Opinion statement
Leishmaniasis, a protozoal infection transmitted by sandfly bite, produces a clinical spectrum of disease ranging from asymptomatic infection to ulcerative skin and mucosal lesions to visceral involvement. Leishmaniasis is endemic in regions of Africa, the Middle East, south Asia, southern Europe, northern South America, and Central America. There has been an increase in imported leishmaniasis into developed, non-endemic countries due to increasing global travel. While pentavalent antimonials have been the mainstay of antileishmanial treatment for decades, newer therapeutic options have become available for all forms of infection, including liposomal amphotericin B, miltefosine, fluconazole, and ketoconazole. For the returning traveler with cutaneous leishmaniasis in the USA, treatment approaches are determined based on infecting species, initial presentation, extent and progression of disease, the advantages and drawbacks of available parenteral and oral drugs, and clinician-consultant experience.
Keywords: Leishmania, Leishmaniasis, Protozoa, Cutaneous, Diagnosis, Treatment, Travelers
Introduction
Epidemiology
Leishmania infection produces three clinical syndromes: visceral (VL), cutaneous (CL), and mucosal leishmania-sis (ML). The clinical epidemiology is divided into two distinct entities—Old World and New World disease—based on transmission geography. Infection is spread by blood-feeding Phlebotomus sandflies in the Old World and Lutzomyia sandflies in the New World. Rarely, infection is transmitted via other methods including blood transfusions [1].
Leishmaniasis affects 12 million people worldwide (Fig. 1), primarily in remote rural regions [2]. There are an estimated 1.5–2 million new cases of CL each year; 90 % of which originate in ten countries: Afghanistan, Iran, Iraq, Saudi Arabia, Algeria, Ethiopia, Sudan, Syria, Brazil, and Peru [3]. CL is caused primarily by Leishmania tropica, Leishmania major, and Leishmania infantum in the Old World and by Leishmania braziliensis, Leishmania guyanensis, Leishmania panamensis, Leishmania peruviana, Leishmania mexicana, and Leishmania amazonensis in the New World [3].
Fig. 1.
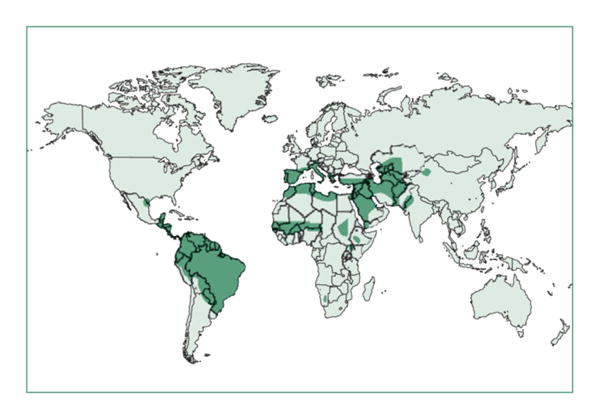
Geographic distribution of regions where cutaneous leishmaniasis (CL) is endemic (reprinted with permission from Reithinger, Lancet Infectious Diseases, 2007) [4].
Clinical presentation
CL typically manifests within several weeks of a sandfly bite with skin lesions developing on exposed body areas. New World CL (NWCL) produces variable clinical manifestations ranging from nodules to ulcerative skin lesions (Fig. 2) which may be accompanied by local lymphadenopathy. Old World CL (OWCL) presents similarly but can also produce plaque-like lesions [6•]. The face, ear, and extremities are most frequently involved. Ulcers are typically painless and enlarge slowly with a granulomatous base and raised margins [7].
Fig. 2.
