Abstract
Measles virus nucleocapsid protein (MVNP) expression in osteoclasts (OCLs) and mutation of the SQSTM1 (p62) gene contribute to the increased OCL activity in Paget’s disease (PD). OCLs expressing MVNP display many of the features of PD OCLs. Interleukin-6 (IL-6) production is essential for the pagetic phenotype, because transgenic mice with MVNP targeted to OCLs develop pagetic OCLs and lesions, but this phenotype is absent when MVNP mice are bred to IL-6−/− mice. In contrast, mutant p62 expression in OCL precursors promotes receptor activator of NF-κB ligand (RANKL) hyperresponsivity and increased OCL production, but OCLs that form have normal morphology, are not hyperresponsive to 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3), nor produce elevated levels of IL-6. We previously generated p62P394L knock-in mice (p62KI) and found that although OCL numbers were increased, the mice did not develop pagetic lesions. However, mice expressing both MVNP and p62KI developed more exuberant pagetic lesions than mice expressing MVNP alone. To examine the role of elevated IL-6 in PD and determine if MVNP mediates its effects primarily through elevation of IL-6, we generated transgenic mice that overexpress IL-6 driven by the tartrate-resistant acid phosphatase (TRAP) promoter (TIL-6 mice) and produce IL-6 at levels comparable to MVNP mice. These were crossed with p62KI mice to determine whether IL-6 overexpression cooperates with mutant p62 to produce pagetic lesions. OCL precursors from p62KI/TIL-6 mice formed greater numbers of OCLs than either p62KI or TIL-6 OCL precursors in response to 1,25-(OH)2D3. Histomorphometric analysis of bones from p62KI/TIL-6 mice revealed increased OCL numbers per bone surface area compared to wild-type (WT) mice. However, micro-quantitative CT (μQCT) analysis did not reveal significant differences between p62KI/TIL-6 and WT mice, and no pagetic OCLs or lesions were detected in vivo. Thus, increased IL-6 expression in OCLs from p62KI mice contributes to increased responsivity to 1,25-(OH)2D3 and increased OCL numbers, but is not sufficient to induce Paget’s-like OCLs or bone lesions in vivo.
Keywords: P62, MVNP, IL-6, PAGET’S DISEASE OF BONE, OSTEOCLASTS
Introduction
The primary cellular abnormality in Paget’s disease (PD) resides in the osteoclast (OCL).(1−3) OCLs are abundant in Paget’s lesions, and are larger, contain increased nuclei/OCL, have increased bone resorbing capacity/OCL, increased 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3) and receptor activator of NF-κB ligand (RANKL) responsivity, and secrete high levels of interleukin 6 (IL-6), compared to normal OCLs.(4,5) Pagetic OCLs frequently express the measles virus nucleocapsid protein (MVNP),(6) which we have shown induces high levels of IL-6 expression in both human and mouse OCLs, and results in the development of pagetic OCLs and pagetic bone lesions in mice in vivo.(7,8) Further, high levels of IL-6 can induce TAF12, a vitamin D receptor (VDR) coactivator, in OCL precursors, which increases their responsivity to 1,25-(OH)2D3. Importantly, knockout of IL-6 in MVNP mice results in loss of their capacity to form pagetic lesions and OCLs,(9) suggests that IL-6 is required for MVNP to induce the development of PD.
There is also a genetic component to the etiology of PD, with up to 5% to 10% of all Paget’s patients carrying a germline mutation in the SQSTM1/p62 gene.(10) Expression of p62P392L, the most frequent mutation in p62 linked to PD in OCL precursors confers hyperresponsivity to RANKL but not 1,25-(OH)2D3, does not increase IL-6, and does not induce hypermultinucleated OCLs that occur in PD. Further, we found that knock-in mice (p62KI) carrying a p62P394L mutation (the murine equivalent of the most common human PD mutation, p62P392L) had modestly increased OCL numbers and developed mild osteopenia, but did not develop pagetic lesions.(11,12) However, when we crossed the p62KI and MVNP mice, the resulting p62KI/MVNP mice developed exuberant bone lesions that closely resembled PD lesions.(9) In addition, OCL precursors isolated from p62KI/MVNP mice were hyperresponsive to both RANKL and 1,25-(OH)2D3, expressed elevated IL-6, and formed hypermultinucleated OCLs that were similar to OCL from PD patients. These results suggest that increasing IL-6 expression in OCLs of p62KI mice may induce pagetic lesions and a pagetic phenotype in p62KI mice in vivo.
