Abstract
This review provides an update of ongoing efforts to expand our understanding of the diversity inherent within the Schistosomatidae, the parasites responsible for causing schistosomiasis and cercarial dermatitis. By revealing more of the species present, particularly among under-studied avian schistosomes, we gain increased understanding of patterns of schistosome diversification, and their abilities to colonize new hosts and habitats. Schistosomes reveal a surprising ability to switch into new snail and vertebrate host species, into new intra-host habitats, and may adopt novel body forms in the process. Often these changes are not associated with deep splits or long branches in their phylogeny, suggesting they are of relatively recent origin. Several hypotheses prompted by the new observations are discussed, helping to focus thinking on processes influencing not only schistosome diversification, but also their pathogenicity and abundance.
Keywords: Schistosomatidae, life cycle, evolution, phylogeny, parasite
Discovering and understanding schistosome diversity is important
Because of their involvement in causing human and animal schistosomaisis [1, 2] and cercarial dermatitis [3, 4], there is an inherent impetus to reveal how many species of schistosomes are in existence, where they occur, and the intermediate and definitive host species they utilize. The more we know about schistosome diversity, the better we are positioned to understand their evolutionary history and potential for future change. We are also better prepared to provide practical means for their identification, and can better gauge the likelihood that some of these species may emerge in new contexts to cause unexpected problems. Also, as many potential host species are suffering declines, our opportunities for finding rare schistosome species would also seem to be dwindling, giving some imperative to this endeavor.
Schistosomes lend themselves to studies of diversity because they are a well-circumscribed monophyletic group (see Glossary) united by some very distinctive features [5, 6, 7]. Like other blood flukes (including the families Sanguinicolidae and Spirorchiidae), members of the Schistosomatidae are digenetic trematodes with two-host life cycles (snail intermediate host and vertebrate definitive host) in which the cercarial stage penetrates an epithelial surface of the definitive host. They live their adult lives in the vascular systems of their hosts, and the intra-vertebrate stages possess distinctive multi-layered membrane surfaces [8].
Schistosomes are distinguished from their blood fluke relatives by an additional distinctive feature; they are dioecious, an attribute rarely abandoned among members of the group. Additionally, they are parasites of birds and mammals. Based on what we know at present, schistosomes represent a distinct lineage, all the members of which live as separate males and females within the vascular system of their endothermic definitive hosts.
A current view of schistosome taxonomy is summarized in Box 1 [6]. Based on named species in the literature, there are four described schistosome genera from mammals and 10 from birds, with about 30 described species from mammals and about 67 from birds. The number of recognized mammalian schistosome genera has decreased by one since Orientobilharzia was incorporated into Schistosoma [9], and two new genera of avian schistosomes have been recognized recently, Allobilharzia from swans [10], and Anserobilharzia from geese [11]. In Box 2, we provide an overview of the new samples of schistosomes mostly collected over the past 10 years that have prompted this review.
Environments of schistosomes: marine, freshwater, even semi-terrestrial?
Basal schistosome genera, Austrobilharzia and Ornithobilharzia, have life cycles based in marine environments (Figure 1). Their snail hosts (Batillaridae, Nassariidae, Littoriniidae, and Potamididae) are marine, and their definitive hosts, mostly charadriiforms (gulls and terns), typically occupy marine environments. Although marine environments have been under-surveyed for schistosomes in general, the diversity of species in the literature for Austrobilharzia and Ornithobilharzia seems to be fairly small, five and three species, respectively, but evidence for cryptic diversity is beginning to emerge [12]. Where the life cycles of the marine basal avian schistosomes are known, the snails involved are inhabitants of mud flats or of rocky intertidal areas and often spend a part of their day out of water, shedding cercariae only when they are re-immersed in water.
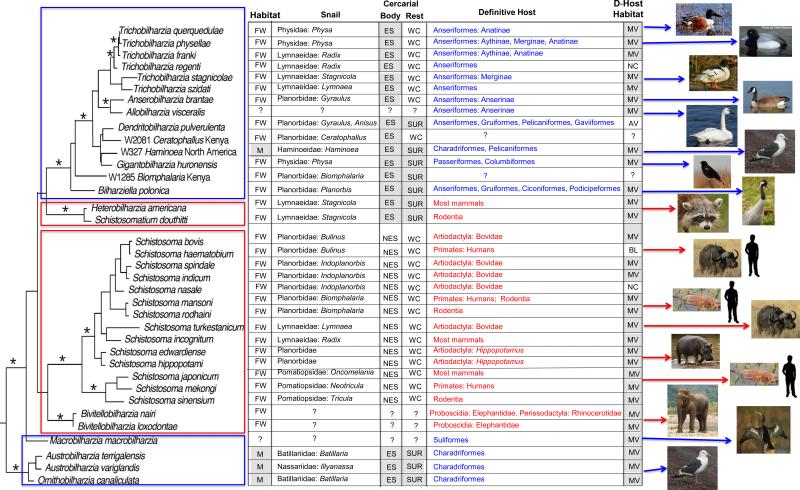
Figure 1. Schistosome phylogeny, including some key attributes for each taxon.
Phylogenetic tree showing the positions of schistosome genera and species, based on Bayesian analysis [11, 28, 29, 90] of the nuclear ribosomal DNA 28S region (1200 bp) for sequences available in GenBank [1, 7, 11, 28, 29]. Mammalian schistosomes are highlighted in red, and avian schistosomes in blue. The tree confirms the robust-bodied marine avian schistosome genera, Austrobilharzia + Ornithobilharzia, as constituting the basal schistosome clade [36]. Two additional well-resolved major branches were retrieved consistently, one constituted by the robust-bodied mammalian parasites Schistosoma spp. + Bivitellobilharzia, and the other by the remaining, largely filiform avian schistosomes (Bilharziella, Dendritobilharzia, Gigantobilharzia, Allobilharzia, Anserobilharzia, + Trichobilharzia). Two additional robust-bodied lineages have not received consistent positional support within the tree. One is comprised of the North American mammalian schistosomes, Heterobilharzia + Schistosomatium. The second is Macrobilharzia, known only as adult worms from anhingas and cormorants. As a consequence of the unstable placement of these two lineages, determination as to whether there have been one or two origins of mammalian schistosomes from avian schistosomes is not yet possible. Also, deeper branches remain without significant nodal support. The table following the tree compares some major features defining the above-mentioned clades of schistosomes, including host use, morphology and behavior in adults and cercariae (see [1] for more details). Shaded gray boxes are those features that are found in the basal clade Austrobilharzia + Ornithobilharzia and thus represent the ancestral features of the family. Asterisks denote significant posterior probabilities (>0.95). Abbreviations: Habitat of the snail hosts (FW, freshwater; M, marine); major morphological and behavioral difference among cercariae, respectively (ES, eye spots present; NES, no eye spots present; WC, swims in water column; SUR, adheres to surface film); habitat of adult schistosomes in the bird or mammalian host (MV, mesenteric veins; NC, nasal cavity; AV, arterial system; BL, bladder).
Although we may be missing lineages of marine schistosomes, or they may once have been more common, the majority of extant schistosomes have freshwater-based life cycles. This is true of all known mammalian schistosomes and most of the avian schistosomes. The growing DAS clade (Figures 1 and 2, Box 2) of avian schistosomes is of interest, for interspersed among the many freshwater lineages is at least one instance in which a marine snail serves as host, a Pacific coast opisthobranch bubble snail, Haminoea [13]. A related schistosome occurs in a species of Haminoea indigenous to the Atlantic coast of North America [14]. Interestingly, species of Haminoea are not close relatives of the species of marine snails involved in transmitting the basal Austrobilharzia and Ornithobilharzia, but in fact are more closely aligned with the pulmonate lineages transmitting the majority of schistosomes [15]. It might seem that a shift from freshwater back to marine environments would be a sufficiently rare event to be marked by a deep split or long branch in the tree; yet, the lineage of schistosome that uses Haminoea snails is no more different from freshwater lineages than the freshwater lineages are from one another [13].