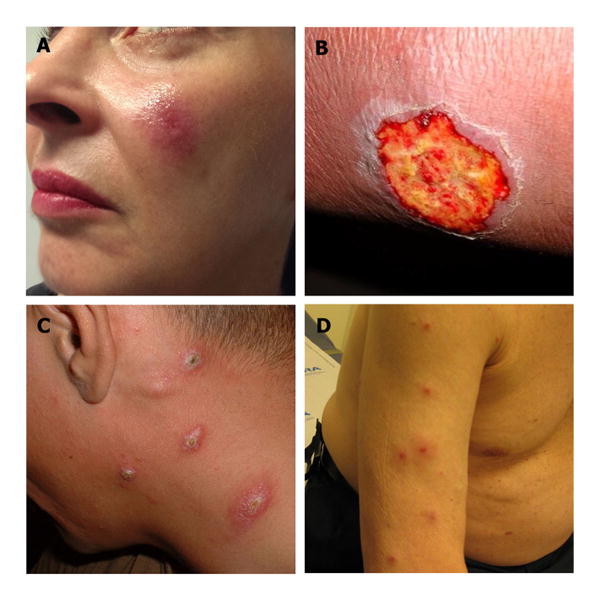
Clinical presentation of cutaneous leishmaniasis (CL). a Ulcerative face lesion in a traveler caused by L. infantum infection acquired in Malta. b Ulcerative L. major infection on leg in a traveler acquired in Israel. c Nodular L. major infection in a US soldier acquired in Iraq (figure reprinted from Murray, Lancet, 2005) [5]. d Disseminated L. infantum infection in a traveler acquired in Greece (courtesy of S. Haber, M.D.).
Depending on the species, CL infection also has the potential to disseminate hematogenously to mucosal surfaces of the nose, mouth, or pharynx and cause ML. ML may develop simultaneously with an initial skin lesion or months to years later, even despite apparently adequate treatment for CL. ML produces chronic nasal congestion and bleeding; ulceration of the nose, mouth, and pharynx; nasal septum perforation; and facial disfigurement. ML is most frequently seen in L. braziliensis infection, although it has also been reported in OWCL [8].
Diagnosis
Patients with non-healing skin lesions and a travel history to an endemic region should be evaluated for CL. Diagnostic confirmation may be made by culture but is principally achieved by direct microscopic detection of Leishmania amastigotes in skin biopsies (Fig. 3); serolog-ic testing is not reliable. With high-level specificity for Leishmania DNA, polymerase chain reaction (PCR) testing of lesion specimens is particularly useful when parasite burden is low and is currently the most accurate method of diagnosis in CL [9].
Fig. 3.
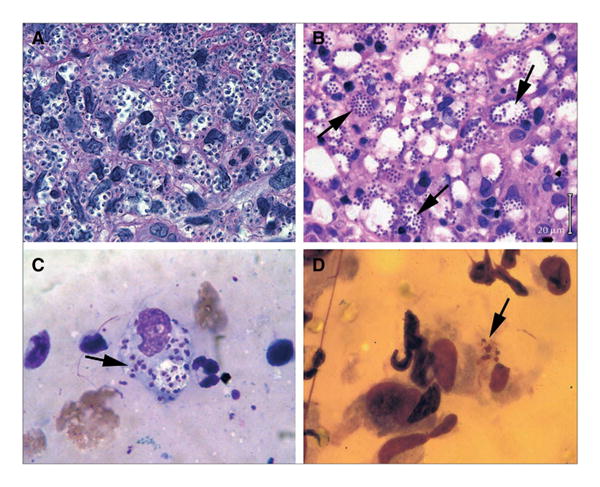
Histologic diagnosis of cutaneous leishmaniasis in hematoxylin/eosin-stained skin lesion biopsies (a, b) and Giemsa-stained lesion scrapings and imprints (c, d). Arrows indicate amastigotes. a, b Biopsies show sheets of amastigotes in a L. major and b L. mexicana infection; in b, note large, parasite-laden macrophage vacuoles seen in L. mexicana infection. c Smear of lesion scraping in probable L. panamensis infection. d Impression smear in L. mexicana infection obtained by pressing glass slide to open ear lesion. Original magnification ×500, except in a, ×1000 (figure reprinted from Murray, Lancet, 2005) [5].
Prognosis
CL is often self-limited, resolving within 6 months without therapy. However, non-healing skin ulcerations involving the face or limbs may cause significant morbidity. Since the complete eradiation of viable organisms seldom occurs, lesions may recur despite prior clinical resolution, and therefore, “cure” is not generally assigned until 6 to 12 months after therapy (Fig. 3).
Prevention
Disease prevention is difficult for the traveler other than avoidance of outdoor activities in endemic areas. There is evidence that using insecticide-impregnated bed nets, curtains, and/or clothing may offer protection. There is currently no available vaccine, although some counties (e.g., Uzbekistan and Sri Lanka) still promote leishmanization, which involves the direct inoculation of viable parasites into areas that are not cosmetically apparent (e.g., the buttocks) [10].
