Abstract
To evaluate the immunotoxicity of trichloroethylene (TCE), we conducted a cross-sectional molecular epidemiology study in China of workers exposed to TCE. We measured serum levels of IL-6, IL-10, and TNF-α, which play a critical role in regulating various components of the immune system, in 71 exposed workers and 78 unexposed control workers. Repeated personal exposure measurements were taken in workers before blood collection using 3 M organic vapor monitoring badges. Compared to unexposed workers, the serum concentration of IL-10 in workers exposed to TCE was decreased by 70% (P = 0.001) after adjusting for potential confounders. Further, the magnitude of decline in IL-10 was >60% and statistically significant in workers exposed to <12 ppm as well as in workers with exposures ≥ 12 ppm of TCE, compared to unexposed workers. No significant differences in levels of IL-6 or TNF-α were observed among workers exposed to TCE compared to unexposed controls. Given that IL-10 plays an important role in immunologic processes, including mediating the Th1/Th2 balance, our findings provide additional evidence that TCE is immunotoxic in humans.
Keywords: trichloroethylene, immunotoxicity, IL-10, TNF-α, IL-6, occupational exposure
Introduction
Trichloroethylene (TCE) is a volatile chlorinated solvent that has been commonly used as a metal degreaser and general purpose solvent in the occupational setting, and has been estimated to be present in about one-third of municipal water supplies in the United States [Jollow et al. 2009]. Exposure to TCE has been reported to result in several adverse health effects in humans, including toxicities involving the skin, kidney, and central nervous and immune systems, and has also been shown to induce autoimmune related effects in occupationally exposed workers and in animal models [United States Environmental Protection Agency, 2011]. TCE is currently classified as a known carcinogen (Group 1) by the International Agency for Research on Cancer (IARC), based on convincing evidence in humans for an association with renal cell carcinoma, and has been associated with several other cancers including non-Hodgkin lymphoma (NHL) in some epidemiologic studies [Guha et al., 2012].
We have previously reported in a cross-sectional study of Chinese factory workers that exposure to TCE results in declines in various markers of immune function, including in total lymphocytes, specific lymphocyte subsets including CD4+ T cells, markers of B-cell activation, and serum immunoglobulins [Lan et al., 2010; Hosgood et al., 2011; Zhang et al., 2013]. Alterations in some of these specific markers have been associated with risk of NHL, indicating that an association between TCE and NHL may be biologically plausible [Grulich et al., 2007; De Roos et al., 2012]. Cytokines are signaling molecules secreted by immune cells and play an important role in regulating immune and inflammatory processes, including maintaining homeostasis between cell-mediated and humoral immune responses. Imbalances in the Th1/Th2 ratio result in immune dysfunction, and have been reported to be associated with autoimmune or atopic conditions, as well as some hematological malignancies including NHL [Lucey et al., 1996].
Tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10) are key cytokines regulating lymphoid development and the immune response, and deregulated concentrations of these cytokines have been associated with NHL and demonstrated to be important prognostic factors in NHL patients [Blay et al., 1993; Kurzrock, 1997; Warzocha et al., 1998]. Further, genetic variation in the IL-10 and TNF genes in particular have emerged as susceptibility loci for overall NHL and for specific NHL subtypes [Rothman et al., 2006]. Given our previous observations that TCE exposure alters immune parameters that are potentially relevant to lymphomagenesis, and some evidence that TCE alters the concentration of other key cytokines in mice [Griffin et al., 2000; Blossom et al., 2007] and in occupationally exposed workers, including IL-2, IL-4, and interferon-gamma (IFN-γ) [Iavicoli et al., 2005], we postulated that TCE exposure may alter the serum concentration of IL-6, IL-10, and TNF- α in our cross-sectional study of occupationally exposed factory workers in Guangdong, China. To the best of our knowledge, this is the first epidemiological study to evaluate levels of these cytokines in relation to TCE exposure in humans.
