Abstract
INTRODUCTION
Carcinosarcomas of the female genital tract, also called malignant mixed müllerian tumors, are aggressive biphasic tumors. Second–line treatment options in the recurrent/persistent setting have yielded marginal responses. Given the potential role of angiogenesis in the gynecological carcinomas, pazopanib, a VEGFR inhibitor, was investigated in the management of patients with recurrent carcinosarcoma of the uterus.
METHODS
Eligible patients had histologically confirmed carcinosarcoma of the uterus, a maximum of two prior lines of therapy, adequate renal, hepatic and hematologic function and a performance status of 0-2. Pazopanib was administered orally at 800 mg. Two dose reductions were allowed. The primary objective was to ascertain the activity of pazopanib as measured by the proportion of patients who survive progression-free for at least six months and the proportion of patients that have objective tumor responses. Secondary objectives included the frequency and severity of adverse events as assessed by CTCAE v4.0.
RESULTS
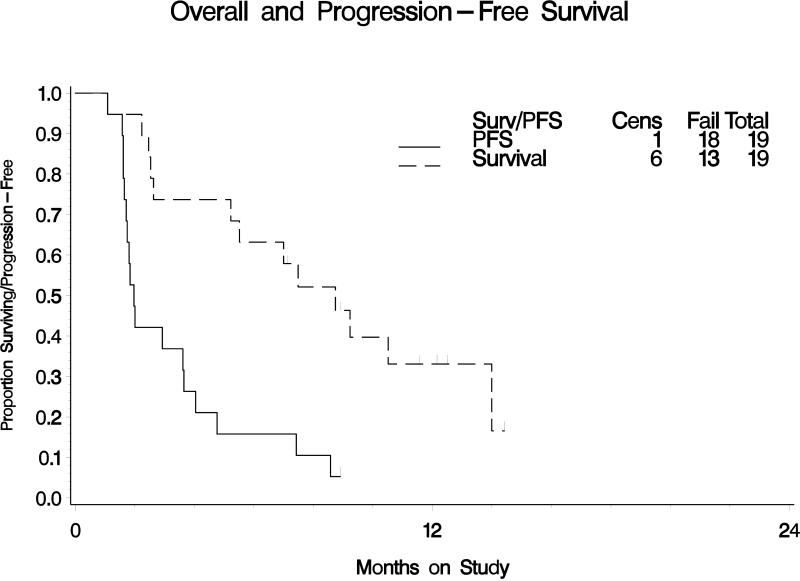
Of the 22 enrolled patients, 19 were eligible and evaluable for toxicity and survival. No patients had a partial or complete response (90% confidence interval [CI]: 0%, 14.6%). Three patients (15.8%) had PFS ≥6 months (90% CI: 4.4%, 35.9%). The median PFS was 2.0 months (first and third quartiles were 1.6 and 4.0 months, respectively). The median overall survival was 8.7 months (first and third quartiles were 2.6 and 14.0 months, respectively).
CONCLUSION
Pazopanib demonstrated minimal activity as a second or third line treatment for advanced uterine carcinosarcoma. Potential clinical trial participation should be discussed with the patients.
Keywords: Carcinosarcoma, uterus, VEGFR inhibitors, angiogenesis
INTRODUCTION
Malignant uterine neoplasms that contain both carcinomatous and sarcomatous elements are designated carcinosarcomas. These neoplasms arise most commonly in the uterus, and with a lesser frequency in the ovary, fallopian tube, cervix or peritoneum. They are regarded as rare aggressive malignancies with a high potential to develop distant metastases and are associated with an overall poor survival.
The histogenesis of this neoplasm has been debated. A number of studies have accumulated evidence suggesting that most of these neoplasms, but not all, are monoclonal in origin rather than true collision tumors [1-8]. Schipf et al investigated the molecular genetic aberrations of both ovarian and uterine carcinosarcoma using comparative genomic hybridization and fluorescence in situ hybridization. In the 30 cases studied, the molecular cytogenetic analysis revealed a high level of homology of chromosomal aberrations between the different tumor components suggesting a monoclonal origin of these tumors [9].
