The aorta is the body’s main conduit for blood flow, passing through the chest and abdomen. When this artery’s wall- thick as a garden hose- weakens, the aorta can dilate abnormally, rupture, and cause life-threatening bleeding. Abdominal aortic aneurysms occur most commonly in individuals between 65 and 75 years old. By contrast, thoracic aortic aneurysms and dissections (TAADs) afflict the young as well and arise primarily from noninflammatory mechanisms that often involve underlying genetic mutations (1, 2). Rupture results from mechanical failure, but what renders the aortic wall vulnerable? It may be that TAADs arise from a failure of cellular mechanosensing.
All large arteries grow and remodel to establish and preserve mechanical homeostasis in response to changing hemodynamic conditions (3, 4). The thoracic aorta (see the figure) is subjected to the largest cyclic circumferential stretch from the distending blood pressure and axial stretch from gross motions of the heart. Like other large arteries, it responds to sustained changes in blood pressure, but its extreme compliance and elastic recoil allow it to accommodate large changes in pressure-driven blood flow without changing the contraction of the smooth muscle cells within the wall.
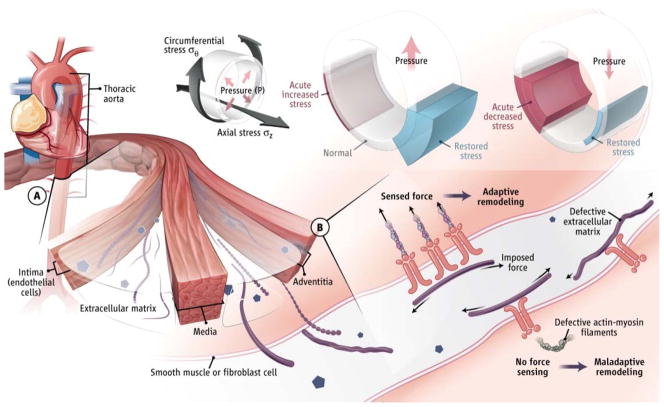
Figure. Aortic mechanobiology.
(A) Cells of the thoracic aortic wall remodel an extracellular matrix (ECM). Endothelial cells (ECs) sense and respond to changes in wall shear stress resulting from blood flow; smooth muscle cells (SMCs) and fibroblasts (FBs) sense and respond to changes in intramural circumferential (σθ) and axial (σz) stresses resulting from blood pressure (P) and motion of the heart. Increases in P increase wall stress but adaptive thickening restores stress to normal values; decreases in P decrease stress but adaptive thinning restores stress.
(B) Cells sense and regulate the ECM via integrins and intracellular contractile structures (actin-myosin filaments). Sensing low versus high stress results in different cell signaling. Misperception of high stress as low stress can result in maladaptive remodeling.
Cells of the aortic wall are embedded in an extracellular matrix that bears most of the stress from blood pressure. Whereas wall stresses are typically 100 to 200 kPa, stresses supported or exerted by cells of the wall are about 3 to 5 kPa (4). This implies that the matrix shields these cells from high stresses. Yet, cells still must sense altered stresses to initiate appropriate remodeling (5, 6). Matrix proteins must also be prestressed when incorporated within existing stressed matrix to promote mechanical homeostasis (4). That is, smooth muscle cells and fibroblasts do not merely secrete collagen fibers; rather, they assemble organized collagen fibrils through force-dependent processes that involve cell adhesion proteins (integrins) and the cytoskeleton (actin and myosin) (7). Hence, cell sensing and regulation of a compliant extracellular matrix are fundamental to maintaining proper thoracic aortic function and structural integrity.
The aortic extracellular matrix consists of myriad proteins, glycoproteins, and glycosaminoglycans, but elastin and collagen play particularly important roles in compliance and recoil, and stiffness and strength, respectively. Smooth muscle cells and fibroblasts sense (5, 6) the mechanical state of this matrix through integrins and the cytoskeleton, and transduction of this state to intracellular signaling pathways allows them to boost the synthesis of matrix components and alter their cytoskeleton in response to cycles of increased mechanical load (from blood pressure) (5, 6). This force-regulated matrix remodeling involves factors that are secreted by cells within the aortic wall. Smooth muscle cells and fibroblasts release transforming growth factor–β (TGF-β), a cytokine that binds to the matrix in latent form and is activated by proteases or integrins through force- dependent processes. Platelet-derived growth factor (PDGF) is also secreted into the matrix and can be released by matrix metalloproteinases (MMPs), which themselves can be influenced by the mechanical state of the matrix. Once released from the matrix, TGF-β and PDGF modulate the expression of cytoskeletal actin and myosin, thereby controlling cellular contractile activity. Another key factor is the peptide hormone angiotensin II, which is released from smooth muscle cells in response to cyclic loading (8) and stimulates cytoskeletal contraction.
