Abstract
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency Medicine 2015 and co-published as a series in Critical Care. Other articles in the series can be found online at http://ccforum.com/series/annualupdate2015. Further information about the Annual Update in Intensive Care and Emergency Medicine is available from http://www.springer.com/series/8901.
Introduction
Despite new promising therapeutic interventions including protective ventilation, prone positioning, use of neuromuscular blockers and conservative fluid balance, acute respiratory distress syndrome (ARDS) remains a devastating disease [1,2]. Mortality rates for ARDS have decreased over time but still remain around 40%, in large part a result of the hemodynamic complications of this syndrome [3]. ARDS has various etiologies and early diagnosis and intervention are key to improving outcomes [4]. Dominant features of ARDS include injury to the alveolar-capillary membrane, which results in severe hypoxemia, decrease in pulmonary compliance, and increase in pulmonary vascular resistance [5,6]. At present, positive-pressure mechanical ventilation is the mainstay of symptomatic treatment for ARDS [1], but may further increase pulmonary hypertension and right ventricular (RV) afterload, leading to acute cor pulmonale and RV failure [6]. Moreover, mechanical ventilation induces additional lung injuries due to overdistention, repeated stretch to the alveoli, atelectotrauma, and increased inflammatory mediator levels [7]. The ARDSNet study reported a reduction in mortality with a ventilation strategy involving limitation of mean tidal volume to 6 ml/kg, as compared with a more traditional tidal volume of 12 ml/kg [1]. However, utilization of lower tidal volumes leads to permissive hypercapnia and most clinicians seldom use very low tidal volumes in practice. Indeed, the need to substantially reduce tidal volume to improve outcome in ARDS patients remains questionable because of the deleterious effects of hypercapnia [8]. In addition, lung injury persists even when tidal volumes are small [9] and further reduction in tidal volume beyond those recommended by ARDSNet may have outcome benefits [10], although not all agree [11]. Thus, modern care for ARDS requires a decision to maximally reduce ventilator settings to ensure lung protection and reduce exacerbation of lung injury while facing the metabolic consequences of this intervention. How can we enhance lung protection in ARDS while not causing metabolic disturbances?
As the discussion about optimization of mechanical ventilation in ARDS patients continues, a new promising adjunct is low-flow partial lung support or extracorporeal CO2 removal (ECCO2R). This approach takes advantage of a concept proposed many years ago [12] which, carried out with modern technology, has been shown to effectively remove metabolically produced CO2 while permitting significant reductions in minute ventilation in preclinical [13,14] and clinical settings [15]. Specifically, combination therapy using reduction in tidal volumes to around 4 ml/kg and concomitant use of ECCO2R has been shown to effectively manage permissive hypercapnia in ARDS [15]. Thus ECCO2R could be an effective strategy in ARDS management and a viable option to combat the deleterious effects of low-tidal volume ventilation, such as permissive hypercapnia.
The purpose of this manuscript is to elaborate on potential applications of ECCO2R as an adjunct to mechanical ventilation for the treatment of ARDS. We discuss the effects of hypercapnia in ARDS and the emerging evidence for the utility of ECCO2R during hypercapnia; as well as the potential role of ECCO2R in optimizing RV-pulmonary artery coupling and RV function in lung failure.
Hypercapnic acidosis: more deleterious than beneficial?