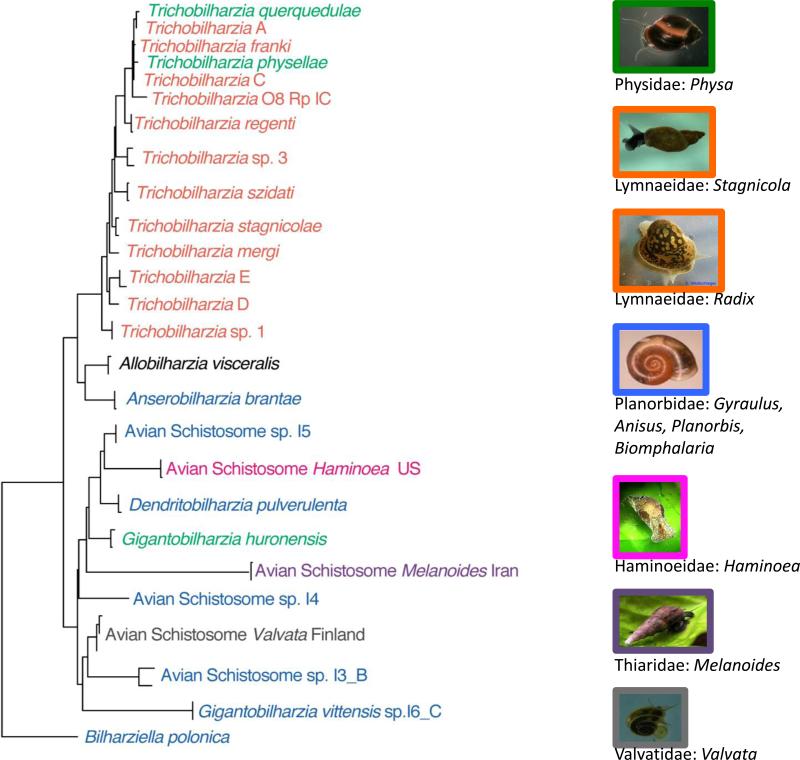
Figure 2. DAS clade phylogeny, emphasizing switches in snail hosts.
Phylogenetic tree showing the positions of genera and species within the main (DAS) clade of avian schistosomes, based on Bayesian analysis [11, 28, 29, 90] of nuclear ribosomal ITS1-5.8S-ITS2 (810 bp) sequences available in GenBank [27, 29, 54, 71, 82]. This tree represents the genetic lineages of avian schistosomes for which snail hosts are also currently known. This includes 7 of the 15 snail families known to support avian schistosomes. We lack sequence data for the avian schistosomes known to use the remaining 8 snail families so they are not depicted here. Lineages are highlighted by color to indicate the snail family used, and images of representatives of the relevant snail families are depicted as well. The snail family name is followed by the snail genus most commonly exploited by that lineage of schistosome. Asterisks denote significant posterior probabilities (>0.95).
Although there is no known land-based schistosome life cycle involving a terrestrial snail, at least some ‘get their backs dry’. In addition to some of the marine schistosomes occupying intertidal snails that may be out of water for a period of time, the human-infecting Schistosoma japonicum uses Oncomelania snails that are ‘amphibious’, often living on moist mud surfaces that can either be dry or submersed. Additionally, human-infecting schistosomes such as S. mansoni and S. haematobium are well known for their ability to survive within their snail hosts when the snails aestivate during periods of drought [16].
Schistosomes and their use of snails
Schistosomes in aggregate have colonized a remarkably broad spectrum of gastropods as first intermediate hosts (Figures 1 and 2). Two major gastropod lineages support schistosomes, the Caenogastropoda and the Heterobranchia [15]. The latter includes two major lineages, classically recognized as opisthobranchs and pulmonates, both of which support schistosomes [17]. To our knowledge, none of the most basal groups of gastropods, including the Patellogastropoda or Vetigastropoda, are hosts for schistosomes.
Mammalian schistosomes use three families of freshwater snails, one caenogastropod (Pomatiopsidae) and two heterobranch pulmonate families (Lymnaeidae and Planorbidae) [7,18]. The natural snail hosts for the elephant schistosomes (Bivitellobilharzia) are unknown but are probably pulmonates [19]. The avian schistosomes have sampled a much broader palette of snails, with representatives from 15 snail families reported infected. These include caenogastropods from marine (Potamididae, Batillariidae, Nassariidae, and Littorinidae) [12, 17, 20-22] and freshwater (Thiaridae, Ampullariidae, Hydrobiidae, and Semisulcospiridae) habitats [17, 23-26]. Heterobranch families supporting avian schistosomes include the freshwater Valvatidae [27] and the marine Haminoeidae [13,14], and pulmonates from freshwater (Physidae, Lymnaeidae, Planorbidae, Chilinidae) [17, 18, 28-30] and marine (Siphonariidae) habitats [31]. Snail hosts for the avian schistosome genera Jilinobilharzia, Macrobilharzia, and Allobilharzia are unknown [28, 32]
The majority of schistosome species are transmitted by the pulmonate families Physidae, Lymnaeidae, and Planorbidae [11, 29, 33]. Within particular snail families, some genera such as Biomphalaria and Indoplanorbis in Planorbidae, and Stagnicola in Lymnaeidae, are known to host species of both avian and mammalian schistosomes [7, 28] .
Also of interest here are particular cases where we might expect that certain groups of gastropods would host schistosomes, yet they do not. For example, whereas several species of avian schistosomes exploit physid snails, no mammalian schistosome has been found to do so [17]. Whereas some lymnaeid snails are commonly exploited as hosts for avian schistosomes (Stagnicola, Lymnaea, and Radix), other lymnaeids, such as the small amphibious species (Fossaria), are not. Within the otherwise heavily exploited family Planorbidae, with some genera such as Bulinus, Indoplanorbis, Planorbis, Biomphalaira, Anisus, and Gyraulus commonly hosting schistosomes [34], other common genera such as Drepanotrema, Planorbula, Helisoma,or Menetus, do not. It is interesting in the case of the latter two genera that they are used as hosts by representatives of the sister group to schistosomes, the turtle blood flukes (Spirorchiidae) [35, 36].
Diversity of cercarial body form and behavior
All schistosome cercariae actively penetrate an epithelial surface of their definitive hosts and have prominent penetration glands appropriate to this task. They all possess a forked tail, with the two furcae being of equal length, and each almost always shorter than half the length of the tail stem (see exception [37]). Other related groups of diplostomoids have furcae that are proportionately longer relative to the length of the tail stem. The overall size of schistosome cercariae varies widely among species, with the smallest having an overall body size (combined body, tail stem, and furcal length) of about 287μm, whereas the largest are up to total length of 1180μm [35]. Particularly variable is the length of the tail stem which varies sixfold among species. Some genera (Austrobilharzia, Schistosoma) are conspicuous for having a body noticeably wider than the tail stem, but in others, such as Trichobilharzia, the body is only slightly wider than the tail stem. Some (Bilharziella) have a tail stem that forms at its base two flanges, which wrap around the posterior margin of the body. With respect to eye spots (Figure 1), all known avian schistosomes possess them, as do the North American mammalian schistosomes (Schistosomatium and Heterobilharzia) [5]. Interestingly, the lack of visible eyespots by no means prevents cercariae of the remaining mammalian species from being responsive to light.
More variable among schistosome cercariae is their behavior (Figure 1) [38, 39]. Some smaller cercariae, such as those of Schistosomatium douthitti, Dendritobilharzia pulverulenta, and the undescribed species from Californian Haminoea are noteworthy for the tenacity by which they adhere, often motionless, to the surface film of water [13, 34]. They tend to be extremely sticky, to the point that they quickly adhere to glass pipettes and are hard to manipulate. By contrast, larger cercariae of Trichobilharzia species are vigorous swimmers and more easily manipulated. They also frequently attach to the surface film using their prominent, protruded ventral sucker. Cercariae of Trichobilharzia physellae, for example, are strongly phototropic and accumulate on the sides of dishes receiving the most direct light (Brant and Loker, unpublished data) [40]. Others repeatedly swim to the surface and passively sink (Schistosoma and Trichobilharzia stagnicolae), followed by another bout of swimming to the surface [38, 40]. Schistosomes differ with respect to the time of day they emerge from their snail hosts, with some species emerging predictably at night (Schistosomatium douthitti), early morning (Schistosoma rodhaini), midday (S. mansoni), later in the afternoon (S. japonicum), and some throughout the day (T. physellae). Time of cercarial release has been well investigated relative to behavioral patterns of known definitive hosts [42, 43].