To test this hypothesis, we generated transgenic mice overexpressing IL-6 in OCLs driven by the tartrate-resistant acid phosphatase (TRAP) promoter (TIL-6 mice), and crossed them with the p62KI mice. OCL precursors from p62KI/TIL-6 mice were hyperresponsive to 1,25-(OH)2D3 and RANKL compared to wild-type (WT). However, although these OCL had increased numbers of nuclei/OCL, the nuclear number was lower than in MVNP mice. Further, p62KI/TIL-6 mice did not form pagetic OCLs or bone lesions in vivo.
PD is characterized by increases in both osteoclast and osteoblast activity; we found that both of these occur in MVNP but not the p62KI mice we generated. These results raise the question of why osteoblast activity is not induced in our previously reported p62KI mice. We found that in contrast to MVNP mice, osteoblasts from p62KI mice expressed much lower levels of Runx2 and osterix, transcription factors necessary for osteoblast differentiation, and higher levels of Dickkopf 1 (DKK1), a Wnt antagonist. Treatment of osteoblast precursors from p62KI mice with IL-6 did not increase Runx2 or osterix and did not decrease DKK1 levels. These results suggest that MVNP expression in OCL induces other factors in addition to IL-6, which are necessary for the development PD lesions in mice.
Subjects and Methods
Generation of TRAP-IL6 transgenic mice
All studies were approved by the Institutional Animal Care and Use Committees at Indiana University School of Medicine, the University of Pittsburgh School of Medicine, and Virginia Commonwealth University. To generate the TRAP-IL-6 transgene construct, a 1.1-kb EcoRI endonuclease fragment containing a human IL-6 cDNA (ATCC cDNA number 67153; American Type Culture Collection [ATCC], Manassas, VA, USA) was inserted into the unique EcoRI site of the pKCR3-mTRAP vector.(13,14) pKCR3-mTRAP contains 1.9 kb of the mouse TRAP gene promoter and 5′-untranslated region (UTR), in addition to rabbit β-globin intron 2 and its flanking exons (for efficient transgene expression). A 4.2-kb injection fragment was then excised from the TRAP-IL-6 construct with Xhol restriction endonuclease, and transgenic mice were generated by standard methods in a CB6F1 (C57Bl/6 × Balb/c) genetic background.(15) p62KI mice carrying a proline-to-leucine mutation at residue 394 (equivalent to human p62P392L) have been described.(11) TRAP-MVNP transgenic mice have also been described.(8)
OCL formation from total transgenic mouse bone marrow
Bone marrow cells flushed from long bones of WT, p62KI, TIL-6, p62KI/TIL-6, or MVNP mice were cultured in 96-well plates (2 × 105 cells/well) with various concentrations of 1,25-(OH)2D3 (Teijin Pharma, Tokyo, Japan) or RANKL (R&D, Minneapolis, MN, USA) as described. The end of cultures, cells were stained for TRAP using a leukocyte acid phosphatase kit (Sigma, St. Louis, MO, USA), and TRAP-positive cells (≥3 nuclei/cell) were scored as OCLs.
OCL formation from purified osteoclast precursors
OCL formation from CD11b+ cells was performed as described.(16) Nonadherent cells were harvested and enriched for CD11b+ mononuclear cells using the Miltenyi Biotec (Auburn, CA, USA) MACS (Magnetic Cell Sorting) system. CD11b+ cells then were cultured in α modified essential medium (α-MEM) containing 10% fetal calf serum (FCS) plus 10 ng/mL of macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA) for 3 days to generate a population of enriched early OCL precursors. These cells were then cultured in α-MEM containing 10% FCS in the presence of 1,25-(OH)2D3 or RANKL for 3 to 4 days to generate OCLs. The cells were then stained for TRAP and TRAP-positive cells (≥3 nuclei/cell) were scored as OCLs.
Bone resorption assays of cultured OCLs
Bone marrow cells were cultured on mammoth dentin slices (Wako, Osaka, Japan) in α-MEM containing 10% FCS and 1,25-(OH)2D3 (1 × 10−8 M) or RANKL (100 ng/mL). After 14 days of culture, the cells were removed, the dentin slices stained with acid hematoxylin, and the areas of dentin resorption determined using image-analysis techniques (NIH ImageJ System).
Immunoblotting of OCL precursor lysates from WT, p62KI, TIL-6, or p62KI/TIL-6 mice
Total proteins were extracted from formed OCL and loaded on SDS gels (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were transferred to nitrocellulose membranes using a semidry blotter (Bio-Rad) and incubated in blocking solution (5% nonfat dry milk in Tris-buffered saline Tween-20 [TBST]) for 1 hour. Membranes were then exposed to primary antibodies overnight at 4°C, and incubated with immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated antibody for 1 hour. The blots were washed and visualized by an Immobilon Western Chemiluminescent detection system (Thermo).