Evidence suggests that the carcinomatous component of carcinosarcomas is the more aggressive element of the cancer. Proliferation indices in both the carcinomatous and sarcomatous tumor component (immunohistochemical Ki-67 staining) revealed a significantly higher proliferation index in the carcinomatous tumor element (mean 70 %) compared to the sarcomatous tumor element (mean 28 %; p<0.001) [9]. Data highlight that the carcinomatous component may be the driving force and that the sarcomatous component is derived from the carcinoma or from a stem cell that undergoes divergent differentiation.
Five-year survival figures for patients with carcinosarcoma are poor [10]. First line treatment of patients with advanced uterine carcinosarcoma with paclitaxel plus carboplatin achieved an objective response rate of 54 % of patients. Progression-free survival (PFS) and overall survival (OS) for the entire cohort was 7.6 months and 14.7 months, respectively [11]. Response rates to second line therapy have been limited. [12-17]. Despite several studies suggesting the activity of various cytotoxic agents, enthusiasm for cytotoxic chemotherapy has been tempered by the rapid emergence of resistance [10, 12-16]. The aggressive nature of this malignancy coupled with a high relapse rate defines a poor clinical course for most patients. As such, a focus on alternative targeted therapeutics has emerged. Huh et al initiated a multi-institutional phase II trial to assess the activity and toxicity of imatinib mesylate in recurrent or persistent uterine carcinosarcoma [18]. Imatinib mesylate had minimal activity as a single agent in unscreened patients.
Independent studies have reported an increase in microvessel density with the progression of a benign to a malignant endometrium [19-22]. These studies have also reported the association of microvessel density with PFS and OS. Wright et al reported a retrospective analysis of patients (n=11) with recurrent uterine neoplasms treated with bevacizumab [23]. Two partial responses were noted in this heavily pretreated cohort and their median progression free intervals were 5.4 months and 8.7 months. Aghajanian et al investigated the role of bevacizumab in the treatment of patients with recurrent/persistent endometrial carcinoma [24]. The median PFS was 4.2 months while the median OS was 10.5 months. Twenty one patients (40.4%) survived progression free for at least 6 months. Nimeiri studied the role of sorafenib, a multi-targeted kinase inhibitor with anti-angiogenic activity, in advanced recurrent endometrial carcinomas and carcinosarcomas. In a group of 39 patients, 5% were reported as having a partial response while 50% had stable disease after two months [25].
Angiogenesis in uterine carcinosarcomas is less well characterized than in endometrial carcinomas. Emoto et al described the localization of the vascular endothelial growth factor (VEGF) and angiopoietin genes in uterine carcinosarcoma [26]. The microvessel density in the epithelial element was found to be higher than that of the mesenchymal element. Ang-2 mRNA was also seen in the vasculature adjacent to the periphery of carcinoma cells. Giatromanolaki et al explored the expression of phosphorylated kinase domain receptor (KDR / VEGFR-2) in endometrial cells utilizing a novel monoclonal antibody that recognized the activated (phosphorylated) form of the KDR (VEGFR-2) receptor [27]. Approximately one-third of patients with endometrial cancer exhibited intense cytoplasmic and nuclear pKDR expression in both cancer cells and peritumoral vessels. This reactivity in the cancer cells was related directly to VEGF and VEGF/KDR complexes. In addition, pKDR expression was associated with poor prognosis. A Phase II GOG study of thalidomide in this patient cohort reported an association between high pre-treatment VEGF- serum levels and poor prognosis in this population [28].
