Abstract
Addictions are often characterized as forms of impulsive behavior. That said, it is often noted that impulsivity is a multidimensional construct, spanning several psychological domains. This review describes the relationship between varieties of impulsivity and addiction-related behaviors, the nature of the causal relationship between the two and the underlying neurobiological mechanisms that promote impulsive behaviors. We conclude that the available data strongly supports the notion that impulsivity is both a risk factor for, and a consequence of, drug and alcohol consumption. While the evidence indicating that subtypes of impulsive behavior are uniquely informative – either biologically or with respect to their relationships to addictions – is convincing, multiple lines of study link “distinct” subtypes of impulsivity to low dopamine D2 receptor function and perturbed serotonergic transmission, revealing shared mechanisms between the subtypes. Therefore, a common biological framework involving monoaminergic transmitters in key frontostriatal circuits may link multiple forms of impulsivity to drug self-administration and addiction-related behaviors. Further dissection of these relationships is needed before the next phase of genetic and genomic discovery will be able to reveal the biological sources of the vulnerability for addiction indexed by impulsivity.
1. Impulsivity
Impulses are strong motivational urges to engage in reward pursuit or consumption and can lead to impulsive behaviors, unless individuals effortfully inhibit or interrupt them.1, 2 Impulsivity refers to a trait-like proclivity to engage in these behaviors, either due to unusually strong impulses or to difficulty with reasoning about or controlling impulsive actions.
Impulsive behaviors are not necessarily pathological and likely reflect the individual’s desire/motivation to obtain high salience outcomes like social dominance,3 high-energy nutrients,4 sex5–7 or other rewards. They are, in that sense, adaptive behaviors that may well have been subject to selection forces that encourage quick exploratory or risk-taking actions, in favor of slower, more deliberative and risk-averse choices. The advantageous nature of a certain degree of impulsive tendencies is likely reflected in the fact that alleles associated with higher propensity for impulsivity are highly conserved in mammals; for example, the dopamine D4 receptor exon 3 variable number tandem repeat polymorphism often linked with impulsive behaviors in humans,8–10 non-human primates11–13 and dogs.14, 15
These behaviors are viewed as pathological when they become intrusive, otherwise disrupt normal life routines, cause clinical distress or lead to harmful behaviors directed at oneself or others16, possibly at the point where there is a failure in the inhibitory self-control mechanisms that are called upon to interrupt or suppress these behaviors.1, 17, 18 Pathological impulsive behaviors are either diagnostic of, or are common sequelae of, a range of psychiatric disorders, including the so-called impulse control disorders, attention deficit/hyperactivity and conduct disorders,19–24 bipolar (manic-depressive) disorder,25 borderline personality disorder26, 27 and (of most relevance to this review) substance-use disorders.22, 23, 28–33 Impulsivity also appears to be a significant major contributor to suicidality in patients with these disorders.34
The relationship of impulsivity to each of these disorders is clinically meaningful (e.g., impulsive behaviors are per se symptoms and directly contribute to psychological distress), but the fact that it features in each of these conditions may be more than simply descriptive. Indeed, these disorders represent a constellation of syndromes that are frequently comorbid with one another, and one hypothesis is that heightened impulsive tendencies represent one potential influence or cause driving the simultaneous presentation of these conditions.21, 30, 35–43
1.1. Impulsivity vs. Compulsivity
Pathological, intrusive behaviors that present in mental disorders can be viewed, alternatively, as being strongly driven by motivational urges to obtain a desired outcome (impulsive) or as repetitive, automatic and outcome-independent actions (compulsive); this distinction roughly maps on to the distinctions between goal-directed and habit-like behavior.44 Because the neural mechanisms that contribute to goal-directed and habitual actions are separable,44, 45 the view that a clinically-impairing behavior in a particular disorder is one or the other is potentially meaningful in terms of underlying pathophysiology. To some degree, many of the problematic behaviors in mental disorders in general, and in substance use disorders in particular, can arguably be viewed as impulsive or compulsive – or perhaps reflecting a transition from heightened impulsivity to heightened compulsivity.30, 31, 46, 47
As noted above, pathological impulsive behaviors may in some, or even many, cases from an erosion of inhibitory, self-control abilities, and this is almost certainly true for compulsive actions, as well. Compulsive behaviors are not necessarily pathological themselves; rather, it is progressive loss of control over habits that defines a pathological course. In that sense, the window into inhibitory control abilities we get from the study of impulsivity and impulse control almost certainly also generalizes in many cases to compulsive behaviors.
1.2. Impulsivity and Drug Addictions
The role for the impulsivity in the initiation, maintenance and relapsing nature of drug-seeking and –taking behaviors, and in clinically impairing drug use disorders, has only received attention comparatively recently. 1, 23, 29, 30, 33, 42 Though impulsivity is not directly named as a symptom of substance use disorders in the DSM-IV rubric,48 the concept of impaired control over impulsive or compulsive drug use features prominently.47 Additionally, a considerable amount of theory suggests that – even if impulsivity is not itself a key feature of end-stage addiction – it likely contributes substantially to the progression towards it.
Impulsivity and/or poor impulse control could have an influence on almost all stages of the life cycle of drug use. Theoretically, they could be linked with heightened probability of initiating drug use, of rapidly escalating drug use, of failing to cut down on drug use once it becomes problematic and of relapsing despite motivation to remain abstinent.22, 23, 28–31, 33, 42, 47, 49–54 These contributions can be better understood through a deeper examination of the causal relationships between impulsivity, drug use and addiction, for example whether impulsive tendencies pre-date the onset of drug use behaviors or whether experience with drug use causes or exacerbates the propensity for impulsivity. These directional linkages will be a major topic of this review.
1.3. Forms of Impulsivity
Before further detailing the relationships between impulsivity and addictions, it is crucial to note that there exist many conceptually and procedurally distinct measures of impulsivity and impulsive behaviors (Table 1). In a highly influential article, Evenden2 details the different theoretical approaches to measuring impulsivity, which have long held that impulsivity and impulsive behavior is not a singular phenomenon. The reasoning for a multi-factorial model of impulsivity starts with its descriptions and definitions. Beginning with the attempts of psychometricians and personality theorists to understand impulsive tendencies (or related phenomena) and extending into the descriptions of clinically-impairing impulsivity,48 there is a substantial variability in the characteristics that are viewed as indicators of it and in the forms in which it manifests. Many of these concepts and definitions were embedded into implementations of laboratory tasks that were designed to operationally measure forms of impulsive behavior (Table 1). One example is the divide between impulsive behavior that seems to reflect compromised ability to inhibit inappropriate behaviors (“impulsive action”) and a tendency to make choices leading to suboptimal immediate or unduly risky outcomes (“impulsive choices”), 20, 32, 55 as demonstrated in Table 1.20, 32, 55
Table 1.
Hypothetical dimensions of impulsivity and associated specific tasks and measures that have been commonly used in research program of multiple labs.
| Domain | Construct | Specific forms | Tasks | Measures | Key reference(s) |
|---|---|---|---|---|---|
| Impulsive action | Inhibitory response control | Action inhibition | Stop signal reaction time task (rats or humans) | Stop signal reaction time (individual estimate of time between Go and Stop cues that supports predetermined rate of successful stopping); longer stop signal reaction times = worse action inhibition | 18 |
| Go/No-go task (rats or humans) | % of commission errors (responding on No-go trials); higher error rates = worse action inhibition | 18 | |||
| Reversal learning (rats or humans) | # of trials required before choice accuracy meets criterion after reversal of contingencies or proportion of erroneous responses to initially-trained stimulus after reversal; more trials before reaching criterion performance or more errors post reversal = poorer action inhibition | 31 | |||
| Waiting | 5CSRTT (rats or humans) | # of target responses made before cues are presented; higher # of premature responses = difficulty waiting | 60 | ||
| DRL (rats) | # of trials in which a response is made before the waiting interval elapses; higher # of trials with early responses = difficult waiting | 280 | |||
| Impulsive choice | Decision-making | Sensitivity of choices to delay | Delay discounting tasks; adjusting amounts (rats or humans) | Preference for a larger, delayed (versus a smaller, immediate) reward as a function of the delay to its delivery after a choice is made. Individual estimates of negative rate of change of preference as delay increases are reflected in modeled variable: k; Larger K values = greater impulsive choice. | 182, 281 |
| Sensitivity of choices to risk | Iowa gambling task (rats or humans) | Preference for net suboptimal high gain/high loss response options over net optimal low gain/low loss options; greater relative preference for high gain/high loss options = more impulsive style of decision-making | 282, 283 | ||
| Probability discounting task (rats) | Preference for a larger vs. smaller reward as a function of the probability that the large reward will not be delivered; greater preference for certain, relative to uncertain, rewards = more impulsive style of decision-making | 284 | |||
| Balloon analogue risk task (humans and rats) | Propensity to engage in serial responding to increase reward accumulation, despite an underlying accruing risk of reward forfeiture; greater average reward pursuit/risk acceptance per trial = more impulsive style of decision-making | 225, 285 |
In the following sections, we will discuss the empirical data linking various forms of impulsive behavior, measured in humans and laboratory animals, to drug-seeking and –taking; our focus is on those measures of impulsivity whose relationship to addiction has been studied sufficiently – including, impulsive action, waiting behavior and delay- and risk-related decision making. We will then turn to the neural circuitry and pharmacological mechanisms that underpin these forms of impulsive behavior. Our discussion is guided by our view that – if these manifestations of impulsive behavior represent distinct psychological processes, they should relate to addiction-related behaviors in different ways; additionally, they should rely upon substantially non-overlapping neural circuitries and depend upon separable molecular and pharmacological substrates. In his review, Evenden drew upon his own, very comprehensive set of behavioral pharmacological studies, arguing that varieties of impulsive behavior can sometimes show different responses to various drug manipulations.2 This review adds to the discussion by addressing whether unique neural mechanisms predict individual variation in subtypes of impulsivity and whether these varieties of impulsivity exhibit distinct relationships to addiction behaviors.
2. Inhibitory Response Control
Inhibitory response control is a cognitive mechanism that enables effortful, goal-directed suppression of motor responses. As noted above, this includes exerting control over both goal-directed, reward-seeking (impulsive) and automatic and/or habitual (compulsive) actions. In either case, failures of inhibitory control can result in hasty actions made with little forethought of the consequences of the behaviors. The proposed significance of inhibitory response control to drug addiction, in particular, is that impairments in this domain might figure centrally into the compromised ability to control drug-seeking and –taking.1, 31, 33, 56–58
2.1. Conventional Tests of Inhibitory Response Control
A number of tests are commonly used in the laboratory to measure inhibitory response control in animals and humans (Table 1). This discussion cannot be comprehensive; rather, it will focus on the tasks most commonly used in the field. These include tests of action inhibition and procedures that emphasize waiting or delaying reward-seeking responses. The following section provides a description of each task and commentary on the advantages/disadvantages of each, as well as on the translational value of the measures.
