Abstract
The current study takes advantage of modern eye-tracking technology and evaluates how individuals allocate their attention when viewing social videos that display an adult model who is gazing at a series of targets that appear and disappear in the four corners of the screen (congruent condition), or gazing elsewhere (incongruent condition). Data demonstrated the feasibility of administrating this experimental paradigm to a diverse sample of healthy adult college students (N = 44). Results revealed that individual differences in gaze allocation were significantly related to a self-report measure evaluating features of the broad autism phenotype, suggesting that individual variation in the broad autism phenotype is related to individual differences in gaze allocation.
Keywords: Broad autism phenotype, Autism spectrum disorder, Response to joint attention, Gaze following, Eye-tracking, Endophenotype
Introduction
Several decades of research have demonstrated that children with autism spectrum disorder (ASD) show characteristic delays in the ability to spontaneously follow the direction of another person's gaze in social situations (responsiveness to others' bids for joint attention, RJA, Loveland and Landry 1986; Mundy et al. 1986; Sigman et al. 2004). In spite of these characteristic delays, children's performance on gaze following tasks has been linked to both children's chronological and mental age (Leekam et al. 1998; Sigman and Ruskin 1999). Generally, children with ASD with mental ages above 4 years successfully follow another person's eye gaze and head turn under experimental conditions. However, subtle differences in spontaneous gaze following may persist throughout development, particularly during real life situations when the environment is more complex than during a controlled laboratory task. Subtle differences in gaze following may also constitute a sub-clinical feature associated with the broad autism phenotype (BAP); (Swanson et al. 2013).
A number of recent studies have revealed that individuals with ASD show normal patterns of reflexive gaze cueing when evaluated using Posner-style laboratory tasks (for a review see Nation and Penny 2008). In this experimental task, participants are shown a centrally located face that gazes either left or right; after a time lag a target stimulus appears on either side of the face and participants are tasked with pressing a key once they have visually located the target stimulus. Participants usually respond faster to valid trials where the central stimulus cues the target location compared to invalid cues (validity effect; Posner 1980). Similarities in the patterns of reaction times to gaze cues between individuals with ASD and matched controls, however, may hide qualitative differences in underlying cognitive mechanisms (Happé 1995). For example, Senju et al. (2004) found that children with ASD showed the validity effect to both social and nonsocial cues even when the cues were counter-informative (i.e., 80 % of the trials were invalid), whereas in typically developing children only reflexive orienting to faces survived this manipulation, indicating a preferential sensitivity to eye gaze in typically developing children, but not in children with ASD. This research suggests that children with ASD rely on the same processing strategies for social and nonsocial cues, whereas typically developing children utilize different underlying processes for social and nonsocial cues. To date, no prospective longitudinal study has been conducted to evaluate whether early qualitative differences in reflexive orienting underlie later delays in spontaneous gaze following (Zwaigenbaum et al. 2007).
One possible interpretation of these seemingly contradictory findings (i.e., persistent delays in spontaneous gaze following despite normal reflexive sensitivity to gaze cues) is that children with ASD fail to use gaze cues to interpret intention, and make predictions about the behavior of other people (Pelphrey et al. 2005). According to such a theory, delays in spontaneous gaze following may be the consequence of a disrupted motivational system where social stimuli are inherently less rewarding. The current study uses eye-tracking to elicit adults's spontaneous (rather than reflexive) gaze following, using dynamic stimuli designed to simulate naturalistic interactions. That is, participants viewed a series of short videos that show a model at the center of the screen who gazes at a series of target objects that appear/disappear in the four corners of the screen (congruent condition). Participants' pattern of eye gaze while viewing these stimuli were then compared to a set of control stimuli, where the model shifts her gaze equally as often, but her gaze is directed at the empty corners of the screen while the target appears in a different corner (incongruent condition). Similar stimuli have been presented in two recent fMRI studies, showing reliable differences in cortical activation (i.e., ventral medial frontal cortex; superior temporal sulcus) between the two experimental conditions (Pelphrey et al. 2003; Williams et al. 2005). Finally, in our own previous research, we used this eye-tracking paradigm to evaluate the gaze patterns of 50 typically developing children between 3 and 9 years of age. Results revealed that children who presented with few parent-reported features of the BAP consistently modulated their eye gaze between the two experimental conditions. In contrast, children who presented with higher levels of sub-clinical symptoms associated with the BAP showed indistinguishable gaze pattern across both conditions (Swanson et al. 2013). The current brief report aims to investigate whether similar associations between individual differences in participant gaze pattern and features of the BAP are also evident in a sample of typical adults.