Treatment
Although attempts are now being made by European investigators [11, 12, 13•], the management of CL, an intrinsically diverse disease, has not been standardized for three reasons: (a) the basic heterogeneity of the disease given that multiple different species cause illness; (b) the fact that clinical manifestation, response to treatment, and outcome vary depending on the geographic region of acquisition; and (c) a general lack of high-quality clinical trials from which to draw guidance.
There are many important factors which play a role in the initial consideration of treatment, including the geographic region where infection was acquired, the species of Leishmania involved, and the extent and location of CL lesions. With patient willingness, observation alone (with adequate wound care) may well be satisfactory if there is evidence of spontaneous regression or well-localized infection caused by L. major (OWCL) and L. mexicana (NWCL). The latter approach is justifiable, as spontaneous healing (albeit with the likelihood of at least some scarring) occurs within 3 to 4 months in 70 and 88 % of localized infections caused by L. major and L. mexicana, respectively [13•]. Widely accepted indications for treatment in all forms of CL are listed in Table 1. Worldwide, the most frequently employed approach to localized CL is direct lesion treatment usually in the form of intralesional injections of antimony; cryotherapy, thermotherapy, and topical paromomycin have also been used (Table 2). In the USA, however, there has been little experience with direct lesion treatment. Furthermore, the goals of systemic treatment in the USA are aimed at attempting to accelerate control of infection to potentially reduce scarring, particularly in cosmetically important areas. The remainder of this review will focus on available systemic treatments used for CL in the USA. In short, these treatments include two parenteral agents, pentavalent antimony (in the form of sodium stibogluconate) and liposomal amphotericin B, and three oral agents, miltefosine and the azoles, fluconazole, and ketoconazole (Fig. 4). There is abundant data showing the efficacy of sodium stibogluconate and miltefosine in CL; clinical experience with liposomal amphotericin B and fluconazole or ketoconazole in CL is limited. Each of these agents has its own individual advantages and drawbacks.
Table 1. Indications for treatment of cutaneous leishmaniasis.
|
Table 2. Treatments for cutaneous leishmaniasis (CL) not available or seldom used in the USA.
| Therapy | Route | Comments | References |
|---|---|---|---|
| Pentamidine | IV | Use limited by adverse reactions. Used for infection caused by L. guyanensis (considered poorly responsive to pentavalent antimony). |
[14••] |
| Amphotericin B deoxycholate | IV | Considered “rescue therapy” in refractory CL. Generally only used in regions where cost of liposomal amphotericin is prohibitive. |
[15] |
| Meglumine antimoniate | IM | Another form of pentavalent antimony available as an intramuscular formulation in Europe (manufactured by Aventis) | [14••] |
| Paromomycin | Topical | Topical drug coformulated with methylbenzethonium appears to increase cure rates in both OWCL and NWCL using a regimen of twice-daily direct application for 21 days. | [16] |
| Imiquimod | Topical | Toll-like receptor immunomodulator (agonist) formulated as a 7.5 % cream. In CL in Peru, direct application every other day for 20 days in combination with pentavalent antimony reportedly more effective than antimony alone and may be useful in refractory disease. |
[17] |
| Intralesional antimony | Local | Provides high concentrations of drug directly in the lesion with minimal toxicity. However, no uniformly accepted protocol for administration and satisfactory treatment requires clinical experience and technical expertise to achieve reasonable and consistent cure rates. Frequently used forlocalized CL in Europe and the UK, often in conjunction with cryotherapy. | [13•, 18] |
| Thermotherapy | Local | Radio frequency waves administered as up to three treatments of 50 °C for 30 s at 7-day intervals appear effective in OWCL and may be as effective as local antimony therapy in NWCL caused by L. braziliensis or L. mexicana. | [19, 20] |
| Cryotherapy | Local | Liquid nitrogen cryotherapy at −195 °C applied weekly to OWCL skin lesions for up to 6 weeks has been used for decades in many regions. In localized OWCL caused by L. major, the combination of superficial cryotherapy plus intralesional antimony may be more effective than either treatment alone. | [13•, 21] |
Fig. 4.