2.1.1. Action Inhibition Tests
In both the stop-signal and go/no-go tasks, subjects are required to make frequent, speeded motor responses to a “go” signal. On a smaller subset of trials, subjects are presented with a “stop” signal, and failures to suppress responding are measured. One key difference between stop signal and go/no-go tasks is the time at which the stop signal is presented.18 In go/no-go tasks, stop signals are either presented in combination or in lieu of the go signal; conversely, in the stop-signal task, stop signals are presented after the go signal is presented, such that subjects must stop and/or inhibit an already initiated response. By parametrically varying the time between the presentation of the go and stop cues, one can create a quantitative, individualized estimate of the time required to inhibit an ongoing response, referred to as the individual’s stop signal reaction time.59 Both procedures have been used extensively in both human and animal research.18
Procedures involving reversal learning can also index the ability of subjects to selectively withhold conditioned responses during learning.31 These tasks usually consist of a phase in which subjects are trained on a set of stimulus-outcome or response-outcome associations. After the initial association is learned, the trained contingencies are unexpectedly changed, or reversed, and subjects must then learn to selectively inhibit the previously reinforced response in order to avoid punishment and/or obtain reward. High rates of responding to the originally trained association are considered to be one index of impulsive (or perhaps compulsive) action.
The adaptability of the reversal learning paradigm has facilitated its use in a variety of species.31 Species-appropriate learning protocols can be used, each configured with a reversal problem and need for response inhibition, making reversal learning one of the more useful measures of inhibitory response control available to translational behavioral neuroscience research.
2.1.2. Tests of Waiting
Tasks such as the 5-Choice Serial Reaction Time Test (5CSRTT)60 and Differential Reinforcement of Low Rates of Responding (DRL) schedules61 have been used to measure the ability to withhold behavior while waiting for the opportunity to obtain a reward. The 5CSRTT, commonly considered the rodent analog of a human Continuous Performance Task, was designed to measure attentional ability by requiring rodents to make spatially-congruent responses following presentation of a brief visual stimulus. However, responses made at inappropriate times, most notably premature responses made during the inter-trial interval, have been proposed to index difficulty with waiting, a form of impulsive responding.60
DRL schedules have been used in rodents to measure the ability to wait to make an action (as opposed to the ability to wait for reinforcement following a choice, as in delay discounting – see below); in these procedures, appetitive reinforcement occurs only if a response is made after a certain, relatively long interval of time has passed.61 Waiting impulsivity is assessed by how frequently early responses are made, and by consequence, how many rewards are forfeited.
While similar in some respects, the forms of waiting impulsivity described here (namely, premature responses in the 5CSRTT and reinforcement rate in the DRL) are differentiated from the measure of waiting behavior captured by delay discounting tasks (Table 1). The former are more highly intertwined with action inhibition – that is, withholding an often reinforced behavior until it is appropriate to emit the behavior – while the latter emphasizes a decision-making process, whereby subjects weigh the merits of a smaller, immediate reward versus a larger, delayed reward, but not inhibition of responses themselves.
2.2. Relationship to Impulsive Temperament
Laboratory measures of inhibitory response control (Table 1) are meant to provide an objective, quantitative measure of variation in impulsivity; here we describe the relationship between response inhibition laboratory tasks and self-reported impulsivity, as indexed by personality scales.
2.2.1. Action Inhibition Tests
The impulsivity subscale of the Eysenck Personality Inventory has been associated with stop signal reaction time scores,62 as have parent/teacher reports of externalizing behaviors in children.63 A weak correlation has been observed between go/no-go performance and Barratt Impulsiveness Scale scores, with no such correlation observed with stop signal inhibition.64 Studies comparing the performance of individuals in reversal-learning tasks with self-reported levels of impulsivity have indicated that difficulty in reversing discrimination problems is associated with heightened levels of self-reported levels of impulsivity.65 Together, these data suggest that the laboratory tasks measure, at least partially, the same forms of impulsivity measured with personality scales.
2.3. Relationship to Substance Use Disorders
The following section deals with the observed relationship between measures of inhibitory response control and drug self-administration in animal models or with subclinical or clinical substance use in humans.
2.3.1. Action Inhibition Tests
Individuals affected by substance use disorders have been repeatedly shown to exhibit impaired stop-signal inhibition66–68, as well as deficits in go/no-go performance.69, 70 These impairments appear to be both an antecedent risk factor for, and consequence of, drug use. Unaffected relatives of substance-dependent probands display longer stop signal reaction times,68, 71 suggesting that impaired response inhibition measured using this task is a preexisting heritable endophenotype for addictions. Conversely, a history of cocaine self-administration is sufficient to elicit deficits in stop signal performance in monkeys.72
In human subjects, reversal-learning performance is impaired in some substance-dependent individuals.73–75 These effects appear to be due, at least in part, to chronic exposure to drugs of abuse, because animal models exposed to drugs in either experimenter-delivered or self-administration paradigms exhibit reversal learning problems as a consequence.76–78 On the other hand, a recent study demonstrated that inbred strains of mice previously shown to exhibit the greatest difficulty inhibiting a response during reversal learning79 also showed the greatest propensity to self-administer cocaine, as compared to strains with relatively normal or good reversal-learning abilities.80
Results from a variety of tests therefore suggest that the relationship between impairments in action inhibition and addiction-related behaviors run in both causal directions, with poor inhibition predicting heightened propensity to self-administer drugs and with drug experience causing an erosion of inhibitory abilities. What remains unknown, to some extent, is whether subjects differ in the direction or magnitude of drug-induced changes in action inhibition as a function of their baseline competency, as well as whether this relationship holds for all drugs of abuse (the data gathered so far have mostly involved the study of stimulants).
2.3.2. Tests of Waiting
Waiting to respond also appears to be impaired in substance use disorders, whether one uses 5CSRTT variants81 or DRL tests.82 These deficits may well proceed the onset of drug use because the 5CSRTT can be used to identify a pattern of waiting impulsivity – present in a minority of rats – that predicts an elevated propensity to self-administer cocaine or nicotine,83, 84 but not heroin83, 85 and with enhanced susceptibility for the development of a compulsive pattern of cocaine seeking.85 Finally, impulsive responding in the 5CSRTT predicts escalation of sucrose intake and susceptibility to cue-induced reinstatement of sucrose seeking, pointing to its ability to predict a dyscontrolled, hyperactive reward-seeking and –taking phenotype that extends beyond drugs of abuse.86 By contrast, neither amphetamine87 or heroin or cocaine self-administration,88 even when coupled with repeated withdrawal episodes, produces lasting changes in premature responding in the 5CSRTT at the group level in rats; strikingly, when analyses are focused only on rats with pre-existing high impulsivity in the 5CSRTT, cocaine self-administration experience actually lessens waiting impulsivity.89
While the predictive value of DRL for self-administration is unknown, repeated, intermittent (experimenter-delivered) administration of cocaine, nicotine, methamphetamine, or amphetamine is sufficient impair waiting in this test,90–93 even when withdrawal periods are imposed in between the final drug administration and test.94
Though 5CSRTT and DRL are viewed from the perspective that they both measure waiting, it is clear that their individual relationships to addiction-like behaviors are different. Waiting in the 5CSRTT predicts self-administration behaviors (much like tests of action inhibition do), but drug experience does not impair waiting in this task at the group level (and, indeed, reduces it in rats with baseline high impulsivity). Waiting in the DRL, on the other hand, is highly sensitive to drug experience. This suggests that, even within one domain of inhibitory response control, different tests exhibit unique relationships to addiction.
2.4. Neural Circuitry of Inhibitory Response Control
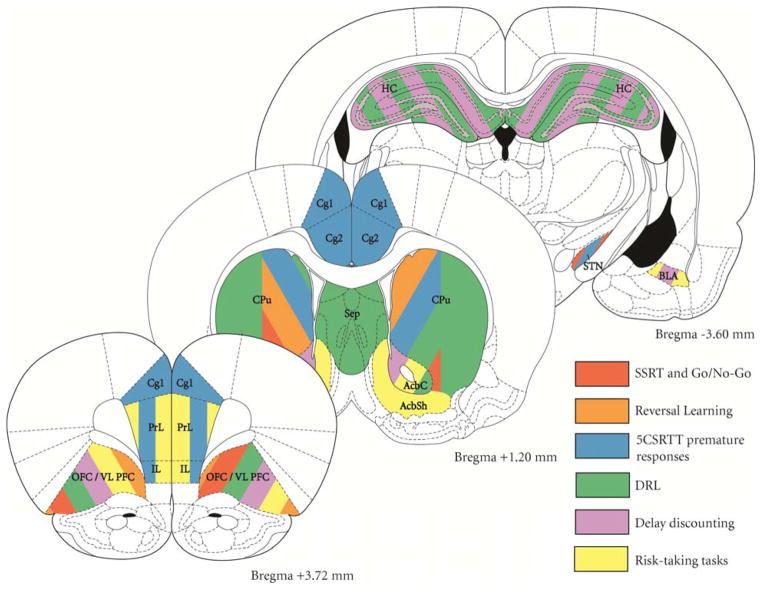
Inhibitory response control is often linked with the integrity of brain systems interconnecting regions of the frontal lobe and basal ganglia. In the following section, we will refer to both lesion and imaging data in animals and humans that help to illuminate the specifics of these anatomy-behavior relationships. In particular, the similarities and differences between the underlying neural circuits responsible for inhibitory control performance in different tasks can help to address the question of whether there is a common biological pathway to impulsive action or whether this phenotype is itself heterogeneously determined and multidimensional. A summary of the experimental studies in rodent models (demonstrating cause-effect relationships) are depicted in Figure 1.
Figure 1. Anatomical localization of brain regions involved in inhibitory response control or delay- or risk-related decision-making.
Figure is color coded according to regions implicated in various laboratory measures of inhibitory response control. Abbreviations: Cg1, Cg2 = Zilles’ areas for anterior cingulate cortex; PrL = prelimbic cortex; IL = infralimbic cortex; OFC/VLPFC = orbitofrontal cortex/ventrolateral prefrontal cortex; Sep = septal nuclei; CPu = caudate/putamen nucleus; AcbC = nucleus accumbens core; AcbSh = nucleus accumbens shell; HC = hippocampus; STN = subthalamic nucleus.
2.4.1. Action Inhibition
Functional neuroimaging studies have repeatedly implicated a circuit comprising ventral parts of the frontal lobe and the basal ganglia in the stop-signal task.24 Neuropsychological studies of brain damaged patients have revealed an important contribution of the right inferior frontal gyrus, in particular, to stop-signal performance.95 In the case of rodent models, lesions of the rodent orbitofrontal cortex,96 but not of the medial prefrontal cortex,96, 97 and the medial,98 but not ventral,97 striatum have been found to slow stop-signal reaction times (Figure 1). It has been proposed that the right inferior frontal gyrus exerts top down influence over striatal regions, possibly via the subthalamic nucleus,99 to mediate response inhibition within the stop-signal task. A very similar pattern of results is found for go/no-go tasks.100–102
The neural mechanisms that govern action inhibition during reversal learning are remarkably similar, if not identical, across species.31 Areas within the corticostriatal circuit that include the orbital regions of frontal cortex and dorsomedial striatum have been identified as being necessary for optimal inhibitory control during reversal-learning tasks.31 Functional MRI studies have also provided support for this hypothesis.103, 104
Collectively, these data support the notion that an at least partially overlapping circuit involving lateral, inferior portions of the frontal lobe and dorsomedial parts of the striatum is implicated in various forms of action inhibition.