Methods
Participants
Forty-four adults were recruited from an urban college located in the Northeastern US. Participants ranged in age from 18 to 50 years (M = 23.58; SD = 7.33; 70 % male), and had completed an average 2.89 (SD = 2.12) years of college. The sample was diverse in terms of ethnicity and race (47.7 % White, 18.2 % Asian, 15.9 % Hispanic, Latino, or Spanish, 6.8 % African American, 2.3 % Middle Eastern, 9.1 % mixed/other) and a substantial proportion of participants (36.7 %) were born outside the US. Many of our participants were first generation college students, with only 41.9 % of their mothers and 46.6 % of their fathers having completed a Bachelors Degree. Informed consent was attained from all participants prior to testing and this study was conducted in accordance of the local Institutional Review Board.
Procedures
Participants were tested during a single laboratory visit lasting 60 min. Participants first completed the Broad Autism Phenotype Questionnaire (BAPQ); (Hurley et al. 2007), presented to participants as the “Personality Styles and Preferences Questionnaire.” Next, participants completed a brief demographic questionnaire and were administration our eye-tracking measure.
Broad Autism Phenotype Questionnaire
The BAPQ, (Hurley et al. 2007) is a self-report questionnaire for adults, evaluating features of the broad autism phenotype. Participants are asked to rate 36 items using a 6 point scale, with responses ranging from very rarely, to very often (see Table 1 for descriptive information). In addition to a total score, the BAPQ provides scores for three subscales that parallel deficits and behaviors common in ASD (i.e., aloof subscale, pragmatic language subscale, rigidity subscale). The current analysis focuses on the BAPQ aloof subscale as it is conceptually related to joint attention. The aloof subscale is intended to tap into “a lack of interest in or enjoyment of social interaction” (Hurley et al. 2007, p. 1681) and items include “I would rather talk to people to get information than to socialize.” Psychometric properties of the BAPQ reveal excellent internal consistency with Cronbach's α scores ranging from 0.80 to 0.95 (Hurley et al. 2007; Sasson et al. 2013). Further, a recent study comparing the BAPQ, social responsiveness scale-A (Constantino, 2002), and autism spectrum quotient (Baron-Cohen et al. 2001) found that the BAPQ was the best of the three measures at quantifying the BAP in terms of internal consistency, criterion validity, and incremental validity in a large population of non-clinical adults (Ingersoll et al. 2011).
Table 1. Descriptive Statistics for BAPQ total and aloof subscale scores and the percentage of our population that scored at or above clinical cutoff as determined by Hurley et al. (2007).
| Variable | Mean (SD) | Range | Clinical cutoff | % Above cutoff |
|---|---|---|---|---|
| BAPQ total score | 2.77 (0.49) | 1.97–4.30 | 3.15 | 13.63 % (n = 6) |
| Aloof subscale | 2.65 (0.74) | 1.33–4.72 | 3.25 | 20.45 % (n = 9) |
| Rigid subscale | 3.04 (0.68) | 2.00–5.00 | 3.50 | 20.45 % (n = 9) |
| Prag. Lang. Subscale | 2.65 (0.52) | 1.75–4.17 | 2.75 | 43.18 % (n = 19) |
Prag. Lang. Subscale pragmatic language subscale
Eye-Tracking Measure of Joint Attention
This eye-tracking measure was developed and described in detail by Swanson et al. (2013). Participants viewed a series of 8 video clips, presented in random order. In the center of the screen, the participant saw the head and face of a model, while targets appeared and disappeared in each of the four corners. Half of the videos were congruent (i.e., the model's gaze and concurrent head turn followed the target on the screen) and intended to engender a joint attention experience, while the other half of the videos were incongruent (i.e., the model's gaze and head were directed elsewhere). See Fig. 1 for a detailed timeline of a sample video.
Fig. 1.