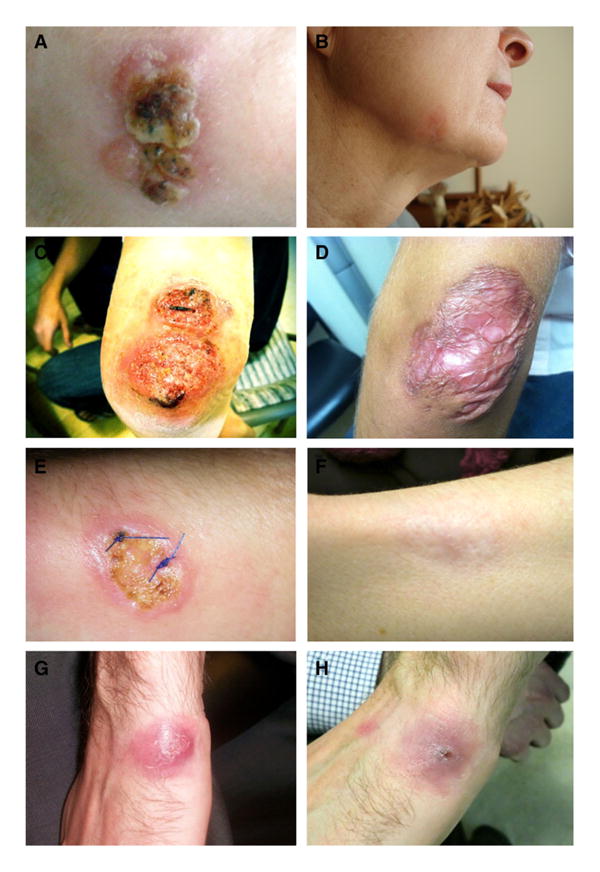
Clinical outcome in treated cutaneous leishmaniasis (CL) in travelers returning to the USA. Images in left column (a, c, e, g) represent pretreatment lesions, while images in right column (b, d, f, h) are posttreatment. a, b Verrucous-like lesion on right chin caused by L. major infection acquired in Namibia, treated with IV pen-tavalent antimony (sodium stibogluconate), with no residual scarring. c, d L. tropica infection on elbow acquired in Egypt, treated with liposomal amphotericin B, with prominent posttreat-ment scarring. e, f L. panamensis infection on forearm acquired in Central America, treated with liposomal amphotericin B, with resolution and minimal scarring. g, h L. major infection on hand acquired in Morocco, responded clinically to initial miltefosine therapy but recurred 3 months later (g). Lesion responded to retreatment with fluconazole (h) and resolved with minimal scarring.
Parenteral therapy
Pentavalent antimony (sodium stibogluconate)
A group of compounds containing the heavy metal antimony; only available in the USA as sodium stibogluconate (Pentostam, GlaxoSmithKline).
Remains the most standard antileishmanial treatment around the world for all forms of CL (as well as for ML and VL in many but not all regions).
Not FDA-approved and only available (free of charge) via an investigational new drug (IND) protocol from the Centers for Disease Control and Prevention (CDC).
Administered at 20 mg/kg via a 10-min intravenous (IV) infusion once daily, usually for 20 days in CL. A 10-day regimen has been used in L. mexicana infection [22].
Clinical data for safety and efficacy far exceeds available data for all other therapeutic options in CL. The antimonials are generally considered to have high efficacy rates (approximately 80–90 % for most Leishmania species) with low rates of relapse [14].
Produces numerous side effects and many patients have difficulty tolerating the full 20-day IV treatment course. As many as 25 % of patients require treatment interruption or discontinuation because of adverse effects including fatigue, fevers, headaches, arthralgias, abdominal pain, nausea and vomiting, pancreatitis, venous thrombosis formation, and phlebotoxicity at the infusion site [23].
Rare but potentially life-threatening cardiac arrhythmias (including ventricular tachycardia, ventricular fibrillation, torsades de pointes) may occur, and EKGs should be performed once weekly [24].
Other adverse signs include hyperamylasemia, elevated serum hepatic enzymes, thrombocytopenia, and leukopenia. Once-weekly blood testing (CBC with platelet count, chemistry profile with amylase) is therefore necessary, and therapy should be interrupted if the level of amylase reaches four times above the upper limit of normal.
Antimony is contraindicated in pregnant women and should be avoided, if possible, in patients older than 55 years of age as well as in patients with preexisting cardiac, hepatic, or renal disease [13•]. Toxicity may also be increased in patients coinfected with HIV.
Since sodium stibogluconate is an investigational drug, approval by an institutional review board (IRB) is typically necessary, and it is often challenging to coordinate up to 20 days of IV treatment. Many home antibiotic infusion companies may not be willing to administer a non-FDA-approved drug, and insurance companies may decline to cover administration costs.
