of Gram-negative resistance. Critical Care Medicine. 2007;35(1):89–95. doi: 10.1097/01.CCM.0000251496.61520.75. Epub 2006/11/18. [DOI] [PubMed] [Google Scholar]
- 3.Kilgore ML, Ghosh K, Beavers CM, Wong DY, Hymel PA, Jr, Brossette SE. The costs of nosocomial infections. Medical care. 2008;46(1):101–4. doi: 10.1097/MLR.0b013e3181468991. Epub 2007/12/29. [DOI] [PubMed] [Google Scholar]
- 4.Chen YY, Chou YC, Chou P. Impact of nosocomial infection on cost of illness and length of stay in intensive care units. Infect Control Hosp Epidemiol. 2005;26(3):281–7. doi: 10.1086/502540. Epub 2005/03/31. [DOI] [PubMed] [Google Scholar]
- 5.Graves N. Economics and preventing hospital-acquired infection. Emerg Infect Dis. 2004;10(4):561–6. doi: 10.3201/eid1004.020754. Epub 2004/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilgore ML, Ghosh K, Beavers CM, Wong DY, Hymel PA, Jr, Brossette SE. The costs of nosocomial infections. Med Care. 2008;46(1):101–4. doi: 10.1097/MLR.0b013e3181468991. Epub 2007/12/29. [DOI] [PubMed] [Google Scholar]
- 7.Graves N. Economics and preventing hospital-acquired infection. Emerging infectious diseases. 2004;10(4):561–6. doi: 10.3201/eid1004.020754. Epub 2004/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachary KC, Bayne PS, Morrison VJ, Ford DS, Silver LC, Hooper DC. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2001;22(9):560–4. doi: 10.1086/501952. Epub 2001/12/06. [DOI] [PubMed] [Google Scholar]
- 9.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136(11):834–44. doi: 10.7326/0003-4819-136-11-200206040-00013. Epub 2002/06/05. [DOI] [PubMed] [Google Scholar]
- 10.Gavazzi G, Krause KH. Ageing and infection. The Lancet infectious diseases. 2002;2(11):659–66. doi: 10.1016/s1473-3099(02)00437-1. Epub 2002/11/01. [DOI] [PubMed] [Google Scholar]
- 11.Meyer KC. The role of immunity in susceptibility to respiratory infection in the aging lung. Respir Physiol. 2001;128(1):23–31. doi: 10.1016/S0034-5687(01)00261-4. Epub 2001/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980;2(5):801–10. doi: 10.1093/clinids/2.5.801. Epub 1980/09/01. [DOI] [PubMed] [Google Scholar]
- 13.Maki DG. Risk factors for nosocomial infection in intensive care. ‘Devices vs nature’ and goals for the next decade. Arch Intern Med. 1989;149(1):30–5. doi: 10.1001/archinte.149.1.30. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 14.Smit J, Korup E, Schonheyder HC. Infections associated with permanent pacemakers and implanted cardioverter-defibrillator devices. A 10-year regional study in Denmark. Scand J Infect Dis. 2010;42(9):658–64. doi: 10.3109/00365548.2010.482943. Epub 2010/05/15. [DOI] [PubMed] [Google Scholar]
- 15.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49(18):1851–9. doi: 10.1016/j.jacc.2007.01.072. Epub 2007/05/08. [DOI] [PubMed] [Google Scholar]
- 16.Schabrun S, Chipchase L. Healthcare equipment as a source of nosocomial infection: a systematic review. J Hosp Infect. 2006;63(3):239–45. doi: 10.1016/j.jhin.2005.10.013. Epub 2006/03/07. [DOI] [PubMed] [Google Scholar]
- 17.Lambert I, Tebbs SE, Hill D, Moss HA, Davies AJ, Elliott TS. Interferential therapy machines as possible vehicles for cross-infection. J Hosp Infect. 2000;44(1):59–64. doi: 10.1053/jhin.1999.0647. Epub 2000/01/14. [DOI] [PubMed] [Google Scholar]
- 18.Rutala WA, Weber DJ. Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis. 2004;39(5):702–9. doi: 10.1086/423182. Epub 2004/09/10. [DOI] [PubMed] [Google Scholar]
