Abstract
Benzene is a ubiquitous chemical in our environment that causes acute leukemia and probably other hematological cancers. Evidence for an association with childhood leukemia is growing. Exposure to benzene can lead to multiple alterations that contribute to the leukemogenic process, indicating a multimodal mechanism of action. Research is needed to elucidate the different roles of multiple metabolites in benzene toxicity and the pathways that lead to their formation. Studies to date have identified a number of polymorphisms in candidate genes that confer susceptibility to benzene hematotoxicity. However, a genome-wide study is needed to truly assess the role of genetic variation in susceptibility. Benzene affects the blood-forming system at low levels of occupational exposure, and there is no evidence of a threshold. There is probably no safe level of exposure to benzene, and all exposures constitute some risk in a linear, if not supralinear, and additive fashion.
Keywords: leukemia, hematology, molecular epidemiology, genetic polymorphism, risk assessment
INTRODUCTION
Benzene is widely used in the United States and ranks in the top 20 chemicals for production volume (see ATSDR Toxicological Profile of Benzene, http://www.atsdr.cdc.gov/toxprofiles/tp3.pdf). It is the primary starting material for chemicals used to make plastics, resins, synthetic fibers, dyes, detergents, drugs, and pesticides. Natural sources of benzene include emissions from fires. Benzene is also a component of crude oil, gasoline, and cigarette smoke. Occupational exposures in the developing world are sometimes very high because of the continuing presence of benzene in industrial solvents and glues. In the United States, workers continue to be exposed to potentially high levels of benzene in the chemical industry, in petroleum refineries, in oil pipelines, on ships and tankers, in auto repair shops, and in bus garages. Shipping may be particularly hazardous because there is little awareness or regulation, and exposures can be considerable. For example, on marine vessels benzene air concentrations typically range from 0.2–2.0 ppm during closed loading and 2–10 ppm during open-loading operations (121). The general public is exposed mainly from mobile sources, such as automobiles. The benzene content of gasoline is, therefore, strictly regulated in the United States and Europe, with limits typically around 1%. The U.S. Environmental Protection Agency (EPA) recently set new regulations that will lower the benzene content in gasoline to 0.62% in 2011 (20).
HISTORICAL OVERVIEW OF THE HEMATOTOXIC EFFECTS OF BENZENE
Benzene is the simplest aromatic chemical and an excellent solvent. Its toxicity to the blood-forming organs was realized soon after its industrial use began. In 1897, Santesson described nine cases of chronic benzene hematotoxicity (88). The hematotoxic effects of benzene were further documented in studies by Selling (90) and Weiskotten (114, 115). This research led Alice Hamilton (35) and others to warn about the occupational dangers of benzene (98).
The first case of benzene-associated leukemia was described by Delore & Borgomano in 1928 (16). Many leukemia cases associated with benzene exposure were reported between 1930 and the 1960s (3, 4, 30, 109), and by 1961 benzene had been identified as one of two industrial leukemogens, the other being ionizing radiation (15). Reports of multiple cases of leukemia and other hematological disorders among shoe workers using benzene as a solvent and in glues were generated by Vigliani and colleagues in Italy (23) and by Aksoy and coworkers in Turkey in the 1960s and 1970s (1, 2), confirming the association with leukemia.
TRADITIONAL EPIDEMIOLOGICAL STUDIES OF THE CARCINOGENIC EFFECTS OF BENZENE EXPOSURE
It was not until 1977 that the first positive finding of increased leukemia risk in an epi-demiological cohort study of workers in the U.S. rubber industry was published, by Infante et al. (43). They reported that workers occupationally exposed to benzene between 1940 and 1949 had at least a fivefold excess risk of all leukemias and a tenfold excess of deaths from myeloid and monocytic leukemias combined compared with controls. The environment of the workers in the study population was not contaminated with solvents other than benzene, showing that benzene must be the cause. This study became known as the Pliofilm study because it investigated workers exposed to benzene in rubber hydrochloride (the Goodyear trade name for which was Pliofilm) manufacturing plants in Ohio. Subsequent follow-ups of this cohort were published by Rinsky and coworkers, with the most recent being in 2002, which reaffirmed the leukemogenic effects of benzene exposure in this cohort (83, 84). Because of its importance as the first epidemiological study to provide quantitative estimates of leukemia risk from benzene exposure, as well as its role in the lowering of the Occupational Safety and Health Administration (OSHA) permissible exposure level to 1 ppm, the Pliofilm study has been the subject of much reanalysis by consultants to the oil and chemical industry (e.g., 73, 74) with the intention of influencing regulatory or legal proceedings as described in detail by Michaels (68). However, subsequent studies in China and Australia have confirmed and expanded on its findings, as described below.
After President Nixon’s visit to China in 1972, China became much more open to trade with the west and became much more industrialized. The manufacturing of shoes and leather goods increased dramatically, along with exposure to benzene through its use as a solvent and as a contaminant in glues. Reports of significant health problems associated with benzene in workplaces in China soon began to appear. These reports led to pioneering studies of benzene-exposed workers in China by Songnian Yin and colleagues of the Chinese Academy of Preventive Medicine (CAPM), who identified more than 500,000 workers exposed to benzene (124). A follow-up survey of 28,460 benzene-exposed and 28,257 unexposed workers from 1972 through 1981 found an increased risk of mortality due to leukemia [standardized mortality ratio (SMR) = 5.7] (123). In 1987, the U.S. National Cancer Institute (NCI) began collaborating with the CAPM team to identify all incident cases of hematologic neoplasms and related disorders in an expanded study cohort of 74,828 benzene-exposed and 35,805 unexposed workers employed from 1972 through 1987 in 12 cities in China (37, 122). The study confirmed increased risks of acute myeloid leukemia (AML) and other malignant and non-malignant hematopoietic disorders associated with benzene exposure and found evidence for hematopoietic cancer risks at levels substantially lower than had previously been established. In contrast to the findings among rubber hydrochloride workers, the NCI-CAPM study showed excess risk at relatively low levels of exposure (<10 ppm average and <40 ppm-years cumulative) but found a relatively modest dose-response effect, with proportionally smaller increases in risk at increasing levels of exposure. The study also reported that workers with 10 or more years of benzene exposure had a relative risk (RR) of developing non-Hodgkin lymphoma (NHL) of 4.2 [95% confidence interval (CI) 1.1–15.9] and an excess risk of myelodysplastic syndromes (MDS) (36, 37, 104). This study considerably expanded the health effects associated with benzene beyond AML and suggested benzene produced effects at levels lower than previously thought. It has again been the subject of much criticism by industry consultants to which the NCI-CAPM investigators have responded (36). They will soon report on an additional 10 years of follow-up through 1997.
Glass and coworkers performed a nested case-control study of lympho-hematopoietic cancer nested within the existing Healthwatch cohort study to examine the role of benzene exposure (26, 28, 36). Cases identified between 1981 and 1999 (n = 79) were age-matched to five control subjects from the cohort. Each subject’s benzene exposure was estimated using occupational histories, local site-specific information, and an algorithm using Australian petroleum industry–monitoring data. This exposure assessment is probably the best of any epidemiological study of benzene to date (25, 27). Matched analyses showed that the risk of leukemia was increased at cumulative exposures above 2 ppm-years and with intensity of exposure of highest exposed job more than 0.8 ppm. Risk increased with higher exposures; for the 13 case-sets with greater than 8 ppm-years cumulative exposure, the odds ratio (OR) was 11.3 (95% CI 2.85–45.1). The risks for acute nonlymphocytic leukemia (ANLL) and chronic lymphocytic leukemia (CLL) were raised for the highest exposed workers. A cumulative exposure of >8 ppm-years was associated with a sevenfold significantly increased risk specifically of ANLL. No association was found between NHL or multiple myeloma and benzene exposure, but this finding may have been due to limited follow-up. The Glass et al. study is important because it found an excess risk of leukemia associated with cumulative benzene exposures and benzene exposure intensities that were considerably lower than reported in previous studies. Furthermore, no evidence was found of a threshold cumulative exposure below which there was no risk. However, it has been suggested that the high incidence of CLL may be due to a surveillance bias (29).
Apart from these three important studies, there have been many other epidemiological studies of the carcinogenicity of benzene, which are too numerous to review here. For reviews, see recent articles in References 10, 48, and 120. The consensus clearly shows that benzene causes AML/ANLL and MDS, even at relatively low doses, and that AML often arises secondary to MDS. However, a series of questions important to the risk assessment of benzene remain (Table 1).
