Abstract
Aim
The purpose of this study was to provide a quantitative assessment of female rat sexual behaviors after acute exposure to the A-ring reduced testosterone metabolite, androstanediol (3α-Diol), through the nucleus accumbens (NA) shell.
Main outcome measures
Quantitative analyses of female rat sexual behaviors and assessment of protein levels for the enzyme glutamic acid decarboxylase isoform 67 (GAD67) and gephyrin, a protein that participates in the clustering of GABA-A receptors in postsynaptic cells, were accomplished.
Methods
Female rats were ovariectomized and primed with estrogen and progesterone to induce sexual behaviors. Females received a 3α-Diol infusion via guided cannula that aimed to the NA shell five minutes prior to a sexual encounter with a stud male. The following parameters were videotaped and measured in a frame by frame analysis: lordosis quotient (LQ), Lordosis rating (LR), frequency and duration of proceptive behaviors (hopping/darting and ear wiggling). Levels of GAD67 and gephyrin were obtained by Western blot analysis two or twenty-four hours after the sexual encounter.
Results
Acute exposure to 3α-Diol in the NA shell enhanced LR, ear wiggling, and hopping/darting but not LQ. Some of these behavioral effects were counteracted by co-infusion of 3α-Diol plus the GABAA-receptor antagonist GABAzine. A transient reduction of GAD67 levels in the NA shell was detected.
Conclusions
The testosterone metabolite 3α-Diol enhances sexual proceptivity, but not receptivity, when infused into the NA shell directly. The GABAergic system may participate in the androgen-mediated enhancement of female rat sexual motivation.
Introduction
The medical management of menopause focuses primarily on the symptoms associated with estrogen deficit (bone loss, enhanced risk of cardiovascular disease, urogenital atrophy, and mood changes). However, net declines in testosterone levels also occurred in postmenopausal women and are associated with reduction in sexual motivation, sexual fantasies, sexual pleasure, sexual arousal, vaginal lubrication, and vasocongestion of erectile tissues [1]. In fact, female androgen insufficiency has been described for women suffering bilateral oophrectomy, chemotherapy, and radiation therapy [1–3]. In women, circulating testosterone levels decreases with age and can attain levels that are below median values for women in their twenties [4]. In recent years, there has been an interest in the clinical use of the testosterone transdermal patch as a hormone replacement therapy for women who are unresponsive to estrogen treatment to manage fatigue, loss of well-being, and reduced libido [5–9].
It remains as a challenge in the field to establish the precise mechanisms of action that mediate the effect of testosterone and its metabolites on human behavior. In the rodent model, testosterone, dihydrotestosterone, its metabolite androstane-3α, 17βdiol (3α-Diol), or synthetic androgens have rewarding affective properties [10–13], thereby influencing affective and cognitive behaviors. More importantly, it has been established that the hedonic effects of testosterone or 3α-Diol can be mediated, at least in part, by the nucleus accumbens (NAc) shell, because subcutaneous administration of 3α-Diol requires intact shell subregion to develop conditioned place preference (CPP), without requirement of the core subregion [13–15]. In addition to the rewarding properties, androgens have been long recognized as important modulators of male and female sexual motivation [16]. In the case of the female rat, several physiological roles for 3α-Diol have been reported in relation to its reproductive cycle. For instance, previous studies have established that 3α-Diol participates in sexual maturation [17]. In addition, 3α-Diol reaches peak levels on the afternoon of proestrus several hours prior to sexual receptivity [18]. Paradoxically, 3α-Diol shows opposite behavioral effects depending on which neural network is infused. Specifically, this compound increases lordosis quotient when infused in the VMH while it attenuates lordotic responses and promotes aggressive-like behaviors when infused in the mPOA [19–20]. As shown with other neurosteroids, 3α-Diol is a positive allosteric modulator of GABAA receptors in these brain regions [21]. With regard to the male rat, the rise in androgen secretion following sexual experience has been associated with an anxiolytic effect of the androgen, which may be mediated by its 5α-reduced metabolite, 3α-Diol [22]. Therefore, the purpose of this study was to provide a quantitative assessment of female rat sexual behaviors after acute exposure to 3α-Diol through the NAc shell.
We have chosen to study the A-ring reduced testosterone metabolite, androstanediol (3α-Diol), when infused into the NAc shell for the following reasons. First, plasma levels of 3α-Diol elevate during the afternoon of proestrus on naturally-cycling female rats [18]. Second, 3α-Diol has hedonic properties as demonstrated in conditioned place preference [14] and self-administration paradigms [11]. Third, within the mesolimbic circuitry of reward, the NAc shell participates in motivational aspects of behavior when challenged with natural rewards [23–24]. Fourth, the GABAergic system modulates female rat sexual behavior [19, 25]. Fifth, more than 90% of the neurons within the NAc are GABAergic, locally connected through chemical [26] and electrical [27] synapses, forming a network that provides fast GABAergic inhibition of nearby cells [28]. Sixth, 5α-reduced steroid metabolites are positive allosteric modulators of GABAA receptors29 that promote fast behavioral changes [13, 19, 29–31].
Based on these facts, we hypothesized that acute exposure to the testosterone metabolite 3α-Diol through the NAc shell modulates distinct components of the female rat sexual repertoire, at least in part, through the GABAergic system. The role of the GABAergic system was evaluated by studying the effect of different GABAergic modulators on receptive and proceptive behaviors. In order to investigate the influence of acute exposure to 3α-Diol on pre- and postsynaptic components of the GABAergic system, immunoblotting experiments with NAc shell crude extract were conducted against glutamic acid decarboxilase 67 (GAD67), which is the rate limiting enzyme for GABA synthesis [32] and gephyrin, a protein responsible for the postsynaptic clustering of GABAA receptors [33].
Methods
Animals
Female Sprague Dawley rats (n=72) weighting 225–250g were purchased from Charles River Laboratories Inc. (Wilmington, MA) and housed in pairs in hanging acrylic cages in a 12 hours light/dark reverse cycle (light off 21: 00 h), with food and water available ad libitum in the Animal Resources Center (ARC) of the XXX. Ovariectomy and brain cannulation surgeries were performed simultaneously at least seven days after arrival. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at XXX.