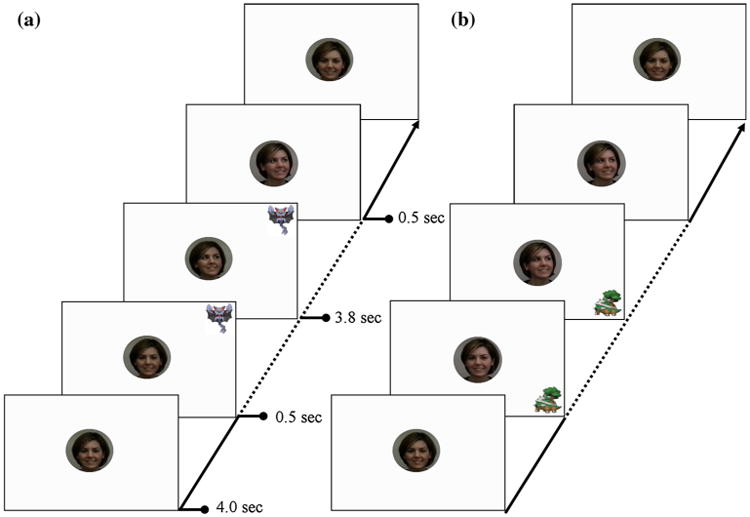
On the left is an example of a congruent condition video, on the right is an example of an incongruent condition video. In both conditions videos begin Phase 1 (4 s): the model looks straight into the camera (simulating the model making eye contact with the participant). Phase 2 (0.5 s): One of eight targets (i.e., popular cartoon characters, © 2011 Nintendo/Pokémon) appears in one of the four corners of the screen. Phase 3 (3.8 s): the model shifts her gaze to a corner and holds her gaze, either congruent or incongruent. Phase 4 (0.5 s): the target disappears while the model continues gazing at the corner. After this last phase, the model shifts her gaze back to the center and the next trial begins
Data Acquisition
Participants completed a standard 5-point calibration during which the eye-tracker collected data and created a physiological 3D model of the eye, later used to calculate gaze data (Tobii Studio, © 2011 TOBII Technology AB). Stimuli presentation and participant eye gaze behavior was recorded using a Tobii T60 eye-tracker with infrared cameras integrated under a 17″ LCD display monitor (Tobii T60, © 2011 TOBII Technology AB). Eye-movements from both eyes were collected at a rate of 60 recordings per second (60 Hz), from an average distance of 60 cm, with an estimated accuracy of 0.4°–0.7° (Tobii Technology AB 2011). Preliminary data processing included gap interpolation of breaks in gaze data shorter than 75 ms, and the application of Tobii I–VT fixation filters (sliding window averaging technique; window length 20 ms, velocity threshold 30 degrees/sec).
Videos covered a rectangular space on the screen of 21.5 by 30.5 cm. Viewed at a distance of 60.0 cm, videos corresponded to a 20.3° by 28.5° visual angle. Face and Target AOIs were created to extend 1 cm past the silhouette of each face and target to compensate for the relative accuracy of the eye-tracker. In terms of average size, Face AOIs were 8.4 cm and circular, while Target AOIs were 7.0 cm and polygons. Viewed at a distance of 60.0 cm, Faces and Targets corresponded to an 8.0° and 6.7° visual angle. The eye-tracking system was completely non-invasive, allowing for free, unconstrained movement of the head and body.
Eye gaze recordings were aggregated by defining scenes (i.e., time windows) and areas of interest (AOI, Boraston and Blakemore 2007). Each scene of interest was defined as the interval between the moments when the target appeared and disappeared on the screen (i.e., phases 2 and 3; Fig. 1). Thus, for each participant, we created 32 total scenes (8 videos, each of which had 4 trials), 16 trials were congruent and 16 incongruent. In addition, we specified two AOIs: (1) Face AOI, or gaze to the model's face, and (2) Target AOI, or gaze to the target. The main metric used to quantify participant eye gaze to the Face and Target AOIs within the scenes was ‘Percent Total Fixation Duration’ (hereto after referred to as ‘% fixation duration’), which measures the sum of the duration of all fixations within a given AOI divided by scene duration (Tobii Studio, Version 2.3.2 © 2011 TOBII Technology AB).