Cellular and metabolic effects
Depending on its degree and duration, permissive hypercapnia has a series of potential adverse effects related to systemic and cerebral vasodilatation, cardiovascular depression, arrhythmia, and increase in gastric hydrogen ion secretion [16]. Until recently, however, cellular and metabolic effects of hypercapnia in ARDS have not been clearly defined. Some investigators have hypothesized that hypercapnia per se might improve outcome in ARDS and have proposed the concept of ‘therapeutic’ hypercapnia [17]. The logic of this approach is that since inflammation contributes to respiratory failure and ARDS and respiratory acidosis has been shown to inhibit several inflammatory mediators [18], it seems reasonable that hypercapnia may be protective in ARDS. In support of this concept, hypercapnia has been demonstrated to attenuate acute lung injury induced by free radicals, pulmonary and systemic ischemia-reperfusion, pulmonary endotoxin, and excessive lung stretch [19]. These effects seem to be due in part to the anti-inflammatory effects of hypercapnia, including attenuation of neutrophil function, reduction in free radicals, decreased oxidant-induced tissue damage, and reduction in the levels of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-8 [20]. However, some of these beneficial effects were likely caused by systemic acidosis rather than hypercapnia per se, because buffering of respiratory acidosis worsened experimental lung injury [21]. Recent studies confirm that CO2 can also act as a signaling molecule via pH-independent mechanisms, leading to deleterious effects in the lung. These effects include inhibition of cell membrane repair, impairment of alveolar fluid clearance, and suppression of innate immunity and host defense [19]. Briva et al. [22] showed that elevated CO2 levels impaired Na,K-ATPase function independently of extra- and intra-cellular acidosis. Taken together, the above reports do not suggest convincingly that hypercapnia could be beneficial and means to mitigate excessive CO2 accumulation in the blood are likely to be useful tools in the arsenal of medical providers.
Hypercapnia, pulmonary hemodynamics and right ventricular function
Hypercapnic acidosis enhances pulmonary vasoconstriction [23]. Several clinical studies demonstrated that hypercapnic acidosis causes an increase in mean pulmonary arterial pressure in ARDS [6]. Acute pulmonary hypertension increases RV afterload [24,25], which individually and collectively with microvascular obstruction, the effects of positive-pressure ventilation, and hypercapnic acidosis exacerbate RV failure in ARDS [6]. Acute cor pulmonale in ARDS patients is associated with high mortality rates [6]. Impaired RV function in early stage ARDS may be under-diagnosed and yet it might be the harbinger of a downward spiral in the patient’s condition [6]. We previously established that pulmonary vascular resistance and RV ejection fraction (RVEF) are poor indicators of RV-arterial performance [24]. RV-arterial coupling is beneficial for cardiovascular performance and can be assessed by the ratio of two elastances: Ees/Ea, where Ees is the RV elastance characterizing the RV system and Ea is the arterial elastance characterizing the pulmonary vascular system. When Ees/Ea is > 1, the system is coupled. However, when Ees/Ea is < 1, the cardio-pulmonary system is uncoupled [24]. Thus, the Ees/Ea ratio reflects the mechano-energetic aspects of RV-vascular coupling. It can be demonstrated that efficiency of energy transfer from the RV to the pulmonary circulatory system is optimal when Ees/Ea = 2 whereas mechanical RV work is maximal when Ees/Ea = 1 [26]. In ARDS patients, increased RV afterload is responsible for increased Ea while Ees may decrease because of hypercapnic acidosis, hypoxia, and often associated sepsis, leading to uncoupling between the right ventricle and the pulmonary circulation, and finally precipitating RV failure (Figure 1) [27]. Therapies should ideally be oriented to restore the coupling between the heart and pulmonary vasculature by avoiding any increase in pulmonary vascular tone as well as depression in RV contractility [27,28]. Alternatively, safe adjuncts to current ARDS management approaches should be considered as we learn more about the pros and cons of hypercapnia in ARDS.
Figure 1.
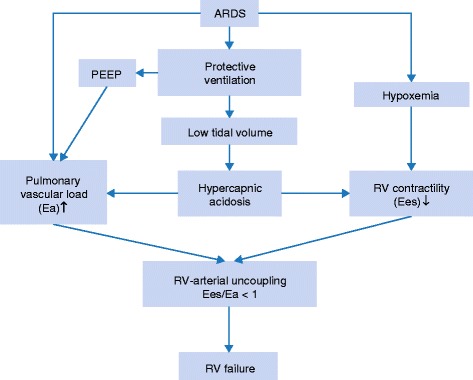
Schematic representation of the key role played by hypercapnic acidosis in right ventricular (RV) failure in patients with acute respiratory distress syndrome (ARDS). PEEP: positive end-expiratory pressure.