Surgeries
In order to have experimental control over plasma levels of estrogen (E) and progesterone (P), animals underwent bilateral ovariectomy (OVX) under a cocktail of xylazine (10 mg/kg, i.p.) and ketamine (80 mg/kg, i.p.) anesthesia as previously described by Waynforth and Flecknell [34]. Because NAc shell it is widely related with addictive and natural reward-associated behaviors, bilateral brain cannulae (10 mm 23-Gauge stainless steel) targeting the NAc medial shell were carried out using a stereotaxic apparatus with the nose bar 5 mm above interaural zero (Stoelting Co. Wood Dale, IL), following coordinates from bregma at AP+3.5, ML+ 1.1 and DV −5.3 according to Paxinos and Watson Atlas [35]. Cannula were held in their place using three 1.59 mm outer diameter stainless steel bone anchor screws (Stoelting Co. Wood Dale, IL) and covered with dental cement (Dentsply Co, Mildford, DE). A stainless steel stylet (30 Ga) was inserted into each cannulae to minimize occlusion and infection. After surgeries, animals received 0.03 mg (i.p.) of buprenorphine (Buprenex®) as analgesic treatment for three consecutive days, and 2 mL of saline immediately after surgery. Stylets were removed and cleaned periodically with 70% ethanol and rinsed with saline prior to reinsertion in order to ensure clean and functional cannulae, and to minimize distress by daily handling of animals after surgery. Animals were allowed to recover for 7 days before behavioral experiments.
Drugs and antibodies
3α-Diol (5α-androstane-3α,17β-Diol), estradiol benzoate (β-estradiol 3-benzoate), progesterone (4-pregnene-3,20-dione), GABAzine (SR-95531), and muscimol (M1523) were purchased from Sigma (St. Louis, MO). 3α-Diol at 1µM [36] was prepared in saline by two successive dilutions from a 10 mM stock-solution in 30% 2-hydroxypropyl-β-cyclodextrin in 0.9% saline; hydrosoluble GABAzine (Sigma, St. Louis, MO). Antibody dilutions were as follows: antiGAD67 1:500 (Chemicon now Millipore, Temecula, CA), antiGephyrin 1:1000 (Sigma, St. Louis, MO), antiGAPDH 1:5000 (Sigma, St. Louis, MO).
Experimental design
Priming
In order to trigger high levels of receptivity OVX/cannulated female rats were injected subcutaneously into the dorsal region of the neck with estradiol benzoate (EB) (10 µg in 0.6 mL of sesame oil) 48 hour prior to behavioral testing and with progesterone (P4) (500 µg in 0.6 mL of sesame oil) 4 hour prior to behavioral testing [35].
Nucleus accumbens shell infusions
Animals were divided into two groups: ‘Behavioral group’ and ‘Inmmunobloting group’. Female rats in the “behavioral group” received bilateral microinfusions of saline, 1µM 3α-Diol, 1mM GABAzine/1µM 3α-Diol [36], 1mM GABAzine [36] into NAc shell in a counterbalanced schedule. Animals from the “immunoblotting group” received saline or 1µM 3α-Diol before a single experimental session. Infusions were made 5 min before the behavioral test via a 12.5 mm 30 gauge stainless steel injectors (Small Parts Inc, Miami, FL) attached by a 0.61 mm outer diameter bore polyethylene tubing to a syringe driven by an infusion pump (Harvard Apparatus, Holliston, MA) at 0.5 µL/min rate for 1 min. which were left in cannula for 1 minute to allow complete drug diffusion and to prevent backflow.
Behavioral chamber
The behavioral chamber consisted of a 75 × 37.5 × 30 cm transparent Plexiglas cage (XXX), divided in half by a removable Plexiglas wall, which had a 5 cm diameter hole in the bottom center. This small hole permitted the passage of the female only so that the female rat had control over the sexual encounter with the stimulus male. Training and behavioral testing were conducted under dim red light illumination (30 watts).
Habituation and training
Animals were habituated to the chamber for 10 min in two different sessions per week before the experimental session. Females were habituated in pairs and they were allowed to go across both sides of the chamber. Males were habituated to the behavioral chamber along with hormone-primed females.
Selection of stimulus males
Copulating (stud) males were selected from a sexually inexperienced group. On the first day of training, pairs of naïve male and stimulus female were restricted to spend time in the male compartment of the behavioral chamber. Males were allowed to mount the female until ejaculation. On subsequent days, the stimulus female was able to move freely between the two compartments of the testing chamber, while males were trained to remain on one side, with a light punishment: the acrylic divider was knocked with the tip of a pen near the gateway between compartments. During subsequent attempts of the male to enter the female compartment, the male was tapped slightly in the snout with a pen following Erskine (1985) [37]. Stud males were selected for this study when the male was able to mount the female within two minutes of her arrival into his compartment. These males displayed a stereotyped mating behavior that included very rapid aggressive-like approaches to the female. Males that consistently got stuck in the opening of the divider between compartments were not selected. Two different males were usually employed to complete a session because it was frequent that males ejaculated before females received the required ten mounts in this experimental design. The replacement of the male was only made when the female returned to her compartment [38].
Sexual behaviors
The female rat sexual response has been traditionally defined as a series of stereotyped responses that are classified as appetitive/proceptive and receptive behaviors. Under this classification, appetitive or proceptive copulatory behaviors such as hopping and darting, ear wiggling and pacing are also known as solicitation behaviors [39]. In contrast, the consummatory phase of copulation is characterized by the lordotic reflex which facilitates penile intromission and intravaginal ejaculation [40]. In this study, each experimental female was placed in the opposite compartment of the stud male. The female had free access to both compartments until she received 10 mounts from the stud male. The test was recorded by a digital video camera (Sony Model DCR-TRV). Test duration was measured with a chronometer. The following parameters were recorded during the session for each female: number of mounts, time to exit and return to male compartment, occurrence of noticeable events, such as rejection to a mount, and test duration. Behavioral sessions were finished at 10 mounts or at 10 minutes. The following parameters were measured: lordosis quotient (LQ) defined as # lordotic responses/10 mounts X 100; lordosis rating defined as the intensity of each lordotic response rated according to a 4-points scale for no, slight, moderate or full dorsiflexion of the back; frequency and duration of proceptive behaviors: ear wiggling and hopping/darting. For accuracy of these measurements, analyses were made with frame by frame analysis (30 frames/sec) [40]. Frequency is defined as the number of events per minute to account for variable test session durations. Rats were tested one day per week for up to 4 weeks in order to complete a counterbalanced protocol that minimized between subject differences on behavioral measures. In a counterbalanced protocol, each female was assayed with all drugs within her own group. For example, subjects 1–5 received 3α-Diol microinjections whereas subjects 6–10 received vehicle injections on week 1. The opposite was true for week 2 for the same batch of animals. This design eliminates an order effect on behavior. Last, the one-week timing interval between behavioral sessions was sufficient to allow recovery from any plausible long-term effects on central drug infusions as reported results stem from pooled data. Our design was possible because it is well established that female rat sexual behaviors are stable through a large number of repeated tests (for review, see [40]).