Results
Preliminary Analysis
All following analyses were completed by fitting mixed models for repeated measures using SAS Proc Mixed (SAS software, Version 9.2 © 2002–2008 SAS Institute Inc.). All models were specified with a random intercept, which is equivalent to a Repeated Measure ANOVA. Denominator degrees of freedom for within-subject variables were computed as number of observations—number of participants—number of predictors. First we evaluated whether missing eye-tracking data systematically biased our statistical analyses. To evaluate the percentage of time during which participants gazed at the screen, we computed the ‘On-task Percentage’. On-task Percentage was measured by summing all time spent in fixations as well as time looking at the screen in between fixations and dividing by the scene duration. Across participants, the mean On-task Percentage ranged between 90.40 and 100.00 % (M = 98.57, SD = 2.19). For all subsequent analyses, trials with an On-task Percentage smaller than 50 % were considered missing (14 trials). Results from fitting a mixed model using SAS Proc Mixed with On-task Percentage as outcome and experimental condition (i.e., congruent vs. incongruent) as a fixed effect showed that the mean On-task Percentage did not differ significantly between the congruent and incongruent condition, F(1, 1,357) = 0.04, p = .83. Likewise, no significant associations were found between the On-task Percentage and participant age or gender, either as a main effect or interaction effects with experimental condition, p > .08.
Second, we investigated relations between target-size and gaze behavior. Across the 8 targets used in this research, targets covered on average 4.02 % of the screen (SD = 0.75 %; range 2.84–4.78 %). Two mixed models were fitted with one of the two dependent variables (% fixation duration to the Target AOI and % fixation duration to the Face AOI). The models were specified with two fixed effects: experimental condition (congruent, incongruent) and target size. Results showed that there was a significant main effect of target size for the Target AOI, F(1, 1,349) = 6.01, p < .05, as well as the Face AOI, F(1, 1,349) = 4.64, p < .05. On average, during trials with larger targets, participants demonstrated more gaze allocation to the Target AOI and less gaze to the Face AOI, as compared to trials with smaller targets. We did not find any evidence for a significant target-size by condition interaction effect (p >. 87). Finally, preliminary analyses revealed no significant associations between either of the two dependent variables and participants' gender or chronological age, p > 30. However, our data revealed that participants belonging to an ethnic/racial minority allocated more attention to the Target AOI, F(1, 42) = 7.35, p < .001, and less attention to the Face AOI, F(1, 42) = 13.97, p < .001, than white participants. The interaction between global participant characteristics (i.e., chronological age, gender, race) and experimental condition was not significant for either of the two dependent variables, p > .25.
Fixation Duration to the Face and Target AOIs
Previous findings from our child sample revealed that the association between children's gaze pattern and features of the broad autism phenotype was the strongest when the receptive aspects of children's social impairments were evaluated (Social Awareness Subscale of the Social Responsiveness Scale, Constantino 2002). This association between children's responsiveness to others' bids for joint attention and their emerging social and linguistic comprehension has been emphasized in the recent developmental and neuro-psychological literature (Mundy and Jarrold 2010). Thus, in the current study, we predicted that individual differences in adult's gaze patterns were most closely associated with social impairments associated with ASD (i.e., the aloof subscale of the BAPQ). Specifically, we predicted that differences in fixation duration (see Data Acquisition) between the congruent and incongruent condition would be more pronounced for participants with low BAPQ aloof scores and less pronounced for individuals with high BAPQ aloof scores. To evaluate this hypothesis, we fit mixed models with either Face or Target AOI as the dependent variable. All models included two main effects (i.e., BAPQ scores, experimental condition), and one interaction effect (i.e., BAPQ × condition). Results from this analysis are reported in Table 2 and revealed significant condition 9 BAPQ aloof subscale interaction effects for both, the % fixation duration to the Face AOI, F(1, 1,348) = 4.22, p < .05, and the % fixation duration to the Target AOI, F(1, 1,348) = 4.19, p <.05. For completeness, similar models were also specified for the BAPQ total score, as well as the two remaining BAPQ subscales (i.e., Rigidity, Pragmatic Language). Results from these models are also reported in Table 2. Models with significant BAPQ × condition interactions effects were re-tested including target size and participant race as covariates, and all models remained significant.
Table 2. Associations between % fixation duration and features of the broad autism phenotype, N = 44.