Schistosomes and their use of definitive hosts
Figure 1 highlights the diversity of definitive host groups employed by schistosomes. With respect to known schistosomes from mammals, all have freshwater-based life cycles. There are, for example, no reports of mammalian schistosomes from marine snails or mammals. Although patterns of host use of schistosomes from mammals have been well-documented [44, 45] and will not be emphasized here, we must also acknowledge that even among well-studied genera such as Schistosoma, there have been descriptions of new species (S. ovuncatum and S. kisumuensis) within the past 11 years [46, 47]. Some mammalian schistosomes such as S. japonicum are extreme generalists with respect to their definitive hosts, whereas other species such as S. haematobium are more confined in their host range, the latter being mostly a human-infectious parasite. We also note a few new observations regarding schistosomes of elephants. Provision of sequence data for the two known species of Bivitellobilharzia, B. loxodontae and B. nairi, from African and Indian elephants, respectively, indicates that they are each other's closest relatives, and affirms Bivitellobilharzia as a well-defined schistosome lineage [48]. The position of Bivitellobilharzia as the sister group of Schistosoma has important implications for interpreting the geographic origin of the latter, medically important genus [28, 49]. Further study of B. nairi has shown for the first time that this schistosome species can also infect the Asian One-Horned Rhinoceros (Rhinoceros unicornis) [50]. The report of B. nairi from two unrelated, large, thick-skinned mammals, Asian elephants and Asian rhinoceroses, raises the possibility that shared ecology or perhaps something associated with large body size per se that we do not fully appreciate (is it a large-host specialist?) may somehow favor acquisition of this species. Unfortunately, the snail host and other details of the life cycle of elephant schistosomes are unknown (Box 3).
Relative to mammalian schistosomes that infect approximately eight orders of mammals, avian schistosomes are recorded so far from at least 10 orders of birds, with Charadriiformes and Anseriformes the most commonly exploited orders. This makes sense because both orders are speciose and prefer aquatic habits. The growing representation of schistosomes from a greater variety of bird species from more geographic areas is bringing into focus patterns in avian schistosome definitive host use. The basal marine avian schistosomes are hosted by charadriiform birds, many of whose members feed in intertidal habitats. The genus Macrobilharzia, the phylogenetic position for which remains unresolved, is found in cormorants and anhingas [28]. As we lack information on the snail hosts of Macrobilharzia, whether the life cycles are based in marine, brackish, or freshwater environments is unknown; anhingas and cormorants can be found in all of these environments.
Within the DAS clade, patterns of definitive host use are becoming more distinct. Species of Bilharziella and Dendritobilharzia are emerging as generalists; both have been reported from at least four orders of aquatic birds (Figure 1). However, it may prove to be the case that some of these birds are better hosts than others. For example, it has been argued that the preferred hosts of D. pulverulenta are diving ducks (Aythinae, Merginae) [51, 52]. Gigantobilharzia huronensis, unlike almost all other known members of the DAS clade, is a parasite of a broad range of passeriform birds [53].
The most prominent clade within DAS is comprised of three genera that are found almost exclusively in anseriform birds (swans, geese, and ducks). Allobilharzia is known only from swans, and Anserobilharzia is known only from geese [11, 29, 32, 54]. The third genus, Trichobilharzia, the most speciose genus in the family, is found almost exclusively in ducks. Trichobilharzia corvi from passeriforms is an exception, but descriptions suggest it belongs in its own genus [29, 55]. There are other cases where named species of Trichobilharzia belong in other genera [29, 55]. Within Trichobilharzia there are several clades specific to certain groups of ducks: (i) T. stagnicolae and T. mergi in mergansers (Merginae) [29, 56]; (ii) T. querquedulae in the ‘blue-winged duck’ clade [57] (Anas clypeata, A. discors, and A. cyanoptera) [29]; (iii) T. physellae in the ecological group of diving ducks that includes ducks in Aythinae and Merginae; (iv) and a common, yet unnamed species of Trichobilharzia in American wigeons [29].
Schistosomes in their intra-definitive host habitats
Adult schistosomes occupy an unusual habitat within their definitive hosts relative to other trematodes. Instead of inhabiting the gut, most schistosomes live in the mesenteric venules. This habitat is also the likely ancestral habitat of schistosomes because this is where adults of the most basal genera of schistosomes, Austrobilharzia and Ornithobilharzia, are found. This habitat offers clear benefits from a nutritional point of view (portal blood is rich in transported nutrients), yet poses challenges: (i) the worms are in constant, direct contact with the immune system and (ii) the eggs must exit the body of the host. As frequently noted, the most striking schistosome feature – dioecy – may be a response to the challenges posed by reaching the smallest venules in the intestinal wall to maximize chances of egg transport out of the body [1, 58-61].
Relatively few adult schistosome species have preferred habitats in other locations. Schistosoma haematobium adults live in the veins of the vesical plexus, which offers an advantageous way to transport eggs out of the host body via the urine, but may entail a trade-off with respect to diminished nutrient levels in veins draining the bladder as opposed to those of the intestine. Adults of a single species of mammalian schistosomes, S. nasale, in cattle and buffaloes, live in veins among the nasal mucosa, whereas several species (at least eight) do the same in avian hosts [18]. For Trichobilharzia regenti, for example, use of this habitat requires a different migration pathway to the egg-laying site, one that involves extensive migration along peripheral nerves and through part of the central nervous system and is potentially pathogenic to the host [62-64]. Again, there would seem to be a considerable advantage to occupying the nasal chamber with respect to egress of eggs, as infected hosts often dip their heads in water, allowing escape of miracidia. This may be countered by the location being less nutritionally beneficial or harder to reach.
The switch to occupation of nasal habitats appears to have happened at least twice in avian schistosomes (S. Brant and E. Loker, unpublished). Note that T. regenti groups with several species with typical egg-laying sites (Figure 1). Nasal schistosomes are not deeply divergent nor do they separate by long branches from species that retain ancestral egg laying habitats, suggesting schistosomes may be relatively adaptable in this regard. Even fewer schistosomes have colonized the arterial system, with only one mammalian species, Schistosoma hippopotami, and one avian species, Dendritobilharzia pulverulenta, implicated. In these cases, the shift to this new habitat type is accompanied by considerable morphological changes [1].
In each of the cases where schistosomes have left the usual, ancestral egg-laying site, why might they have done so? Competition for favorable egg-laying sites with other closely-related species is one possibility [1]. This might tip the balance to favor more distal sites that are nutritionally less favorable. As infection with multiple schistosome species is relatively common in birds [65], this may help to explain why the nasal habitat has been adopted more frequently by avian schistosomes. Another form of competition may occur when males of one species are superior at pairing with females of a different species. The inferior males might then carry their females to locations where such competition is avoided. More wholesale colonization of unusual habitats may be disfavored because their nutritional properties or egg egress properties pose too great a challenge to overcome. Also, with respect to colonization of the bladder, this is not an option for avian schistosomes as birds do not possess one.
Diversity of adult schistosome anatomy
The body forms of male and female schistosome vary considerably, including the extent to which they are sexually dimorphic [1, 59, 66]. How the loss of sexual dimorphism, including loss of a filiform female plus the adoption of an arterial habitat have co-occurred in Dendritobilharzia pulverulenta has been noted previously [1]. Dendritobilharzia is nestled among groups of schistosomes that are venous inhabitants and is not deeply demarcated genetically from other lineages. Dendritobilharzia provides a good example of how adaptable adult schistosome anatomy can be. Another kind of conspicuous change in adult anatomy is represented by Bivitellobilharzia, in which the vitellaria have doubled in representation. The number and arrangement of the testes also varies dramatically between genera in both avian and mammalian species (numbers range from 25-785, with the lower end in both mammalian and avian species, and the higher end exclusively in avian schistosomes) [66].
Within the growing DAS clade, a thread-like, filiform body predominates [29, 54]. Accompanying the filiform body shape is a general loss of pronounced sexual dimorphism (both males and females are filiform), a trend towards reduced elaboration of oral and ventral suckers, a considerable reduction in size of the gynecophoric canal, with some species lacking this iconic schistosome feature, and an increase in number of testes [1]. This same filiform anatomy is maintained by species that adopt a nasal egg-laying site, so does not seem to be driven solely by unique aspects of intestinal egg-laying sites.
Availability of more intact adult avian schistosomes will allow us to address questions such as (i) what are the circumstances that have favored the acquisition of such a distinctive filiform in some avian schistosomes but not others; (ii) why has it not evolved in mammalian species; and (iii) why are there so many species with such a body shape? What is particularly needed is more life history data concerning filiform species. One hypothesis is that these species mature rapidly (within 2-3 weeks) and have transient bouts of mating, which are followed by relatively short periods of egg-laying and then death [1]. Perhaps this better matches the conditions experienced in highly migratory avian hosts, where the worms seek to reproduce quickly, thus depositing their eggs in the same habitats in which their definitive host became infected [67]. Similarly, adoption of different egg-laying sites, increased spatial and temporal subdivision of definitive host and aquatic habitat use (e.g., along migratory flyways) may be factors contributing to genetic differentiation and speciation among the DAS clade.