Table 1.
Current issues in the risk assessment of benzene
| Is it only acute myeloid leukemia that is produced by benzene? |
| What is the mechanism(s) of benzene carcinogenicity? |
| Are there susceptible subpopulations? |
| What is the dose-response curve? Is it linear, and is there a functional threshold? |
IS IT ONLY ACUTE MYELOID LEUKEMIA THAT IS PRODUCED BY BENZENE?
The evidence for other forms of leukemia apart from AML being caused by benzene exposure as well as different forms of NHL has grown steadily over the years. Lymphomas were reported long ago in experimental animals given long-term exposure to benzene. Because all leukemias arise in the stem and progenitor cells of the bone marrow, which are clearly damaged by benzene, there is a biologically plausible basis for suggesting benzene as a causal factor for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML). Some studies of benzene-exposed workers have reported such an increased risk, but the assessment of the association of benzene with these malignancies is hampered mainly by their rarity and is certainly stronger for ALL than CML. Several epidemiological studies, including the above study by Glass et al. (26), have reported an association between benzene exposure and CLL. The main problems in assessing the risk of CLL are the different disease classifications used by investigators over time, the fact that the disease is present with only very low incidence in Asians, and the lack of specific information on CLL in most studies. CLL is now classified as a form of NHL along with multiple myeloma because they are now considered subclassifications of mature B-cell neoplasms (107). Mechanistic and molecular epidemiology studies may contribute to our understanding of the association of benzene with these neoplasms. For example, both CLL and multiple myeloma have precursor forms: Almost all CLL patients are preceded by a monoclonal B-cell lymphocytosis precursor state (54), and monoclonal gammopathy of undetermined significance (MGUS) is a common precursor to myeloma (116). Demonstration that these precursors were elevated in benzene-exposed populations would add support to the hypothesis that benzene was causatively linked to CLL and myelomagenesis, as has recently been shown for certain pesticides (55).
Epidemiological studies on the association between benzene and NHL have produced mixed results. For example, in the NCI-CAPM cohort study discussed above, a relative risk of 4.7 (95% CI 1.2 to 18.1) for NHL was reported (37). In contrast, Sorahan et al. reported a relative risk of 1.00 for NHL in a cohort study of benzene-exposed workers in England and Wales (100). The reasons for these discrepancies are not entirely clear but could be related to differences in study populations, exposure levels, lack of statistical power, and study designs leading to biases such as the healthy worker effect. We systematically reviewed the evidence relating to benzene and NHL and noted problems of bias due to the healthy worker effect (95). We performed formal meta-analysis of studies of NHL and occupational exposure to benzene in work settings other than refineries and formal meta-analysis of NHL and refinery work, a setting that has historically been associated with benzene exposure (101). These were done separately because refinery work can be associated with many chemical exposures other than benzene. In 22 studies of benzene exposure, the summary relative risk for NHL was 1.22 (95% CI 1.02 to 1.47; p = 0.01). When studies that likely included unexposed subjects in the exposed group were excluded, the summary relative risk increased to 1.49 (95% CI 1.12 to 1.97, n = 13), and when studies based solely on self-reported work history were excluded, the relative risk rose to 2.12 (95% CI 1.11 to 4.02, n = 6). In refinery workers, the summary relative risk for NHL in all 21 studies was 1.21 (95% CI 1.00 to 1.46; p = 0.02). When adjusted for the healthy worker effect, this relative risk estimate increased to 1.42 (95% CI 1.19 to 1.69) (101). The finding of elevated relative risks in studies of both benzene exposure and refinery work provides further evidence that benzene exposure is associated with an increased risk of NHL. There are many similarities between cancer chemotherapy drugs and benzene in their abilities to produce both AML and NHL. Both appear to be highly efficient at producing AML with high relative risks and both also produce NHL, but with lower relative risks and a longer latency period than for AML (49). The lower relative risks observed may be due to the fact that NHL is a diverse set of tumors and that benzene and chemotherapy drugs produce only certain subtypes of NHL.
German researchers have concluded that benzene could cause any malignant hemato-logic disease because these diseases all arise from damaged omnipotent stem cells (9). More recently, Beelte et al. convened a committee of experts to evaluate the international literature (10). They concluded that “all kinds of myeloid and lymphoid malignancies including their prestages can be caused by occupational benzene exposure” (p. 197).
EVIDENCE FOR AN ASSOCIATION WITH CHILDHOOD LEUKEMIA
Multiple studies have shown an increase in childhood leukemia risk in relation to air pollution sources emitting benzene, such as gas stations and traffic. For example, a recent nationwide study in France of 765 acute leukemia cases and 1681 controls found that acute leukemia was significantly associated with residence next to gasoline stations or automotive repair garages (OR 1.6, 95% CI 1.2 to 2.2) (12). Furthermore, in a study of the area around Houston, Texas, census tracts with the highest benzene levels, estimated by EPA models, had elevated rates of all leukemias (RR = 1.37; 95% CI 1.05, 1.78), with the association being stronger for AML (117). More studies of pediatric cancers are needed that include estimates of environmental benzene exposure, rather than surrogate exposures such as proximity to gasoline stations or traffic.
Recent mechanistic work adds support to the potential association between benzene exposure and childhood leukemia. Because the genotoxic action of benzene metabolites on pluripotent bone marrow precursor cells appears promiscuous, producing multiple genetic abnormalities, it seems probable that benzene exposure can initiate both AML and ALL by causing the chromosomal rearrangements and mutations that are on the causal pathway to these malignancies. For childhood ALL and AML, studies have shown that the disease is usually initiated in utero because the leukemic translocations and other genetic changes are present in blood spots collected at birth (32, 66, 118, 119). Thus, exposure of the mother, and perhaps the father, to benzene could be just as important as childhood exposures in producing childhood AML and ALL, as has been suggested by epidemiological studies (67, 89, 92, 106). Supporting this hypothesis are animal studies demonstrating that in utero exposure to benzene increases the frequency of micronuclei and DNA recombination events in hematopoietic tissue of fetal and postnatal mice (6, 57). Studies also show that oxygen radicals play a key role in the development of in utero–initiated benzene toxicity through disruption of hematopoietic cell signaling pathways (6). These studies support the idea that genotoxic and nongenotoxic events following benzene exposure may be initiators of childhood leukemia in utero.
MECHANISMS OF BENZENE CARCINOGENICITY: MECHANISMS OF MYELOID LEUKEMIA DEVELOPMENT
AML and MDS are closely related diseases of the bone marrow that arise de novo in the general population or following therapy with alkylating agents, topoisomerase II inhibitors, or ionizing radiation [therapy-related AML and MDS (t-AML and t-MDS)] (75, 76). Occupational exposure to benzene is widely thought to cause leukemias that are similar to t-AML and t-MDS (44, 56, 128). AML and MDS both arise from genetically altered CD34+ stem or progenitor cells in the bone marrow (70) and are characterized by many different types of recurrent chromosome aberrations (71, 76). These aberrations often result in the genetic mutations that produce leukemia. Cytogenetic analysis of chromosome number and structure has therefore become important in diagnosing and treating MDS and AML (71, 76). The chromosome aberrations and gene mutations detected in therapy-related and de novo MDS and AML are very similar, although the frequencies with which they are observed in different subtypes may differ (75). Hence, therapy-related and de novo MDS and AML are considered very similar diseases (75).
At least three cytogenetic subtypes of AML and MDS are commonly observed.
Unbalanced aberrations. Cases with unbalanced chromosome aberrations, primarily 5q–/–5 or 7q–/–7 and +8, represent the first subtype (75, 76). They often present with a complex karyotype and point mutations of p53 or AML1 and are common after therapy with alkylating agents.
Balanced rearrangements. Cases with the recurrent balanced translocations [e.g., t(11q23), t(8;21) and t(15;17)] or inversions [e.g., inv(16)] represent the second subtype and arise, at least in the therapy-related subset, as illegitimate gene recombinations related to the inhibition of topoisomerase II (75).
Normal karyotype. Cases with a normal karyotype comprise the third subtype and often harbor mutations of NPM1, internal tandem duplications of FLT3, and/or point mutations or altered methylation status of C/EBPα(75).