RANKL ELISA assay
Mouse marrow stromal cells were isolated as described(11) and cultured with 1,25-(OH)2D3 for 7 days. Conditioned media from these cultures were harvested at the end of the culture period and the concentration of RANKL present was determined using an ELISA kit for mouse RANKL (R&D), according to the manufacturer’s instructions.
Quantitative microcomputed tomography measurements
The gross morphologic and microarchitectural characteristics of the distal area of the femur and L5 vertebra were examined by quantitative microcomputed tomography (μCT). The L5 vertebrae were used for microquantitative CT (μQCT) to assess the trabecular bone, and the femurs were used to measure mean cortical thickness. A three-dimensional (3D) analysis was done to determine bone volume fraction (BV/TV, %), trabecular number (Tb.N, N/μm2), trabecular thickness (Tb.Th, μm), and trabecular bone spacing (Tb.Sp, μm). Cortical bone also was analyzed in the femur 2 mm below the growth plate, and the same segmentation parameters were used for analysis.
Bone histomorphometric analyses
Mice were given calcein (10 mg/kg) on day 7 and day 2 prior to euthanasia. Lumbar vertebrae from WT, p62KI/TIL-6, or TIL-6 mice were subjected to qualitative histological examination and histomorphometry. The decalcified sections were stained for TRAP, and OCL containing active TRAP were stained red. The undecalcified sections were left unstained for the evaluation of fluorescent labels. The analysis was performed on the cancellous bone/marrow compartment between the cranial and caudal growth plates in the vertebral bodies without lesions using the OsteoMeasure XPTM version 1.01 morphometric programs (OsteoMetrics, Inc., Atlanta, GA, USA). Osteoclasts were defined as TRAP-positive mononuclear and multinuclear cells. Osteoclast surface (Oc.S/BS), cancellous bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), mineralizing surface (MS/BS), mineral apposition rate (MAR), and bone formation rate (BFR/BS) were analyzed—calculated and expressed—according to the recommendations of the ASBMR Nomenclature Committee.(17)
Isolation of primary osteoblasts
After flushing out the bone marrow from tibias and femora of p62KI, MVNP, and WT mice, the tibia and femurs were cultured in α-MEM with 10% FCS for 7 to 10 days. The bones were then placed in 60-mm dishes and the cultures were continued in α-MEM containing 10% FCS until cells growing out of the bones formed a confluent monolayer. The original bone was removed and the outgrowth cells from the bone were treated with 0.25% Trypsin and 0.05% EDTA for 10 minutes at 37°C. These cells were used as primary osteoblasts without further passage. The primary osteoblasts (2 × 105 cells/well in six-well plates) were cultured in α-MEM containing 10% FCS for 3 days and then IL-6 or vehicle was added for 4 additional days. Cell lysates were collected with lysate buffer. This isolation method was previously used to establish the MC3T3-E1 cell line.(18)
von Kossa staining
Primary osteoblasts derived from WT, p62KI, and MVNP mice were cultured in 10% FCS in α-MEM for 3 weeks with the media changed every 3 days. The cells were then fixed with 10% formaldehyde in PBS, and stained with von Kossa stain as described.(19)
Statistical analysis
For all cell culture studies, significance was evaluated using a two-tailed unpaired Student’s t test, with p < 0.05 considered to be significant.
Results
Characteristics of OCLs from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice
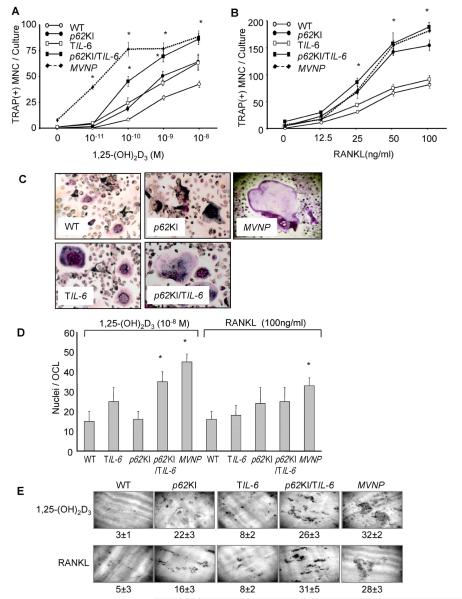
OCL precursors in total marrow cultures from MVNP mice, and to a lesser extent, p62KI/TIL-6 mice, were hyperresponsive to 1,25-(OH)2D3 compared to p62KI, TIL-6, and WT mice, and formed increased numbers of OCLs at 1 × 10−10 to 1 × 10−8 M 1,25-(OH)2D3 (Fig. 1A), suggesting that p62P394L and IL-6 can cooperate to promote an increased osteoclastogenic response to 1,25-(OH)2D3. OCLs from MVNP and p62KI/TIL-6 mice (and to a lesser extent TIL-6 mice) also had increased numbers of nuclei per OCL when treated with 1,25-(OH)2D3, compared to those from WT and p62KI mice (Fig. 1C, D). OCL precursors in marrow cultures from MVNP, p62KI/TIL-6, and p62KI mice also formed increased numbers of OCLs with RANKL treatment compared to TIL-6 and WT mice (Fig. 1B), suggesting that IL-6 does not contribute significantly to RANKL responsivity. Bone resorption in response to 1,25-(OH)2D3 (1 × 10−8 M) and RANKL (100 ng/mL) was comparable in marrow cultures from MVNP and p62KI/TIL6 mice, which were > p62KI > TIL-6 > WT (Fig. 1E).