Discovery-based diversity studies are incubators of new hypotheses about schistosomes
As new parasite lineages or parasite-host associations are uncovered, they provide the raw material to develop new perspectives regarding process to account for the patterns that are seen. Below we provide some examples.
The concentrated transmission hypothesis
One of the surprises originating from our work has been the recovery of different schistosome species (four solely from snails, seven from both snails and birds, and one from mammals) from the relatively arid state of New Mexico in the United States [33]. Although this could be a sampling artifact, one alternative to consider is that the limited number and small size of standing water bodies present in New Mexico have the effect of concentrating definitive host use. This in turn is hypothesized to lead to more snail infections per unit area than would be found in wetter areas with many more and larger snail and definitive host habitats. This is the ‘concentrated transmission’ hypothesis. Another manifestation of the concentrated transmission hypothesis may be the development of hot spots of transmission in habitats subjected to pronounced wet and dry seasons, where diminishing water bodies in the dry season have the effect of greatly focusing definitive host use (including humans), such that transmission and infection become more probable [68-70].
The facilitated host switch hypothesis
Figures 1 and 2 reveal that host switching among schistosomes with respect to their snail hosts has been pronounced [27, 29, 33, 34, 71]. Yet this pattern is somewhat paradoxical because experimental studies with schistosomes show them to be very specific with respect to the snail species they are able to colonize [38, 71, 72]. How does historical host shifting in snails make sense in light of their evident host specificity we see today? One hypothesis to account for this is the ‘facilitated host switch hypothesis’. The basic premise is that some snail species have preexisting trematode infections, and this creates a situation of immunosuppression in the snail, such that the infected snails can later be invaded by trematodes of other species.
This hypothesis builds on the ‘interference theory’ [73], which postulates that trematode infections frequently interfere with snail host defenses, and for which evidence has accumulated in support (see discussion in [1]). A related question is why some lineages of snails, such as Helisoma, have not been found infected with schistosomes. This is intriguing because both avian and mammalian schistosomes colonize the sister group of the helisomes, snails of the genus Biomphalaria. Helisomes are routinely found infected with other trematodes, including the sister group of schistosomes, the spirorchiids. Possibly helisomes are well-protected by the guild of trematodes they already support?
The ‘integrated life cycle hypothesis’
As we learn about more schistosome life cycles, there is a sense that there is a holistic or integrated nature to them that is not fully appreciated. The ‘integrated life cycle hypothesis’ is our attempt to articulate this. It postulates that the biology of a schistosome in the definitive host is not independent of what happens in the intermediate host and vice versa. As a result, only certain combinations of intermediate and definitive hosts permit sufficient reproductive success, production of either cercariae or eggs, for the schistosome to persist. Furthermore, depending on what the respective hosts are, the parasite in one host may exhibit distinctive characteristics that offset constraints imposed by the other host.
A recent impetus for this idea came from considerations of plague (Yersinia pestis) and its transmission by fleas. The plague bacillus may have emerged only 20 to 30 thousand years ago, with flea transmissibility being one of the distinctive features that sets it apart from other Yersinia species. The ability of Y. pestis to achieve very high population densities in the blood of its vertebrate hosts may have been a pre-condition for its ability to colonize fleas, which must acquire a large inoculum to become infected. This in turn has led to its emergence as a distinct species with its own unique flea-borne mode of transmission [74]. What happens in one host influences what can potentially happen in another.
Thoughts about the integrated nature of schistosome life cycles were originally provoked by considerations of S. japonicum, which utilizes a very small intermediate host snail (Oncomelania) that produces relatively few cercariae per day [44]. It is intriguing that the adult stages of S. japonicum produce eggs at a much higher daily rate (partly by producing smaller eggs) than in other members of the genus. The idea then is that the life cycle retains stability by having small rates of cercariae production per infected snail offset by high rates of production of eggs in several host species over long periods of time. Some avian schistosomes such as D. pulverulenta perhaps add support because they use small Gyraulus snails as hosts, in which relatively few cercariae are produced per day. Adults of this species appear to be prolific egg producers as compared to egg outputs of Trichobilharzia or Allobilharzia (estimated by comparing numbers of eggs found in the uterus). See Box 3.
The snail-as-isolating-mechanism hypothesis
Finally, consider the ‘snail-as-isolating-mechanism’ hypothesis as one potentially influencing schistosome diversification. This hypothesis is based on the observation that known species of schistosomes have limited host ranges with respect to their snail hosts. Schistosoma mansoni infects several Biomphalaria species but does not infect any species of Bulinus snails, and vice versa for S. haematobium. Similarly, Trichobilharzia physellae and T. querquedulae infect physid but not lymnaeid snails, and vice versa for T. regenti or T. franki. Schistosomes are not generalists with respect to their snail hosts. Here the basic idea is that once a schistosome has switched into a sufficiently different lineage of snail host, its progeny are no longer able to genetically intermingle with its parental stock that still infect the original snail host. One possibility is that the hybrid progeny, which result, lose their ability to infect either the parental or newly-acquired snail host. In other words, the snails somehow impose strong post-reproductive isolating mechanisms. Although Wright's classic experiments in which hybrid schistosomes had a broader snail host spectrum than either parental species would seem to argue against the poor infectivity of hybrid miracidia postulated above [75]; the snails involved in his experiments were all bulinids, so it may be difficult to apply this result when differences are greater among the snail hosts involved. Fertile hybrids between the relatively distantly related congeners S. mansoni and S. japonicum have been reported, with the hybrids having infectivity for both Oncomelania and Biomphalaria [76]. However, the egg production, hatchability, and infectivity of hybrid miracidia were all low. In each case the morphology and behavior of hybrid progeny resembled that of the maternal parent, suggesting maternal effects or parthenogenesis played a role in the results. Nonetheless, the production of hybrid progeny infective for both snails speaks against our hypothesis, although the low levels of infection argue for it.
This hypothesis was motivated by the observation among species of Trichobilharzia, some of which are found in physids and some in lymnaeid snails, that the genetic distances between these lineages are not great, and the branch lengths in the phylogenetic reconstructions short [29]. This suggests a switch from lymnaeids to physids may have occurred recently. We have not seen evidence of hybrid adults, or of progeny able to infect both physids and lymnaeids, but these may be rare events yet to be revealed.
Future perspectives
One priority for the future is to continue searching for new species and new host associations. This effort needs to couple inextricably with deposition of specimens in museum collections, to provide essential reference points for future studies. Future studies of museum specimens will likely produce spectacular results, including routine provision of complete genome sequences for specimens housed there. The search for new specimens may prove to be especially important when the relevant hosts or habitats may soon be extirpated (Box 3) [77-79].
Another priority for available specimens housed in museum collections is to acquire much more sequence data. The widespread availability of next-generation sequencing platforms makes this entirely feasible. More sequence data will hopefully enable us to resolve deeper branches in the overall schistosome tree, giving improved insight into how schistosomes colonized freshwater by stabilizing the phylogenetic position of pivotal genera such as Macrobilharzia and to determine if schistosomes colonized mammals on one or more occasions by resolving the phylogenetic positions of genera such as Schistosomatium or Heterobilharzia relative to Schistosoma and Bivitellobilharzia. More sequence data will also enable us to understand the genetic traits that have enabled colonization of new hosts or adoption of different body forms.
All that we have discussed above would benefit from a more thorough knowledge of the life histories and population genetics of a greater variety of schistosome species. This is especially needed for avian schistosomes. The limited data in hand suggest they are quite different from mammalian schistosomes with respect to duration of their pre-patent and patent periods [1], the extent to which they provoke strong protective immune responses in their hosts, and the size of their geographic ranges. Host records suggest that a few avian schistosomes have cosmopolitan ranges, no doubt aided by the mobility of their definitive hosts and the ubiquity of some groups of snail hosts.
In conclusion, even for schistosomes – a well-studied group of parasites - there is still much to learn before we fully understand the full spectrum of species and parasite-host interactions, the processes at play in governing their diversification, and how this group is likely to fare in the future. As many of the helminths of the world increasingly come to heel with the implementation of global control programs, it is worth considering that one of the most likely forms of future contact humans will have with schistosomes will come from exposure to the cercariae of avian or non-human mammalian schistosomes in natural habitats (and man-made) used for recreation. Studies of schistosome diversity will help us better understand the species involved, the circumstances favoring human exposure, and help us cope with introductions of schistosomes into new settings where they might suddenly begin causing disease. One species that comes to mind here is Trichobilharzia regenti. Probably related to where the preferred snail host species (Radix peregra, R. lagotis, and R. labiata) occurs [80], this schistosome is commonly recovered from Europe but is unknown in North America. In fact, no nasal schistosome has been recovered from the Western Hemisphere [29]. Given the ability of schistosomulae of this species to cause pathology in experimentally infected ducklings and mice [62], if introduced into North American habitats commonly used for recreation, then an emerging disease problem could conceivably result. The better we understand the spectrum of schistosome species present and their host preferences, the better we can identify and respond to such challenges.