Pazopanib, is a potent competitive, small molecule inhibitor of vascular endothelial growth factor receptor (VEGFR)-1, −2, and −3, platelet-derived growth factor receptor (PDGFR)-α, - β- and c-kit. In preclinical models pazopanib inhibited VEGF-induced VEGFR-2 phosphorylation and was 3- to 400-fold selective for VEGFRs. Pazopanib is approved by the Food and Drug Administration (FDA) for the treatment of soft tissue sarcomas and renal cell carcinoma, and has been evaluated in the management of gynecological cancers [29-32]. Based on the following rationale: (1) the lack of standard second line therapy in patients with advanced carcinosarcoma; (2) the expression of VEGF-mRNA in carcinosarcomas; (3) evidence that angiogenesis plays a role in endometrial carcinomas; and (4) the expression of phosphorylated KDR (VEGFR-2) in endometrial cells, the GOG initiated a phase II study of pazopanib, as second or third-line treatment for patients with recurrent uterine carcinosarcomas.
METHODS
Eligbility
Eligible patients had histologically confirmed recurrent or persistent uterine carcinosarcoma and measurable disease defined by the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) [33]. All patients must have received one prior chemotherapeutic regimen administered after surgical or non- surgical assessment. Patients were allowed to have received one additional cytotoxic regimen for management for recurrent or persistent disease. Patients were not allowed to have received any prior non- cytotoxic or biological therapy. Additional requirements included a GOG performance status of 0-2, adequate bone marrow, renal, hepatic and neurologic function. Patients signed an approved informed consent. The protocol was approved by the Institutional Review Board of each of the participating GOG institutions. Central Pathology review by the GOG Pathology Committee was required. Patients with clinically significant cardiovascular disease, uncontrolled CNS disease, active bleeding and an increased risk of gastrointestinal bleeding or gastrointestinal perforation were considered ineligible.
Pazopanib was administered at 800 mg daily as an oral dose and a cycle was defined as a period of four weeks (28 days). Pazopanib therapy was continued until progressive disease or adverse effects prohibited further therapy. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria Version 4.0 (CTCAE v4) [34]. Hematologic treatment decisions were based on the absolute neutrophil count (ANC) and subsequent cycles of therapy were initiated when the ANC was 1500 cells/mcl and the platelet count was 100,000/mcl. Therapy could be delayed for a maximum of two weeks. Patients who failed to recover adequate counts within this two week period were removed from study. Non- hematologic toxicity assessment for hypertension, peripheral neuropathy, metabolic, endocrine, gastrointestinal disturbances, and cardiac function were reviewed prior to each cycle. Two dose reductions were permitted while on trial. LVEF testing was required prior to enrollment for patients who had received prior anthracycline therapy. Thyroid function tests were also assessed prior to enrollment.
Response assessments were performed by CT imaging every eight weeks. Response and progression were based on RECIST v 1.1.
Statistical methods
The primary objective of this study was to assess the activity of pazopanib in patients with persistent or recurrent carcinosarcoma of the uterus as measured by the proportion of patients achieving a six-month PFS and the proportion of patients who had objective tumor response (complete or partial). Time at risk was assessed from the date of registration onto the study and included all eligible patients who received the drug. The null hypothesis (H0) relating to uninteresting levels of activity was determined from an analysis of a historical dataset based on a similar population of patients where the levels of activity were believed to be inactive to modestly active. The null hypothesis jointly specified that the probability of a patient experiencing a tumor response would be less than or equal to 5% and the probability of a patient experiencing PFS for at least six months would be less than or equal to 15%. Clinically significant differences were a 20% increase in the probability of response (to 25%) or a 20% increase in PFS at six months (to 35%).
To evaluate these hypotheses, a two-stage study design was performed to determine if the study should continue to a second stage of accrual or if the drug should be deemed unworthy of further investigation [35]. The targeted accrual for the first stage was 20 eligible and evaluable patients with a permitted range of 16-23 patients. The cumulative targeted accrual for the second stage was 35 (range from 31-38). With 19 eligible patients, the study required more than one patient with a complete or partial response or more than three patients having PFS for at least six months. The frequency and the severity of adverse events as assessed by CTCAE v4.0 and were tabulated by system affected. Secondary endpoints used to characterize drug efficacy were OS and PFS.