Materials and Methods
Study Population and Exposure Assessment
The study design and exposure assessment protocol for this cross-sectional molecular epidemiology study has been described previously [Lan et al. 2010]. Briefly, six factories were identified in Guangdong, China that used TCE for metal (n = 4), optical lense (n = 1), and circuit board (n = 1) cleaning processes along with four control facilities in the same geographic region that did not use TCE. The exposed factories were selected based on their use of TCE for manufacturing, having low to negligible levels of other chlorinated solvents, and having no detectable benzene, styrene, ethylene oxide, formaldehyde, or epichlorohydrin levels. Exposed workers were frequency matched to unexposed workers by sex and age (±5 years). Any worker with a history of cancer, chemotherapy, radiotherapy, or a previous occupation with notable exposure to benzene, butadiene, styrene, and/or ionizing radiation was excluded from the study. All workers completed a questionnaire that inquired about demographic and lifestyle characteristics, and each worker had a brief physical examination that included an evaluation for evidence of a current respiratory infection. Informed consent was obtained from all subjects and the study was approved by the Institutional Review Boards at the United States National Cancer Institute and the Guangdong National Poison Control Center in China. Two to three personal air exposure measurements using a 3 M organic vapor monitoring badge were collected for a full work shift in exposed workers and in a subgroup of controls on separate work days before blood collection as detailed previously [Lan et al., 2010].
Assay
Following completion of the exposure monitoring period, all subjects were asked to provide a 29 mL peripheral blood sample as described previously [Lan et al., 2010]. Serum concentrations of IL-6, IL-10, and TNF-α from 71 exposed workers and 78 controls were measured using a multiplex high sensitivity human cytokine Milliplex (Billerica, MA) assay for the BioPlex200 (BioRad, Hercules, CA) platform according to the manufacturer's instructions. Samples were assayed in duplicate with 50 μL of serum per well and data were reported in pg/mL. Measurements from blinded quality control replicates interspersed among the samples did not identify outlier batches. A similar percentage of subjects had levels of IL-10 (16.8%) and IL-6 (17.4%) below LOD (0.15 pg/ml for IL-10; 0.10 pg/mL for IL-6), and levels of TNF-α were below LOD (0.05 pg/mL) in 6.0% of subjects. Concentrations of cytokines below LOD were substituted using the LOD/√2. The coefficient of variation for the assays was <13% (IL-6: 10.1%; IL-10: 12.8%; TNF-α: 11.7%) for all measured cytokines and intraclass correlation coefficients were each ≥97%.
Statistical Analysis
Unadjusted summary measures were calculated for IL-6, IL-10, and TNF- α in exposed workers and controls. Linear regression using the natural logarithm of each endpoint was conducted in order to assess differences in the serum concentration of cytokines in exposed workers and controls, as well as to test for a dose-response trend across TCE exposure groups based on the median exposure level in the overall study population (unexposed controls, <12 ppm, ≥12 ppm). All models were adjusted for the matching factors, age (as a continuous variable) and sex. Other potential confounders evaluated in the models included current smoking (yes/no), alcohol consumption (yes/no), BMI, and recent infection (yes/no). These variables were included in the final model if the regression coefficient of the TCE exposure variable was altered by ±10%. Moreover, given our previously published findings that showed significant decreases in lymphocyte counts associated with TCE exposure, and since these cytokines are produced by lymphocyte subsets, we further adjusted the models for total lymphocytes if this variable altered the parameter estimate by ±10% when included in the age and sex adjusted model. All statistical analyses were conducted using SAS v. 9.2 (Cary, NC).
Results and Discussion
Exposed workers were similar to controls with respect to most demographic characteristics, though a higher percentage of exposed workers reported current smoking as compared to controls (Table I). The mean TCE exposure level in the 71 exposed workers was 23.4 ppm, 5.1 ppm in the low exposed group (<12 ppm), and 41.2 ppm in the high exposed group (≥12 ppm; Table I).