New extracorporeal devices for CO2 removal
The premise of intervening with the ventilatory function of the lung stems from early work by Kolobow, Gattinoni and Pesenti, which showed that partial-to-total CO2 removal and so ‘ventilation’ is possible by means of extracorporeal circulation of the blood through a gas exchange membrane [12,29,30]. Of all the available forms of extracorporeal gas exchange, partial lung support, also known as ECCO2R or respiratory dialysis, is the most promising, because it offers unique advantages while carrying a low potential for complications [31,32]. In this context, the recent successes of full extracorporeal membrane oxygenation (ECMO) are also relevant as lung support with full ECMO can replace total lung function to include oxygenation and ventilation. Although full ECMO can also be used for ‘ventilation’ or as an ECCO2R approach, it comes at a higher logistical and economic burden [33] when compared to use of special ECCO2R devices which, in contrast to ECMO, are logistically simpler and do not require dedicated personnel, reducing the cost of care. Although the question about whether various lung support technologies have the potential to avoid mechanical ventilation altogether in select patients remains to be determined [34], partial lung support via ECCO2R at flows of 300–500 ml/min has already been shown to provide replacement of about 50% or more of the ventilatory function of the lung [13] and poses a viable therapeutic adjunct to mechanical ventilation. ECCO2R significantly reduces mechanical ventilator settings while successfully combating hypercapnia and acidosis in humans with ARDS [13,15]. When compared to oxygenation, removal of CO2 from blood can be accomplished at lower blood flows [35]. As a result, less invasive veno-venous devices have been specifically designed for CO2 removal with high gas exchange efficiency at relatively low blood flow rates (300–1,500 ml/min). Theoretically, flow rates as low as 0.5 l/min should be enough to eliminate all the CO2 that the body produces, because a liter of blood with a PaCO2 of 5 kPa contains around 500 ml of CO2 or on average two times more CO2 than the body produces per minute. However, the exact level of CO2 removed will depend on several factors – mainly blood flow through the circuitry and the CO2 level before the membrane [36].
There is an increasing number of modern ECCO2R devices on the market. These devices use 13–17 F veno-venous dual lumen catheters which can be placed percutaneously using the Seldinger technique. The ECCO2R circuitry is heparin-coated, which reduces heparinization requirements. These ECCO2R devices use advanced low impact mechanical pumps to propel the blood and efficient hollow-fiber gas exchangers or membrane lungs. The micropores in the membrane lungs create microscopic blood-gas interfaces allowing efficient gas exchange on a counter-current principle with sweep gas blown through the blood-polymer interface. As micropores also cause plasma leak, non-microporous poly-4-methyl-1-pentene has been recently established as a standard material for gas exchangers, providing better gas exchange, better bio compatibility and less plasma leak compared to older silicone or polypropylene materials [37]. Fibers in the membrane lungs are arranged into a complex mat allowing optimal blood flow and improving gas transfer efficiency by enhancing diffusion. Membranes are also coated with covalently bound heparin to enhance biocompatibility and reduce thrombogenicity. Modern membrane lungs achieve adequate gas exchange with surface areas of 0.67 to 3 m2. Rotary pumps used in modern ECCO2R devices are either centrifugal or diagonal flow pumps designed to minimize blood trauma. To eliminate the need for a drive shaft or bearings and to reduce heating, most advanced centrifugal pump impellors are completely suspended in an electromagnetic field which reduces shear stress. The pump and membrane lung are either separate components or incorporated into a single console. Most importantly, the design and components of the modern veno-venous (VV)-ECCO2R systems reduce the degree of anticoagulation required and minimize the damaging effects of blood coming into contact with foreign surfaces. There are currently four commercially available VV-ECCO2R systems, all approved for use in Europe but none with Food and Drug Administration (FDA) approval status for use in the USA.
The Pump-Assisted Lung Protection (PALP) (Maquet, Rastatt, Germany) system is a low-flow system based on Maquet’s CARDIOHELP® console, which is a portable heart–lung support system. PALP is not an ECMO device and has been designed to serve as a partial lung support device with primary effect on the side of CO2 removal (Figure 2a). However, the PALP can be seamlessly bridged into full ECMO by simply switching out the membrane for a full ECMO oxygenator while using the same operational console which can travel with the patient. The latter is a unique feature of the Maquet system and constitutes a mobile partial lung support to total lung support solution.