Highlights.
An update on discoveries in schistosome diversity, within a phylogenetic framework.
Avian schistosomes are speciose with variation in hosts, habitats, morphology, life history.
Avian schistosome lineages are probably a recent radiation.
Hypotheses to explain schistosome diversification are deliberated.
Box 1. Currently recognized taxonomy for the Family Schistosomatidae.
The most recently published taxonomic treatment is listed as family, subfamily, and genus [6, 9, 10]. Note the addition of a new genus to the Bilharziellinae, Anserobilharzia, was proposed recently [11]. The enigmatic Griphobilharzia amoena from Australian freshwater crocodiles was originally described as a schistosome, but molecular studies suggest it is a spirorchiid [1, 81]; therefore it was not included here.
SCHISTOSOMATIDAE
- Schistosomatinae
- Austrobilharzia – marine birds (5-6 species)
- Bivitellobilharzia – elephants (2 species)
- Heterobilharzia – raccoons, deer, rodents (1 species)
- Macrobilharzia – cormorants and anhingas (2 species)
- Ornithobilharzia – marine birds (1-2 species)
- Schistosomatium – rodents (1 species)
- Schistosoma – many mammals, including humans (26 species)
- Gigantobilharziinae
- Gigantobilharzia – birds (~14 species)
- Dendritobilharzia – birds (2 species)
- Bilharziellinae
- Bilharziella – birds (1 species)
- Trichobilharzia – birds (~35 species)
- Jilinobilharzia – birds (1 species)
- Allobilharzia – birds (1 species)
- Anserobilharzia – birds (1 species)
Box 2. New schistosome samples and resultant phylogenetic trees.
This review considers the many samples of new schistosomes available in the literature over the last 10 years. Collections are mostly from North America and Europe, but all continents except Antarctica have been sampled. Many of the samples are of cercariae recovered from the screening of approximately 20 000 snails, representing 50 species. Adult schistosomes have been obtained from necropsied birds (500 birds of about 70 species). A few specimens were also of eggs from feces of mammals or birds [10-13, 23, 27-29, 32-34, 54, 56, 71, 82]. Routinely, for each specimen acquired, in addition to traditional parasite, host, and locality information, sequence data (28S and ITS, rDNA and cytochrome oxidase 1) were collected, and in some cases parasites were vouched in museum collections for both future morphological and genetic work.
A phylogenetic tree including specimens representing all schistosome genera except Jilinobilharzia [83] is shown in Figure 1 (see legend for details). This tree is based on 28S rDNA, a sequence chosen because it provides good differentiation among the genera of schistosomes, is widely available across different species in GenBank, and can be used in barcoding [11, 28, 29, 82, 84]. Marine and freshwater spirorchiids were used as outgroups [28, 36]. The lineage for which we have the most new information is the derived avian schistosome (DAS) clade, formerly referred to as the BTGD clade [28].
Shown in Figure 2 is an expanded tree for the DAS clade using ITS sequence data since many of the avian schistosomes have only ITS sequence data available for comparison [23, 27, 54, 71, 82, 84, 85]. The only available sequence data for outgroups in GenBank were species of Schistosoma. This tree underscores the greater breadth of snail orders and families used within this clade, as compared to clades of mammalian schistosomes, including Schistosoma (Figure 1). The ITS sequence was chosen because it evolves at a faster rate and therefore provides better discrimination among more closely related taxa and is routinely used in barcoding [86]. Also, in addition to containing the six described genera mentioned above, this tree highlights the presence of unnamed lineages that in our view are sufficiently distinct to represent about seven provisional new genera, potentially raising the number of generic level lineages within the family to 21 [11, 28, 29, 86]. The yardstick used to support these statements is to compare the extent of genetic differentiation among the new and well-defined genera with differences in sequence known to exist between genera that are well-established based on morphological and/or host use differences [11, 13, 28, 29, 32, 33, 82, 86, 87].
Some of these new lineages are based only on DNA sequences recovered from cercariae, and as formal descriptions of new species and genera require the corresponding adults, it may be some time before the often minute, thread-like adult worms representing these lineages can be found and formally named [85] (see Box 3).
Box 3. Outstanding needs and questions.
We need more records of identifiable adult schistosomes from birds, particularly for the new lineages in the DAS clade now known only from cercariae. Obtaining intact adult schistosomes suitable for formal descriptions from birds remains a daunting challenge.
There are a number of schistosomes (Bivitellobilharzia, Jilinobilharzia, Allobilharzia, and Macrobilharzia) for which the molluscan hosts are unknown, even though the adults have been known for decades and often are common. Perhaps there are surprises in store for where the larval stages of these species reside. For some species like the elephant schistosomes, perhaps their snail hosts are rare, or rarely infected. So, even though cercariae are rarely produced, because elephants live so long, they still acquire significant numbers of adult worms. Schistosomes from large, long-lived swans (Allobilharzia) may provide the avian counterpart to this situation. Swans are frequently heavily infected [32], but thus far infections in snails have not been found.
Ideally, a complete genome sequence can be provided for at least one representative of each schistosome genus, an endeavor rendered more feasible by availability of Schistosoma genome sequences [88, 89]. This will help resolve deeper branching patterns in the schistosome tree, clarify issues such as whether mammalian schistosomes arose more than once from avian lineages, and identify key genes involved in colonizing new hosts or habitats.
The hypotheses posed above offer several possibilities for future studies. For example, with respect to the integrated life cycle hypothesis, perhaps the situation of Schistosoma japonicum in which low production of cercariae in snails seems to be offset by high egg production in mammals is too simplistic an interpretation. Factors such as abundance, species diversity, or geographic range of the intermediate or definitive hosts, and trade-offs between the rate and duration of egg production, may override any simple correlation between reproduction in snails and definitive hosts. Other schistosomes are now known which employ small snail hosts such as Valvata or Gyraulus. Maybe these cycles too benefit from prolific reproduction by the corresponding adult worms or, perhaps small snails simply produce more cercariae than we think, or the snails are more abundant such that numbers of infections offset low productivity per infection.
More laboratory studies of the dynamics of avian schistosomes in their natural definitive hosts are needed. These will help answer questions like how quickly they achieve patency, how long they produce eggs, and whether they become permanently immune to reinfection.
Lastly, non-invasive surveys of some exceedingly rare host species such as the pygmy hippopotamus, Sumatran or Javan rhinos, Oriental stork, and Shoe-billed stork, may reveal unique schistosomes that are themselves in danger of extinction. Examination of fecal samples from such hosts for schistosome eggs, or their aquatic habitats for infected snails may yield surprises.
Acknowledgments
The following people are thanked for their contributions to the story of the schistosomes: Veronica Flores, Ramesh Devkota, Erika Gendron, Damien Jouet, Hubert Ferte, and Norman Davis. The University of New Mexico supported this study through National Institutes of Health grants to E.S.L. (1P20RR18754 and R01 AI101438) and a National Science Foundation grant to S.V.B. (DEB 1021427).