| Intercept | Condition ME | BAPQ ME | BAPQ × condition | RoSa | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | Upper limit | % Above cutoff | |
| Dependent variable: % fixation duration to face AOI | ||||||
| BAPQ total | 34.84 (13.49) | −13.51 (4.71)** | −4.36 (4.59) | 3.43 (1.63)* | 3.29 | 11.36 |
| BAPQ aloof | 23.25 (9.11) | −9.90 (3.11)** | −0.19 (3.10) | 2.22 (1.08)* | 3.46 | 11.36 |
| BAPQ rigidity | 43.62 (9.94) | −10.56 (4.46)* | −6.86 (2.99)* | 2.15 (1.41) | – | – |
| BAPQ prag. lang. | 23.59 (13.03) | −6.75 (4.84) | −0.32 (4.81) | 1.04 (1.84) | – | – |
| Dependent variable: % fixation duration to target AOI | ||||||
| BAPQ total | 53.113 (15.69) | 19.84 (7.76)* | 4.86 (5.28) | −4.88 (2.61)+ | 3.39 | 11.36 |
| BAPQ Aloof | 67.18 (10.75) | 15.34 (5.09)** | −0.23 (3.60) | −3.41 (1.66)* | 3.53 | 9.09 |
| BAPQ rigidity | 40.71 (12.366) | 17.58 (7.55)* | 8.49 (3.82)* | −3.70 (2.36) | – | – |
| BAPQ prag. lang. | 67.08 (15.31) | 6.54 (6.77) | −0.19 (5.65) | −0.09 (2.55) | – | – |
ME main effect, SE standard error, RoS region of significance, BAPQ prag. lang BAPQ pragmatic language
p < .05;
p < .01;
p = .06
Region of significance analysis only computed for significant, or marginally significant BAPQ × Condition terms
To further probe the nature of these BAPQ × condition interaction effects, we used a ‘Regions of Significance (RoS)’ approach to identify specific BAPQ values (i.e., upper limits) for which differences between the congruent and incongruent conditions were significant (Preacher et al. 2006). This method is statistically conservative and practically more meaningful than other methods used to analyze continuous moderators (i.e. splitting participants into quartiles). Separate RoS values were computed for the Face AOI and Target AOI. Table 2 reports the RoS values (upper limit) for BAPQ aloof subscales as well as BAPQ total score. In addition, Fig. 2a and b display a graphic illustration of the condition × BAPQ aloof subscale interaction effect for the Face and Target AOI, respectively. For example, participants with BAPQ aloof subscale scores below 3.45 reliably differed in their % fixation duration to the Face AOI between the congruent and incongruent condition. In contrast, participants scoring above this value (n = 5, 11.4 %) did not reliably differ in their % fixation duration to the Face AOI between the congruent and incongruent condition (Fig. 2a). Further, the individuals with the lowest aloof scores spent the most time fixating the target in the congruent condition (when the model is also viewing the target), whereas the individuals with the highest aloof scores spent similar amounts of time fixating the target across congruent and incongruent conditions (Fig. 2b).
Fig. 2.
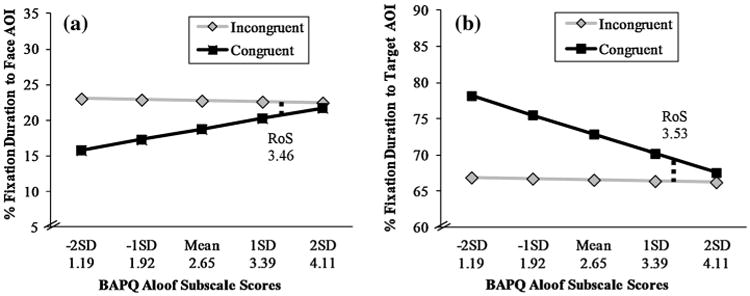
Interaction plots based of two mixed models: a condition × BAPQ aloof subscale interaction predicting % fixation duration to the Face AOI, b condition × BAPQ aloof subscale interaction predicting % fixation duration to the Target AOI. Regions of significance (RoS, upper limits) are indicated with a dotted line
One limitation of modern methods for analyzing repeated measures is that results from fitting mixed models cannot easily be presented graphically in terms of subject-level raw data. Thus, we used simplified, and statistically less powerful traditional methods to generate simple scatter plots. For each participant, we computed two difference scores, representing the degree to which participants modulate their gaze pattern in response to the experimental condition. The first difference score focused on participants' % fixation duration to the Face AOI and was computed as the difference between (1) participants average % fixation duration to the Face AOI during the 16 congruent trials, and (2) participants average % fixation duration to the Face AOI during the 16 congruent trials (congruent minus incongruent). The second difference score was computed identically and focused on the participants' % fixation duration to the Target AOI. Scatter plots representing the association between these two difference scores and participants' BAPQ aloof subscale scores are presented in Fig. 3.
Fig. 3.