The iLA Activve® (Novalung, Germany) is based on the same principle (Figure 2a), but uses a small portable diagonal pump and operational console and has the capacity to run at low or high flow rates (0.5–4.5 l/min). It covers the full range of respiratory support from highly effective CO2 elimination at lower flows to complete oxygenation and ventilation support. This capability is similar to the Maquet system in the sense that the footprint of therapy can be increased from partial to full lung support.
The Hemolung® system (Alung Technologies, Pittsburgh, USA) has a small 0.67 m2 surface area and is the only system specifically designed for CO2 removal and targeting CO2 retention syndromes, such as chronic obstructive pulmonary disease (COPD). The Hemolung integrates blood pump and gas exchange membrane into a single unit (Figure 2b). Blood flows centrally into a rotating core, is radially pumped through a stationary annular fiber bundle, and returns to the patient via an outlet port. The system has not been designed for oxygenation and is generally recommended for COPD patients as a primary indication.
The Decap® system (Hemodec, Salerno, Italy) uses a membrane lung connected in series with a hemodialysis filter and roller pump (Figure 2c). Ultrafiltrate from the filter is returned to the blood stream prior to the membrane lung inflow, allowing additional CO2 removal. Consequently, smaller membrane lungs can be used (0.3 to 1.35 m2) with lower flow rates (< 500 ml/min). This configuration is useful for patients requiring both pulmonary and renal support and is a unique feature of the Decap.
Figure 2.
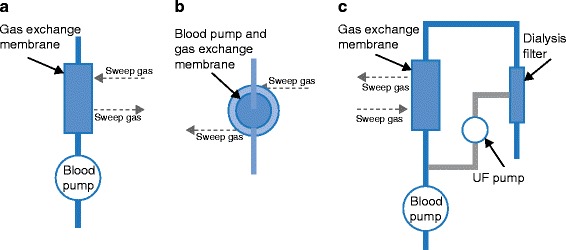
Schematic representation of the three commercially available types of CO 2 removal devices. UF: ultrafiltrate.
Rationale for the use of ECCO2R in ARDS
Experimental evidence
Recent experimental studies have demonstrated that new generations of VV-ECCO2R devices are highly efficient at CO2 removal. A 50% reduction in minute ventilation was obtained in healthy mechanically ventilated swine while maintaining normocarbia using the Hemolung system [13]. In this study, a 15-F dual-lumen catheter was inserted in the external jugular vein and connected to the Hemolung system. Minute ventilation was reduced from 5.6 l/min at baseline to 2.6 l/min 2 h after device insertion and was kept low until the end of the study, while normocarbia (PaCO2 35–45 mm Hg) was maintained. CO2 removal by Hemolung remained steady over 72 h, averaging 72 ± 1.2 ml/min at blood flows of 447 ± 5 ml/min. After insertion, O2 consumption did not change; CO2 production by the lung decreased by 50% and stayed at that level (p < 0.001). Plasma-free hemoglobin did not change during the course of the study signifying the safety of the device with respect to hemolysis [13]. In this study, ECCO2R using the Hemolung permitted significant CO2 removal in a safe and feasible manner while requiring only a partial increase in activated clotting time titrated by continuous heparin infusion.
The hemodynamic effects of CO2 removal seem to be beneficial by decreasing pulmonary hypertension and improving RV-arterial coupling in an experimental model of ARDS. In a recent study, we sought to determine whether low-flow CO2 removal therapy used at an early stage of ARDS could have beneficial hemodynamic effects on the pulmonary circulation. This study was performed in an experimental model of ARDS in pigs. ARDS was obtained by repeated bronchoalveolar lavage (BAL, 0.09% saline solution). Protective ventilation at low tidal volume was then established according to the ARDSNet study. Drainage (12 F) and re-infusion (10 F) cannulae were inserted into the inferior and the superior vena cava, respectively. These cannulae were connected to the PALP system for CO2 removal. ARDS induced severe hypercapnic acidosis with significantly increased pulmonary artery pressure (PAP). After the PALP was started, acidosis was rapidly corrected and normocarbia was maintained despite protective ventilation. PAP significantly decreased and a significant drop in Ea was observed during PALP therapy (Figure 3). Mean blood flow through the PALP was 0.645 l/min and sweep gas flow was 8 l/min. RV-arterial coupling assessed by the ratio of Ees on Ea was improved [38].