Table I. Demographic Characteristics of the Study Population from a Cross-Sectional Molecular Epidemiology Study of TCE in China.
| Control n = 78 | Exposed n = 71 | Low exposed n = 35 | High exposed n = 36 | |
|---|---|---|---|---|
|
|
||||
| Characteristic | (n, %) | (n, %) | <12 ppma (n, %) | ≥12 ppma (n, %) |
| Sex (n, %) | ||||
| Male | 56 (72) | 51 (72) | 21 (60) | 30 (83) |
| Female | 22 (28) | 20 (28) | 14 (40) | 6 (17) |
| Mean age (SD) | 27 (±7) | 25 (±7) | 24 (±5) | 27 (±8) |
| BMI (SD) | 22 (±3) | 22 (±3) | 21 (±3) | 22 (±3) |
| Smoking (n, %) | ||||
| Yes | 29 (37) | 32 (45) | 17 (49) | 15 (42) |
| No | 49 (63) | 39 (55) | 18 (51) | 21 (58) |
| Alcohol use (n, %) | ||||
| Yes | 28 (36) | 25 (35) | 12 (34) | 13 (36) |
| No | 50 (64) | 46 (65) | 23 (66) | 23 (64) |
| Recent infection (n, %) | ||||
| Yes | 16 (21) | 13 (18) | 7 (20) | 6 (17) |
| No | 62 (79) | 58 (82) | 28 (80) | 30 (83) |
| Mean TCE air exposure, ppm (SD) | <0.03 | 23.4 (37.9) | 5.1 (3.7) | 41.2 (46.9) |
| Median TCE air exposure, ppm | <0.03 | 12.3 | 4.0 | 29.3 |
Based on the median exposure level among exposed workers in the overall study population.
Exposure to TCE resulted in a 70% decline in serum IL-10 concentrations relative to controls (P = 0.001 for exposed vs. unexposed workers), adjusted for age, sex, and total lymphocyte counts. In contrast, there were no statistically significant differences in concentrations for IL-6 (P = 0.19 for exposed vs. unexposed workers), adjusted for age and sex, or for TNF-α (P = 0.73 for exposed vs. unexposed workers), adjusted for age, sex, and total lymphocyte counts. Mean serum concentrations of the three cytokines according to TCE exposure levels are shown in Figures 1A–1C. The magnitude of decline in IL-10 levels was >60% for the low and high TCE exposure categories with a 78% decline in workers exposed to <12 ppm of TCE (P = 0.002) and a 62% decline for exposures ≥12 ppm (P = 0.04), compared to unexposed workers and adjusted for age, sex, and total lymphocyte counts (Fig. 1A). There was no evidence for a difference in the IL-10 effect between workers exposed to low and high levels of TCE in analyses restricted to exposed workers (P = 0.31). Further, no significant differences were observed for either IL-6 or TNF-α levels in workers exposed to low or high levels of TCE compared to unexposed controls (Figs. 1B and 1C). Adjustment for additional variables, including current smoking and recent infection, resulted in similar results (data not shown).
Fig. 1.