A critical aspect of this system is that mechanotransduction depends on cell-generated forces and prestressed cytoskeletal structures (9, 10). This is because the entire aortic wall structure, from the matrix across the cell membrane to the cytoskeleton, is physically interconnected and bears force, with multiple components within this linkage contributing to mechanotransduction. Loss of force any- where within this chain can inhibit mechanosensing.
Genetic mutations that predispose patients to TAADs (1, 2) affect structural components of the aortic wall (e.g., collagen type III and fibrillin-1–containing microfibrils), cellular force generation (e.g., α-actin, myosin heavy chain, and myosin light chain kinase), and transmembrane structures that transduce mechanical stimuli (e.g., polycystins). Animal models have also revealed additional genes that encode either structural components of the matrix (e.g., fibulin-4 and biglycan) or mechanosensing signaling molecules (e.g., integrin-linked kinase) whose mutation leads to TAADs. This list thus includes genes that affect both structure and the ability to modify structure in response to changes in mechanical loads. These two aspects are interwoven—mechanosensing requires intact load-bearing structures and assembly of load- bearing structures requires intact mechanosensing. Compromising either component will give rise to similar deficiencies in mechanical homeostasis.
Physiological adaptation of the aortic wall to elevated wall stress includes smooth muscle proliferation (e.g., via PDGF), synthesis of collagen and other matrix components (e.g., via TGF-β), and increased cell signaling through angiotensin II receptors. Together, these pathways promote thickening and reinforcement of the vessel wall (4–6, 11, 12). By contrast, adaptation to reduced wall stress includes smooth muscle cell death, elaboration of proteolytic enzymes, and thinning of the wall. Thus, defects in either force transmission or mechanosensing could lead to the misperception of high stress as low stress, which would trigger the activation of catabolic low-force pathways that reduce load- bearing capability. Mutations that lead to loss of mechanosensing might include those in the actomyosin–focal adhesion kinase signaling pathway that senses loads, in the TGF-β or angiotensin pathways that contribute to force-dependent matrix remodeling, in other mechanosensors such as polycystins, and in the matrix itself (e.g., defective fibrillin-1). Such mutations might diminish force-dependent responses so that they fail to keep up with changing mechanical conditions, or alter the homeostatic “set point” such that remodeling is inappropriate for the actual mechanics. Hence, defective structural proteins will give rise to dysfunctional mechanosensing, which will lead to the formation of defective structures. This may explain why so many distinct mutations generate similar outcomes, and account for the elaboration of MMPs in the face of high stresses. Cells that mistake high stress for low would likely activate the degradative pathways observed in TAADs.
Seen this way, regulatory pathways that govern mechanotransduction and matrix remodeling are potential targets for treating TAADs. For example, inhibiting a kinase that signals low stress could block maladaptive signaling that contributes to weakening of the aortic wall, whereas activating a pathway that signals high stress might increase wall strength. Understanding the mechanobiology of the thoracic aorta in molecular detail is thus essential to future progress in tackling this disorder.
References and Notes
- 1.Milewicz DM, et al. Annu Rev Genomics Hum Genet. 2008;9:283. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay ME, Dietz HC. Nature. 2011;473:308. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dajnowiec D, Langille BL. Clin Sci. 2007;113:15. doi: 10.1042/CS20060337. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey JD. Cell Biochem Biophys. 2008;50:53. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]
- 5.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. Cardiovasc Res. 2012;95:194. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- 6.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Cardiovasc Res. 2010;88:395. doi: 10.1093/cvr/cvq224. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Van Den Diepstraten C, D’Souza SJ, Chan BMC, Pickering JG. Am J Pathol. 2003;163:1045. doi: 10.1016/s0002-9440(10)63464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz MA. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. J Vasc Res. 1998;35:93. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 10.DuFort CC, Paszek MJ, Weaver VM. Nat Rev Mol Cell Biol. 2011;12:308. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuang SQ, et al. Arterioscler Thromb Vasc Biol. 2013;33:2172. doi: 10.1161/ATVBAHA.113.301624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, et al. J Clin Invest. 2014;124:755. doi: 10.1126/science.1253026. [DOI] [PMC free article] [PubMed] [Google Scholar]