- 19.Brennan SA, Walls RJ, Smyth E, Al Mulla T, O'Byrne JM. Tourniquets and exsanguinators: a potential source of infection in the orthopedic operating theater? Acta orthopaedica. 2009;80(2):251–5. doi: 10.3109/17453670902930016. Epub 2009/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC infectious diseases. 2006;6:130. doi: 10.1186/1471-2334-6-130. Epub 2006/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134(1-2):45–54. doi: 10.1016/j.vetmic.2008.09.009. Epub 2008/11/07. [DOI] [PubMed] [Google Scholar]
- 22.von Eiff C, Proctor RA, Peters G. Coagulase-negative staphylococci. Pathogens have major role in nosocomial infections. Postgrad Med. 2001;110(4):63–4. 9–70, 3–6. Epub 2001/10/26. [PubMed] [Google Scholar]
- 23.Chen LF, Anderson DJ, Kaye KS, Sexton DJ. Validating a 3-point prediction rule for surgical site infection after coronary artery bypass surgery. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2010;31(1):64–8. doi: 10.1086/649019. Epub 2009/11/17. [DOI] [PubMed] [Google Scholar]
- 24.Rutala WA, Weber DJ. Surface disinfection: should we do it? J Hosp Infect. 2001;48(Suppl A):S64–8. doi: 10.1016/s0195-6701(01)90017-9. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 25.Hota B, Blom DW, Lyle EA, Weinstein RA, Hayden MK. Interventional evaluation of environmental contamination by vancomycin-resistant enterococci: failure of personnel, product, or procedure? J Hosp Infect. 2009;71(2):123–31. doi: 10.1016/j.jhin.2008.10.030. Epub 2008/12/26. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Annals of internal medicine. 2006;145(8):582–91. doi: 10.7326/0003-4819-145-8-200610170-00007. Epub 2006/10/18. [DOI] [PubMed] [Google Scholar]
- 27.Rutala WA, Gergen MF, Weber DJ. Impact of an oil-based lubricant on the effectiveness of the sterilization processes. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2008;29(1):69–72. doi: 10.1086/524326. Epub 2008/01/04. [DOI] [PubMed] [Google Scholar]
- 28.McDonnell G, Burke P. Disinfection: is it time to reconsider Spaulding? The Journal of hospital infection. 2011;78(3):163–70. doi: 10.1016/j.jhin.2011.05.002. Epub 2011/06/15. [DOI] [PubMed] [Google Scholar]
- 29.Rutala WA, Weber DJ. The benefits of surface disinfection. Am J Infect Control. 2004;32(4):226–31. doi: 10.1016/j.ajic.2004.04.197. Epub 2004/06/04. [DOI] [PubMed] [Google Scholar]
- 30.Falk PS, Winnike J, Woodmansee C, Desai M, Mayhall CG. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol. 2000;21(9):575–82. doi: 10.1086/501806. Epub 2000/09/23. [DOI] [PubMed] [Google Scholar]
- 31.Rittenhouse CD, Siegler BA, Voelker CC, Shouval HZ, Paradiso MA, Bear MF. Stimulus for rapid ocular dominance plasticity in visual cortex. Journal of neurophysiology. 2006;95(5):2947–50. doi: 10.1152/jn.01328.2005. Epub 2006/02/17. [DOI] [PubMed] [Google Scholar]
- 32.Jancin B. Antibiotic-resistant pathogens found on 77% of ECG lead wires. Cardiology News. 2004;2(3):14. [Google Scholar]
- 33.Hung LM, J D, Dillon AK, editors. Field Guide for the Determination of Biological Contamination in the Environmental Samples. Third ed. American Industrial Hygeine Association; 2005. [Google Scholar]
- 34.Albert NM, Hancock K, Murray T, Karafa M, Runner JC, Fowler SB, et al. Cleaned, ready-to-use, reusable electrocardiographic lead wires as a source of pathogenic microorganisms. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2010;19(6):e73–80. doi: 10.4037/ajcc2010304. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 35.Baas LS, Beery TA, Hickey CS. Care and safety of pacemaker electrodes in intensive care and telemetry nursing units. AmJCrit Care. 1997;6(4):302–11. [PubMed] [Google Scholar]
- 36.Brown DQ. Disposable vs reusable electrocardiography leads in development of and cross-contamination by resistant bacteria. Critical care nurse. 2011;31(3):62–8. doi: 10.4037/ccn2011874. Epub 2011/06/03. [DOI] [PubMed] [Google Scholar]