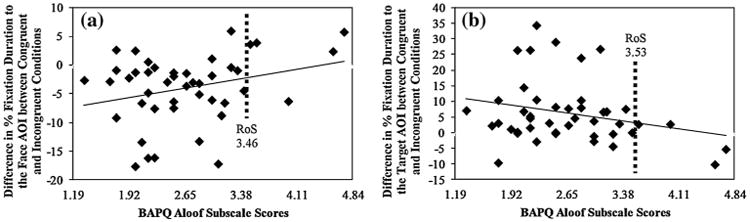
Simplified scatter plots representing the association between a the difference (congruent minus incongruent) between participants' % fixation duration to the Face AOI and their BAPQ aloof subscale scores, and b the difference (congruent minus incongruent) between participants' % fixation duration to the Target AOI and their BAPQ aloof subscale scores
Discussion
Findings from a recent eye-tracking study of typically developing children suggest that individual differences in parent-reported features of the BAP are associated with individual differences in children's patterns of eye gaze while viewing stimuli designed to engender a joint attention experience (Swanson et al. 2013). The current research reveals that similar relations are also evident when evaluating a diverse sample of healthy adult college students. Participants viewed a series of video clips that showed an adult model gazing at a series of targets that appeared and disappeared in the four corners of the screen (congruent condition). Gaze behavior in the congruent condition was compared to a set of control stimuli where the model's gaze was not directed at the appearing/disappearing targets (incongruent condition). Results revealed that adults with few BAP features consistently modulated their gaze pattern between the congruent and incongruent condition. In contrast, this condition-specific modulation was significantly attenuated among adults with higher levels of social impairment.
The pattern of fixation durations observed among adults with few features of BAP (i.e., individuals who scored 1 SD below average on the BAPQ Aloof subscale) revealed that adults consistently allocated less attention to the Face AOI during the congruent than the incongruent condition (21 vs. 27 %, respectively). For comparison, data from our child sample (Swanson et al., 2013) revealed a similar pattern, even though children allocated somewhat higher levels of attention to the Face AOI than adults across both conditions (32 vs. 37 % for the congruent and incongruent condition, respectively). One possible interpretation of longer fixation durations during incongruent videos is that participants may allocate more gaze time to the Face AOI when it is harder for them to interpret the relevant gaze cues (Goldberg and Kotval 1999). Consistent with this interpretation, our data suggests that children require more time to interpret gaze cues than adults; similarly individuals with few social impairments may require more time to interpret gaze cues in the incongruent rather than the congruent condition (e.g., they may check the model's gaze more frequently in the incongruent rather than the congruent condition).
The fixation durations observed among adults with high levels of social impairments (i.e., adults who scored 1 SD above average on the BAPQ Aloof subscale) differed from their more socially adept peers mainly while viewing the congruent videos. While adults with low levels of BAP features showed a marked reduction in gaze allocation to the Face AOI in the congruent when compared to the incongruent condition (21 vs. 27 %, respectively), adults with high levels of BAP features showed relatively high levels of gaze allocation to the Face AOI during both conditions (24 vs. 26 % for the congruent and incongruent condition, respectively). Thus, in the congruent condition, adults with high levels of BAP features allocated more gaze time to the Face AOI than adults with low levels of BAP features. One possible interpretation of this finding is that, in the congruent condition, adults with low levels of BAP features are more likely to follow the model's gaze to the Target AOI than adults with high levels of BAP features. This interpretation is consistent with results from the participants' gaze allocation to the Target AOI. That is, in the congruent condition, adults with lower levels of BAP features allocated more gaze time to the Target AOI than adults with higher levels of BAP features (74 vs. 66 %, respectively). This finding is also consistent with recent research on infants who are at high-risk for developing ASD. That is, Bedford et al. (2012) found that 13 month olds who go on to develop atypically (i.e., ASD, significant delays in cognitive or language development) showed significant deficits in gaze allocation to a congruent target. In the current study, both outcome measures (% fixation duration to the Face AOI and the Target AOI) are highly correlated (intra-class correlations reveal that 64 % of the variance is shared between the two measures). Thus, it is difficult to directly evaluate the causal mechanisms that underlie the current pattern of results. At least two alternate interpretations should be considered. (1) Compared to participants with low BAP features, participants with high BAP features gaze less at the Target AOI during the congruent condition due to a reduced propensity for using gaze cues to interpret the intentions of other people (Pelphrey et al. 2005). (2) Compared to participants with low BAP features, participants with high BAP features gaze more at the Face AOI due to underlying difficulties disengaging from a central stimulus (i.e., the models' face; Landry and Bryson 2004).