Figure 3.
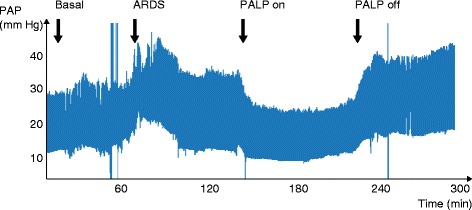
Effects of PALP (‘Pump Assisted Lung Protection’, Maquet, Germany) therapy on systolic pulmonary artery pressure (PAP) in an experimental model of acute respiratory distress syndrome (ARDS).
Other promising approaches for efficient CO2 removal are still in development [39,40]. Novel methods to maximize CO2 removal, such as regional blood acidification which increases the bioavailability of CO2 by unbinding it from the bicarbonate ion in circulating blood, are also under investigation [40].
Clinical evidence
There is accumulating evidence that VV-ECCO2R can effectively reduce PaCO2 in patients with ARDS and that VV-ECCO2R facilitates a lung-protective ventilation strategy by allowing a reduction in tidal volume and inspiratory airway pressures [32,41]. Terragni et al. used VV-ECCO2R to facilitate ‘ultraprotective’ ventilation [15]. They recruited 32 patients with early (< 72 h) ARDS and ventilated them according to the ARDSNet protocol for 72 h, at which point the tidal volume was reduced from 6 to 4 ml/kg in all patients (n = 10) who had a plateau pressure of between 28 and 30 cmH2O, thus facilitating further reductions in plateau pressures. VV-ECCO2R using the Decap device successfully treated the hypercapnic acidosis in all cases and allowed the plateau pressure to be lowered to 25 cmH2O (4 ml/kg tidal volume and higher levels of positive end-expiratory pressure [PEEP]) while mitigating the resultant changes in pH and PaCO2. The study also demonstrated a reduction in bronchoalveolar inflammatory cytokines (IL-6, IL-8, IL-1b, IL-1 receptor antagonist [IL-Ira]) in the Decap group. There were no harmful effects related to the ultra-protective ventilation strategy or the VV-ECCO2R. Although this study was uncontrolled and small, it suggests that there may be benefit to an ultra-protective ventilation strategy facilitated by VV-ECCO2R within 72 hours of diagnosing ARDS. A similar approach was taken by another group using the NovaLung device in arterio-venous configuration. In the prospective randomized Xtravent-study, Bein et al. [42] demonstrated that use of very low tidal volumes (3 ml/kg PBW) combined with extracorporeal elimination of CO2 was feasible without major side effects and might be beneficial in the treatment of patients with severe ARDS. Although that study did not show a mortality benefit, ventilator-free days assessed at 28 and 60 days were significantly higher in the ECCO2R group. Adjunct use of ECCO2R and mechanical ventilation facilitated liberation of patients from excessive sedation and increased levels of spontaneous breathing. Thus, integration of spontaneous breathing into the management of patients with ARDS might be easier and more comfortable with extracorporeal CO2-removal, and a reduced demand for sedative and analgesic medication could be advantageous [42]. Forster et al. [43] demonstrated, in a small series of 10 patients, that low-flow CO2 removal integrated into a renal-replacement circuit could reduce acidosis and decrease vasopressor requirements. The gas exchanger was integrated into the continuous hemodialysis system after the dialysis filter. The authors used a 13.5-F double-lumen catheter placed in the jugular vein. Similarly, we reported a case of refractory hypercapnia in a severely burned adult treated with a simplified VV-ECCO2R technique [44]. We integrated a pediatric oxygenator into a continuous veno-venous hemofiltration circuit. This technique, used for at least 96 h, was feasible and efficiently removed up to 32% of CO2. Future studies are required to determine whether ‘ultraprotective’ ventilation with adjunct use of ECCO2R will improve survival in patients suffering from moderate to severe ARDS. At this time, the effect of ECCO2R on survival in patients with ARDS is accumulating but is not yet conclusive [15,42,45].