RESULTS
Patients and eligibility
Twenty-two patients were enrolled over the period of 12 months. Of these 19 were evaluable and eligible (two patients were ineligible due to the wrong cell type; one patient was enrolled but never treated).
Patient characteristics are listed in Table 1. The median age was 69 years (range, 50-79). Fourteen patients ( 74%) had a performance status of 0 while two (11%) and three (16%) had a performance status of 1 and 2, respectively. Ten patients (53%) had received one prior line of therapy and nine patients (47%) had received two prior lines of treatment. Chemotherapy regimens utilized as prior therapy included carboplatin and paclitaxel, ifosfamide and paclitaxel, ixabepilone, and a combination of liposomal doxorubicin, carboplatin and docetaxel. Fifty- seven percent of patients had been previously treated with radiation. The majority of patient receiving radiation therapy underwent pelvic radiation therapy+/− brachytherapy. Two patients received brachytherapy alone.
Table 1.
Patient Characteristics
| Characteristic | Category | No. of Cases | % of Cases |
|---|---|---|---|
| Age | 50-59 | 1 | 5.3 |
| 60-69 | 11 | 57.9 | |
| 70-79 | 7 | 36.8 | |
| Race | African American | 2 | 10.5 |
| American Indian | 1 | 5.3 | |
| White | 16 | 84.2 | |
| Performance Status | 0 | 14 | 73.7 |
| 1 | 2 | 10.5 | |
| 2 | 3 | 15.8 | |
| Cell Type | Carcinosarcoma-homologous | 8 | 42.1 |
| Carcinosarcoma-heterologous | 7 | 36.8 | |
| Carcinosarcoma, unspecified: MMT | 4 | 21.1 | |
| Grade | Poorly differentiated | 2 | 10.5 |
| Not graded | 17 | 89.5 | |
| Prior Chemotherapy | |||
| 1 | 10 | 52.6 | |
| 2 | 9 | 47.4 | |
| Prior Radiation | No | 10 | 52.6 |
| Yes | 9 | 47.4 | |
| Prior Surgery | Yes | 19 | 100.0 |
Treatment responses
The median number of pazopanib cycles delivered was two (range 1 to 9). No patients (0%) had a partial or complete response (90% confidence interval [CI]: 0%, 14.6%). Three patients (15.8%) had a PFS of ≥6 months (90% CI: 4.4%, 35.9%). The median PFS was 2.0 months (first and third quartiles were 1.6 and 4.0 months, respectively). The objective response rate and the six-month PFS failed to meet the pre-study criteria at completion of the first stage of study accrual; therefore the study was not re-opened to a second stage of accrual. The median OS was 8.7 months (first and third quartiles were 2.6 and 14.0 months, respectively).
Adverse events
Adverse events are listed in Table 2. Six patients (32%) experienced grade 3 toxicities which included anemia, diarrhea, rectal hemorrhage, fatigue, changes in liver function tests, dehydration, hyperglycemia, hyponatremia, headache, memory impairment and hypertension. Grade 2 hypertension was observed in three patients (16 %). Two patients experienced grade 4 toxicities which included including anemia, proctitis, and metabolic abnormalities. Two patients were removed from trial due to toxicity and five patients required dose reductions due to toxicity.
Table 2.