2.4.2. Tests of Waiting
The neural circuitry underlying waiting includes the ventromedial, but not orbital, regions of the frontal cortex (Figure 1), as lesions of the infralimbic and anterior cingulate cortex increase premature responding in the 5CSRTT, while damage to the orbitofrontal, parietal, lateral frontal, or medial frontal cortex (the latter depending on the extent of the lesion) do not.105–107 Moreover, lesions of the medial and lateral striatum, but not the ventral striatum, increase premature responding in the 5CSRTT.108, 109 Like stop signal performance, effective inhibition of anticipatory responding in the 5CSRTT also involves the subthalamic nucleus.110
Waiting in the DRL task is not as sensitive to removal of the ventromedial prefrontal cortex.111, 112 Instead, it appears to depend upon a circuit encompassing ventrolateral frontal regions, the caudate/putamen, nucleus accumbens core, and the septohippocampal system.111, 113–115
From the perspective of brain regions necessary for performance, the tests of waiting again show a discrepancy, with 5CSRTT depending on ventromedial and DRL depending upon ventrolateral frontal cortices. Notably, waiting in the 5CSRTT is linked mechanistically with frontal cortical subregions that are distinct from tests of action inhibition, as well (Figure 1).
2.5. Pharmacological Regulation of Inhibitory Response Control
The following section addresses the question of whether the set of neurotransmitters and/or molecularly-defined neurotransmitter receptor subtypes necessary for various forms of action inhibition and waiting are similar or distinct. As noted above, one key argument in favor of the delineation of these forms of impulsivity from one another has been their differential sensitivity to pharmacological manipulation of monoaminergic systems.2, 55, 116 The following section will focus on monoamine systems, at the expense of discussing other relevant systems like glutamate, GABA, acetylcholine, cannabinoids, etc., because the majority of research in the field has focused on monoamine systems, allowing for a more comprehensive, comparative analysis of different forms of inhibitory response control. The pattern of results discussed below are graphically displayed in Table 2.
Table 2. Effects of pharmacological manipulations of monoaminergic signaling on inhibitory response control.
Upwards arrows indicate that the manipulation increased impulsive responding in the test, while downwards arrows indicate a reduction in impulsive responding in the test. Effects are drawn from studies cited in Section 2.5., 3.5., and 4.4. of the text.
| Serotonin | Dopamine | Norepinephrine | |||||
|---|---|---|---|---|---|---|---|
| Depletion | Reuptake inhibition | -2A antagonist | -2C antagonist | D2 antagonist | Reuptake inhibition | ||
| Action Inhibition | Stop signal reaction time task | No change | No change | Not done | Not done |
|
|
| Reversal learning |
|
|
|
|
|
|
|
| Waiting | 5CSRTT |
|
|
|
|
Variable |
|
| DRL |
|
|
|
Not done | Not done |
|
|
| Delay-related decision making | Delay discounting |
|
|
Not done | Not done | Variable |
|
| Risk-related decision making | Gambling tests |
|
No change | Not done | Not done | Variable | No change |
2.5.1. Serotonin and Action Inhibition
Although depletion of forebrain serotonin levels – presumably impairing serotonergic transmission - has repeatedly been shown to affect go/no-go performance117 and reversal learning,118–121 serotonin appears to play little role in inhibition in the stop signal task.122–124
Elevations in brain serotonin, caused by genetic or pharmacological interference with the serotonin transporter, enhances reversal learning performance,125–128 and variation in the serotonin transporter gene influences inhibitory control during reversal learning in monkeys.129, 130 At least with respect to reversal learning, antagonism of serotonin-2C receptor enhances the ability of rats to learn a spatial reversal task,131, 132 an effect mediated by the orbitofrontal cortex,133 while blockade of serotonin-2A receptors impairs reversal performance.131
2.5.2. Serotonin and Waiting
The literature linking serotonergic transmission to ‘waiting’ is extensive. With respect to the 5CSRTT, genetic or pharmacological disruption of the serotonin transporter or administration of agents that elevate synaptic serotonin levels reduce premature responding,128, 134, 135 while depletion of brain serotonin has the opposite effect.116, 136, 137 Activation of serotonin-1A receptors137 or blockade of serotonin-2C receptors increases premature responding, while -2C agonists or -2A antagonists (the latter given systemically or into the prefrontal cortex) decrease premature responding,138–140 again supporting an oppositional relationship between serotonin-2A and -2C subtypes (albeit the direction of the relationships appear to be opposite to those described for reversal learning and DRL performance).
Waiting in DRL tests is impaired by serotonin depletion141 and is improved by manipulations that elevate serotonergic transmission.61 Systemic administration of serotonin-1A agonists and serotonin-2 antagonists improve waiting in DRL schedules.142, 143 The role for subtypes of serotonin-2 receptors is complex, with systemic activation of serotonin-2C receptors or antagonism of serotonin-2A receptors improving waiting.144, 145 This opposing influence of serotonin-2C and 2A receptors on DRL performance is similar to that observed for 5CSRTT.131
2.5.3. Dopamine and Action Inhibition
Dopaminergic mechanisms play a role in all forms of action inhibition. For example, response inhibition in the stop signal task is impaired after pharmacological blockade of dorsomedial striatal dopamine D2-like receptors, while D1-like receptor blockade in the same brain region increases inhibition in this task.146 Further supporting these results, D2-like receptor stimulation improves stop signal inhibition in humans,147 and individual differences in striatal dopamine D2-like receptor availability, measured with positron emission tomography, vary negatively with the stop signal reaction times.148
Similar results are found for reversal learning. Genetic or pharmacological interference with dopamine D2-like, but not D1-like, receptors impairs inhibition during reversal learning31, 149–151 [but see152], and individual differences in D2-like receptor expression/availability within the striatum positively correlate with reversal learning performance competency in mice and monkeys.79, 153 Finally, variation in the DRD2 gene is linked with reversal learning abilities in humans.154 Overall, these data strongly support the notion that dopaminergic transmission at D2 receptors promotes action inhibition in a confluence of laboratory tests. Given that reductions in striatal D2-like receptor availability have been repeatedly found in individuals with substance use disorders,155 these findings are of particular clinical interest.
Emerging evidence suggests that the serotonergic and dopaminergic influences on action inhibition in the reversal learning task are mechanistically linked. Specifically, we found a relationship between individual differences in the ability to inhibit inappropriate responses in a reversal learning test and brain monoamine transmission in monkeys found a statistical interaction between levels of serotonin in the orbitofrontal cortex and dopamine in dorsal striatum that predicted action inhibition.156 These data indicate that serotonin and dopamine interact to influence response inhibition, rather than simply acting as two distinct and orthogonal influences.
2.5.4. Dopamine and Waiting
Dopamine likely exerts complex effects on waiting in the 5CSRTT. For example, within the medial prefrontal cortex, activation of D1-like receptors leads to reduced anticipatory responding;157 additionally, depletion of dopamine in the ventral striatum decreases, while systemic or local application of amphetamine increases, this behavior.158, 159 Heightened anticipatory responding has been associated with low dopamine content and reduced dopamine D2-like receptor availability in the ventral striatum.83, 160 Pharmacological manipulation of D2-like receptors affects this form of impulsivity and its modulation by amphetamine,159, 161 with baseline impulsivity affecting sensitivity to D2 manipulations.162 Moreover, the core and shell subregions of the nucleus accumbens may exert opposing influences on the relationship between dopamine D2-like receptors and premature responding, with D2-like receptor antagonism increasing and decreasing impulsivity in the shell and core, respectively.163
The evidence for a dopaminergic influence on waiting in the DRL task is limited. However, administration of amphetamine impairs DRL performance, and this effect is blocked by dopamine receptor antagonists.164
2.5.5. Norepinephrine and Action Inhibition
Noradrenergic mechanisms are also implicated in action inhibition. Inhibitors of the norepinephrine transporter have been shown to facilitate action inhibition in the stop signal task in both rats and humans.122, 165–167 Direct injection of atomoxetine or the alpha-2A adrenoceptor agonist guanfacine into the prefrontal cortex is able to alter stop signal inhibition,168 providing evidence that prefrontal norepinephrine regulates response inhibition in this task. Additionally, reversal learning has also been shown to be improved by inhibition of the norepinephrine transporter169, 170 and by stimulation of the alpha-2A adrenoceptor.171
2.5.6. Norepinephrine and Waiting
Similar to the case with stop signal and reversal learning performance, inhibition of the norepinephrine transporter reduces anticipatory responding in the 5CSRTT165, 172 and facilitates waiting in the DRL task.61 It is as of yet unknown why interfering with the norepinephrine transporter produces the most consistent positive effects of various forms of inhibitory response control.173
2.6. Summary
In the preceding section, we reviewed some of the evidence relating measures of action inhibition and waiting (collectively thought to measure aspects of inhibitory response control) to impulsivity and substance abuse behaviors. We also discussed the underlying neural circuitry and neuropharmacology of these measures. What is clear is that action inhibition and tests of waiting do not relate to two distinct patterns of underlying neural circuitry (Figure 1), nor do they exhibit two internally consistent but differentiable patterns of pharmacological response (Table 2). Instead, measures of both action inhibition and waiting can depend upon similar brain regions and neurotransmitter systems, while individually exhibiting idiosyncratic responses to manipulations of particular receptor subtypes. From a big picture perspective, this means that the conceptually-attractive organization of phenotypes related to inhibitory response control show neither strong within category consistency, nor strong between category differences in these biological substrates. Perhaps by comparing this collection of tasks to other even more conceptually distinctive measures of impulsivity (e.g., impulsive choice), further support for the validity of these categories will be revealed; this is the subject of the next two sections of this article.
3. Delay-Related Decision-Making
Reward-seeking behavior is under the control of brain systems that set a value for the outcome being sought. It is almost universally true that the value of rewards decrease (are “discounted”) as a function of the delay to their delivery, but the rate of this discounting of value as a function of delay varies across individuals and species.174 Organisms with rapid discounting rates tend to select actions and responses that lead to immediate outcomes and may therefore engage in a type of impulsive behavior marked by an abnormal preference for immediate gratification, even when it comes at the expense of overall reward receipt. This section deals with the phenomena of delay discounting – which has been conceived of as an independent form of impulsive decision-making, as well as its underlying neurobiology.
3.1. Conventional Tests of Delay-Related Decision-Making
Delay-related decision-making is measured by various delay discounting procedures (Table 1), each of which generally involving choices between an immediately-delivered small reward and a delayed larger reward; either the magnitude or delay can be varied orthogonally to yield equivalent frequencies of selection of the two options (i.e., the indifference point).175 Choice behavior can be plotted against delays, yielding a hyperbolic curve that estimates the devaluation of reward value by virtue of the delay to its receipt.176 In human studies, these can include hypothetical or actual monetary rewards, as well as hypothetical negative health outcomes and drug rewards.175, 177 Studies in non-human subjects often employ procedures in which operant responses are used to obtain a reinforcer (e.g. food, water, or drug), ranging from maze-based tests to variations of choice procedures involving delay to reinforcement.30, 178, 179 In both contexts, despite differences in the type and magnitude of reinforcement and/or delay, the discount functions obtained across species are similar, i.e. hyperbolic in shape; however the discounting rate, or the steepness of the curve, varies.180
3.2. Relationship to Impulsive Temperament
Laboratory measures of the discounting of delayed rewards have been shown to be positively correlated with self-reported measures, as indexed by the Barratt Impulsiveness Scale, in some studies, but not in others; this discrepancy could be due to analyzing total scores versus scores on each subscale of the questionnaire.181 In that sense, sensitivity of choice behavior to delay is not necessarily clearly associated with personality measures of impulsivity.