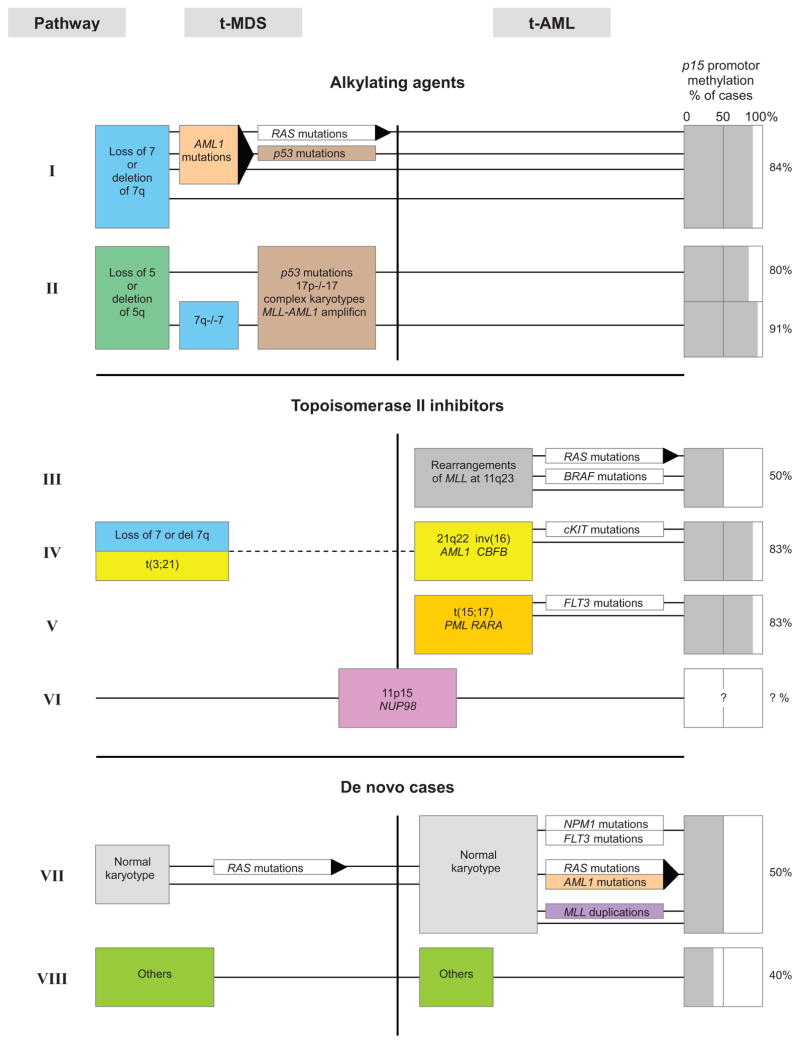
Within these three cytogenetic categories there are at least eight different genetic pathways to MDS and AML, as defined by the specific chromosome aberrations present in each (Pathways I–VIII in Figure 1). As more information is revealed about the molecular cytogenetics of leukemia, it seems likely that numerous other pathways to AML and MDS will be discovered. For example, recent unbiased high-resolution genomic screens have identified many genes that were not previously implicated in AML and which may be relevant for pathogenesis, along with many known oncogenes and tumor suppressor genes (58, 64, 111).
Figure 1.
Genetic pathways to myeloid leukemia (adapted from Reference 76).
An important role for epigenetic changes is also emerging in the development of leukemia. Functional loss of the CCAAT/enhancer binding proteinα(C/EBPα), a master regulatory transcription factor in the hematopoietic system, can result in a differentiation block in granulopoiesis and thus contribute to leukemic transformation (24). Recent work has shown that epigenetic alterations of C/EBPα are a frequent event in AML (34). C/EBPα can also steer miRNA-223 expression, which is vital in granulocytic differentiation (22).
Referring to Figure 1, extensive evidence indicates that benzene can induce AML via Pathways I, II, and IV and demonstrates considerable support for Pathway V. There is some evidence for Pathway III but little information regarding Pathways VI–VIII. Benzene exposure has been associated with higher levels of chromosomal changes commonly observed in AML, including 5q–/–5 or 7q–/–7, +8, and t(8;21) in the blood cells of highly exposed workers (97, 127, 129). Its metabolites also produce these same changes in human cell cultures, including cultures of CD34+ progenitor cells (96, 102). This research provides strong evidence for benzene’s role in the production of AML by Pathways I, II, and IV (Figure 1).
Pathways III, IV, and V are related to the inhibition of the DNA-related enzyme topoisomerase II (topo II), which is essential for the maintenance of proper chromosome structure and segregation. There are different types of topo II inhibitors. Epidophyllotoxins, such as etoposide, cause chromosome damage and kill cells by increasing physiological levels of topoisomerase II-DNA cleavage complexes (17). These drugs are referred to as topoisomerase II poisons to distinguish them from catalytic inhibitors of the enzyme because they convert this essential enzyme to a potent cellular toxin. Other drugs, such as merbarone, act as inhibitors of topo II activity; however, in contrast to etoposide, they do not stabilize topo II-DNA cleavable complexes but are still potent clastogens both in vitro and in vivo (112).
Several studies have shown that benzene in vivo and its reactive benzene metabolites hydroquinone (HQ) and 1,4-benzoquinone (BQ) in vitro inhibit the functionality of topo II and enhance DNA cleavage (13, 60). Bioactivation of HQ by peroxidase to BQ enhances topo II inhibition (19). Indeed, BQ is a more potent topo II inhibitor than is HQ in a cell-free assay system (7, 42). These findings demonstrate that benzene, through its reactive quinone metabolites, can inhibit topo II and probably cause leukemias with chromosome translocations and inversions known to be caused by topo II inhibitors, including AMLs harboring t(21q22), t(15;17), and inv(16) in a manner consistent with Pathways IV and V (69, 75). The evidence for rearrangements of the MLL gene through t(11q23) via Pathway III in benzene-induced leukemia is less convincing but may occur through an apoptotic pathway (108).
AML can arise de novo via Pathways VII and VIII without apparent chromosome abnormalities, but molecular analysis has revealed many genetic changes in these apparently “normal” leukemias, including mutations of NPM1, AML1, FLT3, RAS, and C/EBPα(Figure 1) (21, 64). Research is needed to clarify the ability of benzene and its metabolites to produce mutations of the types found in these leukemias.
The ability of benzene and/or its metabolites to induce epigenetic changes related to the development of leukemia, such as altered methylation status of C/EBPα, is unclear at this time. A recent study reported that hypermethylation in p15 (+0.35%; p = 0.018) and hypomethylation in MAGE-1 (-0.49%; p = 0.049) were associated with very low benzene exposures (~22 ppb) in healthy subjects, including gas station attendants and traffic police officers, although the corresponding effects on methylation were very low (11). Further study of the role epigenetics plays in the hematotoxicity and carcinogenicity of benzene is warranted, including studies of aberrant DNA methylation and altered microRNA expression.
Although benzene and its metabolites are clearly capable of producing multiple forms of chromosomal mutation, including various translocations, deletions, and aneuploidies, these are usually insufficient as a single event to induce leukemia. Other secondary events, such as specific gene mutations and/or other chromosome changes, are usually required (33, 61). Thus, benzene-induced leukemia probably begins as a mutagenic event in the stem or progenitor cell, and subsequent genomic instability allows for sufficient mutations to be acquired in a relatively short time period. Studies have shown that the benzene metabolite HQ is similar to ionizing radiation because it induces genomic instability in the bone marrow of susceptible mice (31). Recent findings showing the importance of DNA repair and maintenance genes, such as WRN, in genetic susceptibility to benzene toxicity also support this mechanism (52, 82).
Thus, benzene exposure can lead to multiple alterations that contribute to the leukemogenic process. Benzene may act by causing chromosomal damage (aneuploidy, deletions, and translocations) through the inhibition of topo II; disrupting microtubules; generating oxygen radicals that lead to point mutations, strand breaks, and oxidative stress; causing immune system dysfunction that leads to decreased immunosurveillance (14, 59); altering stem cell pool sizes through hematotoxicity (45); inhibiting gap-junction intercellular communication (85); and altering DNA methylation and perhaps specific microRNAs. This multimodal mechanism of action suggests that the effects of benzene on the leukemogenic process are not singular and can occur throughout the process. This finding implies that both background and added exposures from occupation and hobbies will have similar impacts on the process and that the effects will be additive. Thus, given the high background exposure to benzene as a combustion by-product in our environment, it seems unlikely that any practical threshold exists, and the effects of each molecule of benzene will be additive in a linear fashion.