In the current sample of 44 adults, 18 % of participants scored above cut off on at least two subscales of the BAPQ, thus meeting clinical cutoff scores proposed by the developers of the measure (Hurley et al. 2007). Available information on the prevalence of features of BAP in the general population are largely unavailable, although Losh et al. (2008) reported that 22 % of Down syndrome parents showed features of the BAP. Similarly, a recent, larger sample of college students (N = 626; Ingersoll et al. 2011) revealed that the average BAPQ scores (both total and subscale scores) were strikingly similar when compared to the current study. Thus, at least in general populations of college students, features of the broad autism phenotype are likely much more common than previously suspected (Rutter 2011).
The literature on spontaneous gaze following in individuals with ASD paints a rather complex picture, revealing both characteristic strengths and delays (Nation and Penny 2008). The current research demonstrates that subtle deficits in gaze following constitute not only a characteristic feature of ASD, but are also associated with sub-clinical features of the broad autism phenotype. Importantly, these associations are not only evident in child samples (Swanson et al., 2013), but also extend to community samples of college students. These findings are important because they suggest that it may be feasible to use eye-tracking measures to define an endophenotype that is both heritable and closely related to the neural circuitry that underlies key aspects of social information processing (Nummenmaa et al. 2012; Pelphrey et al. 2011; Spencer et al. 2011; von dem Hagen et al. 2011). Most current theories suggest that individual genetic risk factors are more closely related to specific features or components of ASD (i.e., endophenotypes) than ASD itself (Abrahams and Geschwind 2008; Geschwind and Alarcón 2006).
Although findings from this research are important, there are limitations to the conclusions that can be drawn. First, findings from the current study revealed as significant association between the BAPQ aloof subscale scores and the participants' gaze pattern while viewing eye-tracking stimuli designed to engender a joint attention experience. However, since our outcome measures relied on a single self-report measure and did not capture traits or disorders that are conceptually unrelated to features of ASD (e.g., internalizing symptoms, learning disabilities), and since our eye-tracking measure did not include a non-social control condition, it is impossible to determine if the identified association is specific, or could be attributed to global characteristics of gaze allocation or developmental outcomes. On the one hand, exploratory results from our own research suggests that individual differences in gaze allocation may also be associated with non-social BAP features such as the BAPQ rigid subscale scores (even though this association was non-significant in the current research, p = .12). On the other hand, recent research on high-risk infants with ASD shows that 7 month olds who go on to develop ASD take slightly longer to shift gaze from a central to a peripheral target, as compared to infants who go on to develop typically (Elison et al. 2013). A second limitation of the current study is that gaze time allocation was the only metric used to capture individual differences in eye gaze pattern. That is, clinical characterizations of ASD typically emphasize not the absence of eye contact, but rather a lack of flexibility and social modulation (Lord et al. 2012). Consistent with this characterization, Freeth and colleagues (Freeth et al. 2010; Freeth et al. 2011) recently reported on a time course analysis of children's gaze behavior, showing that children with ASD differed from matched controls in that they were slower to first fixate the face of the model. Third, results from the Regions of Significance analysis as well as the simplified scatter plots revealed that the number of adults who failed to modulate their gaze between the two experimental conditions was relatively small (n = 4 to n = 5). Thus, future research should specifically target individuals who display signs of the broad autism phenotype (e.g., family members of individuals with ASD, college students who fail ASD-specific screeners).