Adverse Events possibly related to study drug
| Adverse Event | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|
| Leukopenia | 8 | 1 | 0 | 0 | 19 |
| Thrombocytopenia | 2 | 0 | 0 | 0 | 19 |
| Neutropenia | 9 | 1 | 0 | 0 | 19 |
| Anemia | 4 | 1 | 2 | 1 | 19 |
| Other Investigations: (LFTs elevation, creatinine) | 4 | 5 | 1 | 0 | 19 |
| Cardiac | 1 | 0 | 0 | 0 | 19 |
| Gastrointestinal | 11 | 3 | 1 | 1 | 19 |
| General (constitutional) | 9 | 3 | 1 | 0 | 19 |
| Metabolism/nutrition | 3 | 2 | 3 | 1 | 19 |
| Musculoskeletal/connective tissue | 3 | 0 | 0 | 0 | 19 |
| Nervous system | 4 | 1 | 2 | 0 | 19 |
| Psychiatric | 1 | 0 | 0 | 0 | 19 |
| Renal/urinary Gynecological | 0 | 1 | 0 | 0 | 19 |
| 1 | 1 | 0 | 0 | 19 | |
| Respiratory/thoracic/mediastinal | 4 | 0 | 0 | 0 | 19 |
| Skin/subcutaneous | 3 | 3 | 0 | 0 | 19 |
| Vascular disorders (hypertension) | 2 | 3 | 3 | 0 | 19 |
* Gynecological: Pelvic pain: grade 1; Vaginal fistula grade 2
DISCUSSION
Pazopanib is a potent and selective, orally bioavailable, adenosine triphosphate competitive, small molecule inhibitor of VEGFR-1, −2, and −3, platelet-derived growth factor receptor (PDGFR)-α, -β, and c-kit. The rationale for evaluating this drug in recurrent carcinosarcomas was based on the strong association of VEGF-mRNA in carcinosarcomas, evidence that angiogenesis plays a role in endometrial cancer , the expression of phosphorylated KDR (VEGFR-2) in endometrial cells, a recent report of thalidomide noting an association between pre-treatment VEGFA and prognosis in this population and the positive expression of c-kit or PDGFR-β in 88% of patients studied with recurrent or persistent carcinosarcoma of the uterus [18,26,27,28].
Pazopanib demonstrated minimal clinical activity with no objective responses observed and only 15.8% of patients’ progression free at six months. Despite the disappointing performance of this agent, the role of angiogenesis inhibitors in uterine carcinosarcoma remains undefined. Other anti- angiogenic agents therapeutics such angiopoietin inhibitors and vascular disrupting agents have not been explored in carcinosarcoma. Central to the success of these agents may be the identification of a marker that predicts response. Angiogenic signatures identifying high- risk patients or those that may benefit from the addition of anti-angiogenic therapies in other diseases such as high- grade serous ovarian carcinoma have been published and are currently being explored prospectively in clinical trials [36, 37]. A predictive biomarker for anti-vascular treatment in uterine carcinosarcoma has not been identified. In recent years, focus has shifted to understanding the molecular aberrations that drive the pathogenesis of various tumors. In carcinosarcoma this disease is made difficult by the rare and heterogenous nature of the disease. Previous studies exploring genetic aberrations have highlighted characteristic and frequent chromosomal amplifications observed on chromosomes 8q and 20q [9]. Other amplifications have been noted in the MDM2 and ERBB2 gene [9]. Growdon and colleagues recently identified mutational profiles that may be potential drivers of disease [38]. The authors identified in a cohort of 52 patients several gene mutations including TP53 (23%), PI3KCA (19%), KRAS (15%), CTNNB1 (4%) and NRAS (2%). Recently reported by the Cancer Genome Atlas Research Network was an integrated genomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies [39]. This resulted in classification of endometrial cancers into four categories: POLE ultra mutated, microsatellite instability hyper mutated, copy-number low, and copy-number high. A similar analysis is planned specifically for patients with carcinosarcoma of the uterus.
Identification of mutation(s) that drive tumorigenesis coupled with the identification of signaling pathway cross-talks confer the greatest potential for a successful targeted approach to treatment. In light of the Growdon data, the MAPK and PI3K/AKT/mTOR pathway may be a logical target in the study of carcinosarcoma [39-42].
In this phase II study, pazopanib failed to achieve objective responses or disease stabilization in a sufficient number of patients to be considered a potentially active agent in uterine carcinosarcoma. The overall goal remains to target this uncommon disease early in its evolution utilizing agents that target potential driver mutations. Potential clinical trial participation should be discussed with the patients.
HIGHLIGHTS.