METABOLISM OF BENZENE AND ITS RELEVANCE TO BENZENE CARCINOGENICITY
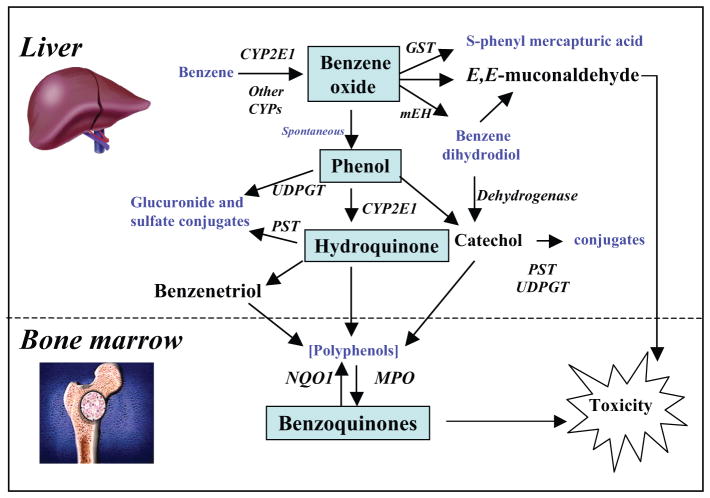
Benzene must be metabolized to become carcinogenic (86, 99). Its metabolism is summarized in Figure 2. The initial step involves cytochrome P450 (CYP)-dependent oxidation of benzene to benzene oxide, which exists in equilibrium with its tautomer oxepin. Most benzene oxide spontaneously rearranges to phenol (PH), which is either excreted or further metabolized to HQ and 1,4-BQ. The remaining benzene oxide is either hydrolyzed to produce catechol (CA) and 1,2-BQ or reacts with glutathione to produce S-phenylmercapturic acid (SPMA). Metabolism of oxepin is thought to open the aromatic ring, yielding the reactive muconaldehydes and E,E-muconic acid (MA). Human exposures to benzene at air concentrations between 0.1 and 10 ppm result in urinary metabolite profiles with 70%–85% PH, 5%–10% each of HQ, MA, and CA, and less than 1% of SPMA (47). Benzene oxide, the BQs, muconaldehydes, and benzene diol epoxides (formed from CYP oxidation of benzene dihydrodiol) are electrophiles that readily react with peptides and proteins (8, 39, 65, 110) and can thereby interfere with cellular function (94). It remains unclear what role these different metabolites play in benzene carcinogenicity, but BQ formation from HQ via myeloperoxidase in the bone marrow may be key (94). Considerable evidence indicates that this pathway plays an important role in BQ formation because the BQ-detoxifying enzyme NQO1 protects mice against benzene-induced myelodysplasia (46, 62) and protects humans against benzene hematotoxicity (87). However, this protection does not rule out adverse effects from other metabolites.
Figure 2.
Simplified metabolic scheme for benzene showing major pathways and metabolizing enzymes leading to toxicity. CYP2E1, cytochrome P450 2E1; GST, glutathione-S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1; MPO, myeloperoxidase; UDPGT, uridine diphosphate glucuronyl transferase; PST, phenol sulfotransferase; mEH, microsomal epoxide hydrolase.
Benzene is most likely metabolized initially to PH and MA via two enzymes rather than just one CYP enzyme, and the putative high-affinity enzyme is active primarily below 1 ppm (79). Because CYP2E1 is the primary enzyme responsible for mammalian metabolism of benzene (72, 105), it is reasonable to assume that the low-affinity enzyme is responsible for benzene metabolism mainly at higher levels of exposure. CYP2F1 and CYP2A13 are reasonable candidates for the high-affinity metabolic enzymes, which are active at environmental levels of exposure below 1 ppm (77, 79, 91). Interestingly, these CYPs are highly expressed in the human lung. Despite much research, more work is needed to elucidate the different roles of multiple metabolites in benzene toxicity and the pathways that lead to their formation.
EMERGING ROLE OF THE ARYL HYDROCARBON RECEPTOR
The aryl hydrocarbon receptor (AhR) is known mainly as the mediator for the toxicity of certain xenobiotics. However, this transcription factor has many important biological functions, and emerging evidence indicates that it has a significant role in the regulation of hematopoietic stem cells (HSCs) (40, 93). AhR expression may be necessary for the proper maintenance of quiescence in HSCs, and AhR downregulation is essential for the stem cells to “escape” from quiescence and undergo subsequent proliferation (93). This hypothesis implicates the AhR as a negative regulator of hematopoiesis to curb excessive proliferation. This, in turn, prevents the premature exhaustion of HSCs and sensitivity to genetic alterations, thus preserving HSC function over the organism’s life span. However, AhR dysregulation may result in the altered ability of HSCs to sense appropriate signals in the bone marrow microenvironment, leading to hematopoietic disease.
Inoue and colleagues have shown that AhR-knockout (KO) mice do not show any hematotoxicity after benzene exposure (125). Follow-up studies showed that mice that had been lethally irradiated and repopulated with marrow cells from AhR-KO mice essentially did not have signs of benzene-induced hematotoxicity (41). The most likely explanation for these findings is that the absence of AhR removes HSCs from their quiescent state and makes them susceptible to DNA damage from benzene exposure and subsequent cell death through apoptosis. Further research is needed to examine the effects of benzene and its metabolites on cycling and quiescent HSCs.
SUSCEPTIBLE SUBPOPULATIONS
Aksoy (1) reported striking variation in benzene toxicity among workers with comparable levels of occupational exposure. The reasons underlying this variation are unknown. Part of the variation may be caused by biological factors such as gender, age, genetics, and amount of adipose tissue, with the remainder being due to environmental influences such as routes of exposure, physical activity, coexposures, smoking, alcohol consumption, and dietary habits.
Studies to date have identified a number of single-nucleotide polymorphisms (SNPs) in candidate genes that appear to confer susceptibility to benzene hematotoxicity. The first ones identified were related to metabolism, including polymorphisms in cytochrome P450 2E1 (CYP2E1), NAD(P)H:quinone oxidoreductase 1 (NQO1), myeloperoxidase (MPO), glutathione-S-transferases (GSTs), and microsomal epoxide hydrolase (mEH) in Figure 2. The role of metabolizing enzyme polymorphisms was reviewed by Dougherty et al. (18) in 2008. They concluded that the polymorphisms produced a modest effect on the biomarkers of benzene exposure and effect analyzed in 22 studies; GSTM1 and GSTT1 showed some consistent associations.
In a study of 1395 SNPs in 411 cancer-related genes on lowered white blood cell (WBC) counts in benzene-exposed workers, highly significant findings were clustered in genes (BLM, TP53, RAD51, WDR79, and WRN) that play a critical role in DNA repair and genomic maintenance (52). In vitro functional studies revealed that deletion of SGS1 in yeast, equivalent to lacking BLM and WRN function in humans, caused reduced cellular growth in the presence of the toxic benzene metabolite HQ, and knockdown of WRN increased susceptibility of human lymphoid TK6 and myeloid HL60 cells to HQ toxicity (52, 82). Thus, SNPs in genes involved in DNA repair and genomic maintenance play an important role in susceptibility to benzene-induced hematotoxicity. Other possible associations with DNA repair and genome maintenance include the recent findings that polymorphisms in the p53-dependent genes p21 and p14(ARF) may play a role in susceptibility to chronic benzene poisoning (103).
The other class of genetic polymorphisms associated with benzene toxicity is in cytokine and chemokine genes. Associations have been reported with SNPs in VEGF, IL-1A, IL-4, IL-10, IL-12A, VCAM1, and lowered WBC counts (53) and with an SNP in TNF-alpha and chronic benzene poisoning (63). Additional studies are needed to confirm these associations.
Thus, genetic polymorphisms that confer susceptibility to benzene toxicity should be taken into account when assessing the risks of benzene exposure. Select combinations of genetic polymorphisms may increase susceptibility of individuals and/or population subgroups. However, gene-gene interactions are not yet analyzed in well-designed studies that incorporate multiple biological end points and multiple genes, and a genome-wide study is needed to truly assess the role of genetic variation in conferring susceptibility.