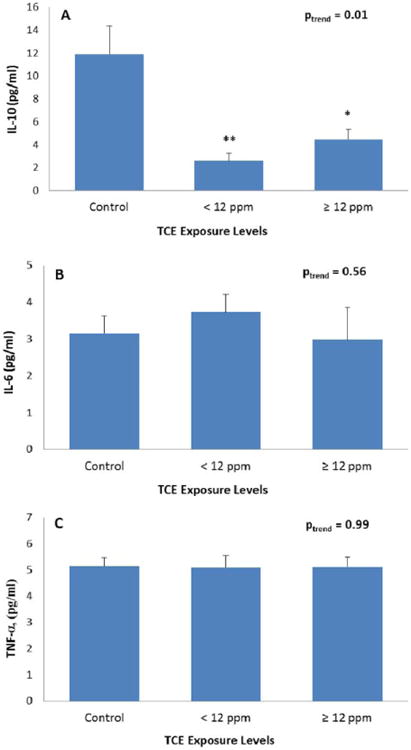
A–C: Mean serum concentrations of cytokines in workers according to exposure categories of trichloroethylene (control, <12 ppm, ≥12 ppm). Twelve ppm was the median TCE exposure level of the exposed subjects. P-values compare low exposed workers and high exposed workers to controls and are indicated as *P < 0.05; **P < 0.01. Levels of IL-10 and TNF-α were adjusted for age, sex, and total lymphocyte counts, and IL-6 was adjusted for age and sex. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To the best of our knowledge, this is the first study to consider the effect of TCE on serum levels of IL-6, IL-10, or TNF-α in healthy adult workers. Our finding of a reduction in IL-10 levels in exposed workers, which was independent of smoking status, declines in lymphocyte counts, and other demographic factors that may influence serum cytokine levels, provides further indication that TCE exposure can alter immune functioning at relatively low exposure levels.
IL-10 is an immunoregulatory cytokine and reduced levels of IL-10 may in part favor pro-inflammatory conditions, as this cytokine has been demonstrated to suppress chronic inflammation through apoptotic effects on developing macrophages and mast cells [Bailey et al., 2006]. Several lines of evidence have emerged suggesting that TCE exposure, in addition to resulting in immunosuppression as characterized by declines in lymphocytes and CD4+ T cells in particular [Lan et al., 2010], may result in the dysregulation of cell-mediated and humoral immune responses. Indeed, an accelerated autoimmune response, characterized by alterations in cytokine profiles, have been observed in animal models following early exposure to relatively low doses of TCE, and epidemiologic evidence in humans has suggested a role of TCE in the development of autoimmune disease, particularly for scleroderma in men [Griffin et al., 2000; Cooper et al., 2009].
Although few studies evaluating changes in cytokine levels in relation to TCE exposure have been conducted in humans, some studies in mice exposed to various levels of TCE or its metabolites have indicated an elevation in the type 1 cytokine IFN-γ following initial exposure [Griffin et al., 2000; Blossom et al., 2007], with one study observing a simultaneous decrease in secretion of the type 2 cytokine IL-4 from activated CD4+ T cells following 4 weeks of TCE exposure [Griffin et al., 2000]. A similar immune response was observed in the one previous occupational study conducted in TCE exposed factory workers, as levels of the type 1 cytokines IFN-γ and IL-2 were significantly increased in these workers whereas the levels of the anti-inflammatory cytokine IL-4 were reduced relative to unexposed workers [Iavicoli et al., 2005]. Our results showing a decrease in IL-10 levels in exposed workers are consistent with this type of immune response, given that IL-10 is involved in the inhibition of Th1 cytokine production and T-cell proliferation [Kidd, 2003].
IL-10 levels, as well as genetic variation in the IL-10 gene, have been extensively studied with respect to cancer risk and prognosis [Kidd, 2003; Howell and Rose-Zerilli, 2006]. Whereas IL-10 may contribute to a reduction in immune surveillance, leading to a poorer cancer prognosis, other evidence suggests that IL-10 may also have immunostimulating properties leading to tumor regression [Mocellin et al., 2005], suggesting that an increase as well as a reduction in IL-10 concentration may be relevant to cancer risk. For NHL in particular, higher blood levels of IL-10 and IL-10 polymorphisms that lead to increased IL-10 expression have been associated with a poorer prognosis in NHL patients [Blay et al., 1993; el-Far et al., 2004; Lech-Maranda et al., 2004], while other evidence has indicated that IL-10 SNPs associated with lower production of IL-10 are associated with aggressive forms of NHL and less favorable prognosis in NHL patients [Cunningham et al., 2003; Bogunia-Kubik et al., 2008]. Thus, our finding of reduced IL-10 levels in TCE-exposed workers provides some biologic plausibility for the suggestive epidemiologic evidence implicating a role for TCE in lymphomagenesis, but further studies are needed to evaluate additional immune markers and the underlying mechanism in occupationally exposed workers in order to characterize the full extent of the immunotoxicity resulting from TCE exposure.