Clinical evidence suggests that angiogenesis plays a role in endometrial cancer
Pazopanib, a small molecule inhibitor of VEGFR, has limited activity in the management of carcinosarcoma of the uterus
Mutational Profiles and Chromosomal amplifications may profile potential driver of disease
Figure 1. Overall and Progression-Free Survival.
Kaplan – Meier estimate of progression free survival (PFS) and overall survival (OS) for all eligible and evaluable patients on the study
Table 3.
Objective Responses (OR) and Progression Free Survival (PFS) at 6 months
| Characteristic | Category | No. of Cases | % of Cases |
|---|---|---|---|
| Response | Increasing Disease | 10 | 52.6 |
| Stable Disease | 7 | 36.8 | |
| Indeterminate | 2 | 10.5 | |
| PFS > 6 Months | No | 16 | 84.2 |
| Yes | 3 | 15.8 | |
| Numebr of cycles of Treatment | 1 | 1 | 5.3 |
| 2 | 11 | 57.9 | |
| 3 | 1 | 5.3 | |
| 4 | 3 | 15.8 | |
| 8 | 1 | 5.3 | |
| 9 | 2 | 10.5 | |
| Alive | Without progression | 1 | 5.3 |
| With progression | 5 | 26.3 | |
| Dead | From disease | 12 | 63.2 |
| From neither treatment nor disease | 1 | 5.3 |
Acknowledgments
This study was supported by the National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469) and the Gynecological Oncology Study Group Statistical Office (CA 37517). The following Gynecologic Oncology institutions participated in this study: Abington Memorial Hospital, Columbus Cancer Council/Ohio State, Walter Reed Army Medical Center, Memorial Sloan Kettering Cancer Center, Women and Infants’ Hospital, University of Oklahoma, Washington University Medical Center, The Cleveland Clinic Foundation, Rush University Medical Center, University of North Carolina School of Medicine and Duke University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The co-authors have no conflicts of interest to declare.
REFERENCES
- 1.Auerbach HE, LiVolsi VA, Merino MJ. Malignant mixed Mullerian tumors of the uterus. An immunohistochemical study. Int J Gynecol Path. 1988;7:123–30. doi: 10.1097/00004347-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 2.De Brito PA, Silverberg SG, Orenstein JM. Carcinosarcoma (malignant mixed mullerian (mesodermal) tumor of the female genital tract: immunohistochemical and ultrastructural analysis 28 cases. Hum Path. 1993;24:132–42. doi: 10.1016/0046-8177(93)90291-n. [DOI] [PubMed] [Google Scholar]
- 3.Chung MT, Mukai K, Teshima S, Kishi K, Shimosato Y. Expression of various antigens by different components of uterine mixed Mullerian tumors. An immunohistochemical study. Acta Pathol Jpn. 1988;38:35–45. doi: 10.1111/j.1440-1827.1988.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 4.Costa MJ, Khan R, Judd R. Carcinoma (malignant mixed mullerian {mesodermal tumor] of the uterus and ovary. Correlation of clinical, pathologic and immunohistochemical features in 29 cases. Arch Pathol Lab Med. 1991;115:583–90. [PubMed] [Google Scholar]
- 5.Emoto M, Iwasaki H, Kikuchi M, Ishiguro M, Kubota T, Izumi H, et al. Two cell lines established from mixed mullerian tumors of the uterus. Morphological, immunocytochemical and cytogenetic analyses. Cancer. 1992;69:1759–68. doi: 10.1002/1097-0142(19920401)69:7<1759::aid-cncr2820690718>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Geisinger KR, Dabbs DJ, Marshall RB. Malignant mixed Mullerian tumors. An ultrastructural and immunohistochemical analysis with histogenetic considerations. Cancer. 1987;59:1781–90. doi: 10.1002/1097-0142(19870515)59:10<1781::aid-cncr2820591017>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Meis JM, Lawrence WD. The immunohistochemical profile of malignant mixed mullerian tumor. Overlap with endometrial adenocarcinoma. Am J Clin Pathol. 1990;94:1–7. doi: 10.1093/ajcp/94.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Micci F, Teixeira MR, Haugom L, Krisrtensen G, Abeler VM, Heim S. Genomic aberrations in carcinosarcomas of the uterine corpus. Genes Chromosomes Cancer. 2004;40:229–46. doi: 10.1002/gcc.20038. [DOI] [PubMed] [Google Scholar]