3.3. Relationship to Substance Use Disorders
Heightened propensity to discount delayed rewards has been reported in humans with a long-term history of consumption of various drugs of abuse.182 In humans, the discount of delayed rewards is positively correlated with family history of drug use disorders, suggesting that it may represent a potential risk factor.183 Similarly, steep delay discounting is associated with self-reported early onset of alcohol use184 and smoking initiation in adolescents.185
Similarly, preclinical studies in rats show that this greater delay discounting can predict greater alcohol, cocaine, and methamphetamine self-administration rates, escalation of cocaine intake, increased nicotine-seeking during abstinence and greater vulnerability to cue-induced nicotine reinstatement.84, 186–190 A propensity for steep delay discounting may also be a consequence of drug use, with exposure to stimulant drugs increases delay discounting in rodents,191, 192 and drug-paired contextual stimuli can also elicit a state of impulsive decision-making behavior, as rats exhibit an increase in delay discounting in a cocaine-paired context.193 In this sense, delay-related decision-making phenotypes relate to drug-taking behaviors in humans and animals in a manner not unlike the tests of impulsive action inhibition.
3.4. Neural Circuitry of Delay-Related Decision-Making
The neural circuitry involved in delay-related decision making is shown in Figure 1, in comparison with the brain regions implicated in inhibitory response control. Activity in both the ventromedial and dorsolateral PFC, as well as the posterior cingulate cortex, is associated with the discounted value of future rewards in human subjects.194–196 Moreover, heightened discounting is observed in patients with ventromedial PFC or medial orbitofrontal cortex damage.197 However, in rats, medial PFC lesions do not alter discounting,198 while lesions of the orbitofrontal cortex yield equivocal results – increasing delay discounting in one study while decreasing it in another;199, 200 in humans, greater lateral orbitofrontal cortex activity is associated with less discounting.201
Activity in the anterior cingulate cortex and lateral PFC is associated with response time during delay discounting, potentially reflecting the degree of cognitive control over impulsive choices.202, 203 Indeed, disruption of lateral PFC function via repetitive transcranial magnetic stimulation increases choice for immediate rewards over larger delayed rewards.204
Additionally, the nucleus accumbens core, hippocampus, and basolateral amygdala, have also been implicated in choice tasks, as damage to these areas impairs performance in delay discounting.198, 200, 205 While their specific contributions are less well understood, these regions are thought to represent potential future outcomes of decisions.206
Peters and Buchel206 have proposed that these areas can be viewed as distinct neural networks, based on the aspect of decision-making they are involved in: a valuation network - representing the subjective worth of discounted rewards - comprised of the ventromedial PFC, medial orbitofrontal cortex, ventral striatum, and posterior cingulate cortex; a cognitive control network - reflecting decision conflict and degree of exertion of cognitive control - comprised of the anterior cingulate cortex and lateral PFC; and a prospection network - reflecting prediction, affect, and prospection processes in decision-making and comprised of the medial temporal lobe (namely hippocampus and amygdala), with the ventromedial PFC and posterior cingulate cortex being involved in both valuation and prospection networks.
3.5. Pharmacological Regulation of Delay-Related Decision-Making
The sensitivity of delay-related decision-making to various pharmacological manipulations of monoamine transmission are shown and compared with the effects on impulsive action in Table 2. Serotonin neurotransmission has often been implicated in delay-related decision-making. In general, lesion-induced decreases in serotonin result in an increased preference for the small-immediate- reward, while pharmacologically-induced serotonin release increases preference for the large-but-delayed reward.207–209 However, other studies have also shown serotonin antagonism to decrease delay discounting and forebrain serotonin depletion to have no effect on performance.116, 178 No studies have explored the role for -2A and -2C subtype serotonin receptors in delay-related decision making.
Pharmacologically-induced decreases in dopamine activity also yield mixed results in delay discounting behavior. Antagonism of both D1 and D2 receptors increases discounting of delayed rewards in some studies, while having no effect in others.210–212 Similarly, increasing dopamine levels via L-DOPA administration has been shown to increase delay discounting; however amphetamine and dopamine reuptake inhibitors, can either decrease or have no effect on delay discounting in rodents and humans, respectively.212–215 Inconsistent relationships between dopamine and delay discounting may be due to individual differences in baseline dopaminergic signaling,216 as differences in baseline availability of dopamine autoreceptors in the substantia nigra/ventral tegmental area predict increased discounting.217 Consistent with its effects on multiple forms of action inhibition, the noradrenaline reuptake inhibitor atomoxetine reduces choice for small immediate rewards in rats.165
3.6. Summary
The proclivity to choose actions that lead to immediate gratification, at the expense of other – arguably more rational and optimal – behaviors is thought to reflect a distinct aspect of impulsivity than do phenotypes linked with impulsive action. The preceding section dealt with the relationship between delay discounting and substance abuse behaviors, as well as the underlying neural circuitry and neuropharmacological mechanisms that mediate this form of impulsive choice. While differences in mechanism between inhibitory response control and delay discounting have been reported, it is also clear that the two dimensions of impulsivity share, at least in part, biological influences. The next section will add to this comparison by considering yet another conceptually distinctive dimension of impulsivity related to risk assessment, preference and aversion.
4. Risk-Related Decision-Making
The risks associated with choices also figure into decision-making. Many decisions that involve procuring a potential reward also engender risks of reward loss or even explicitly negative consequences (punishment). It is of substantial interest why some individuals are prone to make poor quality decisions, sometimes repeatedly and after having directly experienced the negative consequences of their behavior, as in the case of individuals diagnosed with drug or alcohol dependence;48 indeed, risky decision-making may represent a core phenotype associated with substance dependence, and therefore may also be a target for intervention.
4.1. Conventional Tests of Risk-Related Decision-Making
A variable set of tasks have been used to capture behavioral sensitivity to risk (some of which are described in Table 1). These tests all feature choices for high-value reward-seeking in the face of risk of forfeiture or punishment, as compared to choice for low-value reward-seeking that is more certain or “safer”.
The Iowa Gambling Task218 is a game wherein subjects may sequentially choose from any of four decks of cards. Each card is associated with a gain or a loss in points/money. Commonly, two decks yield larger rewards but are also subject to a high rate of large penalties, while the other two yield small rewards but fewer penalties. The payout schedules are arranged such that across the testing session, it is ultimately disadvantageous to select the high reward/penalty decks. The common dependent variable is the difference between the numbers of advantageous versus disadvantageous choices. Generally, control (unaffected) persons start out by selecting the disadvantageous deck but update their behavior appropriately as the task progresses and their experience with the outcomes accumulates.
The Rat Gambling Task219 was designed to mimic the structure of the Iowa Gambling task. In the rat version, animals are given a limited amount of time to select from four options. As with the decks in the Iowa Gambling Task, there are two choices with larger potential rewards (more sugar pellets) but also the chance of a large penalty (a long time-out period). The other two choices deliver smaller rewards, but also have shorter time-out penalties. Since time to perform the task is limited, optimal performance involves selection of the smaller reward but shorter time penalty options.
The Risky Decision-Making Task220, 221 is a rodent task that operates similarly to the gambling task described above but that implements stronger aversive punishment. Here, rats must choose between either a small reward “safe” lever or a large reward “risky” lever, which sometimes results in an electric foot shock.
The Probabilistic Discounting Task222 also requires rats to choose between two levers. The small/certain lever guarantees the delivery of one food reward pellet, while the large/risky lever may deliver four pellets with a given probability. Typically, the probability of reward delivery for the large/risky lever descends across four trial blocks from certain (100%) to unlikely (12.5%), producing a shift in optimal choice across the session and allowing for a parametric assessment of risky decision-making.
The Betting Task223 is designed to assess sensitivity to betting magnitudes when outcomes are actually probabilistically equivalent, a form of irrational choice bias in the face of risk. Here, rats may again choose between safe and risky levers. The latter delivers either twice the value of the safe lever or no reward at 50:50 odds; thus both levers have equal utility, but the size of the “bet” can be varied to identify wager-sensitive and insensitive rats.
The Balloon Analog Risk Task (BART) is a computerized task224 that measures sequential economic risk-taking behavior. In this task, the subject is presented with a picture of a balloon on a computer screen and is given the option to press two buttons. One button inflates (or “pumps”) the balloon, and each inflation results in the accrual of a small amount of reward (monetary or a points system). Subjects may choose to press the other “cash out” button at any time to add the earned rewards to their guaranteed “bank”. With every pump, however, there is a chance that the balloon will burst on the screen, and reward for that trial is forfeited. In the task, optimal performance consists of pumping the balloon enough to maximize reward, while avoiding over-accumulating risk. The main dependent variable studied is the mean number of pumps produced on non-burst trials.
The Rat Balloon Analogue Risk Task225 operates similarly to the human version and is adapted for use in operant boxes. Here rats press on one lever to accumulate food rewards that can be cashed out and received at any time by pressing on a second lever. However, a certain risk is applied such that an additional accumulation press may result in forfeiture of accumulated reward for that trial and a time-out.
The Cambridge Gambling Task226, 227 is a computerized task wherein subjects are asked to place bets on the location of a hidden token. Unlike in other tasks, the odds of guessing correctly are presented to the subject explicitly by varying the ratio of colors among “boxes” that may contain the token, and subjects are free to choose the size of their wager. Common outcomes measured include the speed of decision-making, frequency of making less probable choices, risk tolerance (the mean wager), and risk adjustment (the degree to which subjects vary their wager size based on the parametrically varied explicit odds).
4.2. Relationship to Substance Use Disorders
If the general hypothesis that drug users are more risk-prone is true, quantified levels of risk-taking propensity theoretically should discriminate between drug dependent individuals and controls. Some tasks report this discrimination while others do not in certain populations, possibly due to the nature of optimal performance in the tasks, as will be detailed below.
The Iowa Gambling Task and Cambridge Gambling Task have been used to identify differences in decision-making between individuals with substance use problems and controls. In the Iowa Gambling Task, heavy users of cannabis, stimulants, alcohol tend to suboptimally perform as compared to matched controls by failing to update their choice behavior and instead continuing to select disadvantageous decks across the test session.228–230 This pattern of behavior may be linked with insensitivity to the future consequences of disadvantageous choices231, 232 or insensitivity to negative reinforcement.233 Similarly, in the Cambridge Gambling Task, stimulant, alcohol, marijuana and opiate abusers select suboptimal risky bets and had increased deliberation times in some circumstances compared to healthy controls.227, 234, 235
Data from the Balloon Analogue Risk Task, however, is mixed in terms of its value as predictive of drug use problems. Among young adults and adolescents, greater risk-taking in the BART is associated with increased use of alcohol and other drugs.224, 236, 237 Alternatively, other studies have found that young adult tobacco users take less risk in the BART than non-smoking controls,238 and that among a large sample of adults with alcohol use problems, greater risk-taking in the BART predicted less severe clinical symptomatology.239, 240 Dean et al.238 have suggested that this negative relationship may be due to the risk-taking being confounded with delay discounting since task performance requires persistence and patience; furthermore, unlike the other tasks described here, reduced risk-taking in the BART is, in the economic sense, a suboptimal strategy since diminished risk-taking ultimately results in smaller earnings.