Glossary
- 28S rDNA (large ribosomal subunit)
a region of nuclear DNA commonly used to estimate phylogenetic relationships and barcoding, especially of invertebrates
- Aestivate
to remain dormant by inactivity and lowered metabolic rate, as when habitats dry out for freshwater snails
- Anseriform birds
an order of birds that contains swans, geese, ducks, and screamers
- Arterial system
large system of blood vessels that carry oxygenated blood from the heart to the organs
- Barcoding
a taxonomic method that uses DNA sequence of a particular gene to identify an organism to a particular species. The goal is not classification but simply to identify an unknown sample in terms of a known classification
- Basal clade
a relative term in phylogenetic analyses referring to the earliest clade to branch in a larger clade. It appears at the base of a clade
- Bayesian analysis
a method of calculating the statistical probability that the probability of one treatment is superior based on the observed data and prior beliefs
- Blood flukes
a monophyletic clade of digenetic trematode flatworms that share a feature of living in the venous or arterial system of their vertebrate hosts. There are three families, Sanguinicolidae in fishes, Spirorchiidae in turtles and crocodiles, and Schistosomatidae in birds and mammals
- BTGD clade
a clade of avian schistosomes that was defined by the included genera, Bilharziella, Trichobilharzia, Gigantobilharzia, and Dendritobilharzia, since superseded by DAS clade (see Glossary)
- Bulinids
a term referring to snails of the genus Bulinus, in the family Planorbidae
- Cercariae
produced by asexual reproduction, the usually free-swimming larval stage of a digenetic trematode that emerges from the first intermediate host and can only survive if it can enter either a second intermediate host or a definitive host
- Clade
see monophyletic
- Derived avian schistosome clade (DAS)
This expands on the BTGD clade to be more inclusive of current results to include two additional genera, Allobilharzia in swans, and Anserobilharzia in geese
- Digenetic trematodes
a subclass of parasitic flatworms with complex life cycles, with a sexually reproducing adult in a definitive host, and asexually-reproducing larvae in a first intermediate host, which is almost always a mollusk
- Dioecious
having male and female reproductive organs in separate individuals of the same species
- Dioecy
see dioecious
- Diplostomids
referring a superfamily of digenetic trematodes, Diplostomoidea (includes the families Cyathocotylidae, Diplostomatidae, and Strideidae), that are commonly found in birds and mammals
- Endothermic
an organism that self generates and maintains a constant body temperature
- Eyespots
a concentrated area of light sensitive pigment cells
- Filiform
resembling a filament or thread
- First intermediate host
the host in which asexual reproduction occurs in the digenetic trematode life cycle. It is almost always a mollusk (usually a snail), and rarely an annelid
- Furca(e)
refers to one of the two forks in the tail of a schistosome cercaria. The two furcae are of equal length
- Gastropod
a class in the Phylum Mollusca that includes snails and slugs
- GenBank
An on-line database of publicly available DNA sequence data maintained by the National Center for Biotechnology Information (NCBI), a part of the National Institutes of Health (NIH)
- Genetic lineages
lineages defined by DNA sequence features, such as a gene or portion of a gene, rather than phenotypic features
- Gynecophoric canal
a ventral groove of varying lengths in males, specific to members of the family Schistosomatidae, where the filiform female resides for short or long periods
- Internal transcribed spacer region (ITS)
a region of non-coding nuclear ribosomal DNA used commonly to estimate phylogenetic relationships and for barcoding, especially of invertebrates. This is in large part due to the high copy number of this region and high amount of variation among closely related species
- Lineage
two or more groups that share a common ancestor. See monophyletic
- Mesentery
the connective tissue among the small and large intestines
- Mesenteric veins
veins found in the mesentery, which collect to form the hepatic portal vein
- Mesenteric venules
a small blood vessel that receives blood from the capillaries and transports it to the mesenteric veins
- Miracidia
the ciliated, (usually) free-living larval stage of a digenetic trematode that hatches from the egg. It can only survive by penetrating the first intermediate host
- Monophyletic
two or more groups that share a common ancestor, and are also united by a single node based on shared characters and includes all its descendants. Another term that can be used is a clade
- Museum vouchers
a specimen and all its associated data that are preserved in a museum. The voucher is proof of the existence of a species or object at a particular time and space and ensures a defined reference point for future research
- Passeriform birds
the largest order of birds that contains more than half of all bird species. This order contains, for example, finches, sparrows, wrens, robins, warblers, and blackbirds
- Patent period
for schistosomes, the interval during which eggs are passed by the definitive host
- Phylogeny
a hypothesis about the evolutionary history of a group of organisms
- Portal blood
blood coming from the hepatic portal vein that transports blood from the intestines, spleen, pancreas, and gallbladder to the liver
- Posterior probabilities
In Bayesian statistics, the posterior probability of a random event is the conditional probability that is assigned after the relevant evidence is taken into account
- Pre-patent period
for schistosomes, the time between first infection of the definitive host and the time when the eggs of the parasite are first passed
- Pulmonate
gastropods that belong to the subclass Pulmonata that include terrestrial snails and slugs as well as some freshwater and marine snails that breathe air using lung-like sacs
- Schistosomulae
an immature schistosome following penetration of the cercariae into skin and blood vessels of its bird or mammal host
- Sexual dimorphism
difference between males and females of the same species, such as size, ornamentation, and behavior
- Speciose
a group comprised of many species
- Spirorchiids
members of a family (Spirorchiidae) of digenetic trematodes that live in the venous system of turtles and crocodiles. They are closely related to Schistosomatidae and Sangunicolidae
- Vesical plexus
a network of veins that are distributed to the bladder and adjacent reproductive organs
- Vitellaria
a group of glands the secrete yolk around the fertilized ovum in some invertebrates. Their arrangement and position is often a diagnostic feature for digenetic trematodes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loker ES, Brant SV. Diversification, dioecy and dimorphism in schistosomes. Trends in Parasitology. 2006;22:521–528. doi: 10.1016/j.pt.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B. Schistosomiasis. Infectious Disease Clinics of North America. 2012;26:383–397. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Kolářová L. Schistosomes causing cercarial dermatitis: a mini-review of current trends in systematics and a host specificity and pathogenicity. Folia Parasitologica. 2007;54:81–87. [PubMed] [Google Scholar]
- 4.Soldánová M, et al. Swimmer's itch: etiology, impact, and risk factors in Europe. Trends in Parasitology. 2013;29:65–74. doi: 10.1016/j.pt.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Snyder SD, Loker ES. Evolutionary relationships among the Schistosomatidae (Platyhelminthes:Digenea) and an Asian origin for Schistosoma. Journal of Parasitology. 2000;86:283–288. doi: 10.1645/0022-3395(2000)086[0283:ERATSP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Khalil LF. Schistosomatoidea. In: Gibson DI, Jones A, Bray RA, editors. Keys to the Trematoda. CABI Publishing; 2002. pp. 419–432. [Google Scholar]
- 7.Lockyer AE, et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126:203–224. doi: 10.1017/s0031182002002792. [DOI] [PubMed] [Google Scholar]
- 8.McLaren DJ, Hockley DJ. Blood flukes have a double outer membrane. Nature. 1977;269:147–149. doi: 10.1038/269147a0. [DOI] [PubMed] [Google Scholar]
- 9.Aldhoun JA, Littlewood DTJ. Orientobilharzia Dutt & Srivastava, 1955 (Trematoda: Schistosomatidae), a junior synonym of Schistosoma Weinland, 1858. Systematic Parasitology. 2012;82:81–88. doi: 10.1007/s11230-012-9349-8. [DOI] [PubMed] [Google Scholar]