- 9.Schipf A, Mayr D, Kirchner T, Diebold J. Molecular genetic aberrations of ovarian and uterine carcinosarcoma- a CGH and FISH study. Virchows Arch. 2008;452:259–68. doi: 10.1007/s00428-007-0557-6. [DOI] [PubMed] [Google Scholar]
- 10.Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee TC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as postsurgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–85. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell MA, Filiaci VL, Rose PG, Mannel RS, Hanjani P, DeGeest K, et al. Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. J Clin Oncol. 2010;28:2727–31. doi: 10.1200/JCO.2009.26.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, et al. Phase III trial of Ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:526–31. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- 13.Ramondetta LM, Burke TW, Jhingran A, Schmandt R, Bevers MW, Wolf JF, et al. A phase II trial of cisplatin, Ifosfamide, and mesna in patients with advanced or recurrent uterine malignant mixed müllerian tumors with evaluation of potential molecular targets. Gynecol Oncol. 2003;90:529–36. doi: 10.1016/s0090-8258(03)00332-9. [DOI] [PubMed] [Google Scholar]
- 14.Miller DS, Blessing JA, Schilder J, Munkarah A, Lee YC. Phase II evaluation of topotecan in carcinosarcoma of the uterus: A Gynecological Oncology Group study. Gynecol Oncol. 2005;98:217–21. doi: 10.1016/j.ygyno.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Curtin JP, Blessing JA, Soper JT, DeGeest K. Paclitaxel in the treatment of carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol. 2001;83:268–70. doi: 10.1006/gyno.2001.6256. [DOI] [PubMed] [Google Scholar]
- 16.Hoskins PJ, Le N, Ellard S, Lee U, Martin LA, Swenerton KD, et al. Carboplatin and paclitaxel for advanced or recurrent uterine malignant mixed mullerian tumors. The British Columbia Cancer agency experience. Gynecol Oncol. 2008;108:58–62. doi: 10.1016/j.ygyno.2007.08.084. [DOI] [PubMed] [Google Scholar]
- 17.Pectasides D, Pectasides E, Papaxoinis G, Xiros N, Sykiotis C, Papchristodoulou A, et al. Combination chemotherapy with carboplatin, paclitaxel, and pegylated liposomal doxorubicin for advanced or recurrent carcinosarcoma of the uterus: Clinical experience of a single institution. Gynecol Oncol. 2008;110:299–303. doi: 10.1016/j.ygyno.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Huh WK, Sill MW, Darcy KM, Elias KM, Hoffman JS, Boggess JF, et al. Efficacy and safety of imatinib Mesylate (Gleevec®) and immunohistochemical expression of c-KIT and PDGFR-β in a Gynecologic Oncology Phase II Trial in women with recurrent or persistent carcinosarcomas of the uterus. Gynecol Oncol. 2010;117:248–54. doi: 10.1016/j.ygyno.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Kamat AA, Merritt WM, Coffey D, Lin YG, Patel PR, Broaddus R, et al. Clinical and Biological Significance of Vascular Endothelial Growth Factor in Endometrial Cancer. Clin Can Res. 2007;13:7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama Y, Sato S, Futagami M, Fukusji Y, Sakamoto T, Umemoto M, et al. Prognostic significance of vascular endothelial growth factor and its receptors in endometrial carcinoma. Gynecol Oncol. 2000;77:413–8. doi: 10.1006/gyno.2000.5802. [DOI] [PubMed] [Google Scholar]
- 21.Obermair A, Tempfer C, Wasicky R, Kaider A, Hefler L, Kainz C. Prognostic significance of tumor angiogenesis in endometrial cancer. Obstet Gynecol. 1999;93:367–71. doi: 10.1016/s0029-7844(98)00417-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee CN, Cheng WF, Chen CA, Chu JS, Hsieh CY, Hsieh FJ. Angiogenesis of endometrial carcinomas assessed by measurement of intratumoral blood flow, microvessel density, and vascular endothelial growth factor levels. Obstet Gynecol. 2000;96:615–21. doi: 10.1016/s0029-7844(00)00976-5. [DOI] [PubMed] [Google Scholar]