Data from rodent models examining the effects of acute drug-of-abuse exposure on risky decision-making have yielded mixed results. Morphine and ethanol did not have significant effects on behavior in the Risky Decision-Making Task.220 Acute nicotine administration241 and amphetamine222 increased selection of the large/risky lever in the Probabilistic Discounting Task where the risk is for a time-out. On the other hand, a lower dose of nicotine and higher dose range of amphetamine decreased selection of the risky lever in the Risky Decision-Making Task, where subjects risk a foot shock.220, 242 While non-linear effects of drugs on behavior are not atypical, it appears here that the nature of punishment (reward forfeiture versus active shock) may also modulate the effect of these drugs on choice behavior.
4.3. Neural Circuitry of Risk-Related Decision-Making
Interpretation of neuroimaging data obtained from patients and controls during tests of risk-related decision-making is a significant challenge as broad neural networks are involved and the designs of some tasks limit their implementation in event-related fMRI analysis. However, animal models offer the opportunity for controlled investigation of relevant circuitry. For a more detailed review of the neural circuitry implicated in specific components of risk-related decision-making in addiction, see Diekof, Falkai & Gruber.243 Below we outline recent human and rodent data that implicates a frontal-striatal network in regulation of behavior in these tasks; these results are presented graphically in Figure 1.
The Iowa Gambling Task was initially implemented in patients with ventromedial PFC damage;218 these individuals exhibit remarkably poor performance in the task, despite being unaffected in many other intellectual dimensions. Pharmacologic inactivation studies in rats demonstrated the roles of the basolateral amygdala and orbitofrontal cortex in performance of the Rat Gambling Task.244 Functional disconnection studies have also shown the importance of communication between the basolateral amygdala and either the nucleus accumbens or prefrontal cortex in choice behavior in the Probabilistic Discounting Task.245
Similar regions are identified even when probabilities of outcomes are known as in the Cambridge Gambling Task. Here, patients with ventromedial PFC damage consistently bet more than healthy controls, and those with insula damage failed to appropriately decrease their bets as the odds of winning decreased.246 Furthermore, in healthy adolescents, increased risk-taking in the Cambridge Gambling Task is associated with diminished ventral striatal response to reward anticipation235.
Neuroimaging of control and substance-using subjects performing the BART has implicated a partially overlapping network of brain regions. Brain regions implicated in risk acceptance include anterior insula, anterior cingulate, dorsolateral PFC and deactivations of the ventromedial PFC.247–249 Activity in the amygdala was found to promote risk aversion after loss in the BART.249, 250 Pharmacologic inactivation studies in rats have confirmed a role for the ventromedial PFC and orbitofrontal cortex in aspects of risk-taking in the rat-BART.225 Furthermore, striatal D2/D3 dopamine receptor density was negatively correlated with the degree to which dorsolateral PFC activation was modulated by risk-taking,250 highlighting the interaction between these systems in updating potential reward values and guiding goal-directed behavior.
4.4. Pharmacological Regulation of Risk-Related Decision-Making
Data on the neuropharmacological basis of risk-related decision making is emerging; some of the results discussed below are also presented in Table 2 for comparison purposes. The hypothesized role of serotonin in the decision-making process involves its regulation of the affective and behavioral responses to negative feedback.251 Serotonergic depletion produces a pattern of impaired decision-making in the Cambridge Gambling task similar to that observed in substance use disorders.227 On the other hand, a serotonin-1A agonist (8-OH-DPAT) impaired performance in the Rat Gambling Task219 while a selective serotonin reuptake inhibitor (citalopram) had no effect,252 suggesting that the relationship between serotonin and optimal decision-making is not necessarily linear.
Dopamine, however, may influence non-affective aspects of decision-making related to learning and evaluating risk and reward levels. Although the effects are not uniform, pharmacologic stimulation and suppression of the dopamine system using systemic D1 and D2 receptor agonists and antagonists can bias choice behavior in some rodent tasks.219, 221, 222, 252 These effects may involve activity at D1 dopamine receptors (but not D2) in the nucleus accumbens.253 Microdialysis measurement of dopamine efflux during the Probabilistic Discounting Task in the prefrontal cortex and nucleus accumbens respectively suggests that the former encodes relative reward rate or availability while the latter encodes an integration of reward rate, uncertainty and preference, and decision information.254 Additionally, striatal D2/D3 density as assessed by micro-PET in rats was negatively correlated with wager-sensitivity in The Betting Task,223 implicating this system in irrational choice bias in the face of uncertainty. In sum, aggregate data indicates that dopamine signaling in brain regions implicated in decision-making and learning processes influence behavior in these risky decision-making tasks, but the precise mapping of signals at specific receptor subtypes in these networks onto specific decision-making related functions remains to be resolved.
Finally, there have been few studies investigating the influence of the norepinephrine system. In particular, the norepinephrine reuptake inhibitor atomoxetine alone did not alter decision-making in the Rat Gambling Task, but it did increase choice of the disadvantageous lever when combined with a specific dopamine reuptake inhibitor.252
4.5. Summary
Risk-related decision-making appears to predict susceptibility for substance use disorders in humans and addiction-related behaviors in animals, but the progression of drug experience appears to alter these relationships, producing task specific changes in impulsive decision-making. While less is known about the underlying mechanistic basis of risky decision-making, from the neural circuitry and pharmacological perspectives, it is evident that this domain at least partially shares a neural circuitry and neuropharmacology with inhibitory response control and delay discounting (Figure 1; Table 2). A great deal more work needs to be conducted to compare test of risky decision-making to one another and to the other dimensions of impulsivity discussed in sections 2 and 3.
5. Impulsivity in Addiction: Multi-Dimensional?
In the preceding sections, we presented some of the data linking certain manifestations of impulsive behavior (Table 1) to drug-seeking and –taking, and we provided a survey of the involvement of frontostriatal (Figure 1) and monoaminergic mechanisms (Table 2) to these relationships. Perhaps the strongest conclusion to be drawn from this work is that the relationship between impulsivity and addiction-related behaviors is very strong. In human subjects, there appears to be a robust association between both self-report measures of impulsivity, laboratory tests of impulsive behavior and recreational and clinically-impairing patterns of drug and alcohol abuse and dependence. Deficits in action inhibition, waiting, delay discounting and risk-related decision-making are all found in various populations affected by substance use disorders, and burgeoning evidence suggests that some of these deficits (including impairments in action inhibition, delay discounting and risky decision-making) predate the onset of pathological drug-taking (and possibly anticipate all drug use). Research in animal models expands on this data by providing definitive evidence that individual differences in the propensity to engage in impulsive behavior predicts aspects of drug self-administration behaviors, and experience with the pharmacological effects of drugs, over the long-term, can cause abnormalities in action inhibition, waiting, delay discounting and risk-related decision-making. For all these reasons, and despite some inconsistency in the literature, impulsive behaviors do appear to play a key role in the various stages of substance use, abuse and dependence.
As indicated in the introduction, a dominant hypothesis in the field is that there are various forms of impulsive behavior that may each contribute in unique ways to understanding addictions. This hypothesis emerged mostly because of descriptive differences between the purported subtypes (e.g., inhibiting an inappropriate behavior seems conceptually very different than reasoning about a delayed reward or risky outcome). Stronger evidence for this notion might include: 1) independent determination of individual differences in performance of various tests of impulsivity in both humans and animals, 2) unique relationships between each measures of impulsivity and addiction (i.e., each phenotype is predictive of or affected by exposure to drugs of abuse in dissociable ways) and 3) different underlying neurobiological mechanisms that configured each for of impulsive behavior.
Relatively few studies have conducted a systematic effort to examine patterns of co-variation in forms of impulsive behavior in animals or humans, and even fewer have conducted a well-designed study. In rats, two separate studies suggest that action inhibition in the 5CSRTT does not predict delay discounting ability. 255, 256 In human subjects, behavioral measures of action inhibition and delay-related decision-making did not correlate with one another or with subjective self-report measures.255 Caution should be taken with interpreting these results, however, since comparing two phenotypes to one another often magnifies the differences and minimizes the similarities. Moreover, this approach cannot separate differences in task from differences in the construct(s) intended to be measured. Instead, an ideal design for studies of this type involves multiple assessments of both related and unrelated phenotypes. Additionally, inbred rodent or human twin designs are required to separate shared genetic influences that span across tasks from other factors (see256).
The extent to which different phenotypes related to impulsivity uniquely predict liability for addiction-related behaviors has not been entirely explored, but the available data indicates that a number of the measures are linked with a heritable susceptibility for drug use in humans and animals. Tests of action inhibition (stop signal inhibition,257, 5CSRTT83, 84 and reversal learning80), delay discounting32 and risky decision-making258 all appear to have a predictive value, in this regard. While few studies have directly compared their predictive value using standardized procedures, one such study that did suggested that action inhibition and delay discounting predicted dissociable aspects of drug self-administration.84
The evidence that each of these measures predict elevated propensity to self-administer drugs is not consistent with the idea that the tests are uniquely predictive, though clearly more systematic work needs to be done to evaluate the notion that putative subtypes of impulsivity are associated with different dimensions of drug self-administration behaviors, and again, more sophisticated designs must be undertaken before it can be determined whether these correlations are genetically-mediated, and whether shared genetic factors underscore the relationship between different measures of impulsivity and addictions.
The underlying biological mechanisms (both circuitry and molecular mechanisms) necessary for performance of various tests of impulsivity is not identical, but a core set of frontostriatal circuits is repeatedly implicated in various types of impulsivity and decision-making. As shown in Figure 1, there is a distinction between measures of impulsive behavior that rely upon more lateral, orbital regions of the frontal cortex and ventromedial, limbic portions. At the neuropharmacological level, there are also important distinctions, with responses to acute challenge with monoaminergic drugs and/or responses to chronic administration of addictive drugs being task-specific (Table 2).
But one cannot ignore the remarkable similarity, at the same time. For example, it is true that various forms of impulsivity, including 5CSRTT, reversal learning and delay discounting, all predict enhanced acquisition of stimulant self-administration.80, 83, 187–190, 259 Moreover, the neutral circuitry underlying the tasks contains a great deal of overlap, particularly in the medial orbital regions of the frontal cortex and dorsomedial regions of the striatum (Discussed above and summarized in Figure 1). And finally, there is consistency of evidence that low dopamine D2 receptor function and alterations in serotonin -2A and -2C receptors in many forms of impulsivity discussed here.2, 31, 79, 83, 139, 155, 162, 163, 212, 217, 260–265
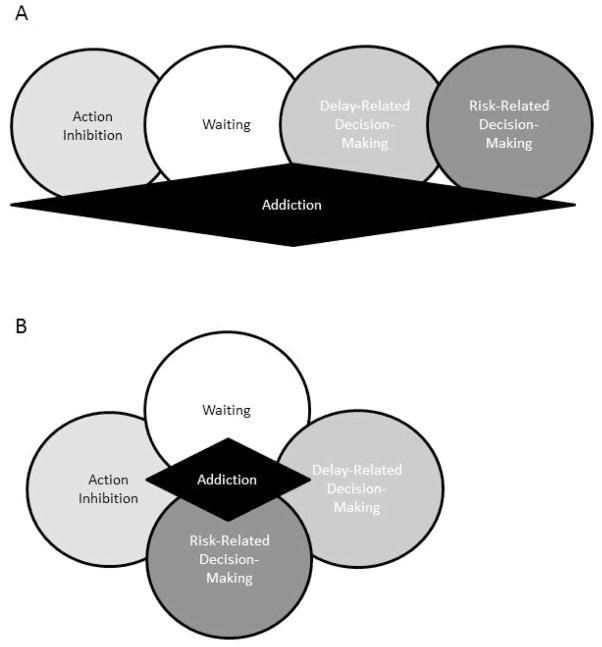
How can one resolve the facts that these arguably separable forms of impulsivity: 1) each have qualitatively similar patterns of predictive value of substance use disorders in humans and drug self-administration in animals, 2) each depend upon partially overlapping neural circuitries and 3) each are sensitive in similar ways to monoaminergic manipulations? Figure 2 shows two potential models that could explain these facts; these two models are certainly not the only possibilities but may be instructive in any case. One model (Figure 2A) suggests that each of the forms of impulsivity share some variance and mechanism with one another but that each shares a unique set of variance and mechanism with addictions. This model says that the correspondences in Figure 2 and Table 2 are instructive about the relationships between forms of impulsivity but not about their individual relationships to addictions. It suggests that other mechanisms, perhaps one unknown to use, explain these individual predictive relationships.