Fig. 1.
Osteoclast formation in whole bone marrow cultures from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice. (A) OCL formation by treatment of 1,25-(OH)2D3. Data are expressed as the mean ± SD (n = 4); *p < 0.01, significantly different from OCLs formed with the same treatment in WT mouse cultures. (B) OCL formation by treatment of RANKL. Data are expressed as the mean ± SD (n = 4); *p < 0.01, significantly different from OCLs formed with the same treatment in WT mouse cultures. (C) Phenotype of OCLs formed from mouse bone marrow cultures. OCLs formed by 1,25-(OH)2D3 (1 × 10−8 M) were stained for TRAP. Magnification ×100. (D) Nuclei per OCL. The nuclear numbers per OCL were randomly counted in 25 OCLs formed in 1 × 10−8 M 1,25-(OH)2D3 or 100 ng/mL RANKL-treated cultures as in A and B. Data are expressed as the mean ± SD (n = 25); *p < 0.01, significantly different from OCLs formed with the same treatment in WT mouse cultures. (E) Bone resorption capacity of OCLs. Bone marrow cells were cultured for 7 days with 1,25-(OH)2D3 (1 × 10−8 M) or RANKL (100 ng/mL) on mammoth dentin slices. Values represent the amount of dentin surface resorption (%), mean ± SD (n = 4). WT = wild = type; OCL = osteoclast; 1,25-(OH)2D3 = 1,25-dihydroxyvitamin D3; TRAP = tartrate-resistant acid phosphatase; RANKL = receptor activator of NF-κB ligand.
OCL formation by highly purified populations of OCL precursors
OCL formation assays by highly purified populations of OCL precursors showed that only OCL precursors from TIL-6, p62KI/TIL-6, and MVNP mice were hyperresponsive to 1,25-(OH)2D3, compared to WT and p62KI derived cultures (Fig. 2A), demonstrating that the increased 1,25-(OH)2D3 responsivity seen in total marrow cultures from the p62KI mice (Fig. 1A) resulted from effects of stromal cells in these cultures. However, the relative OCL formation in response to RANKL by pure populations of OCL precursors was identical to that from the total marrow cultures; ie, OCL precursors from p62KI, p62KI/TIL-6, and MVNP and mice were hyperresponsive to RANKL compared to those from TIL-6 or WT mice (Fig. 2B). The nuclear number per OCL also showed the same pattern of results as seen in OCLs formed from whole marrow cultures (Fig. 2C). These results are consistent with our previous results showing that mutant p62 contributes to RANKL hyperresponsivity directly in OCLs, whereas its contribution to increased 1,25-(OH)2D3 responsivity is mediated through effects on stromal cells.(11) In contrast, IL-6 contributes to 1,25-(OH)2D3 responsivity directly in OCLs, but does not appear to have an affect on OCL response to RANKL.
Fig. 2.
Osteoclast formation formed by CD11b+ cells from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice. (A) OCL formation by 1,25-(OH)2D3. Data are expressed as the mean ± SD (n = 4); *p < 0.01, significantly different from OCLs formed with the same treatment in WT mouse cultures. (B) OCL formation by RANKL. Data are expressed as the mean ± SD (n = 4); *p < 0.01, significantly different from OCLs formed with the same treatment in WT mouse cultures. (C) Nuclei per OCL. The nuclear number per OCL was determined by randomly scoring 25 OCLs formed in 1 × 10−8 M 1,25-(OH)2D3 or 100 ng/mL of RANKL-treated cultures. Data are expressed as the mean ± SD (n = 25); *p < 0.01, significantly different from OCLs formed with the same treatment in WT mouse cultures. (D) TAF12 expression in OCLs. CD11b+ mononuclear cells were treated with 10 ng/mL of M-CSF for 3 days, then cultured with RANKL (100 ng/mL) for 4 days and cell lysates were collected. TAF12 expression was analyzed by immunoblot using antibodies recognizing TAF12 (ProteinTech). TFIIB was used as a loading control. WT = wild-type; RANKL = receptor activator of NF-κB ligand; OCL = osteoclast; 1,25-(OH)2D3 = 1,25-dihydroxyvitamin D3; M-CSF = macrophage colony-stimulating factor; TFIIB = transcription factor IIB.