- 10.Kolářová L, et al. Allobilharzia visceralis gen. nov., sp. nov. (Schistosomatidae-Trematoda) from Cygnus cygnus (L.) (Anatidae). Parasitology International. 2006;55:179–186. doi: 10.1016/j.parint.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Brant SV, et al. Anserobilharzia gen. n. (Digenea, Schistosomatidae) and redescription of A. brantae (Farr & Blankemeyer, 1956) comb. n. (syn. Trichobilharzia brantae), a parasite of geese (Anseriformes). Zootaxa. doi: 10.11646/zootaxa.3670.2.5. in press. [DOI] [PubMed] [Google Scholar]
- 12.Al-Kandari WY, et al. Molecular identification of Austrobilharzia species parasitizing Cerithidea cingulata (Gastropoda: Potamididae) from Kuwait Bay. Journal of Helminthology. 2012;86:470–478. doi: 10.1017/S0022149X11000733. [DOI] [PubMed] [Google Scholar]
- 13.Brant SV, et al. Cercarial dermatitis transmitted by an exotic marine snail. Emerging Infectious Diseases. 2010;16:1357–1365. doi: 10.3201/eid1609.091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leigh WH. The morphology of Gigantobilharzia huttoni (Leigh, 1953) an avian schistosome with marine dermatitis-producing cercariae. Journal of Parasitology. 1955;44:262–269. [PubMed] [Google Scholar]
- 15.Ponder WF, Linderg DR. Towards a phylogeny of gastropod molluscs: an analysis using morphological features. Zoological Journal of the Linnaean Society. 1997;119:83–265. [Google Scholar]
- 16.Badger LI, Oyerinde JPO. Effect of aestivation of Biomphalaria pfeifferi on the survival and infectivity of Schistosoma mansoni cercariae. British Journal of Biomedical Science. 2004;61:138–141. doi: 10.1080/09674845.2004.11732659. [DOI] [PubMed] [Google Scholar]
- 17.Blair D, et al. Evolutionary relationships between trematodes and snails emphasizing schistosomes and paragonimids. Parasitology. 2001;123:S229–S243. doi: 10.1017/s003118200100837x. [DOI] [PubMed] [Google Scholar]
- 18.Horák P, et al. Biology of the schistosome genus Trichobilharzia. Advances in Parasitology. 2002;52:155–233. doi: 10.1016/s0065-308x(02)52012-1. [DOI] [PubMed] [Google Scholar]
- 19.Vogel H, Minning W. Bilharziose bei Elefanten. Archiv fur Schiffs und Tropenhygiene. 1940;44:562–574. [Google Scholar]
- 20.Miller HM, Jr., Northup FE. The seasonal infestations of Nassa obsoleta (Say) with larval trematodes. The Biological Bulletin. 1926;50:490–509. [Google Scholar]
- 21.Penner LR. The biology of a marine dermatitis producing schistosome cercaria from Batillaria minima. Journal of Parasitology. 1953;39:19–20. [Google Scholar]
- 22.Chu GWTC, Cutress CE. Austrobilharzia variglandis (Miller and Northup, 1926) Penner, 1953, (Trematoda: Schistosomatidae) in Hawaii with notes on its biology. Journal of Parasitology. 1954;40:515–524. [PubMed] [Google Scholar]
- 23.Karamian M, et al. Parasitological and molecular study of the furcocercariae from Melanoides tuberculata as a probable agent of cercarial dermatitis. Parasitology Research. 2011;108:955–962. doi: 10.1007/s00436-010-2138-x. [DOI] [PubMed] [Google Scholar]
- 24.Leedom WS, Short RB. Cercaria pomaceae sp. n., a dermatitis-producing schistosome cercaria from Pomacea paludosa, the Florida apple snail. Journal of Parasitology. 1981;67:257–261. [Google Scholar]
- 25.Szidat L. Investigaciones sobre Cercaria chascomusi n.sp. agente causal de una nueva enfermedad humana en la Argentina: ‘La Dermatitis de los banistas de la laguna Chascomus’. Boletin del Museo Argentino de Ciencias Naturales. 1958;18:1–13. [Google Scholar]
- 26.Ito J. Studies on the morphology and life cycle of Pseudobilharziella corvi Yamaguti, 1941 (Trematoda: Schistosomatidae). Japanese Journal of Medical Science and Biology. 1960;13:53–58. doi: 10.7883/yoken1952.13.53. [DOI] [PubMed] [Google Scholar]
- 27.Aldhoun JA, et al. Schistosomes in the north: a unique finding from a prosobranch snail using molecular tools. Parasitology International. 2009;58:314–317. doi: 10.1016/j.parint.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Brant SV, et al. An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including DNA sequence from single life cycle stages. Journal of Parasitology. 2006;92:77–88. doi: 10.1645/GE-3515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brant SV, Loker ES. Molecular systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. Journal of Parasitology. 2009;95:941–963. doi: 10.1645/GE-1870.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martorelli SR. Sobre una cercaria de la familia Schistosomatidae (Digenea) parasita de Chilina gibbosa Sowerby, 1841 en el Lago Pellegrini, Provincia de Rio Negro, Republica Argentina. Neotropica. 1984;30:97–106. [Google Scholar]
- 31.Ewers WH. A new intermediate host of schistosome trematodes from New South Wales. Nature. 1961;190:283–284. doi: 10.1038/190283b0. [DOI] [PubMed] [Google Scholar]
- 32.Brant SV. The occurrence of the avian schistosome Allobilharzia visceralis Kolářová, Rudolfová, Hampl et Skírnisson, 2006 (Schistosomatidae) in the tundra swan, Cygnus columbianus (Anatidae), from North America. Folia Parasitologica. 2007;54:99–104. doi: 10.14411/fp.2007.013. [DOI] [PubMed] [Google Scholar]
- 33.Brant SV, Loker ES. Schistosomes in the southwest United States and their potential for causing cercarial dermatitis or ‘swimmer's itch. Journal of Helminthology. 2009;83:191–198. doi: 10.1017/S0022149X09308020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brant SV, et al. New intermediate host records for the avian schistosomes Dendritobilharzia pulverulenta, Gigantobilharzia huronensis and Trichobilharzia querquedulae from North America. Journal of Parasitology. 2011;97:946–949. doi: 10.1645/GE-2743.1. [DOI] [PubMed] [Google Scholar]
- 35.Combes C. Atlas mondial des cercaires. Memoires du Museum National d'Histoire Naturelle Serie A, Zoologie. 1980;115:1–235. [Google Scholar]
- 36.Snyder SD. Phylogeny and paraphyly among tetrapod blood flukes (Digenea: Schistosomatidae and Spirorchiidae). International Journal for Parasitology. 2004;34:1385–1392. doi: 10.1016/j.ijpara.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Neuhaus W. Der Einfluss des Zwischenwirtes auf die Gestalt der Cercarie von Trichobilharzia szidati Neuhaus1951und ihre systematische Kennzeichnung. Zoologischer Anzeiger. 1952;148:275–285. [Google Scholar]
- 38.Haas W. Parasitic worms: strategies of host finding, recognition and invasion. Zoology. 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- 39.Prokofiev VV, Galaktionov KV. Strategies of search behaviour in trematode cercariae. Trudy Zoologicheskogo Instituta. 2009;313:308–318. [Google Scholar]
- 40.McMullen DB, Beaver PC. Studies on schistosome dermatitis. IX. The life cycles of three dermatitis-producing schistosomes from birds and a discussion of the subfamily Bilharziellinae (Trematoda: Schistosomatidae). American Journal of Hygiene. 1945;42:128–154. [Google Scholar]
- 42.Theron A. Early and late shedding patterns of Schistosoma mansoni cercariae – ecological significance in transmission to human and murine hosts. Journal of Parasitology. 1984;70:652–655. [PubMed] [Google Scholar]
- 43.Steinauer ML, et al. Interactions between natural populations of human and rodent schistosomes in the Lake Victoria region of Kenya: A molecular epidemiological approach. PLoS Neglected Tropical Diseases. 2008;2:e222. doi: 10.1371/journal.pntd.0000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loker ES. A comparative study of the life histories of mammalian schistosomes. Parasitology. 1983;87:343–369. doi: 10.1017/s0031182000052689. [DOI] [PubMed] [Google Scholar]
- 45.Rollinson D, Southgate VR. The genus Schistosoma: a taxonomic appraisal. In: Rollinson D, Simpson AJG, editors. The Biology of Schistosomes: From Genes to Latrines. Academic Press; 1987. pp. 1–49. [Google Scholar]
- 46.Attwood SW, et al. Schistosoma ovuncatum n. sp. (Digenea: Schistosomatidae) from northwest Thailand and the historical biogeography of southeast Asian Schistosoma Weinland, 1858. Systematic Parasitology. 2002;51:1–19. doi: 10.1023/a:1012988516995. [DOI] [PubMed] [Google Scholar]
- 47.Hanelt B, et al. Schistosoma kisumuensis n. sp. (Digenea: Schistosomatidae) from murid rodents in the Lake Victoria Basin, Kenya and its phylogenetic position within the S. haematobium species group. Parasitology. 2009;136:987–1001. doi: 10.1017/S003118200900643X. [DOI] [PubMed] [Google Scholar]
- 48.Brant SV, et al. Molecular phylogenetics of the elephant schistosome Bivitellobilharzia loxodontae (Trematoda: Schistosomatidae) from the Central African Republic. Journal of Helminthology. 2013;87:102–107. doi: 10.1017/S0022149X1200003X. [DOI] [PubMed] [Google Scholar]