Figure 2. Hypothetical models possibly explaining the relationships between forms of impulsive behavior and addiction.
One model (A) proposes that each manifestation of impulsivity shares unique variance and mechanism with addiction behaviors. Another (B) suggests that these varieties of impulsivity share a small but measureable amount of variance and mechanism between them and that it is this overlap that is shared between them and addictions.
Another model (Figure 2B) suggests that the various forms of impulsivity once again share a portion of their variance and mechanism with one another AND with addiction-related behaviors. It suggests that these tasks may well only share limited amounts of variance and mechanism but that this small similarity is exactly what links them each to addictions. In this case, the brain mechanisms they share in common should also be biomarkers often found in clinical drug addictions. It is notable, in this regard, that relatively low brain dopamine D2 receptor availability is both a biomarker for impulsive tendencies1, 31 and for substance use disorders.155, 266 Moreover, drug addictions have repeatedly been linked to structural and functional abnormalities in the same circuits implicated in impulsivity, namely the ventrolateral frontal cortex, ventromedial frontal cortex and limbic regions of the striatum.54, 266–271 From this perspective, a core set of neuroadaptations involving (perhaps) orbital and ventromedial frontal cortical dysfunction, reductions in brain dopamine D2 receptor signaling and/or altered serotonergic transmission that results from genetic or early environmental mechanisms OR from experience with the pharmacological effects of drugs of abuse result in a pattern of impulsive action and choice that are due to shared mechanism. This is an important hypothesis to test, whether it proves to be correct or incorrect.
6. New Directions for Research on Impulsivity and Addictions
One of the most promising areas of work described above is the research that identifies aspects of impulsive behavior as a quantitative indicator of liability for the initiation of substance use, of its transition to a more compulsive form of substance abuse and of successful treatment of substance dependence. Because impulsivity predates substance use in many circumstances and because impulsivity is itself a heritable trait,272 the discovery of genetic influences on impulsive behavior is crucial. Using human subjects, studies of related individuals (particularly monozygotic and dizygotic twins) can be used to model the genetic relationships between putatively distinct forms of impulsive behaviors and substance use, abuse and dependence. In the pre-clinical laboratory, isogenic rodent strains (inbred rats and mice)256, 273 or pedigreed non-human primates274, 275 can also be used to separate genetic from shared environmental factors. Moreover, advanced lines of mice – including recombinant inbred mice,276, 277 the hybrid mouse diversity panel278 and the diversity outcross279 – are now available for identifying the genetic and genomic factors that may be unique to, or shared between, measures of impulsivity that each predict susceptibility for drug self-administration and addiction-related behaviors. Because of the advent of highly sophisticated genetics resources that can uncover biological influences never before considered, it is crucial that the relationship between dimensions of impulsivity and addictions be dissected at the behavioral and neural circuitry level. By doing so, we may make progress towards understanding the concept of susceptibility for addictions at all levels of analysis – from genes to cells to circuits to behavior.
References and Literature Cited
- 1.Jentsch JD, Pennington ZT. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 3.Fairbanks LA, et al. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. American journal of primatology. 2004;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, et al. The addictive dimensionality of obesity. Biological psychiatry. 2013;73:811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayaki J, Anderson BJ, Stein MD. Sexual risk-taking mediates the association between impulsivity and acquisition of sexually transmitted infections among hazardously drinking incarcerated women. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2012;21(Suppl 1):S63–71. doi: 10.1111/j.1521-0391.2012.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings JA, et al. Male rats that differ in novelty exploration demonstrate distinct patterns of sexual behavior. Behavioral neuroscience. 2013;127:47–58. doi: 10.1037/a0031528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charnigo R, et al. Sensation seeking and impulsivity: combined associations with risky sexual behavior in a large sample of young adults. Journal of sex research. 2013;50:480–488. doi: 10.1080/00224499.2011.652264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes EE, et al. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes JJ, et al. The molecular genetics of executive function: role of monoamine system genes. Biological psychiatry. 2011;69:e127–143. doi: 10.1016/j.biopsych.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? European journal of pharmacology. 2000;410:183–203. doi: 10.1016/s0014-2999(00)00815-3. [DOI] [PubMed] [Google Scholar]
- 11.James AS, et al. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14358–14364. doi: 10.1523/JNEUROSCI.4508-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey JN, et al. The association of DRD4 and novelty seeking is found in a nonhuman primate model. Psychiatric genetics. 2007;17:23–27. doi: 10.1097/YPG.0b013e32801140f2. [DOI] [PubMed] [Google Scholar]
- 13.Fairbanks LA, et al. Maternal and offspring dopamine D4 receptor genotypes interact to influence juvenile impulsivity in vervet monkeys. Psychological science. 2012;23:1099–1104. doi: 10.1177/0956797612444905. [DOI] [PubMed] [Google Scholar]
- 14.Hejjas K, et al. Association of polymorphisms in the dopamine D4 receptor gene and the activity-impulsivity endophenotype in dogs. Animal genetics. 2007;38:629–633. doi: 10.1111/j.1365-2052.2007.01657.x. [DOI] [PubMed] [Google Scholar]
- 15.Wan M, et al. DRD4 and TH gene polymorphisms are associated with activity, impulsivity and inattention in Siberian Husky dogs. Animal genetics. 2013;44:717–727. doi: 10.1111/age.12058. [DOI] [PubMed] [Google Scholar]
- 16.Moeller FG, et al. Psychiatric aspects of impulsivity. The American journal of psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 17.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences. 2014 doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 19.Evenden J. Impulsivity: a discussion of clinical and experimental findings. Journal of psychopharmacology. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- 20.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clinical psychology review. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience and biobehavioral reviews. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins TW, et al. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends in cognitive sciences. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in neurobiology. 2013 doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Swann AC. Impulsivity in mania. Current psychiatry reports. 2009;11:481–487. doi: 10.1007/s11920-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 26.van Reekum R, et al. Impulsivity, defensive functioning, and borderline personality disorder. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 1996;41:81–84. doi: 10.1177/070674379604100204. [DOI] [PubMed] [Google Scholar]
- 27.Links PS, Heslegrave R, van Reekum R. Impulsivity: core aspect of borderline personality disorder. Journal of personality disorders. 1999;13:1–9. doi: 10.1521/pedi.1999.13.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Jentsch JD. Impulsivity in Animal Models for Drug Abuse Disorders. Drug discovery today. Disease models. 2008;5:247–250. doi: 10.1016/j.ddmod.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, biochemistry, and behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 33.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 34.Courtet P, et al. The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies? Translational psychiatry. 2011;1:e7. doi: 10.1038/tp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahlstedt C, Thorell LB, Bohlin G. Heterogeneity in ADHD: neuropsychological pathways, comorbidity and symptom domains. Journal of abnormal child psychology. 2009;37:551–564. doi: 10.1007/s10802-008-9286-9. [DOI] [PubMed] [Google Scholar]
- 36.Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In vivo. 2010;24:761–769. [PubMed] [Google Scholar]
- 37.Coffey SF, et al. Impulsivity and risk-taking in borderline personality disorder with and without substance use disorders. Personality disorders. 2011;2:128–141. doi: 10.1037/a0020574. [DOI] [PubMed] [Google Scholar]
- 38.Karakus G, Tamam L. Impulse control disorder comorbidity among patients with bipolar I disorder. Comprehensive psychiatry. 2011;52:378–385. doi: 10.1016/j.comppsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Fawcett J. Diagnosis, Traits, States, and Comorbidity in Suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- 40.Kooij JJ, et al. Distinguishing comorbidity and successful management of adult ADHD. Journal of attention disorders. 2012;16:3S–19S. doi: 10.1177/1087054711435361. [DOI] [PubMed] [Google Scholar]
- 41.Latalova K, et al. Comorbidity bipolar disorder and personality disorders. Neuro endocrinology letters. 2013;34:1–8. [PubMed] [Google Scholar]
- 42.Winstanley CA, et al. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism, clinical and experimental research. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalley JW, et al. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacology, biochemistry, and behavior. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behavioural brain research. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 46.Belin D, et al. Addiction: failure of control over maladaptive incentive habits. Current opinion in neurobiology. 2013 doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology. 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Association, A. P. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. American Psychiatric Publishing, Inc; Washington, DC: 2000. [Google Scholar]
- 49.Robinson TE, Berridge KC. Addiction. Annual review of psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 50.Winstanley CA. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Annals of the New York Academy of Sciences. 2007;1121:639–655. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- 51.Adinoff B, et al. Impulsivity, neural deficits, and the addictions: the “oops” factor in relapse. J Addict Dis. 2007;26(Suppl 1):25–39. doi: 10.1300/J069v26S01_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain research. Brain research reviews. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 53.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews. Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. British journal of pharmacology. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American journal of psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Groman SM, Jentsch JD. Identifying the molecular basis of inhibitory control deficits in addictions: neuroimaging in non-human primates. Current opinion in neurobiology. 2013;23:625–631. doi: 10.1016/j.conb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of experimental psychology. Human perception and performance. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 60.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 61.O’Donnell JM, Marek GJ, Seiden LS. Antidepressant effects assessed using behavior maintained under a differential-reinforcement-of-low-rate (DRL) operant schedule. Neuroscience & Biobehavioral Reviews. 2005;29:785–798. doi: 10.1016/j.neubiorev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 62.Logan GD, Schachar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8:60–64. [Google Scholar]
- 63.Kooijmans R, Scheres A, Oosterlaan J. Response inhibition and measures of psychopathology: a dimensional analysis. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2000;6:175–184. doi: 10.1076/chin.6.3.175.3154. [DOI] [PubMed] [Google Scholar]
- 64.Aichert DS, et al. Associations between trait impulsivity and prepotent response inhibition. Journal of clinical and experimental neuropsychology. 2012;34:1016–1032. doi: 10.1080/13803395.2012.706261. [DOI] [PubMed] [Google Scholar]
- 65.Franken IHA, et al. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Research. 2008;158:155–163. doi: 10.1016/j.psychres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 67.Monterosso JR, et al. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and alcohol dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Ersche KD, et al. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. The American journal of psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive behaviors. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 70.Lane SD, et al. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am J Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- 71.Acheson A, et al. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug and alcohol dependence. 2011;117:198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S, Heitz RP, Bradberry CW. A touch screen based Stop Signal Response Task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. Journal of neuroscience methods. 2009;177:67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ersche KD, et al. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghahremani DG, et al. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. Journal of Psychopharmacology. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- 76.Izquierdo A, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jentsch JD, et al. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 78.Schoenbaum G, et al. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. The European journal of neuroscience. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 79.Laughlin RE, et al. Genetic dissection of behavioral flexibility: reversal learning in mice. Biological psychiatry. 2011;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cervantes MC, Laughlin RE, Jentsch JD. Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voon V, et al. Measuring “Waiting” Impulsivity in Substance Addictions and Binge Eating Disorder in a Novel Analogue of Rodent Serial Reaction Time Task. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubio G, et al. Varieties of impulsivity in males with alcohol dependence: the role of Cluster-B personality disorder. Alcoholism, clinical and experimental research. 2007;31:1826–1832. doi: 10.1111/j.1530-0277.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 83.Dalley JW, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diergaarde L, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biological psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 85.Dalley Jeffrey W, Everitt Barry J, Robbins Trevor W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 86.Diergaarde L, et al. Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose-associated stimuli. Behavioral Neuroscience. 2009;123:794–803. doi: 10.1037/a0016504. [DOI] [PubMed] [Google Scholar]