We recently reported that IL-6 induces expression of TAF12, a novel coactivator of VDR-mediated transcription that is increased in OCLs from PD patients and MVNP mice.(20) Therefore, we determined if TAF12 expression was increased in OCLs from p62KI, TIL-6, and p62KI/TIL-6 mice. OCLs formed by highly purified OCL precursors from TIL-6 and p62KI/TIL-6 mice expressed elevated levels of TAF12 compared to WT (Fig. 2D). In contrast, TAF12 was not increased in OCLs from p62KI mice.
RANKL expression by marrow stromal cells derived from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice
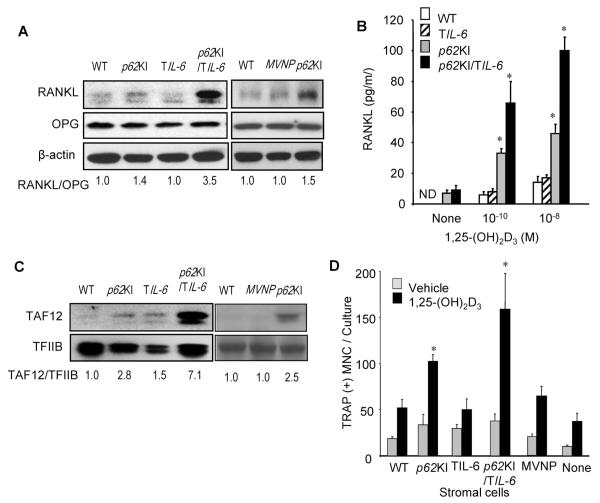
We previously found that marrow stromal cells from p62KI but not MVNP mice have increased expression of TAF12 which resulted in enhanced RANKL production by the stromal cells when treated with low concentrations of 1,25-(OH)2D3.(7,8). Therefore, we measured RANKL production by stromal cells from p62KI, TIL-6, p62KI/TIL-6, MVNP, and WT mice. Stromal cells from p62KI/TIL-6 and p62KI mice treated with 1,25-(OH)2D3 produced increased levels of RANKL when treated with 1 × 10−10 M 1,25-(OH)2D3 (Fig. 3A). Interestingly, p62KI/TIL-6 stromal cells produced twofold more secreted RANKL than p62KI stromal cells (Fig. 3B). Both the RANKL/osteoprotegerin (OPG) ratio (Fig. 3A) and TAF12 levels (Fig. 3C) were markedly increased in stromal cells from p62KI/TIL-6 mice but not in MVNP mice. These results demonstrate that high levels of IL-6 produced by TIL-6 mice also induce TAF12 in marrow stromal cells, which enhances their responsivity to 1,25-(OH)2D3 and results in increased RANKL production by stromal cells from p62KI/TIL-6 mice treated with 1,25-(OH)2D3.
Fig. 3.
Support of OCL formation by marrow stromal cells from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice. (A) RANKL and OPG expression. Stromal cells from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice were cultured with 1,25-(OH)2D3 (1 × 10−8 M) for 2 days, the cell lysates were collected, and the levels of RANKL and OPG were determined by Western blot analysis using anti-RANKL and anti-OPG antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The ratio of RANKL/OPG expression levels from Western blots were quantitated by densitometry using WT cultures as 1.0. (B) RANKL production by mouse marrow stromal cells. Mouse marrow stromal cells were cultured with 1,25-(OH)2D3 for 7 days. Conditioned media from these cultures were harvested at the end of the culture period and the concentration of RANKL present was determined. The data is shown as mean ± SD (n = 4); *p < 0.01 compared with WT cells cultured with the same concentration of 1,25-(OH)2D3. (C) TAF12 expression in marrow stromal cells. Stromal cells were cultured with 10% FCS in IMDM for 3 days and then cell lysates were collected. TAF12 expression was analyzed by immunoblot using a polyclonal antibody recognizing TAF12. TFIIB was used as a loading control. (D) Support of OCL formation by marrow stromal cells. Stromal cells from WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP mice were cocultured with CFU-GM derived from WT mouse bone marrow in the presence of 1 × 10−8 M 1,25-(OH)2D3 for 7 days. The cells were then fixed and stained for TRAP, and the TRAP-positive OCLs were counted. Results are expressed as the mean ± SD (n = 4); *p < 0.01 compared with results in WT cultures. OCL = osteoclast; WT = wild-type; RANKL = receptor activator of NF-κB ligand; OPG = osteoprotegerin; 1,25-(OH)2D3 = 1,25-dihydroxyvitamin D3; FCS = fetal calf serum; IMDM = Iscove’s Modi fied Dulbecco’s Media; TFIIB = transcription factor IIB; CFU-GM = colony-forming unit–granulocytemacrophage; TRAP = tartrate-resistant acid phosphatase.