Western blot analysis of GAD67 and gephyrin
Fifty (50) animals were infused with saline or 3α-Diol (1µM) and tested on a sexual encounter as specified above. A total of 32 animals were decapitated two hours after the behavioral test (16 saline- and 16 3α-Diol -treated females) while 18 animals were decapitated 24 hours after the test (9 saline- and 9 3α-Diol -treated females). Animals in this group were assayed after a single intrashell infusion of saline or 3α-Diol (1µM) to minimize tissue damage. Punches were collected from the shell zones surrounding the site which the cannulae occupied. Infusion sites were distributed along the rostro-caudal medial shell. Brains were excised quickly and frozen on dry ice for tissue preparation. Tissue was extracted following the anatomical demarcation of +2.2 mm through +1mm from bregma [41]. Samples for animals decapitated two hours after the behavioral session (saline versus 3α-Diol) consisted of four samples of four brains each whereas the twenty-four hour samples (saline versus 3α-Diol) consisted of three samples of three brains each. Differences in sample size were due to animal loss during experiments. Brains were placed in a 3 mm slices-acrylic rat brain matrix (Zivic Laboratories Inc, Pittsburgh, PA). NAc shell region was removed bilaterally with a 19 Ga- Neuro Punches (Fine Science Tools Inc, Foster City, CA) and punches were suspended in 15X its weight in volume of buffer containing protease inhibitor cocktail (Protease Arrest, Calbiochem, La Jolla, Ca). Tissue was homogenized for crude extract using a manual mixer pellet PSTLS 0.5 mL (Kontes Glass Co., Vineland, NJ) and the protein concentration was estimated by using the Bradford method. Extracts were denatured with Laemmli Buffer [62.5 mM Tris-HCl, pH 6.8, 2% dodecyl sulfate (SDS), 160 mM dithiothreitol, 0.001% bromophenol blue, 6M urea] and heated for 5 min at 95°C. The samples were loaded with 6 µg protein onto a 7.5% SDS-polyacrylamide gel and electrophoresed at 100 V for 1.5 h. The proteins were transferred to polyvinylidene difluoride (PVDF) membranes Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA) by semidry transfer (Trans-blot SD Bio Rad, Hercules, CA) at 15 V for 1 h.
Immunoblot analysis
PVDF Membranes were blocked at room temperature in 5% non-fat dry milk in Tris-buffered saline-Tween 20 (TBS-T; 50 mM Tris base, 20 mM NaCl, 0.1% Tween 20) for 1 h. Membranes were incubated with rabbit antiGAD 67 affinity purified polyclonal antibody (1:500, Chemicon, Temecula, CA) or rabbit gephryrin affinity purified polyclonal antibody (1:1000, Chemicon, Temecula, CA) diluted in PBS and 3% non-fat dry milk in TBS-T, respectively. Membranes were washed with TBS-T and incubated for 1 h at room temperature in horseradish peroxidase conjugated anti-rabbit antibody (1:5000; Sigma). The housekeeping protein GAPDH was used as loading controls for the western blots (monoclonal antibody GAPDH of, 1:5000, (Chemicon, Temecula, CA), horseradish peroxidase conjugated anti-mouse antibody 1: 5,000). Membranes were rinsed in TBS-T and the immunolabeled proteins were detected by enhanced chemoluminescence SuperSignal™ West Dura extended Duration Substrate (Pierce, Rockford, IL). Immunoreactive bands were visualized using a VersaDoc (Bio-Rad-Laboratories, Hercules, CA) imaging system, and quantification was carried out using the Quantity One software program. Each membrane was stripped twice at room temperature for 10 min with Restore Western Blot Stripping Buffer (Pierce, Rockford IL) for reprobing with all three antibodies. After blotting, membranes were placed between two Whatman™ filter papers and conserved at 4°C until the next blot. Western blots were then, achieved in the following order: (1) GAD67 expression, (2) 1st stripping (3) reblot with GAPDH antibody (4) 2nd stripping and finally (5) reblot for Gephyrin expression. This order was chosen to prevent interference or cross-reactivity between polyclonal antibodies.
Histology
At the end of behavioral experiments, rats were decapitated and brains were rapidly removed, placed on dry ice, and stored at −80° C until coronal sections were made in a CM 1900 Leica cryostat. Each 40 µm frozen slice was mounted on gelatin-coated slide, defatted and stained with cresyl violet stain (Sigma-Aldrich St. Louis). A Ziess, Axiokop light microscope with Zeiss Axiocam camera was used to verify cannulae placement and the site of injection according to the rat brain atlas [35].
Inclusion criteria
Two criteria were used to determinate the inclusion of data for analysis: first, females that accepted mounts from males with at least an LQ score of 60 [42]; second, bilateral microinfusions reached the NAc shell upon histological verification.
Statistical Analysis
The parameters of the mating tests are expressed as mean ± standard error of the mean (SEM). Kruskal-Wallis one way analysis of ranks followed by Dunn’s method was employed. Statistical significance was attained at p ≤ 0.05.
Results
Microinfusion sites and dose-response relationships for hormonal priming
Histological verification for the infusion site confirmed that 78% of our bilateral brain cannulation surgeries that aim at the Nucleus accumbens (NAc) shell were successful (Figure 1). The optimal hormonal priming doses for estrogen and progesterone were established before testing the effects of intrashell microinfusion of 3α-Diol on female sexual behaviors. Three different priming schedules were tested; 50% =5 µg of estradiol benzoate (EB) + 250 µg of P4, 75%= 7.5 µg of EB+ 375 µg of P4, and 100%= 10 µg of EB + 500 µg of P4, 75%= 7.5 µg of EB+ 375 µg of P4, and 100%= 10 µg of EB + 500 µg of P4. In this dose-response study, half of the animals for each dose were exposed to vehicle infusion while the other half was exposed to 3α-Diol. Analyses of the lordosis quotient (LQ) and lordosis rating (LR), revealed that 3α-Diol did not enhance LQ’s at any of the priming dosages whereas the schedule of 10 µg of EB + 500 µg of P4 priming elicited robust display of solicitation behavior in OVX-primed female rats in presence of 1µM 3α-Diol. A significant increase of lordosis rating (LR) after central infusion of 3α-Diol was observed (p ≤.001; data not shown). Similarly, a significant increase in the duration of motivational aspects of female sexual behaviors (hopping/darting and ear wiggling) after intrashell infusion of 1µM 3α-Diol was observed (p≤ 0.05; data not shown). However, no differences were detected on the lordosis quotient (LQ) after 3α-Diol infusion in the NAc shell. Therefore, all subsequent experiments were performed with a 10 µg of EB + 500 µg of P4 priming regime.