- 87.Dalley JW, et al. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- 88.Dalley JW, et al. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology. 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- 89.Caprioli D, et al. Baseline-dependent effects of cocaine pre-exposure on impulsivity and D2/3 receptor availability in the rat striatum: possible relevance to the attention-deficit hyperactivity syndrome. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1460–1471. doi: 10.1038/npp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balcells-Olivero M, Richards JB, Seiden LS. Sensitization to amphetamine on the differential-reinforcement-of-low-rate 72-s schedule. Psychopharmacology. 1997;133:207–213. doi: 10.1007/s002130050393. [DOI] [PubMed] [Google Scholar]
- 91.Lobarinas E, Lau CE, Falk JL. Sensitization of operant behavior to oral cocaine with increasing- and repetitive-dose regimens. Behavioural pharmacology. 1999;10:15–26. doi: 10.1097/00008877-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 92.Sabol KE, Richards JB, Yung K. The effects of high-dose methamphetamine in the aging rat: differential reinforcement of low-rate 72-s schedule behavior and neurochemistry. The Journal of pharmacology and experimental therapeutics. 2000;294:850–863. [PubMed] [Google Scholar]
- 93.Kirshenbaum AP, et al. Differential-reinforcement-of-low-rate-schedule performance and nicotine administration: a systematic investigation of dose, dose-regimen, and schedule requirement. Behavioural pharmacology. 2008;19:683–697. doi: 10.1097/FBP.0b013e328315ecbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peterson JD, Wolf ME, White FJ. Impaired DRL 30 performance during amphetamine withdrawal. Behavioural Brain Research. 2003;143:101–108. doi: 10.1016/s0166-4328(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 95.Aron AR, et al. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 96.Eagle DM, et al. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cerebral cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 97.Eagle DM, Robbins TW. Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behavioural Brain Research. 2003;146:131–144. doi: 10.1016/j.bbr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 98.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 99.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aron AR, Poldrack RA. The Cognitive Neuroscience of Response Inhibition: Relevance for Genetic Research in Attention-Deficit/Hyperactivity Disorder. Biological psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Rubia K, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 102.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 103.D’Cruz AM, et al. Human reversal learning under conditions of certain versus uncertain outcomes. NeuroImage. 2011;56:315–322. doi: 10.1016/j.neuroimage.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghahremani DG, et al. Neural components underlying behavioral flexibility in human reversal learning. Cerebral cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chudasama Y, et al. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural Brain Research. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 106.Muir JL, Everitt BJ, Robbins TW. The Cerebral Cortex of the Rat and Visual Attentional Function: Dissociable Effects of Mediofrontal, Cingulate, Anterior Dorsolateral, and Parietal Cortex Lesions on a Five-Choice Serial Reaction Time Task. Cerebral cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- 107.Passetti F, Chudasama Y, Robbins TW. The Frontal Cortex of the Rat and Visual Attentional Performance: Dissociable Functions of Distinct Medial Prefrontal Subregions. Cerebral cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- 108.Murphy ER, et al. Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. European Journal of Neuroscience. 2008;28:353–363. doi: 10.1111/j.1460-9568.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rogers RD, et al. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behavioral neuroscience. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- 110.Baunez C, Robbins TW. Bilateral Lesions of the Subthalamic Nucleus Induce Multiple Deficits in an Attentional Task in Rats. European Journal of Neuroscience. 1997;9:2086–2099. doi: 10.1111/j.1460-9568.1997.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 111.Neill DB. Frontal-striatal control of behavioral inhibition in the rat. Brain research. 1976;105:89–103. doi: 10.1016/0006-8993(76)90925-2. [DOI] [PubMed] [Google Scholar]
- 112.Cho YH, Jeantet Y. Differential involvement of prefrontal cortex, striatum, and hippocampus in DRL performance in mice. Neurobiology of learning and memory. 2010;93:85–91. doi: 10.1016/j.nlm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 113.Clark CV, Isaacson RL. Effect of bilateral hippocampal ablation on DRL performance. Journal of comparative and physiological psychology. 1965;59:137–140. doi: 10.1037/h0021599. [DOI] [PubMed] [Google Scholar]
- 114.Glick SD, Cox RD. Differential effects of unilateral and bilateral caudate lesions on side preferences and timing behavior in rats. Journal of comparative and physiological psychology. 1976;90:528–535. doi: 10.1037/h0077234. [DOI] [PubMed] [Google Scholar]
- 115.Pothuizen HHJ, et al. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. European Journal of Neuroscience. 2005;22:2605–2616. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- 116.Winstanley CA, et al. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 117.Harrison AA, Everitt BJ, Robbins TW. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behavioural brain research. 1999;100:99–112. doi: 10.1016/s0166-4328(98)00117-x. [DOI] [PubMed] [Google Scholar]
- 118.Clarke HF, et al. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 119.Clarke HF, et al. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lapiz-Bluhm MD, et al. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clarke H, et al. Cognitive Inflexibility after Prefrontal Serotonin Depletion Is Behaviorally and Neurochemically Specific. Cerebral cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 122.Bari A, et al. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology. 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clark L, et al. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology. 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 124.Eagle DM, et al. Serotonin depletion impairs waiting but not stop-signal reaction time in rats: implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology. 2009;34:1311–1321. doi: 10.1038/npp.2008.202. [DOI] [PubMed] [Google Scholar]
- 125.Brigman JL, et al. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cerebral cortex. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nonkes LJ, et al. Serotonin transporter knockout rats show improved strategy set-shifting and reduced latent inhibition. Learning & memory. 2012;19:190–193. doi: 10.1101/lm.025908.112. [DOI] [PubMed] [Google Scholar]
- 127.Nonkes LJ, Maes JH, Homberg JR. Improved cognitive flexibility in serotonin transporter knockout rats is unchanged following chronic cocaine self-administration. Addiction biology. 2013;18:434–440. doi: 10.1111/j.1369-1600.2011.00351.x. [DOI] [PubMed] [Google Scholar]
- 128.Homberg JR, et al. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. European Journal of Neuroscience. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- 129.Izquierdo A, et al. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vallender EJ, et al. Polymorphisms in the 3′ UTR of the serotonin transporter are associated with cognitive flexibility in rhesus macaques. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B:467–475. doi: 10.1002/ajmg.b.30835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 132.Nilsson SR, et al. Reduced activity at the 5-HT(2C) receptor enhances reversal learning by decreasing the influence of previously non-rewarded associations. Psychopharmacology. 2012;224:241–254. doi: 10.1007/s00213-012-2746-5. [DOI] [PubMed] [Google Scholar]
- 133.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats’ performance differently in a five-choice serial reaction time task. Psychopharmacology. 1992;106:228–234. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- 135.Baarendse PJ, Vanderschuren LMJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology. 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- 137.Carli M, Samanin R. The 5-HT1A receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: Role of presynaptic 5-HT1A receptors. Psychopharmacology. 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- 138.Fletcher PJ, et al. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology. 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 139.Winstanley CA, et al. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- 140.Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. Journal of Psychopharmacology. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- 141.Fletcher PJ. Effects of combined or separate 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei on responding maintained by a DRL 20s schedule of food reinforcement. Brain research. 1995;675:45–54. doi: 10.1016/0006-8993(95)00037-q. [DOI] [PubMed] [Google Scholar]
- 142.Marek GJ, Li AA, Seiden LS. Evidence for involvement of 5-hydroxytryptamine1 receptors in antidepressant-like drug effects on differential-reinforcement-of-low-rate 72-second behavior. Journal of Pharmacology and Experimental Therapeutics. 1989;250:60–71. [PubMed] [Google Scholar]
- 143.Marek GJ, Seiden LS. Effects of selective 5-hydroxytryptamine-2 and nonselective 5-hydroxytryptamine antagonists on the differential-reinforcement-of-low-rate 72-second schedule. Journal of Pharmacology and Experimental Therapeutics. 1988;244:650–658. [PubMed] [Google Scholar]
- 144.Martin JR, et al. 5-HT2C Receptor Agonists: Pharmacological Characteristics and Therapeutic Potential. Journal of Pharmacology and Experimental Therapeutics. 1998;286:913–924. [PubMed] [Google Scholar]
- 145.Ardayfio PA, et al. The 5-Hydroxytryptamine2A Receptor Antagonist R-(+)-α-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) Attenuates Impulsivity after Both Drug-Induced Disruption (Dizocilpine) and Enhancement (Antidepressant Drugs) of Differential-Reinforcement-of-Low-Rate 72-s Behavior in the Rat. Journal of Pharmacology and Experimental Therapeutics. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- 146.Eagle DM, et al. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nandam LS, et al. Dopamine D(2) receptor modulation of human response inhibition and error awareness. Journal of cognitive neuroscience. 2013;25:649–656. doi: 10.1162/jocn_a_00327. [DOI] [PubMed] [Google Scholar]
- 148.Ghahremani DG, et al. Striatal dopamine D/D receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee B, et al. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- 150.Kruzich PJ, et al. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cognitive, affective & behavioral neuroscience. 2006;6:86–90. doi: 10.3758/cabn.6.1.86. [DOI] [PubMed] [Google Scholar]
- 151.Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- 152.Izquierdo A, et al. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behavioural brain research. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 153.Groman SM, et al. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jocham G, et al. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Volkow ND, et al. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Groman SM, et al. Monoamine levels within the orbitofrontal cortex and putamen interact to predict reversal learning performance. Biological psychiatry. 2013;73:756–762. doi: 10.1016/j.biopsych.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- 158.Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: Implications for theories of selective attention and arousal. Behavioural Brain Research. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- 159.Pattij T, et al. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology. 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- 160.Moreno M, et al. Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1198–1208. doi: 10.1038/npp.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernando AP, et al. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology. 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moreno M, et al. Divergent effects of D2/3 receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology. 2013;228:19–30. doi: 10.1007/s00213-013-3010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Besson M, et al. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cheng RK, Liao RM. Dopamine receptor antagonists reverse amphetamine-induced behavioral alteration on a differential reinforcement for low-rate (DRL) operant task in the rat. The Chinese journal of physiology. 2007;50:77–88. [PubMed] [Google Scholar]
- 165.Robinson ES, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- 166.Chamberlain SR, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biological psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 167.Chamberlain SR, et al. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bari A, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Seu E, Jentsch JD. Effect of acute and repeated treatment with desipramine or methylphenidate on serial reversal learning in rats. Neuropharmacology. 2009;57:665–672. doi: 10.1016/j.neuropharm.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Seu E, et al. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology. 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci. 1997;111:883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- 172.Economidou D, et al. Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bickel WK, et al. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug and alcohol dependence. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Madden GJ, et al. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and clinical psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- 176.Mazur JE, Coe D. Tests of transitivity in choices between fixed and variable reinforcer delays. J Exp Anal Behav. 1987;47:287–297. doi: 10.1901/jeab.1987.47-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 178.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 179.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 180.de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebraska Symposium on Motivation. Nebraska Symposium on Motivation. 2004;50:19–55. [PubMed] [Google Scholar]