There are clinical trials planned for several of the new ECCO2R devices. The rationale for adjunct use of ECCO2R will depend on the clinical situation in each individual patient. However, with the new Berlin definition of ARDS, the therapeutic window for ECCO2R in ARDS may be expanded [46]. Because the intensity of therapeutic intervention increases proportionally to the level of hypoxemia, adjunct use of ECCO2R will likely be considered at earlier stages of ARDS, for example when the PaO2/FiO2 ratio is < 200. At that time, ECCO2R could be initiated in combination with tidal volume reduction in order to achieve ultraprotective ventilation. This approach will need to be tested in prospective randomized fashion but the preliminary evidence suggests that, at least in some patients with slow ARDS progression, this early intervention may be of use. A few areas of concern remain for both ECCO2R and ECMO alike, including cannula thrombosis, need to exchange membranes due to thrombosis and pump malfunction [45]. Intense research is oriented toward solution of these problems and major improvements in anticoagulation protocols and updates to clinical practice guidelines are expected as the results of this research become available. In our opinion, alternative anticoagulation approaches, such as the work by Cardenas et al. utilizing regional citrate anticoagulation, could provide a promising solution to future ECCO2R approaches, especially in line with the tendency for developing modular therapeutic solutions permitting concomitant lung and renal interventions [39,47]. Other novel approaches are emerging with respect to heparin-free antibody-based interventions to the coagulation cascade as a means to induce thromboprotection during extracorporeal circulation [48]. Specific anticoagulation requirements for low-flow systems must be studied systematically and will be the cornerstone of further acceptance of ECCO2R as well as full ECMO into daily practice, especially in patients with ARDS due to multiple trauma and burns, in whom heparinization is not desired.
Conclusion
ARDS remains a life-threatening condition with long-term consequences in survivors. Protective ventilation reduces alveolar stress and strain and clearly improves mortality. However, these beneficial effects are tempered by the fact that low tidal volume ventilation induces hypercapnic acidosis responsible for deleterious effects. Uncoupling between impaired RV function and increased pulmonary vascular tone enhanced by hypercapnic acidosis and positive pressure ventilation is a starting point in the downward spiral of ARDS patients. New generation ECCO2R therapy can be seen as a low impact and safe ‘respiratory dialysis’ allowing control of hypercapnia and acidosis. ECCO2R should be considered as a therapeutic adjunct in moderate to severe ARDS, combined with further decrease in tidal volume. Recent major technological improvements in devices make them simpler, safer, less invasive and more efficient, requiring lower blood flow rates and smaller access cannulas with reduced anticoagulation requirements. However, while the efficiency of modern ECCO2R devices has been clearly demonstrated in experimental and clinical settings, current evidence on their impact on survival in ARDS is just accumulating and more data will be needed before these techniques can be incorporated into routine use.
Declarations
Publication of this article was funded by the Leon Fredericq Foundation of the University of Liege.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- BAL
Bronchoalveolar lavage
- ECCO2R
Extracorporeal CO2 removal
- ECMO
Extracorporeal membrane oxygenation
- FDA
Food and drug administration
- IL
Interleukin
- PALP
Pump-assisted lung protection
- PAP
Pulmonary artery pressure
- PEEP
Positive end-expiratory pressure
- RV
Right ventricular
- RVEF
RV ejection fraction
- TNF
Tumor necrosis factor
- VV
Veno-venous
Footnotes
Competing interests
PM and BL have received supply of equipment (CO2 removal device, medical disposables) and funding by Maquet for an experimental study on extracorporeal CO2 removal theapy in 2013 and 2014. PM and AB have received travel and lodging support for conferences.
Contributor Information
Philippe Morimont, Email: ph.morimont@chu.ulg.ac.be.
Andriy Batchinsky, Email: andriy.batchinsky.i.vol@mail.mil.
Bernard Lambermont, Email: b.lambermont@chu.ulg.ac.be.