Figure 1.
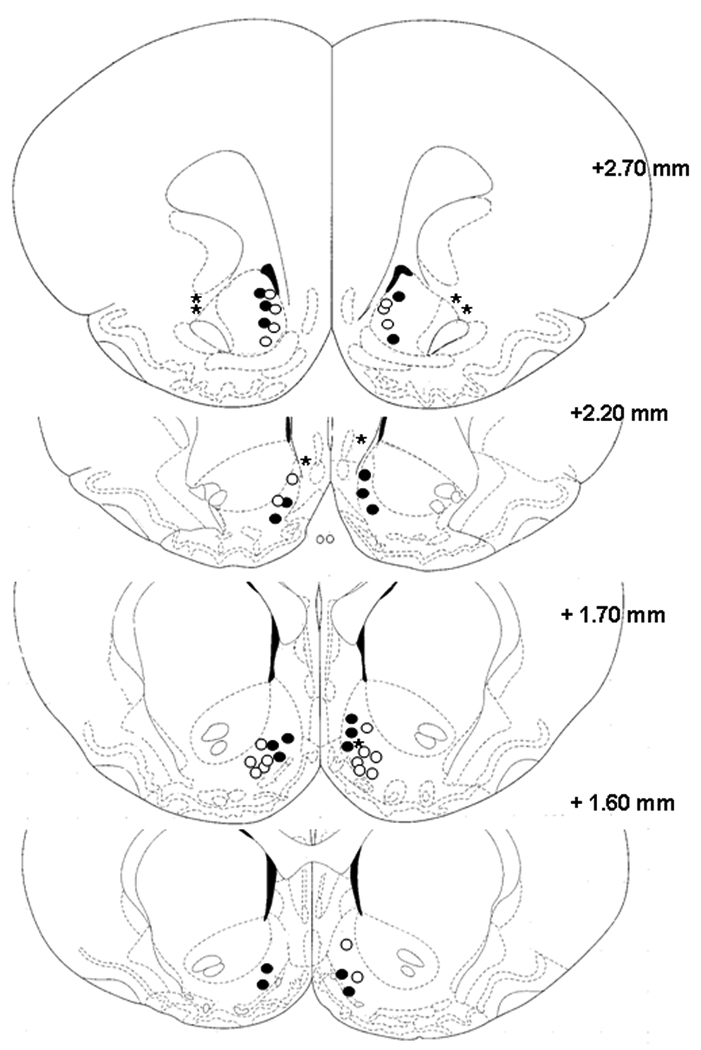
Pharmacological profile of 3α-Diol effects on female rat sexual behavior when infused in the NAc shell
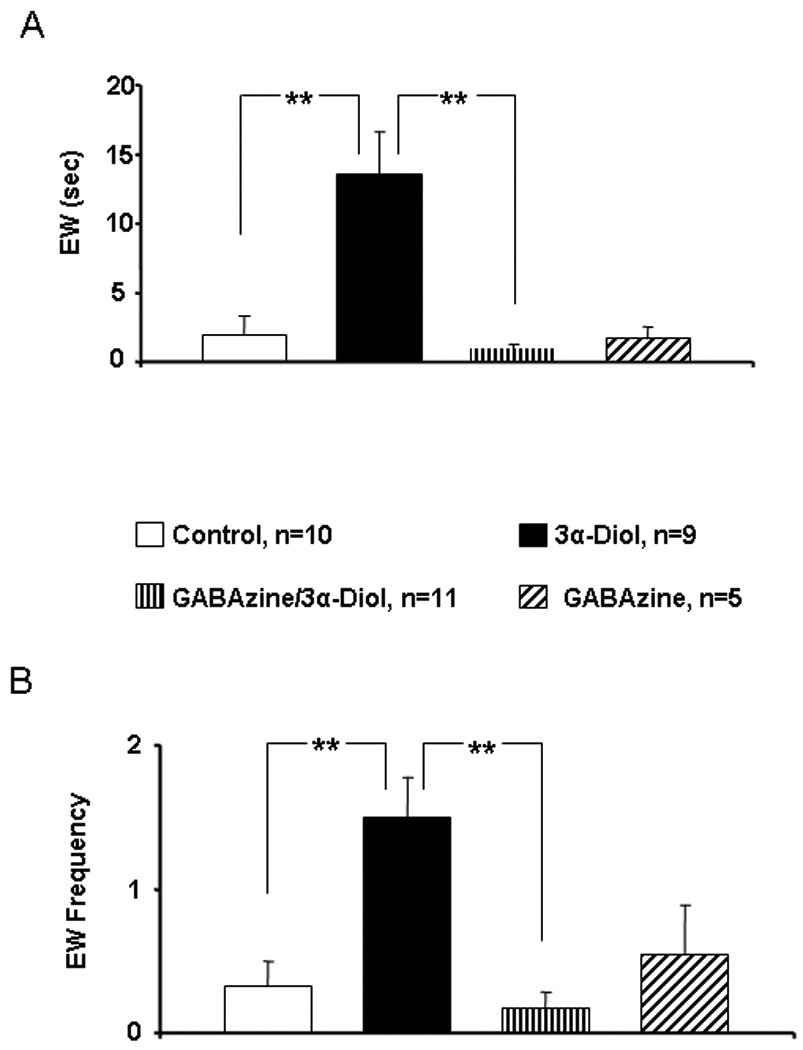
OVX- EB + P4 primed female rats received counterbalanced infusion of saline (control), 3α-Diol (1µM), co-infusion of the GABAA receptor selective antagonist GABAzine (1mM) plus 3α-Diol (1µM), or GABAzine alone (1mM) into the NAc shell five minutes prior to a sexual encounter with a stud male. There was no effect of treatment on LQ score (Figure 2A). However, infusion of 3α-Diol induced an increase of lordosis rating (LR) by approximately 25% when compared to all other treatments within the group (p ≤ 0.001; Figure 2B) whereas co-infusion of 3α-Diol plus GABAzine produced a LR comparable to the saline group and statistically different from the 3α-Diol group (p ≤ 0.001). GABAzine alone did not modify LR score when compared to control. Similarly, infusion of 3α-Diol in the NAc shell induced a significant increase in hopping/darting duration (Figure3A, p ≤ 0.01) and hopping/darting frequency (Figure 3B, p ≤ 0.001). Co-infusion of 3α-Diol plus GABAzine resulted in a hopping/darting frequency comparable to the control group and statistically different from the 3α-Diol group (p ≤ 0.001). Last, intrashell microinfusion of 3α-Diol increased the duration (Figure 4A) and the frequency (Figure 4B) of ear wiggling when compared to the control group (p ≤ 0.001) and to the 3α-Diol plus GABAzine group (p ≤ 0.001).