Acknowledgments
We would like to thank the Hunter College community for participating in this study. We are grateful to the past and present members of the Communication and Play Lab for their continued support, especially Gayle Serlin who helped collect data for this study. This study was supported by a Grant-In-Aid of Research from the National Academy of Sciences, administered by Sigma Xi. The Scientific Research Society, and a Doctoral Student Research Grant from the Graduate Center of City University of New York awarded to MRS. This experiment was carried out by MRS as part of her doctoral dissertation.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from Asperger Syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, et al. Precursors to social and communication difficulties in infants at-risk for autism: Gaze following and attentional engagement. Journal of Autism and Developmental Disorders. 2012;42:2208–2218. doi: 10.1007/s10803-012-1450-y. [DOI] [PubMed] [Google Scholar]
- Boraston Z, Blakemore SJ. The application of eye-tracking technology in the study of autism. Journal of Physiology-London. 2007;581(3):893–898. doi: 10.1113/jphysiol.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. The social responsiveness scale. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Botteron KN, Dager SR, Estes AM, Evans AC. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. American Journal of Psychiatry. 2013 doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeth M, Chapman P, Ropar D, Mitchell P. Do gaze cues in complex scenes capture and direct the attention of high functioning adolescents with ASD? Evidence from eye-tracking. Journal of Autism and Developmental Disorders. 2010;40(5):534–547. doi: 10.1007/s10803-009-0893-2. [DOI] [PubMed] [Google Scholar]
- Freeth M, Ropar D, Mitchell P, Chapman P, Loher S. Brief report: How adolescents with ASD process social information in complex scenes. Combining evidence from eye movements and verbal descriptions. Journal of Autism and Developmental Disorders. 2011;41(3):364–371. doi: 10.1007/s10803-010-1053-4. [DOI] [PubMed] [Google Scholar]
- Geschwind D, Alarcón M. Finding genes in spite of heterogeneity: Endophenotypes, and expression profiling in autism. In: Moldin SO, Rubenstein JLR, editors. Understanding autism: From basic neuroscience to treatment. Boca Raton, FL: CRC Press; 2006. pp. 75–93. [Google Scholar]
- Goldberg JH, Kotval XP. Computer interface evaluation using eye movements: Methods and constructs. International Journal of Industrial Ergonomics. 1999;24(6):631–645. doi: 10.1016/S0169-8141(98)00068-7. [DOI] [Google Scholar]
- Happé FGE. The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Development. 1995;66(3):843–855. doi: 10.2307/1131954. [DOI] [PubMed] [Google Scholar]
- Hurley RSE, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders. 2007;37(9):1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, Hopwood C, Wainer A, Brent Donnellan M. A comparison of three self-report measures of the broader autism phenotype in a non-clinical sample. Journal of Autism and Developmental Disorders. 2011;41(12):1646–1657. doi: 10.1007/s10803-011-1192-2. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Targets and cues: Gaze-following in children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham S, Bishop SL. Autism diagnostic observation schedules, second edition (ADOS-2) manual (part I): modules. Torrance, CA: Western Psychological Services; 2012. pp. 1–4. [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(4):424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16(3):335–349. doi: 10.1007/bf01531663. [DOI] [PubMed] [Google Scholar]
- Mundy P, Jarrold W. Infant joint attention, neural networks and social cognition. Neural networks: The Official Journal of the International Neural Network Society. 2010;23(8–9):985. doi: 10.1016/j.neunet.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contributions of nonverbal-communication measures. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology. 2008;20(1):79. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Engell AD, von dem Hagen E, Henson RNA, Calder AJ. Autism spectrum traits predict the neural response to eye gaze in typical individuals. NeuroImage. 2012;59(4):3356–3363. doi: 10.1016/j.neuroimage.2011.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris J, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: The influence of context. Neuropsychologia. 2003;41(2):156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980 Feb;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- Rutter M. Progress in understanding autism: 2007–2010. Journal of Autism and Developmental Disorders. 2011;41(4):395–404. doi: 10.1007/s10803-011-1184-2. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Lam KSL, Childress D, Parlier M, Daniels JL, Piven J. The broad autism phenotype questionnaire: Prevalence and diagnostic classification. Autism Research. 2013:n/a–n/a. doi: 10.1002/aur.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry. 2004;45(3):445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):221–233. doi: 10.1002/mrdd.20046. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64(1):1–142. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Suckling J, Calder AJ, Bullmore ET, Baron-Cohen S. A novel functional brain imaging endophenotype of autism: the neural response to facial expression of emotion. Translational Psychiatry. 2011;1:e19. doi: 10.1038/tp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M, Serlin G, Siller M. Broad autism phenotype in typically developing children predicts performance on an eye-tracking measure of joint attention. Journal of Autism and Developmental Disorders. 2013;43(3):707–718. doi: 10.1007/s10803-012-1616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobii Technology AB. Technical report. Stockhold; 2011. Accuracy and precision test report: Tobii T 60 eye-tracker. Retrieved from http://www.tobii.com/Global/Analysis/Training/Metrics/Tobii_T60_Eye_Tracker_Accuracy_and_Precision_Test_Report.pdf. [Google Scholar]
- von dem Hagen EAH, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cerebral Cortex. 2011;21(3):493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Perra O, Perrett DI, Whiten A. An fMRI study of joint attention experience. NeuroImage. 2005;25(1):133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high-risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]