- 23.Wright JD, Powell MA, Rader JS, Mutch DG, Gibb RK. Bevacizumab Therapy in Patients with Recurrent Uterine Neoplasms. Anticancer Res. 2007;27:3525–8. [PubMed] [Google Scholar]
- 24.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. A phase II evaluation of bevacizumab in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2011;29:2259–65. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimeiri H, Oza AM, Morgan RJ, Huo D, Elit L, Knost JA, et al. A phase II study of sorafenib in advanced /recurrent uterine carcinoma: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol. 2010;117:37–40. doi: 10.1016/j.ygyno.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emoto M, Charnock-Jones DS, License DR, Ishiguro M, Kawai M, Yanaihara A, et al. Localization of the VEGF and angiopoietin genes in uterine carcinosarcoma. Gynecol Oncol. 2004;9593:474–82. doi: 10.1016/j.ygyno.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Giatromanolaki A, Koukourakis M, Turley H, Sivridis E, Harris AL, Gatter KC, et al. Phosphorylated KDR expression in endometrial cancer cells relates to HIF1-α/VEGF pathway and unfavorable prognosis. Modern Pathol. 2006;19:701–7. doi: 10.1038/modpathol.3800579. [DOI] [PubMed] [Google Scholar]
- 28.McMeekin DS, Sill MW, Benbrook D, Darcy KM, Stearns-Kurosawa DJ, Eaton L, et al. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: a GOG study. Gynecol Oncol. 2007;105:508–16. doi: 10.1016/j.ygyno.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organization for research and treatment of cancer-soft tissue and bone sarcoma (EORTC study 62043). J Clin Oncol. 2009;27:3126–32. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 30.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 31.Monk BJ, Lopez M, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–9. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 32.Friedlander M, Hancock KC, Benigno B, Messing MJ, Stringer CA, Matthys GM, et al. Pazopanib (GW 786034) is active in women with advanced epithelial ovarian, fallopian tube, and peritoneal cancers: results of a phase II study. Gynecol Oncol. 2010;119:32–7. [Google Scholar]
- 33.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. May 29, 2009NIH publication # 09-7473
- 35.Sill MW, Yothers G. A method for utilizing bivariate efficacy outcome measures to screen agents for activity in 2-stage phase II clinical trials, Technical Report 06-08. Department of Biostatistics Website: http://sphhp.buffalo.edu/biostat/research/techreports/index.php.
- 36.Bentink S, Haibe-Kains B, Risch T, Fan JB, Hirsch M, Holton K, et al. Angiogenic mRNA and micrRNA gene expression signature predicts a novel subtype of serous ovarian cancer. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhaek RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, et al. Prognostically relevant gene signatures of high grade serous carcinoma. The J Clin Invest. 2013;123:517–25. doi: 10.1172/JCI65833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Growdon WB, Roussel BN, Scialabba VL, Foster R, Dias-Santagata DD, Iafrate AJ, et al. Tissue-specific signatures of activating PI3KCA and RAS mutation sin carcinosarcomas of gynecologic origin. Gynecol Oncol. 2011;121:212–7. doi: 10.1016/j.ygyno.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 39.The Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinomas. Nature. 2012;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Nicolantonio F, Arena S, Tabernero J, Grosso S, Molinari F, Macarulla T, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858–66. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe R, Wei L, Huang J. mTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med. 2011;52:497–500. doi: 10.2967/jnumed.111.089623. [DOI] [PubMed] [Google Scholar]