Figure 2.
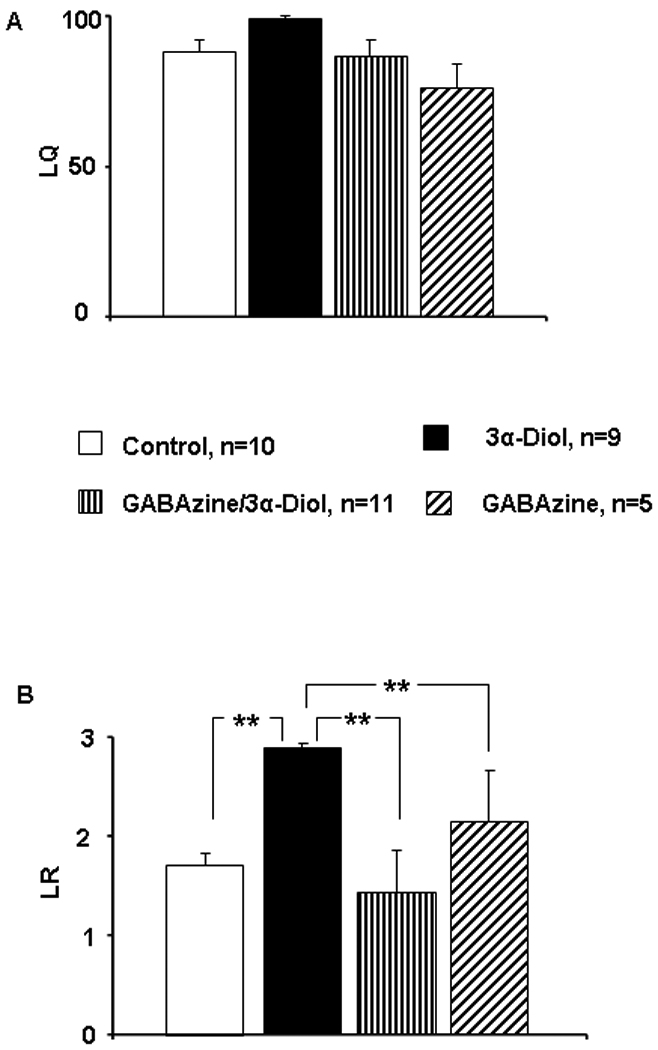
Figure 3.
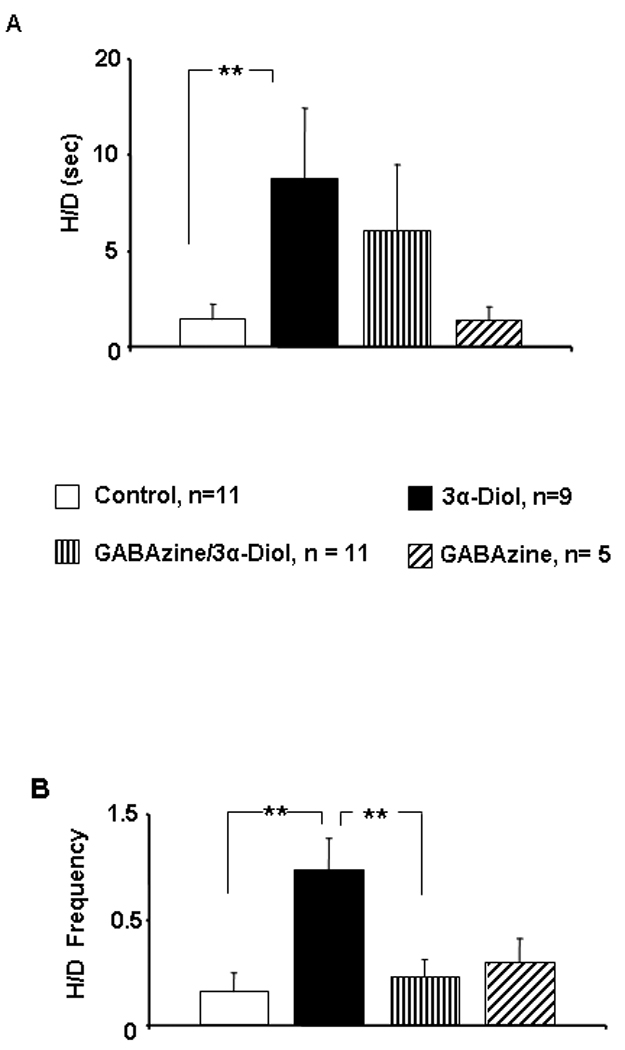
Figure 4.
GAD67 and gephrin levels after infusion of 3α-Diol in the NAc shell
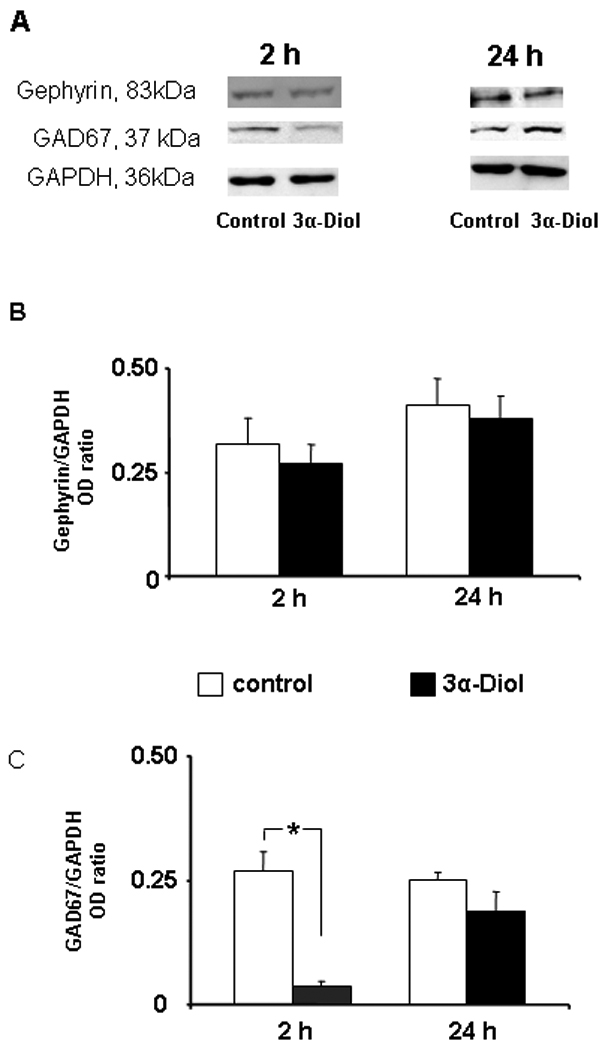
In order to investigate the influence of acute exposure to 3α-Diol on pre- and postsynaptic components of the GABAergic system, immunoblotting experiments were conducted with NAc shell crude extract against GAD67 and gephyrin. A representative gel for the expression of gephryrin, GAD67, and GAPDH in the NAc shell two or twenty-hours after infusion of saline or 3α-Diol followed by behavioral testing is shown in Figure 5A. No differences were detected on gephryn for any time point (Figure 5B). However, an 80% decrease in GAD67 levels on the two-hour time point was detected in the 3α-Diol group (Figure 5C, p ≤ 0.05). GAD67 returned to baseline levels on the 24 hour time point (Figure 5C).