WHAT IS THE DOSE-RESPONSE CURVE? IS IT LINEAR AND IS THERE A FUNCTIONAL THRESHOLD IN THE LOW-DOSE REGION?
Although there is undoubtedly a causal link between benzene exposure and leukemia, the shape of the exposure-response relationship is controversial, particularly at low doses at or below 1 ppm in air. Indeed, when considering regulatory actions, litigation, and potential clean-up costs in the billions of dollars, this uncertainty represents a major challenge for environmental toxicology and epidemiology. Recent action by the U.S. EPA to reduce cancer risks from mobile sources underscores this point (see 20). In justifying its decision to lower the benzene content of gasoline, the EPA cited studies pointing to supralinear (greater-than-proportional) production of benzene-related protein adducts at air concentrations below 1 ppm (80, 81). Such behavior would likely result from saturation of the metabolism of benzene to benzene oxide-oxepin. Because the EPA had previously assumed that human benzene metabolism proceeded according to nonsaturating (first-order) kinetics at exposure concentrations well above 10 ppm, saturation of metabolism below 1 ppm “could lead to substantial underestimation of leukemia risks” in the general population (20).
Traditional epidemiology is unlikely to determine the shape of the dose-response curve for benzene-induced leukemia in the low-dose region, although the Glass et al. study shows effects at 1–2 ppm in air and no sign of a threshold. Chronic animal toxicity studies are also unlikely to be informative for two reasons: (a) no accepted animal model of benzene-induced leukemia exists at the present time, and (b) low-dose studies would require a prohibitively large number of animals. In situations like this, where traditional epidemiology and toxicology are of limited value, investigators have proposed that nontumor data such as biological markers (biomarkers) be employed in the risk-assessment process (5).
The most appropriate biomarker of leukemia risk appears to be lowered WBC counts because this factor has been associated with an increased risk of hematological malignancies. Ward et al. (113) found no evidence of a threshold for hematotoxic effects of benzene and suggested that exposure to <5 ppm benzene could result in hematologic suppression. Occupational exposure decreased WBC count in petrochemical workers exposed to <10 ppm benzene (126), and Qu et al. reported that depressions in blood cell counts in benzene-exposed Chinese workers were not only exposure dependent, but also significantly different in the lowest exposed group (at or below 0.25 ppm) compared with unexposed subjects (78). In a large study of more than 400 workers, hematotoxicity occurred in workers exposed to <1 ppm benzene (51). Further analysis of this data showed a linear monotonicity of the association between lowered blood cell counts and benzene exposure by spline regression analyses (50). Thus, the literature shows that benzene affects the blood-forming system at low levels of occupational exposure, at or below 1 ppm, and that there is no evidence of a threshold. As a result, the threshold limit value has recently been lowered by the ACGIH to 0.5 ppm, and various government agencies and scientific bodies have recommended the 8-hour time-weighted average standard be lowered to 0.1 ppm. The latest research indicates that there is likely no safe level of exposure to benzene and that all exposures constitute some risk in a linear, if not supralinear, and additive fashion. Public health agencies should act accordingly.
Acknowledgments
This work was supported by NIH grants RO1ES06721, P42ES04705, and U54ES01611. I am grateful to my many collaborators over the years including, but not limited to, Luoping Zhang, Nat Rothman, Stephen Rappaport, Qing Lan, Songnian Yin, Guilan Li, Roel Vermeulen, Alan Hubbard, Zhiying Ji, Cliona McHale, Weihong Guo, Min Shen, Matthew Forrest, John Curry, Laura Gunn, Richard Hayes, Mustafa Dosemeci, David Eastmond, Vangala Subrahmanyam, Prema Kolachana, Nina Holland, and David Ross. I am grateful to Dr. Bernard Goldstein for many helpful comments and his encouragement and to Surakshya Dhakal, William Brockett and Minerva Reyes for library and administrative support.
Glossary
- EPA
Environmental Protection Agency
- CAPM
Chinese Academy of Preventive Medicine
- NCI
National Cancer Institute
- AML
acute myeloid leukemia
- NHL
non-Hodgkin lymphoma
- MDS
myelodysplastic syndromes
- OR
odds ratio
- HQ
hydroquinone
- SNP
single nucleotide polymorphism
Footnotes
DISCLOSURE STATEMENT
The author has received consulting and expert testimony fees from lawyers representing both plaintiffs and defendants in cases involving claims related to exposure to benzene. The author has also received consulting fees from the governments of Australia, Norway, and the United States.
LITERATURE CITED
- 1.Aksoy M. Benzene hematotoxicity. In: Aksoy M, editor. Benzene Carcinogenicity. Boca Raton, FL: CRC Press; 1988. pp. 59–112. [Google Scholar]
- 2.Aksoy M. Hematotoxicity and carcinogenicity of benzene. Environ Health Perspect. 1989;82:193–97. doi: 10.1289/ehp.8982193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aksoy M, Dincol K, Akgun T, Erdem S, Dincol G. Haematological effects of chronic benzene poisoning in 217 workers. Br J Ind Med. 1971;28:296–302. doi: 10.1136/oem.28.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksoy M, Dincol K, Erdem S, Akgun T, Dincol G. Details of blood changes in 32 patients with pancytopenia associated with long-term exposure to benzene. Br J Ind Med. 1972;29:56–64. doi: 10.1136/oem.29.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albertini R, Clewell H, Himmelstein MW, Morinello E, Olin S, et al. The use of non-tumor data in cancer risk assessment: reflections on butadiene, vinyl chloride, and benzene. Regul Toxicol Pharmacol. 2003;37:105–32. doi: 10.1016/s0273-2300(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 6.Badham HJ, Winn LM. In utero exposure to benzene disrupts fetal hematopoietic progenitor cell growth via reactive oxygen species. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp242. In press. [DOI] [PubMed] [Google Scholar]
- 7.Baker RK, Kurz EU, Pyatt DW, Irons RD, Kroll DJ. Benzene metabolites antagonize etoposide-stabilized cleavable complexes of DNA topoisomerase IIalpha. Blood. 2001;98:830–33. doi: 10.1182/blood.v98.3.830. [DOI] [PubMed] [Google Scholar]
- 8.Bechtold WE, Willis JK, Sun JD, Griffith WC, Reddy TV. Biological markers of exposure to benzene: S-phenylcysteine in albumin. Carcinogenesis. 1992;13:1217–20. doi: 10.1093/carcin/13.7.1217. [DOI] [PubMed] [Google Scholar]
- 9.Beelte S, Haas R, Germing U, Jansing P-J. Practice of recognizing benzene-caused occupational diseases in 2006. Med Klin. 2008;103:553–60. doi: 10.1007/s00063-008-1090-3. (In German) [DOI] [PubMed] [Google Scholar]
- 10.Beelte S, Haas R, Germing U, Jansing P-J. Paradigm change in the assessment of myeloid and lymphoid neoplasms associated with occupational benzene exposure. Med Klin. 2009;104:197–203. doi: 10.1007/s00063-009-1032-8. (In German) [DOI] [PubMed] [Google Scholar]
- 11.Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–80. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 12.Brosselin P, Rudant J, Orsi L, Leverger G, Baruchel A, et al. Acute childhood leukaemia and residence next to petrol stations and automotive repair garages: the ESCALE study (SFCE) Occup Environ Med. 2009;66:598–606. doi: 10.1136/oem.2008.042432. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Eastmond DA. Topoisomerase inhibition by phenolic metabolites: a potential mechanism for benzene’s clastogenic effects. Carcinogenesis. 1995;16:2301–7. doi: 10.1093/carcin/16.10.2301. [DOI] [PubMed] [Google Scholar]
- 14.Cho JY. Suppressive effect of hydroquinone, a benzene metabolite, on in vitro inflammatory responses mediated by macrophages, monocytes, and lymphocytes. Mediat Inflamm. 2008;2008:298010. doi: 10.1155/2008/298010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronkite EP. Evidence for radiation and chemicals as leukemogenic agents. Arch Environ Health. 1961;3:297–303. doi: 10.1080/00039896.1961.10663025. [DOI] [PubMed] [Google Scholar]
- 16.Delore P, Borgomano C. Leucemie aigue au cours de l’intoxication benzenique: sur l’origine toxique de certaines leucemies aigues et leur relations avec les anemies graves. J Med Lyon. 1928;9:227–33. [Google Scholar]
- 17.Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 2009;37:738–48. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougherty D, Garte S, Barchowsky A, Zmuda J, Taioli E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure—a literature review. Toxicol Lett. 2008;182:7–17. doi: 10.1016/j.toxlet.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Eastmond DA, Mondrala ST, Hasegawa L. Topoisomerase II inhibition by myeloperoxidase-activated hydroquinone: a potential mechanism underlying the genotoxic and carcinogenic effects of benzene. Chem Biol Interact. 2005;153–54:207–16. doi: 10.1016/j.cbi.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Environ. Prot. Agency. Control of hazardous air pollutants from mobile sources. 2007 http://www.epa.gov/fedrgstr/EPA-AIR/2007/February/Day-26.