- 49.Webster BL, Littlewood TJ. Mitochondrial gene order change in Schistosoma (Platyhelminthes: Digenea: Schistosomatidae. International Journal for Parasitology. 2012;42:313–321. doi: 10.1016/j.ijpara.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Devkota R, et al. Sharing schistosomes: the elephant schistosome Bivitellobilharzia nairi also infects the greater one-horned rhinoceros (Rhinoceros unicornis) in Chitwan National Park, Nepal. Journal of Helminthology. 2013 doi: 10.1017/S0022149X12000697. in press. [DOI] [PubMed] [Google Scholar]
- 51.Vande Vusse FJ. Host parasite relations of Dendritobilharzia pulverulenta (Trematoda: Schistosomatidae) and anatids. Journal of Parasitology. 1979;65:894–897. [Google Scholar]
- 52.Vande Vusse FJ. A review of the genus Dendritobilharzia Skrjabin and Zakharow 1920 (Trematoda: Schistosomatidae). Journal of Parasitology. 1980;66:814–822. [PubMed] [Google Scholar]
- 53.Najim AT. Life history of Gigantobilharzia huronensis Najim, 1950 – a dermatitis producing bird blood fluke (Trematoda: Schistosomatidae). Parasitology. 1956;46:443–469. doi: 10.1017/s0031182000026597. [DOI] [PubMed] [Google Scholar]
- 54.Jouet D, et al. Avian schistosomes in French aquatic birds: molecular approach. Journal of Helminthology. 2009;83:181–189. doi: 10.1017/S0022149X09311712. [DOI] [PubMed] [Google Scholar]
- 55.Blair D, Islam KS. The life cycle and morphology of Trichobilharzia australis n. sp. (Digenea: Schistosomatidae) from the nasal blood vessels of the black duck (Anas superciliosa) in Australia, with a review of the genus Trichobilharzia. Systematic Parasitology. 1983;5:89–117. [Google Scholar]
- 56.Kolářová L, et al. Trichobilharzia mergi sp. nov. (Trematoda: Digenea: Schistosomatidae), a visceral schistosome of Mergus serrator (L.) (Aves: Anatidae). Parasitology International. 2013;62:300–308. doi: 10.1016/j.parint.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Johnson KJ, Sorenson MD. Phylogeny and biogeography of the dabbling ducks (Genus: Anas): A comparison of molecular and morphological evidence. The Auk. 1999;116:792–805. [Google Scholar]
- 58.Basch PF. Why do schistosomes have separate sexes? Parasitology Today. 1990;6:160–163. doi: 10.1016/0169-4758(90)90339-6. [DOI] [PubMed] [Google Scholar]
- 59.Despres L, Maurice S. The evolution of dimorphism and separate sexes in schistosomes. Proceedings of the Royal Society of London B Biological Sciences. 1995;262:175–180. [Google Scholar]
- 60.Platt TR, Brooks DR. Evolution of the schistosomes (Digenea: Schistosomatoidea): the origin of dioecy and colonization of the venous system. Journal of Parasitology. 1997;83:1035–1044. [PubMed] [Google Scholar]
- 61.Beltran S, Boissier J. Male-biased sex ratio: why and what consequences for the genus Schistosoma? Trends in Parasitology. 2010;26:63–69. doi: 10.1016/j.pt.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Hrádková K, Horák P. Neurotropic behavior of Trichobilharzia regenti in ducks and mice. Journal of Helminthology. 2002;76:137–141. doi: 10.1079/JOH2002113. [DOI] [PubMed] [Google Scholar]
- 63.Chanová M, Horák P. Terminal phase of bird schistosomiasis caused by Trichobilharzia regenti (Schistosomatidae) in ducks (Anas platyrhynchos f. domestica). Folia Parasitologica. 2007;54:105–107. [PubMed] [Google Scholar]
- 64.Horák P, et al. Penetration of Trichobilharzia cercariae into mammals: dangerous or negligible event? Parasite. 2008;15:299–303. doi: 10.1051/parasite/2008153299. [DOI] [PubMed] [Google Scholar]
- 65.Spakulová M, et al. The karyotype of Trichobilharzia regent Horák, Kolářová, and Dvořák, 1998 (Digenea: Schistosomatidae), a nasal schistosome in Central Europe. Parasitology Research. 2001;87:479–483. doi: 10.1007/s004360100395. [DOI] [PubMed] [Google Scholar]
- 66.Morand S, Muller-Graf CD. Muscles or testes? Comparative evidence for sexual competition among dioecious blood parasites (Schistosomatidae) of vertebrates. Parasitology. 2000;120:45–56. doi: 10.1017/s0031182099005235. [DOI] [PubMed] [Google Scholar]
- 67.Bourns TKR, et al. Migration and development of Trichobilharzia ocellata (Trematoda: Schistosomatidae) in its duck hosts. Canadian Journal of Zoology. 1973;51:1021–1030. doi: 10.1139/z73-148. [DOI] [PubMed] [Google Scholar]
- 68.Ozumba NA, et al. Endemicity, focality and seasonality of transmission of human schistosomiasis in Amagunze village, eastern Nigeria. Journal of Helminthology. 1989;63:206–212. doi: 10.1017/s0022149x00008993. [DOI] [PubMed] [Google Scholar]
- 69.Agbolade OM, et al. Human urinary schistosomiasis transmission foci and period in an endemic town of Liebu North, southwest Nigeria. Tropical Biomedicine. 2004;21:15–22. [PubMed] [Google Scholar]
- 70.Hassan AO, et al. Human water contact activities and urinary schistosomiasis around Erinle and Eko-ende dams. Global Advanced Research Journal of Medicine and Medical Sciences. 2012;1:077–084. [Google Scholar]
- 71.Aldhoun JA, et al. Bird schistosomes in planorbid snails in the Czech Republic. Parasitology International. 2012;61:250–259. doi: 10.1016/j.parint.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Rollinson D. Natural history, ecology and schistosome transmission. In: Toledo R, Fried B, editors. Biomphalaria Snails and Larval Trematodes. Springer New York; 2011. pp. 57–79. [Google Scholar]
- 73.Lie KJ. Survival of Schistosoma mansoni and other trematode larvae in the snail Biomphalaria glabrata – a discussion of the interference theory. Tropical and Geographical Medicine. 1982;34:111–122. [PubMed] [Google Scholar]
- 74.Chouikha I, Hinnebusch JB. Yersinia-flea interactions and the evolution of the arthropod-bourne transmission route of plague. Current Opinion in Microbiology. 2012;15:239–246. doi: 10.1016/j.mib.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright CA. Snail susceptibility or trematode infectivity? Journal of Natural History. 1974;8:545–548. [Google Scholar]
- 76.Fan PC, Lin LH. Hybridization of Schistosoma mansoni and Schistosoma japonicum in mice. Southeast Asian Journal of Tropical Medicine and Public Health. 2005;36:89–96. [PubMed] [Google Scholar]
- 77.Tsangaras K, et al. Museums and disease: Using tissue archive and museum samples to study pathogens. Annals of Anatomy. 2012;194:58–73. doi: 10.1016/j.aanat.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Hartigan A, et al. Museum material reveals frog parasite emergence after the invasion of the cane toad in Australia. Parasites & Vectors. 2010;3 doi: 10.1186/1756-3305-3-50. DOI:10.1186/1756-3305-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoberg EP, et al. Why museums matter: a tale of pinworms (Oxyuroidea: Heteroxynematidae) Among Pikas (Ochotona princeps and O. collaris) in the American West. Journal of Parasitology. 2009;95:490–501. doi: 10.1645/GE-1823.1. [DOI] [PubMed] [Google Scholar]
- 80.Hunova K, et al. Radix spp.: Identification of trematode intermediate hosts in the Czech Republic. Acta Parasitologica. 2012;57:273–284. doi: 10.2478/s11686-012-0040-7. [DOI] [PubMed] [Google Scholar]
- 81.Platt TR, et al. Griphobilharzia amoena n. gen., n. sp. (Digenea: Schistosomatidae), a parasite of the freshwater crocodile Crocodylus johnstoni (Reptilia: Crocodylia) from Australia, with the erection of a new subfamily, Griphobilharziinae. Journal of Parasitology. 1991;77:65–68. [Google Scholar]
- 82.Jouet D. Molecular diversity of Trichobilharzia franki in two intermediate hosts (Radix auricularia and Radix peregra): a complex of species. Infection and Genetics and Evolution. 2010;10:1218–1227. doi: 10.1016/j.meegid.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Lui Z, Bai G. On bird schistosomes from Jilin Province: Jilinobilharzia crecci gen. nov., sp. nov. (Schistodomatidae: Bilharziellinae) with a discussion on the taxonomy of the subfamily Bilharziellinae. Acta Zoologica Sincia. 1976;22:385–392. [Google Scholar]
- 84.Santoferrara L, et al. Utility of genetic markers and morphology for species discrimination within the order Tintinnida (Ciliophora, Spirotrichea). Protist. 2013;164:24–36. doi: 10.1016/j.protis.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Rudolfová JA, et al. Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitology Research. 2005;95:79–89. doi: 10.1007/s00436-004-1262-x. [DOI] [PubMed] [Google Scholar]
- 86.Vilas R, et al. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology. 2005;131:839–846. doi: 10.1017/S0031182005008437. [DOI] [PubMed] [Google Scholar]
- 87.Detwiler JT, et al. Revealing cryptic parasite diversity in a definitive host: echinostomes in muskrats. Journal of Parasitology. 2012;98:1148–1155. doi: 10.1645/GE-3117.1. [DOI] [PubMed] [Google Scholar]
- 88.Berriman M, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–U65. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young ND, et al. Whole-genome sequence of Schistosoma haematobium. Nature Genetics. 2012;44:221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 90.Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]