Next we examined if WT, p62KI, TIL-6, p62KI/TIL-6, and MVNP stromal cells differentially supported OCL formation. Stromal cells were cocultured with colony-forming unit–granulocytemacrophage (CFU-GM)-derived cells (OCL precursors) from WT mice with 1 × 10−8 M 1,25-(OH)2D3 or vehicle for 7 days. As shown in Fig. 3D, stromal cells derived from p62KI/TIL-6 mice, and to a lesser extent p62KI mice, had an increased capacity to support OCL formation in response to 1,25-(OH)2D3. Because stromal cells from MVNP mice do not express increased TAF12, they did not increase OCL formation when cocultured with WT OCL precursors treated with 1,25-(OH)2D3 (Fig. 3D).
Bone phenotype of p62KI/TIL-6 mice
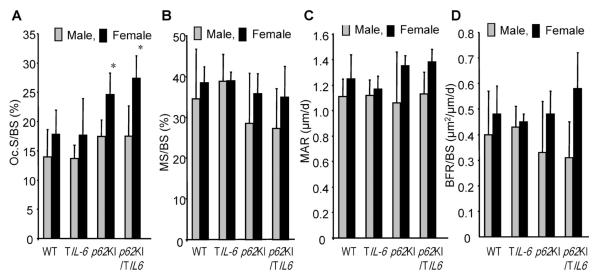
To determine whether coexpression of mutant p62 and IL-6 in the bone promote the development of pagetic lesions, we examined lumbar vertebral bone from p62KI, TIL-6, p62KI/TIL-6, and WT mice at 12 months of age by qualitative histology and histomorphometry, and femurs and L5 vert6ebra by μCT. No pagetic lesions were found in the lumbar vertebrae of any of these mice. Further, μQCT histomorphometric analysis revealed no significant differences between mice of any of the four genotypes in bone structural variables (cancellous BV/TV, Tb.N, Tb.Wi, Tb.Sp) (Table 1), nor in the mineralized surface (Md.Pm), MAR, and BFR (Fig. 4). Only OCL numbers per bone surface were significantly increased in both p62KI and p62KI/TIL-6 mice (Fig. 4).
Table 1.
Structural Histomorphometric Variables of WT, TIL-6, p62KI, and p62KI/TIL-6 Mice
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT (n = 7) |
TIL-6 (n = 7) |
p62KI (n = 6) |
p62KI/TIL-6 (n = 3) |
WT (n = 7) |
TIL-6 (n = 10) |
p62KI (n = 7) |
p62KI/TIL-6 (n = 10) |
|
| BV/TV (%) | 13.4±2.1 | 13.1±5.9 | 13.5±5.8 | 14.6±4.5 | 13.3±5.6 | 11.9±4.3 | 10.2±2.5 | 11.2±3.9 |
| Tb.Th (μm) | 31.4±3.1 | 33.1±5.6 | 36.2±8.5 | 33.1±5.0 | 33.3±4.5 | 35.5±5.3 | 35.5±3.6 | 37.1±9.8 |
| Tb.N (1/mm2) | 4.3±0.4 | 3.8±1.1 | 3.7±1.3 | 4.4±0.9 | 4.0±1.6 | 3.5±1.7 | 2.9±0.6 | 3.0±0.1 |
| Tb.Sp (μm) | 204.4±22.3 | 248.1±86.0 | 265.4±121.9 | 203.0±48.1 | 239.6±73.0 | 312.0±169.1 | 327.1±84.3 | 313.0±84.2 |
Structural variables for the lumbar vertebral bodies from 12-month-old WT, p62KI, TIL-6, and p62KI/TIL-6 mice. Data are expressed as mean ± SD. No significant differences between WT and other mice in all the variables.
WT = wild-type; BV/TV = cancellous bone volume; Tb.Th = trabecular thickness; Tb.N = trabecular number; Tb.Sp = trabecular separation.
Fig. 4.
Histomorphometric analysis of WT, TIL-6, p62KI, and p62KI/TIL-6 lumbar vertebra from 12-month-old. (A) OC.Pm, (B) Md.Pm, (C) MAR, and (D) BFR for WT, TIL-6, p62KI, and p62KI/TIL-6 mice are shown. Data represent mean ± SD for WT (7 male, 7 female), p62KI (6 male, 7 female), TIL-6 (7 male, 10 female), and p62KI/TIL-6 (3 male, 10 female) mice per group. *p < 0.01 significant differences between WT and p62KI/TIL-6 mice were detected. WT = wild-type; OCL = osteoclast; OC.Pm = OCL surface; Md.Pm = mineralized surface; MAR = mineral apposition rate; BFR = bone formation rate.