- 21.Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–85. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 22.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, et al. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–31. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Forni AM, Cappellini A, Pacifico E, Vigliani EC. Chromosome changes and their evolution in subjects with past exposure to benzene. Arch Environ Health. 1971;23:385–91. doi: 10.1080/00039896.1971.10666024. [DOI] [PubMed] [Google Scholar]
- 24.Frohling S, Dohner H. Disruption of C/EBPalpha function in acute myeloid leukemia. N Engl J Med. 2004;351:2370–72. doi: 10.1056/NEJMp048241. [DOI] [PubMed] [Google Scholar]
- 25.Glass DC, Gray CN, Adams GG, Manuell RW, Bisby JA. Validation of exposure estimation for benzene in the Australian petroleum industry. Toxicol Ind Health. 2001;17:113–27. doi: 10.1191/0748233701th099oa. [DOI] [PubMed] [Google Scholar]
- 26.Glass DC, Gray CN, Jolley DJ, Gibbons C, Sim MR, et al. Leukemia risk associated with low-level benzene exposure. Epidemiology. 2003;14:569–77. doi: 10.1097/01.ede.0000082001.05563.e0. [DOI] [PubMed] [Google Scholar]
- 27.Glass DC, Gray CN, Jolley DJ, Gibbons C, Sim MR. Health Watch exposure estimates: Do they underestimate benzene exposure? Chem Biol Interact. 2005;153–54:23–32. doi: 10.1016/j.cbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Glass DC, Gray CN, Jolley DJ, Sim MR, Fritschi L. Benzene exposure and leukemia. Epidemiology. 2004;15:510–11. [Google Scholar]
- 29.Goldstein B. Benzene exposure and leukemia. Epidemiology. 2004;15:509–10. doi: 10.1097/01.ede.0000129523.30311.49. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein BD. Benzene toxicity. Occup Med. 1988;3:541–54. [PubMed] [Google Scholar]
- 31.Gowans ID, Lorimore SA, McIlrath JM, Wright EG. Genotype-dependent induction of transmissible chromosomal instability by gamma-radiation and the benzene metabolite hydroquinone. Cancer Res. 2005;65:3527–30. doi: 10.1158/0008-5472.CAN-04-4242. [DOI] [PubMed] [Google Scholar]
- 32.Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3:639–49. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- 33.Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–33. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hackanson B, Bennett KL, Brena RM, Jiang J, Claus R, et al. Epigenetic modification of CCAAT/enhancer binding protein alpha expression in acute myeloid leukemia. Cancer Res. 2008;68:3142–51. doi: 10.1158/0008-5472.CAN-08-0483. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton A. Benzene (benzol) poisoning. General review. Arch Pathol Lab Med. 1931;11:434. [Google Scholar]
- 36.Hayes RB, Songnian Y, Dosemeci M, Linet M. Benzene and lymphohematopoietic malignancies in humans. Am J Ind Med. 2001;40:117–26. doi: 10.1002/ajim.1078. [DOI] [PubMed] [Google Scholar]
- 37.Hayes RB, Yin SN, Dosemeci M, Li GL, Wacholder S, et al. Benzene and the dose-related incidence of hematologic neoplasms in China. Chinese Academy of Preventive Medicine–National Cancer Institute Benzene Study Group. J Natl Cancer Inst. 1997;89:1065–71. doi: 10.1093/jnci/89.14.1065. [DOI] [PubMed] [Google Scholar]
- 38.Hayes RB, Yin SN, Rothman N, Dosemeci M, Li G, et al. Benzene and lymphohematopoietic malignancies in China. J Toxicol Environ Health A. 2000;61:419–32. doi: 10.1080/00984100050166442. [DOI] [PubMed] [Google Scholar]
- 39.Henderson AP, Bleasdale C, Delaney K, Lindstrom AB, Rappaport SM, et al. Evidence for the formation of Michael adducts from reactions of (E,E)-muconaldehyde with glutathione and other thiols. Bioorg Chem. 2005;33:363–73. doi: 10.1016/j.bioorg.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Hirabayashi Y, Inoue T. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem Pharmacol. 2009;77:521–35. doi: 10.1016/j.bcp.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Hirabayashi Y, Yoon BI, Li GX, Fujii-Kuriyama Y, Kaneko T, et al. Benzene-induced hematopoietic toxicity transmitted by AhR in wild-type mouse and nullified by repopulation with AhR-deficient bone marrow cells: time after benzene treatment and recovery. Chemosphere. 2008;73:S290–94. doi: 10.1016/j.chemosphere.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Hutt AM, Kalf GF. Inhibition of human DNA topoisomerase II by hydroquinone and p-benzoquinone, reactive metabolites of benzene. Environ Health Perspect. 1996;104(Suppl 6):1265–69. doi: 10.1289/ehp.961041265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Infante PF, Rinsky RA, Wagoner JK, Young RJ. Leukaemia in benzene workers. Lancet. 1977;2:76–78. doi: 10.1016/s0140-6736(77)90074-5. [DOI] [PubMed] [Google Scholar]
- 44.Irons RD, Stillman WS. The process of leukemogenesis. Environ Health Perspect. 1996;104(Suppl 6):1239–46. doi: 10.1289/ehp.961041239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irons RD, Stillman WS, Colagiovanni DB, Henry VA. Synergistic action of the benzene metabolite hydroquinone on myelopoietic stimulating activity of granulocyte/macrophage colony-stimulating factor in vitro. Proc Natl Acad Sci USA. 1992;89:3691–95. doi: 10.1073/pnas.89.9.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iskander K, Jaiswal AK. Quinone oxidoreductases in protection against myelogenous hyperplasia and benzene toxicity. Chem Biol Interact. 2005;153–54:147–57. doi: 10.1016/j.cbi.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, et al. Modeling human metabolism of benzene following occupational and environmental exposures. Cancer Epidemiol Biomark Prev. 2006;15:2246–52. doi: 10.1158/1055-9965.EPI-06-0262. [DOI] [PubMed] [Google Scholar]
- 48.Kirkeleit J, Riise T, Gjertsen BT, Moen BE, Bratveit M, Øystein B. Effects of benzene on human ° hematopoiesis. Open Hematol J. 2008;2:87–102. [Google Scholar]
- 49.Krishnan B, Morgan GJ. Non-Hodgkin lymphoma secondary to cancer chemotherapy. Cancer Epidemiol Biomark Prev. 2007;16:377–80. doi: 10.1158/1055-9965.EPI-06-1069. [DOI] [PubMed] [Google Scholar]
- 50.Lan Q, Vermeulen R, Zhang L, Li G, Rosenberg PS, et al. Benzene exposure and hematotoxicity: response. Science. 2006;312:998–99. doi: 10.1126/science.312.5776.998b. [DOI] [PubMed] [Google Scholar]
- 51.Lan Q, Zhang L, Li G, Vermeulen R, Weinberg RS, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306:1774–76. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan Q, Zhang L, Shen M, Jo WJ, Vermeulen R, et al. Large-scale evaluation of candidate genes identifies associations between DNA repair and genomic maintenance and development of benzene hematotoxicity. Carcinogenesis. 2009;30:50–58. doi: 10.1093/carcin/bgn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lan Q, Zhang L, Shen M, Smith MT, Li G, et al. Polymorphisms in cytokine and cellular adhesion molecule genes and susceptibility to hematotoxicity among workers exposed to benzene. Cancer Res. 2005;65:9574–81. doi: 10.1158/0008-5472.CAN-05-1419. [DOI] [PubMed] [Google Scholar]