Summary: Pentavalent antimony, in the form of sodium stibogluconate, is historically the most frequently used first-line therapy for CL, given its long clinical experience. It is administered at 20 mg/kg daily via IV infusion for 20 days. It frequently produces adverse reactions and is often difficult for patients to tolerate.
Liposomal amphotericin B
Available as AmBisome (Astellas Pharma) and FDA-approved for VL but not for CL or ML.
Based on safety, tolerance, and remarkably high-level efficacy in VL, the use of IV liposomal amphotericin B has been reasonably extrapolated to CL and ML [25••]. However, published clinical experience with treatment in CL and ML remains limited.
The FDA-approved dosing regimen for VL includes a total dose of 21 mg/kg given IV over a 21-day period. Regimens in CL typically employ this same total dose (21 mg/kg), but the treatment period is usually abbreviated. One likely effective regimen consists of 3 mg/kg per day for seven consecutive days [26].
Some clinicians administer a liter of IV saline prior to the 2-h infusion of liposomal amphotericin B in an attempt to reduce the likelihood of potential nephrotoxicity.
Although usually well-tolerated, side effects of therapy include infusion-related fever and chills and may also include hypokalemia, anemia, and renal insufficiency. Blood counts and serum chemistries should be monitored twice weekly while on treatment.
Liposomal amphotericin B is considered safe for use in pregnancy (category B).
Liposomal amphotericin B treatment is expensive due to the cost of the drug itself in addition to the cost of IV administration. Insurance companies may decline to cover this treatment since CL is not one of the drug's FDA-approved indications.
Summary: liposomal amphotericin B is highly effective therapy for VL, and its use has been extrapolated to CL. It is generally administered as a total dose of 21 mg/kg divided over seven consecutive days.
Oral therapy
Miltefosine
Available as Impavido (Knight Therapeutics) and FDA-approved in 2014 as the first drug designated for the treatment of CL and ML in the USA. Also, FDA-approved for VL.
A phospholipid compound developed in the 1980s for use as an anticancer drug; recent studies have also demonstrated some success in treating infections caused by free-living amoeba, including Naegleria fowleri and Acanthamoeba species [27].
Clinical cure responses in NWCL range from 50 and 91 %, depending on the species involved and region of acquisition; experience in OWCL has demonstrated cure rates of 86–92 % [28].
Available in 50-mg capsules and administered at 2.5 mg/kg/day (up to 150 mg/day) in two or three divided doses for 28 days [29••].
Miltefosine is generally well tolerated; however, it regularly produces headaches, nausea, vomiting, and/or diarrhea in a significant percentage of patients, typically during the first week of treatment only [23].
Hepatotoxicity and nephrotoxicity are unusual [30], but serum chemistry profiles should be tested weekly during therapy.
Teratogenicity has been demonstrated in rats, and use in pregnancy (category D) or in breastfeeding is contraindicated. Effective contraception must be maintained during and for up to 3 months after treatment.
Pricing for a 28-day course of miltefosine in the USA has not yet been established.
Summary: miltefosine is a well-tolerated oral systemic therapy for CL and ML and is administered at 2.5 mg/kg/day (up to 150 mg/day) for 28 days.
Azoles (ketoconazole and fluconazole)
These structurally related triazoles demonstrate antileishmanial activity in patients with both OWCL and NWCL. However, published clinical experience with these two agents is limited.
There is some evidence to support the use of high-dose fluconazole for the treatment of OWCL caused by L. major [31•] and NWCL caused by L. braziliensis [32].
Ketoconazole, at 600 mg once daily for 28 days, appears to be active in L. panamensis and L. mexicana infection acquired in Panama and Guatemala [33].
Adverse effects for these azoles include gastrointestinal symptoms and hepatotoxicity [34•]. Serum chemistries should be monitored weekly for high-dose regimens.
Summary: although reported clinical experience is limited, there is evidence to consider the use of high-dose fluconazole and ketoconazole as oral agents in the treatment of CL caused by selected species.
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Daniel P. Eiras declares that he has no conflict of interest.
Laura A. Kirkman declares that she has no conflict of interest.