Figure 5.
Discussion
We have found that central administration of the testosterone metabolite 3α-Diol into NAc shell enhances the lordosis rating (LR) and increases the frequency and duration of proceptive behaviors (hopping/darting and ear wiggling) without affecting the lordosis quotient (LQ). Lordosis is the motor reflex responsiveness of the female rat during intromission by the male. All hormone schedules that were used in this study for priming enabled sexual receptivity. This was evident by the fact that lordosis quotient (LQ) of 85 was obtained with 50% of maximal priming doses. An LQ of at least 60% is commonly accepted as positive for the presence of receptivity [38]. Given that sexual receptivity in experimental females was stable, as assessed with LQ, we are certain that changes in other components of the sexual response were not due to receptivity. In fact, a comparison of lordosis rating and proceptive behaviors (hopping/darting and ear wiggling) allowed us to detect dose-dependent effect on proceptive behaviors. By testing three different schedules, we determined that the dose of 10 µg EB + 500 µg P4 (100%) produced the highest level of motivational parameters, a finding consistent with the literature [38– 39].
Although a ceiling effect for the capacity of 3α-Diol to enhance LQs cannot be ruled out, it is well known that rodent sexual behaviors are amenable to housing conditions, handling, and lighting periods [43–45]. Any of these variables could have affected baseline lordotic responses. Nevertheless, our results suggest that 3α-Diol effects on female rat sexual behavior through the NAc shell targets sexual motivation rather than sexual receptivity. In fact, a body of work shows that 3α-Diol enhances lordosis through VMN whereas it attenuates lordotic responses when infused in mPOA [19–20]. In addition, it shortens the period of sexual receptivity [18]. These seemingly paradoxical effects were precisely the basis for our study. We hypothesized that the net effect of 3α-Diol on female rat sexual behavior will be dependent on the precise timing at which distinct neural circuitries are modulated by this compound.
The late and influential animal sex researcher Mary Erskine proposed that the ring-A reduced 3α-Diol synthesized from ovarian testosterone, reaches peak levels during the afternoon of proestrus to abbreviate the period of the sexual receptivity [38]. Our results now show that an enhancement of sexual-incentive motivation can be attained by 3α-Diol within the nucleus accumbens shell. Therefore, the neurochemical signal that mediates sexual reward could be the same signal that “turns off” sexual receptivity. It is well-established that 3α-Diol can reach physiologically relevant levels in the brain during proestrus [46, 47]. The emergent scenario is that 3α-Diol mediates, at least in part, the rewarding effects of testosterone [30]. In fact, it has been shown that OVX female rats self-administer 3α-Diol [11]. Other A-reduced steroid metabolites have been implicated as strong modulators of female rat sexual behaviors. For instance, a recent study demonstrated that brain levels of dihydroprogesterone (DHP) and 5α-pregnan-3α-ol-20-one were increased in intact proestrus rats after paced- mating copulation [47]. Therefore, A-reduced steroid metabolites seem to provide a fast mechanism to modulate female rat sexual behaviors. With regard to paced-mating copulation, it is important to highlight that in contrast to Long-Evans female rats, Sprague-Dawley and Wistar female rats do not display paced mating behavior in a semi-natural arena (Anders Ågmo, personal communication). In this study, females spent about 85% of the time in the male compartment which precluded our analyses for drug effects on paced mating behavior. Although it is a tenet in the field that only pacing- mated female rats are able to develop CPP [48] following a rise in dopamine levels in the NAc [49], the preference for the male compartment displayed by Sprague Dawley female rats could be indicative for greater incentive motivation [50]. In support of this interpretation, it has been reported that CPP can be apparent in non-paced mating conditions [51]. Specifically, they proposed that the time elapsed between females that received an ejaculation and the time for the next intromission is critical for maintaining the reinforcing properties of the sexual encounter. In this study, the male that ejaculated was replaced by other stud male only when the female left his compartment, thus keeping intact the post-ejaculatory interval in the presence of the male.
There is powerful evidence that A-reduced steroid metabolites modulate neuronal activity predominantly through GABAergic neurotransmission [19, 21, 52]. We expected that 3α-Diol treatments would up-regulate gephryrin to strengthen GABAergic postsynaptic plasticity. However, we were not able to detect changes at 2h and 24h intervals. The simplest explanation would be that we lost the time point to detect a change. Conversely, it is known that gephyrin binds γ2-subunit to anchor GABAA receptors at the postsynaptic site [53]. But, very recent evidence reported that there are no GABAA receptors having high sensitivity for neurosteroids co-expressing δ and γ2-subunit in the striate [54]. Therefore, the lack of effect of 3α-Diol in modulating gephryrin expression in this study can be explained, in part, by the lack of GABAA-R with appropriate subunits that are required for anchorage of the receptor within the NAc shell.
GAD67 levels decrease when GABA levels rise [55]. GAD67, more predominantly cytosolic holo-GAD is a sensitive and fast regulator of GABA synthesis. As such, changes in the expression of GAD67 may exert more significant changes in GABA homeostasis in response to external agents [56]. Further experiments are required to understand if the 3α-Diol–mediated down-regulation of GAD67 is a consequence of the 3α-Diol enhancement of GABAergic neurotransmission, of the sexual encounters, or whether these are separate events. In vivo microdialysis studies can shed light on this issue. In addition, further experiments are required to determine the exact mechanism(s) responsible for the diminution of GAD67 levels. The monitoring of the conversion of full-length GAD67 into truncated and less active forms of tGAD at various time points is desirable [57].