- 54.Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360:659–67. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landgren O, Kyle RA, Hoppin JA, Beane Freeman LE, Cerhan JR, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 2009;113:6386–91. doi: 10.1182/blood-2009-02-203471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larson RA, Le Beau MM. Therapy-related myeloid leukaemia: a model for leukemogenesis in humans. Chem Biol Interact. 2005;153–54:187–95. doi: 10.1016/j.cbi.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Lau A, Belanger CL, Winn LM. In utero and acute exposure to benzene: investigation of DNA double-strand breaks and DNA recombination in mice. Mutat Res. 2009;676:74–82. doi: 10.1016/j.mrgentox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B, Li YQ, Yang LJ, Chen SH, Yu W, et al. Decreased T-cell receptor excision DNA circles in peripheral blood mononuclear cells among benzene-exposed workers. Int J Immunogenet. 2009;36:107–11. doi: 10.1111/j.1744-313X.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 60.Lindsey RH, Jr, Bender RP, Osheroff N. Effects of benzene metabolites on DNA cleavage mediated by human topoisomerase II alpha: 1,4-hydroquinone is a topoisomerase II poison. Chem Res Toxicol. 2005;18:761–70. doi: 10.1021/tx049659z. [DOI] [PubMed] [Google Scholar]
- 61.Lobato MN, Metzler M, Drynan L, Forster A, Pannell R, Rabbitts TH. Modeling chromosomal translocations using conditional alleles to recapitulate initiating events in human leukemias. J Natl Cancer Inst Monogr. 2008;39:58–63. doi: 10.1093/jncimonographs/lgn022. [DOI] [PubMed] [Google Scholar]
- 62.Long DJ, 2nd, Gaikwad A, Multani A, Pathak S, Montgomery CA, et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002;62:3030–36. [PubMed] [Google Scholar]
- 63.Lv L, Kerzic P, Lin G, Schnatter AR, Bao L, et al. The TNF-α 238A polymorphism is associated with susceptibility to persistent bone marrow dysplasia following chronic exposure to benzene. Leuk Res. 2007;31:1479–85. doi: 10.1016/j.leukres.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 64.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–66. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDonald TA, Waidyanatha S, Rappaport SM. Production of benzoquinone adducts with hemoglobin and bone-marrow proteins following administration of [13C6]benzene to rats. Carcinogenesis. 1993;14:1921–25. doi: 10.1093/carcin/14.9.1921. [DOI] [PubMed] [Google Scholar]
- 66.McHale CM, Wiemels JL, Zhang L, Ma X, Buffler PA, et al. Prenatal origin of childhood acute myeloid leukemias harboring chromosomal rearrangements t(15;17) and inv(16) Blood. 2003;101:4640–41. doi: 10.1182/blood-2003-01-0313. [DOI] [PubMed] [Google Scholar]
- 67.McKinney PA, Alexander FE, Cartwright RA, Parker L. Parental occupations of children with leukaemia in west Cumbria, north Humberside, and Gateshead. Br Med J. 1991;302:681–87. doi: 10.1136/bmj.302.6778.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michaels D. Doubt Is Their Product: How Industry’s Assault on Science Threatens Your Health. New York: Oxford Univ. Press; 2008. [Google Scholar]
- 69.Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352:1529–38. doi: 10.1056/NEJMoa042715. [DOI] [PubMed] [Google Scholar]
- 70.Morgan GJ, Alvares CL. Benzene and the hemopoietic stem cell. Chem Biol Interact. 2005;153–54:217–22. doi: 10.1016/j.cbi.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 71.Mrozek K, Bloomfield CD. Clinical significance of the most common chromosome translocations in adult acute myeloid leukemia. J Natl Cancer Inst Monogr. 2008;39:52–57. doi: 10.1093/jncimonographs/lgn003. [DOI] [PubMed] [Google Scholar]
- 72.Nedelcheva V, Gut I, Soucek P, Tichavská B, Týnkova L, et al. Metabolism of benzene in human liver microsomes: individual variations in relation to CYP2E1 expression. Arch Toxicol. 1999;73:33–40. doi: 10.1007/s002040050583. [DOI] [PubMed] [Google Scholar]
- 73.Paustenbach DJ, Price PS, Ollison W, Blank C, Jernigan JD, et al. Reevaluation of benzene exposure for the Pliofilm (rubberworker) cohort (1936–1976) J Toxicol Environ Health. 1992;36:177–231. doi: 10.1080/15287399209531633. [DOI] [PubMed] [Google Scholar]
- 74.Paxton MB. Leukemia risk associated with benzene exposure in the Pliofilm cohort. Environ Health Perspect. 1996;104(Suppl 6):1431–36. doi: 10.1289/ehp.961041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–48. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–49. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 77.Powley MW, Carlson GP. Cytochromes P450 involved with benzene metabolism in hepatic and pulmonary microsomes. J Biochem Mol Toxicol. 2000;14:303–9. doi: 10.1002/1099-0461(2000)14:6<303::AID-JBT2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 78.Qu Q, Shore R, Li G, Jin X, Chen LC, et al. Hematological changes among Chinese workers with a broad range of benzene exposures. Am J Ind Med. 2002;42:275–85. doi: 10.1002/ajim.10121. [DOI] [PubMed] [Google Scholar]
- 79.Rappaport SM, Kim S, Lan Q, Vermeulen R, Waidyanatha S, et al. Evidence that humans metabolize benzene via two pathways. Environ Health Perspect. 2009;117:946–52. doi: 10.1289/ehp.0800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rappaport SM, Waidyanatha S, Qu Q, Shore R, Jin X, et al. Albumin adducts of benzene oxide and 1,4-benzoquinone as measures of human benzene metabolism. Cancer Res. 2002;62:1330–37. [PubMed] [Google Scholar]
- 81.Rappaport SM, Waidyanatha S, Yeowell-O’Connell K, Rothman N, Smith MT, et al. Protein adducts as biomarkers of human benzene metabolism. Chem Biol Interact. 2005;153–54:103–9. doi: 10.1016/j.cbi.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 82.Ren X, Lim S, Smith MT, Zhang L. Werner syndrome protein, WRN, protects cells from DNA damage induced by the benzene metabolite hydroquinone. Toxicol Sci. 2009;107:367–75. doi: 10.1093/toxsci/kfn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rinsky RA, Hornung RW, Silver SR, Tseng CY. Benzene exposure and hematopoietic mortality: a long-term epidemiologic risk assessment. Am J Ind Med. 2002;42:474–80. doi: 10.1002/ajim.10138. [DOI] [PubMed] [Google Scholar]
- 84.Rinsky RA, Smith AB, Hornung R, Filloon TG, Young RJ, et al. Benzene and leukemia. An epidemiologic risk assessment. N Engl J Med. 1987;316:1044–50. doi: 10.1056/NEJM198704233161702. [DOI] [PubMed] [Google Scholar]
- 85.Rivedal E, Witz G. Metabolites of benzene are potent inhibitors of gap-junction intercellular communication. Arch Toxicol. 2005;79:303–11. doi: 10.1007/s00204-004-0638-0. [DOI] [PubMed] [Google Scholar]
- 86.Ross D. The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J Toxicol Environ Health A. 2000;61:357–72. doi: 10.1080/00984100050166361. [DOI] [PubMed] [Google Scholar]
- 87.Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C–>T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997;57:2839–42. [PubMed] [Google Scholar]
- 88.Santesson GG. Uber chronische Vergiftungen mit steinkohlen Benzin. Vier todes falle. Arch Hyg. 1897;31:336–76. [Google Scholar]
- 89.Scélo G, Metayer C, Zhang L, Wiemels JL, Aldrich MC, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environ Health Perspect. 2009;117:133–39. doi: 10.1289/ehp.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selling L. Benzol as a leucotoxin. Studies on the degeneration and regeneration of the blood and haematopoietic organs. Johns Hopkins Hosp Rep. 1916;17:83–148. [Google Scholar]
- 91.Sheets PL, Yost GS, Carlson GP. Benzene metabolism in human lung cell lines BEAS-2B and A549 and cells overexpressing CYP2F1. J Biochem Mol Toxicol. 2004;18:92–99. doi: 10.1002/jbt.20010. [DOI] [PubMed] [Google Scholar]
- 92.Shu XO, Stewart P, Wen WQ, Han D, Potter JD, et al. Parental occupational exposure to hydrocarbons and risk of acute lymphocytic leukemia in offspring. Cancer Epidemiol Biomark Prev. 1999;8:783–91. [PubMed] [Google Scholar]