- 181.Baumann AA, Odum AL. Impulsivity, risk taking, and timing. Behavioural processes. 2012;90:408–414. doi: 10.1016/j.beproc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bickel WK, et al. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Acheson A, et al. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcoholism, clinical and experimental research. 2011;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kollins SH. Delay discounting is associated with substance use in college students. Addictive behaviors. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 185.Audrain-McGovern J, et al. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perry JL, et al. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacology, biochemistry, and behavior. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behavioural pharmacology. 1995;6:810–814. [PubMed] [Google Scholar]
- 188.Perry JL, et al. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 189.Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Anker JJ, et al. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology, biochemistry, and behavior. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gipson CD, Bardo MT. Extended access to amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology. 2009;207:391–400. doi: 10.1007/s00213-009-1667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mendez IA, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124:470–477. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xie X, et al. Role of nicotinic acetylcholine receptors in the effects of cocaine-paired contextual stimuli on impulsive decision making in rats. Psychopharmacology. 2012;223:271–279. doi: 10.1007/s00213-012-2715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron. 2008;59:161–172. doi: 10.1016/j.neuron.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kalenscher T, et al. Single units in the pigeon brain integrate reward amount and time-to-reward in an impulsive choice task. Current biology : CB. 2005;15:594–602. doi: 10.1016/j.cub.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 196.Sripada CS, et al. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Human brain mapping. 2011;32:1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:16429–16436. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cardinal RN, et al. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 199.Mobini S, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 200.Winstanley CA, et al. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Boettiger CA, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pine A, et al. Encoding of marginal utility across time in the human brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9575–9581. doi: 10.1523/JNEUROSCI.1126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hoffman WF, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Figner B, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nature neuroscience. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 205.Cheung TH, Cardinal RN. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC neuroscience. 2005;6:36. doi: 10.1186/1471-2202-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Peters J, Buchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in cognitive sciences. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 207.Denk F, et al. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- 208.Bizot J, et al. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400–412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- 209.Poulos CX, Parker JL, Le AD. Dexfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption. Behavioural pharmacology. 1996;7:395–399. doi: 10.1097/00008877-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 210.Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. Journal of clinical psychopharmacology. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- 211.Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- 212.van Gaalen MM, et al. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biological psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 213.Isles AR, Humby T, Wilkinson LS. Measuring impulsivity in mice using a novel operant delayed reinforcement task: effects of behavioural manipulations and d-amphetamine. Psychopharmacology. 2003;170:376–382. doi: 10.1007/s00213-003-1551-6. [DOI] [PubMed] [Google Scholar]
- 214.Pine A, et al. Dopamine, time, and impulsivity in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Experimental and clinical psychopharmacology. 2008;16:113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yacubian J, et al. Gene-gene interaction associated with neural reward sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Buckholtz JW, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bechara A, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 219.Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- 220.Mitchell MR, et al. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Simon NW, et al. Dopaminergic modulation of risky decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- 223.Cocker PJ, et al. Irrational choice under uncertainty correlates with lower striatal D(2/3) receptor binding in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15450–15457. doi: 10.1523/JNEUROSCI.0626-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lejuez CW, et al. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 225.Jentsch JD, et al. Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharmacology. 2010;35:1797–1806. doi: 10.1038/npp.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Deakin J, et al. Risk taking during decision-making in normal volunteers changes with age. J Int Neuropsychol Soc. 2004;10:590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- 227.Rogers RD, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 228.Bechara A, et al. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 229.van der Plas EAA, et al. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. Journal of clinical and experimental neuropsychology. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Verdejo-Garcia A, et al. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cantrell H, et al. Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcoholism, clinical and experimental research. 2008;32:1398–1407. doi: 10.1111/j.1530-0277.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stout JC, et al. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- 233.Thompson LL, et al. Negative reinforcement learning is affected in substance dependence. Drug Alcohol Depend. 2012;123:84–90. doi: 10.1016/j.drugalcdep.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fishbein DH, et al. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain research. Cognitive brain research. 2005;23:119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 235.Schneider S, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. The American journal of psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 236.Fernie G, et al. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug and Alcohol Dependence. 2010;112:54–61. doi: 10.1016/j.drugalcdep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 237.Lejuez CW, et al. Differences in impulsivity and sexual risk behavior among inner-city crack/cocaine users and heroin users. Drug Alcohol Depend. 2005;77:169–175. doi: 10.1016/j.drugalcdep.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 238.Dean AC, et al. Is all risk bad? Young adult cigarette smokers fail to take adaptive risk in a laboratory decision-making test. Psychopharmacology. 2011;215:801–811. doi: 10.1007/s00213-011-2182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ashenhurst JR, Jentsch JD, Ray LA. Risk-Taking and Alcohol Use Disorders Symptomatology in a Sample of Problem Drinkers. Experimental and clinical psychopharmacology. 2011;19:361–370. doi: 10.1037/a0024412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Courtney KE, et al. The relationship between measures of impulsivity and alcohol misuse: an integrative structural equation modeling approach. Alcoholism, clinical and experimental research. 2012;36:923–931. doi: 10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mendez IA, et al. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology. 2012;224:489–499. doi: 10.1007/s00213-012-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Simon NW, et al. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 244.Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2197–2204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.St Onge JR, et al. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Clark L, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain : a journal of neurology. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schonberg T, et al. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an FMRI investigation of the balloon analog risk task. Frontiers in neuroscience. 2012;6:80. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fecteau S, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. Journal of Neuroscience. 2007;27:6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Galván A, et al. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kohno M, et al. Risk-Taking Behavior: Dopamine D2/D3 Receptors, Feedback, and Frontolimbic Activity. Cerebral cortex. 2013 doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cools R, Roberts A, Robbins TW. Serotoninergic regulation of emotional and behavioural control processess. Trends in Cognitive Science. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 252.Baarendse PJ, Winstanley CA, Vanderschuren LJ. Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology. 2013;225:719–731. doi: 10.1007/s00213-012-2857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:715–728. doi: 10.1038/npp.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.St Onge JR, et al. Dynamic fluctuations in dopamine efflux in the prefrontal cortex and nucleus accumbens during risk-based decision making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:16880–16891. doi: 10.1523/JNEUROSCI.3807-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Broos N, et al. The relationship between impulsive choice and impulsive action: a cross-species translational study. PloS one. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Richards JB, et al. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes, brain, and behavior. 2013;12:490–502. doi: 10.1111/gbb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ersche KD, et al. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 258.Lejuez CW, et al. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcoholism, clinical and experimental research. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Belin D, et al. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lee B, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Simon NW, et al. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. The European journal of neuroscience. 2013;37:1779–1788. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Anastasio NC, et al. Serotonin (5-hydroxytryptamine) 5-HT(2A) receptor: association with inherent and cocaine-evoked behavioral disinhibition in rats. Behavioural pharmacology. 2011;22:248–261. doi: 10.1097/FBP.0b013e328345f90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ardayfio PA, et al. The 5-hydroxytryptamine2A receptor antagonist R-(+)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. The Journal of pharmacology and experimental therapeutics. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- 264.Pattij T, et al. Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behavioural brain research. 2003;141:137–145. doi: 10.1016/s0166-4328(02)00345-5. [DOI] [PubMed] [Google Scholar]
- 265.Winstanley CA, et al. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 266.Volkow ND, et al. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of learning and memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 267.Goldstein RZ, et al. The neurocircuitry of impaired insight in drug addiction. Trends in cognitive sciences. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Thompson PM, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.London ED, et al. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 270.London ED. Studying addiction in the age of neuroimaging. Drug and alcohol dependence; Marian W. Fischman Lecture given at the 2008 meeting of CPDD; 2009. pp. 182–185. [DOI] [PubMed] [Google Scholar]
- 271.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 272.Niv S, et al. Heritability and longitudinal stability of impulsivity in adolescence. Behavior genetics. 2012;42:378–392. doi: 10.1007/s10519-011-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dudek BC, Underwood KA. Selective breeding, congenic strains, and other classical genetic approaches to the analysis of alcohol-related polygenic pleiotropisms. Behavior genetics. 1993;23:179–189. doi: 10.1007/BF01067423. [DOI] [PubMed] [Google Scholar]
- 274.Jasinska AJ, et al. A non-human primate system for large-scale genetic studies of complex traits. Human molecular genetics. 2012;21:3307–3316. doi: 10.1093/hmg/dds160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jasinska AJ, et al. Systems biology of the vervet monkey. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2013;54:122–143. doi: 10.1093/ilar/ilt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Threadgill DW, et al. The collaborative cross: a recombinant inbred mouse population for the systems genetic era. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2011;52:24–31. doi: 10.1093/ilar.52.1.24. [DOI] [PubMed] [Google Scholar]
- 277.Crabbe JC, et al. Use of recombinant inbred strains for studying genetic determinants of responses to alcohol. Alcohol Alcohol Suppl. 1994;2:67–71. [PubMed] [Google Scholar]
- 278.Ghazalpour A, et al. Hybrid mouse diversity panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mammalian genome : official journal of the International Mammalian Genome Society. 2012;23:680–692. doi: 10.1007/s00335-012-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chesler EJ. Out of the bottleneck: the Diversity Outcross and Collaborative Cross mouse populations in behavioral genetics research. Mammalian genome : official journal of the International Mammalian Genome Society. 2013 doi: 10.1007/s00335-013-9492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.O’Donnell JM, Marek GJ, Seiden LS. Antidepressant effects assessed using behavior maintained under a differential-reinforcement-of-low-rate (DRL) operant schedule. Neuroscience and biobehavioral reviews. 2005;29:785–798. doi: 10.1016/j.neubiorev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 281.Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology. 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- 282.Bechara A, et al. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends in cognitive sciences. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154. [DOI] [PubMed] [Google Scholar]
- 283.de Visser L, et al. Rodent versions of the iowa gambling task: opportunities and challenges for the understanding of decision-making. Frontiers in neuroscience. 2011;5:109. doi: 10.3389/fnins.2011.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Floresco SB, et al. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, affective & behavioral neuroscience. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- 285.Lejuez CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]