Henry W. Murray declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14(12):e1032–9. doi: 10.1016/j.ijid.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues JC, Godinho JL, de Souza W. Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell Biochem. 2014;74:1–42. doi: 10.1007/978-94-007-7305-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Goto H, Lauletta Lindoso JA. Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin North Am. 2012;26(2):293–307. doi: 10.1016/j.idc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 5.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366(9496):1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 6•.Murray HW. Leishmaniasis in the United States: treatment in 2012. Am J Trop Med Hyg. 2012;86(3):434–40. doi: 10.4269/ajtmh.2012.11-0682. This paper reviews the new treatment options available to U.S. clinicians who are treating patients who contracted leishmaniasis abroad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon M, Benenson S, Baum S, Schwartz E. Tropical skin infections among Israeli travelers. Am J Trop Med Hyg. 2011;85(5):868–72. doi: 10.4269/ajtmh.2011.10-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jirmanus L, Glesby MJ, Guimarães LH, Lago E, Rosa ME, Machado PR, et al. Epidemiological and clinical changes in American integumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg. 2012;86(3):426–33. doi: 10.4269/ajtmh.2012.11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirian S, Oryan A, Hatam GR, Panahi S, Daneshbod Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch Pathol Lab Med. 2014;138(2):235–40. doi: 10.5858/arpa.2013-0098-OA. [DOI] [PubMed] [Google Scholar]
- 10.McCall LI, Zhang WW, Ranasinghe S, Matlashewski G. Leishmanization revisited: immunization with a naturally attenuated cutaneous Leishmania donovani isolate from Sri Lanka protects against visceral leishmaniasis. Vaccine. 2013;31(10):1420–5. doi: 10.1016/j.vaccine.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 11.Blum J, Buffet P, Visser L, Harms G, Bailey MS, Caumes E, et al. LeishMan recommendations for treatment of cutaneous and mucosal leishmaniasis in travelers. J Travel Med. 2014;21(2):116–29. doi: 10.1111/jtm.12089. 2014. [DOI] [PubMed] [Google Scholar]
- 12.Hodiamont CJ, Kager PA, Bart A, de Vries HJ, van Thiel PP, Leenstra T, et al. Species-directed therapy for leishmaniasis in returning travellers: a comprehensive guide. PLoS Negl Trop Dis. 2014;8(5):e2832. doi: 10.1371/journal.pntd.0002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Morizot G, Kendjo E, Mouri O, Thellier M, Pérignon A, Foulet F, et al. Travelers with cutaneous leishmaniasis cured without systemic therapy. Clin Infect Dis. 2013;57(3):370–80. doi: 10.1093/cid/cit269. This study by the Cutaneous Leishmaniasis French Study Group reviewed clinical outcomes of various treatment paradigms for CL and described clinical criteria for starting systemic versus topical/local therapy. [DOI] [PubMed] [Google Scholar]
- 14••.Neves LO, Talhari AC, Gadelha EP, Silva Júnior RM, Guerra JA, Ferreira LC, et al. A randomized clinical trial comparing meglumine antimoniate, pentamidine and amphotericin B for the treatment of cutaneous leishmaniasis by Leishmania guyanensis. An Bras Dermatol. 2011;86(6):1092–101. doi: 10.1590/s0365-05962011000600005. This study is one of the largest randomized control studies in recentyears to compare clinical outcomes in parenteral therapy for NWCL. [DOI] [PubMed] [Google Scholar]
- 15.Mueller Y, Nguimfack A, Cavailler P, Couffignal S, Rwakimari JB, Loutan L, et al. Safety and effectiveness of amphotericin B deoxycholate for the treatment of visceral leishmaniasis in Uganda. Ann Trop Med Parasitol. 2008;102(1):11–9. doi: 10.1179/136485908X252142. [DOI] [PubMed] [Google Scholar]
- 16.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 2013;368(6):524–32. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- 17.Khalili G, Dobakhti F, Mahmoudzadeh-Niknam H, Khaze V, Partovi F. Immunotherapy with Imiquimod increases the efficacy of glucantime therapy of Leishmania major infection. Iran J Immunol. 2011;8(1):45–51. [PubMed] [Google Scholar]
- 18.David CV, Craft N. Cutaneous and mucocutaneous leishmaniasis. Dermatol Ther. 2009;22(6):491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 19.Bumb RA, Satoskar AR. Radiofrequency-induced heat therapy as first-line treatment for cutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2011;9(6):623–5. doi: 10.1586/eri.11.50. [DOI] [PubMed] [Google Scholar]