- 93.Singh KP, Casado FL, Opanashuk LA, Gasiewicz TA. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 2009;77:577–87. doi: 10.1016/j.bcp.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith MT. The mechanism of benzene-induced leukemia: a hypothesis and speculations on the causes of leukemia. Environ Health Perspect. 1996;104(Suppl 6):1219–25. doi: 10.1289/ehp.961041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith MT, Jones RM, Smith AH. Benzene exposure and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomark Prev. 2007;16:385–91. doi: 10.1158/1055-9965.EPI-06-1057. [DOI] [PubMed] [Google Scholar]
- 96.Smith MT, Zhang L, Jeng M, Wang Y, Guo W, et al. Hydroquinone, a benzene metabolite, increases the level of aneusomy of chromosomes 7 and 8 in human CD34-positive blood progenitor cells. Carcinogenesis. 2000;21:1485–90. [PubMed] [Google Scholar]
- 97.Smith MT, Zhang L, Wang Y, Hayes RB, Li G, et al. Increased translocations and aneusomy in chromosomes 8 and 21 among workers exposed to benzene. Cancer Res. 1998;58:2176–81. [PubMed] [Google Scholar]
- 98.Snyder R. Benzene and leukemia. Crit Rev Toxicol. 2002;32:155–210. doi: 10.1080/20024091064219. [DOI] [PubMed] [Google Scholar]
- 99.Snyder R. Xenobiotic metabolism and the mechanism(s) of benzene toxicity. Drug Metab Rev. 2004;36:531–47. doi: 10.1081/dmr-200033445. [DOI] [PubMed] [Google Scholar]
- 100.Sorahan T, Kinlen LJ, Doll R. Cancer risks in a historical UK cohort of benzene exposed workers. Occup Environ Med. 2005;62:231–36. doi: 10.1136/oem.2004.015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steinmaus C, Smith AH, Jones RM, Smith MT. Meta-analysis of benzene exposure and non-Hodgkin lymphoma: Biases could mask an important association. Occup Environ Med. 2008;65:371–78. doi: 10.1136/oem.2007.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stillman WS, Varella-Garcia M, Irons RD. The benzene metabolite, hydroquinone, selectively induces 5q31- and -7 in human CD34+CD19- bone marrow cells. Exp Hematol. 2000;28:169–76. doi: 10.1016/s0301-472x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 103.Sun P, Qiu Y, Zhang Z, Wan J, Wang T, et al. Association of genetic polymorphisms, mRNA expression of p53 and p21 with chronic benzene poisoning in a Chinese occupational population. Cancer Epidemiol Biomark Prev. 2009;18:1821–28. doi: 10.1158/1055-9965.EPI-09-0140. [DOI] [PubMed] [Google Scholar]
- 104.Travis LB, Li CY, Zhang ZN, Li DG, Yin SN, et al. Hematopoietic malignancies and related disorders among benzene-exposed workers in China. Leuk Lymphoma. 1994;14:91–102. doi: 10.3109/10428199409049654. [DOI] [PubMed] [Google Scholar]
- 105.Valentine JL, Lee SS, Seaton MJ, Asgharian B, Farris G, et al. Reduction of benzene metabolism and toxicity in mice that lack CYP2E1 expression. Toxicol Appl Pharmacol. 1996;141:205–13. doi: 10.1006/taap.1996.0277. [DOI] [PubMed] [Google Scholar]
- 106.van Steensel-Moll HA, Valkenburg HA, van Zanen GE. Childhood leukemia and parental occupation. A register-based case-control study. Am J Epidemiol. 1985;121:216–24. doi: 10.1093/oxfordjournals.aje.a113992. [DOI] [PubMed] [Google Scholar]
- 107.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 108.Vaughan AT, Betti CJ, Villalobos MJ, Premkumar K, Cline E, et al. Surviving apoptosis: a possible mechanism of benzene-induced leukemia. Chem Biol Interact. 2005;153–154:179–85. doi: 10.1016/j.cbi.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 109.Vigliani EC, Saita G. Benzene and leukemia. N Engl J Med. 1964;271:872–76. doi: 10.1056/NEJM196410222711703. [DOI] [PubMed] [Google Scholar]
- 110.Waidyanatha S, Rappaport SM. Investigation of cysteinyl protein adducts of benzene diolepoxide. Chem Biol Interact. 2005;153–54:261–66. doi: 10.1016/j.cbi.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 111.Walter MJ, Payton JE, Ries RE, Shannon WD, Deshmukh H, et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proc Natl Acad Sci USA. 2009;106:12950–55. doi: 10.1073/pnas.0903091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Roy SK, Eastmond DA. Differential cell cycle-specificity for chromosomal damage induced by merbarone and etoposide in V79 cells. Mutat Res. 2007;616:70–82. doi: 10.1016/j.mrfmmm.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 113.Ward E, Hornung R, Morris J, Rinsky R, Wild D, et al. Risk of low red or white blood cell count related to estimated benzene exposure in a rubberworker cohort (1940–1975) Am J Ind Med. 1996;29:247–57. doi: 10.1002/(SICI)1097-0274(199603)29:3<247::AID-AJIM4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 114.Weiskotten HG, Gibbs GBF, Boggs EO, Templeton ER. The action of benzol. VI Benzol vapor leucopenia (rabbit) J Med Res. 1920;41:425–38. [PMC free article] [PubMed] [Google Scholar]
- 115.Weiskotten HG, Shwartz SC, Steinsland HS. The action of benzol on blood and blood forming tissues. J Med Res. 1916;35:63–69. [PMC free article] [PubMed] [Google Scholar]
- 116.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whitworth KW, Symanski E, Coker AL. Childhood lymphohematopoietic cancer incidence and hazardous air pollutants in southeast Texas, 1995–2004. Environ Health Perspect. 2008;116:1576–80. doi: 10.1289/ehp.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 119.Wiemels JL, Xiao Z, Buffler PA, Maia AT, Ma X, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99:3801–5. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 120.Wilbur S, Wohlers D, Paikoff S, Keith L, Faroon O. ATSDR evaluation of health effects of benzene and relevance to public health. Toxicol Ind Health. 2008;24:263–398. doi: 10.1177/0748233708090910. [DOI] [PubMed] [Google Scholar]
- 121.Williams PR, Robinson K, Paustenbach DJ. Benzene exposures associated with tasks performed on marine vessels (circa 1975 to 2000) J Occup Environ Hyg. 2005;2:586–99. doi: 10.1080/15459620500339147. [DOI] [PubMed] [Google Scholar]
- 122.Yin SN, Hayes RB, Linet MS, Li GL, Dosemeci M, et al. An expanded cohort study of cancer among benzene-exposed workers in China. Benzene Study Group. Environ Health Perspect. 1996;104(Suppl 6):1339–41. doi: 10.1289/ehp.961041339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yin SN, Li GL, Tain FD, Fu ZI, Jin C, et al. Leukaemia in benzene workers: a retrospective cohort study. Br J Ind Med. 1987;44:124–28. doi: 10.1136/oem.44.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin SN, Li Q, Liu Y, Tian F, Du C, Jin C. Occupational exposure to benzene in China. Br J Ind Med. 1987;44:192–95. doi: 10.1136/oem.44.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoon BI, Hirabayashi Y, Kawasaki Y, Kodama Y, Kaneko T, et al. Aryl hydrocarbon receptor mediates benzene-induced hematotoxicity. Toxicol Sci. 2002;70:150–56. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- 126.Zhang B. Investigation of health status in workers exposed to low-level benzene. Chin J Prev Med. 1996;30:164–66. In Chinese. [PubMed] [Google Scholar]
- 127.Zhang L, Eastmond DA, Smith MT. The nature of chromosomal aberrations detected in humans exposed to benzene. Crit Rev Toxicol. 2002;32:1–42. doi: 10.1080/20024091064165. [DOI] [PubMed] [Google Scholar]
- 128.Zhang L, Rothman N, Li G, Guo W, Yang W, et al. Aberrations in chromosomes associated with lymphoma and therapy-related leukemia in benzene-exposed workers. Environ Mol Mutagen. 2007;48:467–74. doi: 10.1002/em.20306. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L, Rothman N, Wang Y, Hayes RB, Yin S, et al. Benzene increases aneuploidy in the lymphocytes of exposed workers: a comparison of data obtained by fluorescence in situ hybridization in interphase and metaphase cells. Environ Mol Mutagen. 1999;34:260–68. [PubMed] [Google Scholar]