References
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 3.Squara P, Dhainaut JF, Artigas A, Carlet J. Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intensive Care Med. 1998;24:1018–1028. doi: 10.1007/s001340050710. [DOI] [PubMed] [Google Scholar]
- 4.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancio LC, Batchinsky AI, Dubick MA, et al. Inhalation injury: pathophysiology and clinical care proceedings of a symposium conducted at the Trauma Institute of San Antonio, San Antonio, TX, USA on 28 March 2006. Burns. 2007;33:681–692. doi: 10.1016/j.burns.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Lheritier G, Legras A, Caille A, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med. 2013;39:1734–1742. doi: 10.1007/s00134-013-3017-6. [DOI] [PubMed] [Google Scholar]
- 7.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 8.Ijland MM, Heunks LM, van der Hoeven JG. Bench-to-bedside review: hypercapnic acidosis in lung injury – from ‘permissive’ to ‘therapeutic’. Crit Care. 2010;14:237. doi: 10.1186/cc9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 10.Hager DN, Krishnan JA, Hayden DL, Brower RG. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 12.Pesenti A, Pelizzola A, Mascheroni D, et al. Low frequency positive pressure ventilation with extracorporeal CO2 removal (LEPPV-ECCO2R) in acute respiratory failure (ARF): technique. Trans Am Soc Artif Intern Organs. 1981;27:263–266. [PubMed] [Google Scholar]
- 13.Batchinsky AI, Jordan BS, Regn D, et al. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39:1382–1387. doi: 10.1097/CCM.0b013e31820eda45. [DOI] [PubMed] [Google Scholar]
- 14.Batchinsky AI, Chung K, Cannon J, Cancio LC. Respiratory dialysis is not extracorporeal membrane oxygenation. The authors answer. Extracorporeal membrane oxygenation and respiratory dialysis: our expending tool box. Crit Care Med. 2011;39:2788–2789. doi: 10.1097/CCM.0b013e318232cebe. [DOI] [Google Scholar]
- 15.Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 16.Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med. 1994;150:1722–1737. doi: 10.1164/ajrccm.150.6.7952641. [DOI] [PubMed] [Google Scholar]
- 17.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill – too little of a good thing? Lancet. 1999;354:1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 18.O’Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. Bench-to-bedside review: permissive hypercapnia. Crit Care. 2005;9:51–59. doi: 10.1186/cc2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadasz I, Hubmayr RD, Nin N, Sporn PH, Sznajder JI. Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol. 2012;46:417–421. doi: 10.1165/rcmb.2011-0395PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curley G, Contreras MM, Nichol AD, Higgins BD, Laffey JG. Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology. 2010;112:462–472. doi: 10.1097/ALN.0b013e3181ca361f. [DOI] [PubMed] [Google Scholar]
- 21.Nichol AD, O’Cronin DF, Howell K, et al. Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit Care Med. 2009;37:2953–2961. doi: 10.1097/CCM.0b013e3181b028ce. [DOI] [PubMed] [Google Scholar]
- 22.Briva A, Vadasz I, Lecuona E, et al. High CO2 levels impair alveolar epithelial function independently of pH. PLoS One. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stengl M, Ledvinova L, Chvojka J, et al. Effects of clinically relevant acute hypercapnic and metabolic acidosis on the cardiovascular system: an experimental porcine study. Crit Care. 2013;17:R303. doi: 10.1186/cc13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimont P, Lambermont B, Ghuysen A, et al. Effective arterial elastance as an index of pulmonary vascular load. Am J Physiol Heart Circ Physiol. 2008;294:H2736–H2742. doi: 10.1152/ajpheart.00796.2007. [DOI] [PubMed] [Google Scholar]
- 25.Viitanen A, Salmenpera M, Heinonen J. Right ventricular response to hypercarbia after cardiac surgery. Anesthesiology. 1990;73:393–400. doi: 10.1097/00000542-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Sagawa KML, Suga H, Sunagawa K. Cardiovascular interaction. In: Sagawa K, editor. Cardiac Contraction and the Pressure–Volume Relationship. New York: Oxford University Press; 1988. pp. 232–298. [Google Scholar]