- 20.Bumb RA, Prasad N, Khandelwal K, Aara N, Mehta RD, Ghiya BC, et al. Long-term efficacy of single-dose radiofrequency-induced heat therapy vs. intralesional antimonials for cutaneous leishmaniasis in India. Br J Dermatol. 2013;168(5):1114–9. doi: 10.1111/bjd.12205. [DOI] [PubMed] [Google Scholar]
- 21.López L, Robayo M, Vargas M, Vélez ID. Thermotherapy an alternative for the treatment of American cutaneous leishmaniasis. Trials. 2012;13:58. doi: 10.1186/1745-6215-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195(12):1846–51. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, et al. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011;118(2):87–96. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Monge-Maillo B, López-Vélez R. Therapeutic options for old world cutaneous leishmaniasis and new world cutaneous and mucocutaneous leishmaniasis. Drugs. 2013;73(17):1889–920. doi: 10.1007/s40265-013-0132-1. [DOI] [PubMed] [Google Scholar]
- 25••.Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, et al. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;83(5):1028–33. doi: 10.4269/ajtmh.2010.10-0171. This review of patients treated at the Walter Reed Army Medical Center demonstrated high efficacy (84%) of liposomal amphotericin B against various species of Leishmania. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon M, Pavlotzky F, Barzilai A, Schwartz E. Liposomal amphotericin B in comparison to sodium stibogluconate for Leishmania braziliensis cutaneous leishmaniasis in travelers. J Am Acad Dermatol. 2013;68(2):284–9. doi: 10.1016/j.jaad.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol. 2006;53(2):121–6. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 28.Machado PR, Penna G. Miltefosine and cutaneous leishmaniasis. Curr Opin Infect Dis. 2012;25(2):141–4. doi: 10.1097/QCO.0b013e3283509cac. [DOI] [PubMed] [Google Scholar]
- 29••.Rubiano LC, Miranda MC, Muvdi Arenas S, Montero LM, Rodríguez-Barraquer I, Garcerant D, et al. Nonin-feriority of miltefosine versus meglumine antimoniate for cutaneous leishmaniasis in children. J Infect Dis. 2012;205(4):684–92. doi: 10.1093/infdis/jir816. This paper presents data from a recent randomized noninferiority study comparing oral miltefosine to antimony in the treatment of NWCL. Based on this and other trials in Colombia and Brazil, miltefosine was approved by the FDA for the indication of CL, ML, and VL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorlo TP, van Thiel PP, Schoone GJ, Stienstra Y, van Vugt M, Beijnen JH, et al. Dynamics of parasite clearance in cutaneous leishmaniasis patients treated with miltefosine. PLoS Negl Trop Dis. 2011;5(12):e1436. doi: 10.1371/journal.pntd.0001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Emad M, Hayati F, Fallahzadeh MK, Namazi MR. Superior efficacy of oral fluconazole 400 mg daily versus oral fluconazole 200 mg daily in the treatment of cutaneous leishmania major infection: a randomized clinical trial. J Am Acad Dermatol. 2011;64(3):606–8. doi: 10.1016/j.jaad.2010.04.014. This paper presents data from a randomized trial in Iran which evaluated the efficacy of high-dose fluconazole for the treatment of L. major OWCL, and demonstrated an 81% rate of complete resolution after 6 weeks of treatment. [DOI] [PubMed] [Google Scholar]
- 32.Sousa AQ, Frutuoso MS, Moraes EA, Pearson RD, Pompeu MM. High-dose oral fluconazole therapy effective for cutaneous leishmaniasis due to Leishmania (Vianna) braziliensis. Clin Infect Dis. 2011;53(7):693–5. doi: 10.1093/cid/cir496. [DOI] [PubMed] [Google Scholar]
- 33.Saenz RE, Paz H, Berman JD. Efficacy of ketoconazole against Leishmania braziliensis panamensis cutaneous leishmaniasis. Am J Med. 1990;89(2):147–55. doi: 10.1016/0002-9343(90)90292-l. [DOI] [PubMed] [Google Scholar]
- 34•.Ramanathan R, Talaat KR, Fedorko DP, Mahanty S, Nash TE. A species-specific approach to the use of non-antimony treatments for cutaneous leishmaniasis. Am J Trop Med Hyg. 2011;84(1):109–17. doi: 10.4269/ajtmh.2011.10-0437. This case series from the National Institutes of Health evaluated efficacy and tolerability of non-antimony-based treatments for CL, including oral miltefosine, ketoconazole, and liposomal amphotericin B. [DOI] [PMC free article] [PubMed] [Google Scholar]