Expression of OCL fusion molecules in OCL precursors
Because OCL precursors from MVNP mice and pagetic patients expressing MVNP form OCLs with increased nuclei per OCL, we measured the expression levels of several fusion molecules in MVNP, p62KI, and WT OCL precursors treated with IL-6 for 4 days. OCLs formed from MVNP mice with or without IL-6 treatment had elevated expression of dendritic cell-specific transmembrane protein (DC-STAMP) compared with those from p62KI and WT mice (Fig. 5). The expression levels of the d2 isoform of the v-ATPase V0 domain (ATP6v0d2) and a distintegrin and a metalloproteinase domain-8 (ADAM8) were only modestly elevated in MVNP OCL (Fig. 5).
Fig. 5.
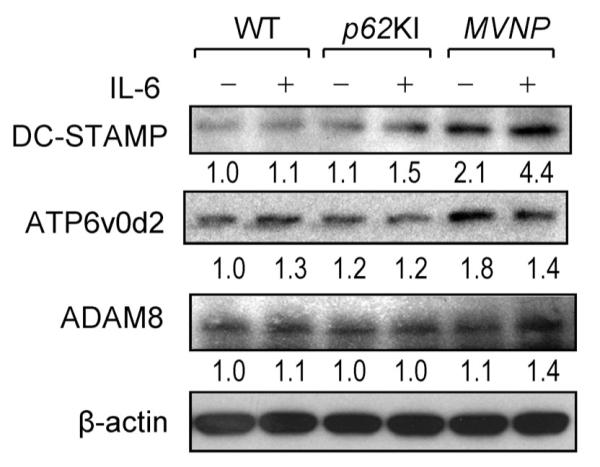
The expression of fusion molecules in WT, p62KI, and MVNP OCL precursors. CD11b+ mononuclear cells were treated with 10 ng/mL M-CSF for 3 days, then treated with or without mouse IL-6 (10 ng/mL) (R&D) and mouse IL-6 receptor (10 ng/mL) (R&D) for 4 days. Cell lysates were analyzed by immunoblot using antibodies recognizing DC-STAMP (Cosmo Bio Co. Ltd, Tokyo, Japan), ATP6v0d2 (Abnova Co., Taipei, Taiwan), ADAM8 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (Abcam, Cambridge, MA, USA) as a loading control. WT = wild-type; OCL = osteoclast; M-CSF = macrophage colony-stimulating factor; IL-6 = interleukin 6; DC-STAMP = dendritic cell-speci fic transmembrane protein; ATP6v0d2 = the d2 isoform of the v-ATPase V0 domain; ADAM8 = a distintegrin and a metalloproteinase domain-8.
Effect of IL-6 on osteoblast differentiation
Our previous data demonstrated that IL-6 was required to increase bone formation and induce a pagetic phenotype in MVNP mice.(4) It was thus our hypothesis that increasing IL-6 expression in OCLs of p62KI mice would result in development of a pagetic bone lesions in p62KI/TIL-6 mice. We therefore examined the effects of IL-6 on osteoblast differentiation by primary osteoblasts from p62KI, MVNP, and WT mice.
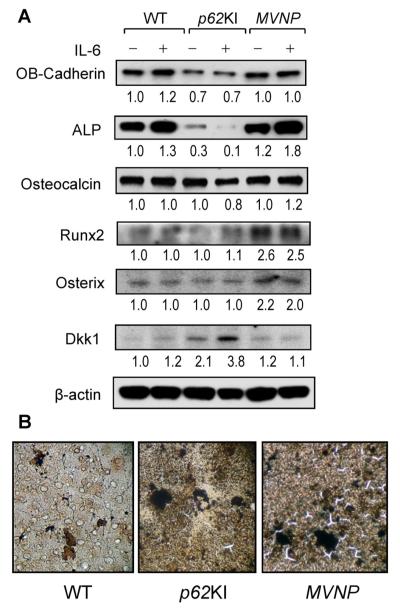
We found that there was a twofold increase in the levels of Runx2 and Osterix in osteoblasts from MVNP mice compared with WT and p62KI mice. These parameters were not affected by IL-6 treatment of WT, MVNP, or p62KI osteoblasts (Fig. 6A). In contrast, IL-6 treatment of MVNP osteoblasts modestly enhanced alkaline phosphatase (ALP) expression. The levels of osteocalcin expression were not different in WT, p62KI, and MVNP osteoblasts and IL-6 did not increase osteocalcin expression (Fig. 6A). Because high expression levels of Dickkopf 1 (Dkk1) can inhibit osteoblast differentiation,(21) we measured Dkk1 levels in WT, p62KI, and MVNP osteoblasts. Dkk1 expression in p62KI osteoblasts was elevated twofold and increased to 3.8-fold with IL-6 treatment (Fig. 6A). In contrast, MVNP and WT osteoblasts had much lower levels of Dkk1 that were not affected by IL-6 treatment (Fig. 6A). Osteoblast (OB)-cadherin and ALP were decreased in the p62KI mice compared to WT and MVNP, and were inversely correlated with Dkk1 expression, which was elevated in p62KI compared to WT and MVNP; RUNX2 and osterix were induced twofold in MVNP mice.