- 27.Morimont P, Lambermont B, Desaive T, et al. Right ventriculoarterial coupling in acute respiratory distress syndrome (ARDS) and expected benefits of CO2 removal therapy. J Crit Care. 2013;28:e30. doi: 10.1016/j.jcrc.2013.07.010. [DOI] [Google Scholar]
- 28.Weber T, Tschernich H, Sitzwohl C, et al. Tromethamine buffer modifies the depressant effect of permissive hypercapnia on myocardial contractility in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;162:1361–1365. doi: 10.1164/ajrccm.162.4.9808092. [DOI] [PubMed] [Google Scholar]
- 29.Kolobow T, Gattinoni L, Tomlinson T, Pierce JE. An alternative to breathing. J Thorac Cardiovasc Surg. 1978;75:261–266. [PubMed] [Google Scholar]
- 30.Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2:292–294. doi: 10.1016/S0140-6736(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 31.Terragni P, Maiolo G, Ranieri VM. Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care. 2012;18:93–98. doi: 10.1097/MCC.0b013e32834f17ef. [DOI] [PubMed] [Google Scholar]
- 32.Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung. Crit Care Med. 2010;38:S549–S554. doi: 10.1097/CCM.0b013e3181f1fe0c. [DOI] [PubMed] [Google Scholar]
- 33.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 34.Del Sorbo L, Ranieri VM. We do not need mechanical ventilation any more. Crit Care Med. 2010;38:S555–S558. doi: 10.1097/CCM.0b013e3181f20d5b. [DOI] [PubMed] [Google Scholar]
- 35.Barrett KBS, Boitano S, Brooks H. Ganong’s Review of Medical Physiology. 23. New York: Mc Graw Hill; 2003. pp. 587–607. [Google Scholar]
- 36.Park M, Costa EL, Maciel AT, et al. Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS One. 2013;8:e54954. doi: 10.1371/journal.pone.0054954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toomasian JM, Schreiner RJ, Meyer DE. A polymethylpentene fiber gas exchanger for long-term extracorporeal life support. ASAIO J. 2005;51:390–397. doi: 10.1097/01.mat.0000169111.66328.a8. [DOI] [PubMed] [Google Scholar]
- 38.Morimont P, Desaive T, Guiot J, et al. Effects of veno-venous CO2 removal therapy on pulmonary circulation in an ARDS model. Intensive Care Med Exp. 2014;2:45. doi: 10.1186/2197-425X-2-S1-P45. [DOI] [Google Scholar]
- 39.Scaravilli V, Kreyer S, Linden K, et al. Modular extracorporeal life support: effects of ultrafiltrate recirculation on the performance of an extracorporeal carbon dioxide removal device. ASAIO J. 2014;60:335–341. doi: 10.1097/MAT.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 40.Zanella A, Mangili P, Giani M, et al. Extracorporeal carbon dioxide removal through ventilation of acidified dialysate: an experimental study. J Heart Lung Transplant. 2014;33:536–541. doi: 10.1016/j.healun.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Cove ME, MacLaren G, Federspiel WJ, Kellum JA. Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care. 2012;16:232. doi: 10.1186/cc11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forster C, Schriewer J, John S, Eckardt KU, Willam C. Low-flow CO(2) removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care. 2013;17:R154. doi: 10.1186/cc12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rousseau AF, Damas P, Renwart L. Use of a pediatric oxygenator integrated in a veno-venous hemofiltration circuit to remove CO: A case report in a severe burn patient with refractory hypercapnia. Burns. 2014;40:e47–e50. doi: 10.1016/j.burns.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald M, Millar J, Blackwood B, et al. Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care. 2014;18:222. doi: 10.1186/cc13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 47.Cardenas VJ, Jr, Miller L, Lynch JE, Anderson MJ, Zwischenberger JB. Percutaneous venovenous CO2 removal with regional anticoagulation in an ovine model. ASAIO J. 2006;52:467–470. doi: 10.1097/01.mat.0000227743.07743.5d. [DOI] [PubMed] [Google Scholar]
- 48.Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]