In summary, we found that the serum concentration of IL-10 was markedly reduced in workers exposed to TCE compared to controls, and that these declines occurred in workers exposed to <12 ppm as well as ≥12 ppm of TCE. Conversely, no change in IL-6 or TNF-α levels according to TCE exposure status was evident. Given that immunologic alterations are suspected to play a role in lymphomagenesis, and IL-10 plays an important role in immunologic processes, our findings provide additional evidence that TCE is immunotoxic in humans and some support for the biologic plausibility that TCE may be associated with NHL. However, our findings require replication in larger studies and in other exposed populations.
Footnotes
Author Contributions: Q.L., L.Z., R.V., X.T., M.T.S., H.H., and N.R. conceived of and designed the study. Q.L., R.V., L.Z., X. T., M.S., W.H., C.Q., Y.G., Z.J., B.R., S.L., L.L., and N.R. were involved in subject enrollment, exposure assessment and/or on-site biological sample processing components of the study. B.A.B., M.S., D.H., W.G., F.Y., M.P., N.R., and Q.L. were involved in data preparation and/or analysis. R.B. conducted the lab analyses. B.A.B. prepared the manuscript draft and figures with input from Q.L., L.Z., R.V., and N.R. All authors reviewed the manuscript.
References
- Bailey DP, Kashyap M, Bouton LA, Murray PJ, Ryan JJ. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J Leukoc Biol. 2006;80:581–589. doi: 10.1189/jlb.0405201. [DOI] [PubMed] [Google Scholar]
- Blay JY, Burdin N, Rousset F, Lenoir G, Biron P, Philip T, Banchereau J, Favrot MC. Serum interleukin-10 in non-Hodgkin's lymphoma: A prognostic factor. Blood. 1993;82:2169–2174. [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Gilbert KM. Chronic exposure to a trichloroethylene metabolite in autoimmune-prone MRL+/+ mice promotes immune modulation and alopecia. Toxicol Sci. 2007;95:401–411. doi: 10.1093/toxsci/kfl149. [DOI] [PubMed] [Google Scholar]
- Bogunia-Kubik K, Mazur G, Wrobel T, Kuliczkowski K, Lange A. Interleukin-10 gene polymorphisms influence the clinical course of non-Hodgkin's lymphoma. Tissue Antigens. 2008;71:146–150. doi: 10.1111/j.1399-0039.2007.00984.x. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Makris SL, Nietert PJ, et al. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect. 2009;117:696–702. doi: 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LM, Chapman C, Dunstan R, Bell MC, Joske DJ. Polymorphisms in the interleukin 10 gene promoter are associated with susceptibility to aggressive non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44:251–255. doi: 10.1080/1042819021000035590. [DOI] [PubMed] [Google Scholar]
- De Roos AJ, Mirick DK, Edlefsen KL, LaCroix AZ, Kopecky KJ, Madeleine MM, Magpantay L, Martinez-Maza O. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Res. 2012;72:4733–4743. doi: 10.1158/0008-5472.CAN-12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Far M, Fouda M, Yahya R, el-Baz H. Serum IL-10 and IL-6 levels at diagnosis as independent predictors of outcome in non-Hodgkin's lymphoma. J Physiol Biochem. 2004;60:253–258. doi: 10.1007/BF03167070. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR. Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology. 2000:46123–137. doi: 10.1016/s0162-3109(99)00164-2. [DOI] [PubMed] [Google Scholar]
- Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:405–408. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- Guha N, Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K Monograph IARC. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012;13:1192–1193. doi: 10.1016/s1470-2045(12)70485-0. [DOI] [PubMed] [Google Scholar]