The NAc has two main subregions, core and shell, and functional implications had been described in relation with anatomical and neurochemical differentiation of the NAc. In this regard, it has been found that the blockade of glutamatergic inputs to core subregion, but not shell, reduced exploratory locomotion [58]. In addition, the NAc core projects into the dorsolateral ventral pallidum, which, in turns, projects to the globus pallidus, and the substantia nigra pars reticulate [59]. Therefore, this circuitry is interconnected with motor structures associated to response-reinforcement learning [60–61]. In contrast, the shell subregion projects into the subcortical limbic system: medial VP, VTA and the hypothalamus, which is particularly sensitive to natural reward [62–63], as well as addictive drugs [64]. Many studies from drug reward have shown that self-administration of psychostimulants is mediated by the medial shell, but not core [65–66]. The NAc shell conforms part of the so-called mesolimbic circuitry of reward, a circuitry that is clearly involved in natural rewards and drug addiction [49, 64]. In fact, dopamine levels within the NAc shell are regulated by the GABAergic system and they provide a point of neurochemical convergence for the rewarding aspects of sex versus drug addiction. Increases in dopamine levels in female rat NAc have been reported after sexual activity [67] and after paced-mating [49]. In fact, the increase of NAc dopamine depends on the timing of copulatory stimuli [68].
Taken together, our results suggest that an endogenous androgen favors the display of proceptive behaviors in female sexual behavior accompanied by plastic changes in the GABAergic system within the NAc shell. Therefore, the role of NAc shell on the female rat sexual response must be re-examined with regard to sexual motivation. In fact, a recent model postulates the involvement of the mesolimbic circuitry on hormone-mediated reward processes [69]. Our results are consistent with emergent ideas on how neural networks outside the limbic circuitry can impinge upon distinct components of the sexual repertoire. It is of great interest that a testosterone metabolite enhances female rat sexual proceptivity through a mesolimbic neural network. We cannot ask female rats how it feels during an experimental sexual encounter but we can certainly uncover the neurochemical complexities that underlie their sexual behaviors, which could be favored by androgens and its 5α-reduced metabolites.
Acknowledgments
This research was made possible by Grant Number RR15565 to JCJ (2001–06) and DID UACh S-2010-09 to ESM. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. We dedicate this work to the memory of Dr. Mary Erskine.
Footnotes
Conflict of Interest: None
References
- 1.Bachmann G, Bancroft J, Braunstein G, Burger H, Davis S, Dennerstein L, Goldstein I, Guay A, Leiblum S, Lobo R, Notelovitz M, Rosen R, Sarrel P, Sherwin B, Simon J, Simpson E, Shiften J, Spark R, Traish A. Female androgen insufficiency: The Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 2.Shwenkhagen A. Hormonal changes in menopause and implications on sexual health. J Sex Med. 2007;4(Suppl 3):220–226. doi: 10.1111/j.1743-6109.2007.00448.x. [DOI] [PubMed] [Google Scholar]
- 3.Davison S, Bell R, Donath S, Montalto J, Davis S. Androgen levels in adult females: change with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 4.Genazzani AR, Pluchino N, Freschi L, Ninni F, Luisis M. Androgens and the brain. Maturitas. 2007;57:27–30. doi: 10.1016/j.maturitas.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Shifren JL, Davis SR, Moreau M. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause. 2006;13:770–779. doi: 10.1097/01.gme.0000243567.32828.99. [DOI] [PubMed] [Google Scholar]
- 6.Kingsberg S, Shifren J, Wekselman RN, Rodenberg C, Koochaki P, DeRogatis L. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001–1008. doi: 10.1111/j.1743-6109.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 7.Kingsberg SA, Simon JA, Goldstein I. The current outlook for testosterone in the management of hypoactive sexual desire disorder in postmenopausal women. J Sex Med. 2008;5(Suppl 4):182–193. doi: 10.1111/j.1743-6109.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 8.Panzer C, Guay A. Testosterone replacement therapy in naturally and surgically menopausal women. J Sex Med. 2009;6:8–18. doi: 10.1111/j.1743-6109.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 9.van der Made F, Bloemers J, Yassem WE, Kleiverda G, Everaerd W, van Ham D, Olivier B, Koppeschaar H, Tuiten A. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med. 2009;6:777–790. doi: 10.1111/j.1743-6109.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 10.Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacol Biochem Behav. 2000;65:327–332. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- 11.Jorge JC, Velázquez KT, Ramos-Ortolaza DL, Lorenzini I, Marrero J, Maldonado-Vlaar CS. A testosterone metabolite is rewarding to ovariectomized female rats. Behavioral Neurosci. 2005;119:1222–1226. doi: 10.1037/0735-7044.119.5.1222. [DOI] [PubMed] [Google Scholar]
- 12.Parrilla-Carrero J, Figueroa O, Lugo A, García-Sosa R, Brito-Vargas P, Cruz B, Rivera M, Barreto-Estrada JL. The anabolic steroids testosterone propionate and nandrolone, but not 17alpha-methyltestosterone, induce conditioned place preference in adult mice. Drug Alcohol Depend. 2009;100:122–127. doi: 10.1016/j.drugalcdep.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frye CA, Rhodes ME, Rosellini R, Svare B. The nucleus accumbens as a site of action for rewarding properties of testosterone and it's alpha-reduced metabolites. Pharmacol Biochem Behav. 2002;74:119–137. doi: 10.1016/s0091-3057(02)00968-1. [DOI] [PubMed] [Google Scholar]
- 14.Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3α-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res Rev. 2001;37:162–171. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 15.Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5α-reduced metabolite 3α-Androstanediol. Pharmacol Biochem Behav. 2007;86:354–367. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: Implications for the biological basis of sexual motivation. Behav Neurosci. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein B, Yehud S, Shani J, Goldhaber G. Suppression of luteinizing hormone release by 5α-androstane-3α,17βdiol and its 3β epimer in immature ovariectomized rats. J Endocrinol. 1976;70:25–30. doi: 10.1677/joe.0.0700025. [DOI] [PubMed] [Google Scholar]
- 18.Erskine MS. Serum 5α-androstane-3α,17β-diol increases in response to paced coital stimulation in cycling female rats. Biol Reprod. 1987;37:1139–1148. doi: 10.1095/biolreprod37.5.1139. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA, Duncan JE, Basham M, Erskine MS. Behavioral effects of 3 alpha-androstanediol. II: Hypothalamic and preoptic area actions via a GABAergic mechanism. Behav Brain Res. 1996;79:119–130. doi: 10.1016/0166-4328(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgen in modulating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 21.Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3 alpha-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996;79:109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- 22.Edinger KL, Frye CA. Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol Behav. 2007;92:443–453. doi: 10.1016/j.physbeh.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Stratford T. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide In nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 25.Luine VN, Wu V, Hoffmsn C, Renner KJ. GABAergic regulation of lordosis: Influence of gonadal hormones on turnover of GABA and interaction of GABA with 5-HT. Neuroendocrinology. 1999;69:438–445. doi: 10.1159/000054447. [DOI] [PubMed] [Google Scholar]
- 26.Goto Y, O'Donnell P. Network synchrony in the nucleus accumbens in vivo. J Neurosci. 2001;27:4498–4504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onn SP, Grace AA. Amphetamine alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci. 2000;20:2332–2345. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taverna T, van Dongen YC, Groenewegen HJ, Pennartz MA. Direct physiological evidence for synaptic connectivity between medium-sized spine neurons in rat nucleus accumbens in situ. J Neurophysiol. 2004;91:1111–1121. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- 29.Frye CA, Walf AA, Petralia SM. Progestins´s effects on sexual behaviour of female rats and hamsters involving D1 and GABA(A) receptors in the ventral tegmental area may be G-protein-dependent. Behav Brain Res. 2006;172:286–293. doi: 10.1016/j.bbr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:31–50. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- 31.Raddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3alpha-androstanediol. Neuroreport. 2004;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- 32.Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- 33.Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Yu W, Daniels SB, Cantino ME. De Blas AL Synaptic and extrasynaptic GABA-A receptor and gephyrin clusters. Prog Brain Res. 2002;36:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- 34.Waynforth BB, Flecknell PA. Experimental and surgical technique in the rat. San Diego: Academic Press; 1992. pp. 276–278. [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinate. 2nd Ed. San Diego: Academic Press Inc.; 1986. [Google Scholar]
- 36.Pérez-Acevedo N, Lathroum L, Jorge JC. The neurosteroid 3αDIOL modulates place preference when infused in the basolateral amygdala according to sex. Behav Neurosci. 2006;120:632–640. doi: 10.1037/0735-7044.120.3.632. [DOI] [PubMed] [Google Scholar]
- 37.Erskine MS. Effect of paced coital stimulation of estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- 38.Erskine MS. Solicitation behavior in the estrous female rat: A review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 39.Ellingsen E, Ǻgmo A. Sexual-incentive motivation and paced sexual behavior in female rats after treatment with drugs modifying dopaminergic neurotransmission. Pharmacol Biochem Behav. 2004;77:431–445. doi: 10.1016/j.pbb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Ågmo A. Sexual motivation-an inquiry into events determining the occurrence of sexual behavior. Behav Brain Neurosci. 1999;105:129–150. doi: 10.1016/s0166-4328(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 41.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 42.Guarraci FA, Benson A. "Coffee, tea and me": moderate doses of caffeine affect sexual behavior in female rats. Pharmacol Biochem Behav. 2005;82:522–530. doi: 10.1016/j.pbb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Fox MW. Environmental influences on behavior of domesticated and laboratory animals. Adv Vet Sci Comp Med. 1971;15:45–65. [PubMed] [Google Scholar]
- 44.Erskine MS, Kornberg E. Stress and ACTH increase circulation concentrations of 3α-androstanediol in female rats. Life Sci. 1992;5:2065–2071. doi: 10.1016/0024-3205(92)90157-k. [DOI] [PubMed] [Google Scholar]
- 45.Balfour ME, Yu L, Coolen LM. Sexual and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- 46.Erskine MS. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav Neurosci. 1992;106:690–697. doi: 10.1037//0735-7044.106.4.690. [DOI] [PubMed] [Google Scholar]
- 47.Frye CA, Paris JJ, Rhodes M. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social, increased 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez I, Paredes RG. Only self-paced mating is rewarding in rats of both sexes. Horm Behav. 2001;40:510–517. doi: 10.1006/hbeh.2001.1712. [DOI] [PubMed] [Google Scholar]
- 49.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–65. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 50.López HH, Wurzel G, Ragen B. The effect of acute bupropion on sexual motivation and behavior in the female rat. Pharmacol Biochem Behav. 2007;87:369–379. doi: 10.1016/j.pbb.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Meerts H, Clarck A. Female rats exhibit a conditioned place preference for nonpaced mating. Horm Behav. 2007;51:89–94. doi: 10.1016/j.yhbeh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 53.Schwizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Lüsher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 54.Sassoè-Pognetto M, Follesa P, Panzanelli P, Perazzinni A, Porcu P, Sogliano C, Cherchi C, Concas A. Fluctuations in brain concentrations of neurosteroids are not associated to changes in gephyrin levels. Brain Res. 2007;1169:1–8. doi: 10.1016/j.brainres.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 55.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 56.Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD67 protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- 57.Wei J, Davis KM, Wu H, Wu KY. Protein phosphorylation of human brain glutamic acid decarboxylase GAD65 and GAD67 and its physiological implications. Biochemistry. 2004;43:6182–6189. doi: 10.1021/bi0496992. [DOI] [PubMed] [Google Scholar]
- 58.Maldonado-Irizarry CS, Kelley AE. Excitotoxic lesions of the core and shell subregions of the nucleus accumbens differentially disrupt body weight regulation and motor activity in rat. Brain Res Bull. 1995;38:551–559. doi: 10.1016/0361-9230(95)02030-2. [DOI] [PubMed] [Google Scholar]
- 59.Corbit LH, Murbit JL. Balleine The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sellings LHL, Clarke PBS. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Behav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 64.McFarland K, Davidge S, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lecca D, Piras G, Driscoll P, Giorgi O, Corda MG. A differential activation of dopamine output in the shell and core of the nucleus accumbens is associated with the motor responses to addictive drugs: a brain dialysis study in Roman high- and low-avoidance rats. Neuropharmacology. 2004;46:688–699. doi: 10.1016/j.neuropharm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- 67.Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- 68.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;27:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato SM, Schulz KM, Sisk CL, Wood RI. Adolescents and androgens, receptors and rewards. Horm Behav. 2008;53:647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