- Hosgood HD, 3rd, Zhang L, Tang X, Vermeulen R, Qiu C, Shen M, Smith MT, Ge Y, Ji Z, Xiong J, He J, Reiss B, Liu S, Xie Y, Guo W, Galvan N, Li L, Hao Z, Rothman N, Huang H, Lan Q. Decreased numbers of CD4(+) naive and effector memory T cells, and CD8(+) naive T cells, are associated with trichloroethylene exposure. Front Oncol. 2011;1:53. doi: 10.3389/fonc.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WM, Rose-Zerilli MJ. Interleukin-10 polymorphisms, cancer susceptibility and prognosis. Fam Cancer. 2006;5:143–149. doi: 10.1007/s10689-005-0072-3. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Marinaccio A, Carelli G. Effects of occupational trichloroethylene exposure on cytokine levels in workers. J Occup Environ Med. 2005;47:453–457. doi: 10.1097/01.jom.0000161728.23285.66. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Bruckner JV, McMillan DC, Fisher JW, Hoel DG, Mohr LC. Trichloroethylene risk assessment: A review and commentary. Crit Rev Toxicol. 2009;39:782–797. doi: 10.3109/10408440903222177. [DOI] [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- Kurzrock R. Cytokine deregulation in hematological malignancies: Clinical and biological implications. Clin Cancer Res. 1997;3(12 Pt 2):2581–2584. [PubMed] [Google Scholar]
- Lan Q, Zhang L, Tang X, Shen M, Smith MT, Qiu C, Ge Y, Ji Z, Xiong J, He J, Reiss B, Hao Z, Liu S, Xie Y, Guo W, Purdue MP, Galvan N, Xin KX, Hu W, Beane Freeman LE, Blair AE, Li L, Rothman N, Vermeulen R, Huang H. Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis. 2010;31:1592–1596. doi: 10.1093/carcin/bgq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech-Maranda E, Baseggio L, Bienvenu J, Charlot C, Berger F, Rigal D, Warzocha K, Coiffier B, Salles G. Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood. 2004;103:3529–3534. doi: 10.1182/blood-2003-06-1850. [DOI] [PubMed] [Google Scholar]
- Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: A counterpoint. J Leukoc Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, Spinelli JJ, Willett E, De Sanjose S, Cocco P, Berndt SI, Brennan P, Brooks-Wilson A, Wacholder S, Becker N, Hartge P, Zheng T, Roman E, Holly EA, Boffetta P, Armstrong B, Cozen W, Linet M, Bosch FX, Ennas MG, Holford TR, Gallagher RP, Rollinson S, Bracci PM, Cerhan JR, Whitby D, Moore PS, Leaderer B, Lai A, Spink C, Davis S, Bosch R, Scarpa A, Zhang Y, Severson RK, Yeager M, Chanock S, Nieters A. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: A report from the InterLymph Consortium. Lancet Oncol. 2006;7:27–38. doi: 10.1016/S1470-2045(05)70434-4. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. Toxicological Review of Trichloroethylene. [Accessed December 2012];2011 Available at: http://www.epa.gov/iris/toxreviews/0199tr/0199tr.pdf.
- Warzocha K, Ribeiro P, Bienvenu J, Roy P, Charlot C, Rigal D, Coiffier B, Salles G. Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin's lymphoma outcome. Blood. 1998;91:3574–3581. [PubMed] [Google Scholar]
- Zhang L, Bassig BA, Mora JL, Vermeulen R, Ge Y, Curry JD, Hu W, Shen M, Qiu C, Ji Z, Reiss B, McHale CM, Liu S, Guo W, Purdue MP, Yue F, Li L, Smith MT, Huang H, Tang X, Rothman N, Lan Q. Alterations in serum immunoglobulin levels in workers occupationally exposed to trichloroethylene. Carcinogenesis. 2013;34:799–802. doi: 10.1093/carcin/bgs403. [DOI] [PMC free article] [PubMed] [Google Scholar]