Fig. 6.
Osteoblast differentiation markers in osteoblasts derived from WT, p62KI, and MVNP mice. (A) Expression of osteoblast differentiation markers. Primary osteoblasts (2 × 105 cells/35-mm dish) were cultured with or without 10 ng/mL of IL-6 for 4 days in 10% FCS in α-MEM. Cell lysates were analyzed by immunoblot using antibodies recognizing OB-Cadherin (Cell Signaling, Beverly, MA, USA), alkaline phosphatase (Millipore, Billerica, MA, USA), Runx2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Osterix (Abcam, Cambridge, MA, USA), osteocalcin (Millipore), Dkk1 (Cell Signaling), and β-actin (Abcam) as loading control. (B) Calcification in vitro. Osteoblasts were cultured in 10% FCS with α-MEM for 3 weeks as described in Subjects and Methods. The cells were stained with von Kossa stain (×100). WT = wild-type; IL-6 = interleukin 6; FCS = fetal calf serum; α-MEM = α modified essential medium.
We then measured the mineral deposition capacity of the osteoblasts by von Kossa staining. MVNP osteoblast cultures showed increased numbers of calcified areas compared with cultures of p62KI and WT osteoblasts (Fig. 6B). These results suggest that the osteoblast differentiation capacity of osteoblast from p62KI mice is much lower than osteoblast from MVNP mice.
Discussion
Both environmental elements and genetic causes both contribute to PD. We found that the expression of both MVNP and the SQSTM1 (p62) mutation P392L in OCLs contribute to the increased OCL activity in PD, and we have reported that p62P392L knock-in mice do not develop pagetic lesions unless MVNP is also present. When MVNP is present with the p62KI mutation, mice develop exuberant pagetic lesions very similar to those seen in patients with PD of bone. However, Daroszewska and colleagues(22) reported that a similar p62P394L knock-in mouse develops small focal lesions which showed increases in bone turnover with increased bone resorption and formation, disruption of the normal bone architecture, and an accumulation of woven bone. The basis for the differences in these two knock-in models is unclear at this time but demonstrate that the capacity of mutant p62 to induce pagetic lesions in vivo is variable. MVNP, but not p62KI, mice have increased IL-6 production and loss of IL-6 blocks the effects of MVNP in PD.(9,11,12) These results suggest p62KI in combination with high IL-6 in OCL may result in PD. To address this question, we generated p62KI/TIL-6 transgenic mice by breeding p62KI mice to TIL-6 mice in which overexpression of IL-6 is driven by the TRAP promoter, and characterized their OCLs and bone phenotype.
OCL precursors from p62KI/TIL-6 mice formed OCL that expressed an intermediate pagetic phenotype in vitro (Fig. 1). The OCLs were hyperresponsive to 1,25-(OH)2D3 and RANKL, formed OCL with increased bone resorbing capacity and elevated levels of TAF12 but developed only modest multinuclearity (Figs. 1, 2) compared to MVNP mice.
In contrast, OCL precursors from p62KI and WT mice were not hyperresponsive to 1,25-(OH)2D3, expressed normal levels of TAF12, and formed normal OCLs.(11) Only OCL precursors from p62KI/TIL-6 were hyperresponsive to RANKL, whereas both p62KI/TIL-6 and TIL-6 cells had increased STAT3 signaling (Fig. 2D). p62KI and WT OCL had normal ratios of nuclei/OCL when treated with 1,25-(OH)2D3 or RANKL. These results suggested that expression of IL-6 in p62KI OCL precursors is required for OCLs to express a pagetic phenotype in vitro, and that high levels of IL-6 enhances OCL precursor fusion in p62KI mice. The enhanced OCL precursor fusion in MVNP mice most likely reflects the increased expression of DC-STAMP(23,24) in their OCL precursors, which was enhanced by IL-6 treatment (Fig. 5). DC-STAMP appears to be increased selectively in MVNP OCL precursors compared with other fusion molecules associated with increased OCL precursor fusion (eg, D44, CD48, and ADAM8), and was not increased significantly in p62KI and WT OCL precursors (Fig. 5). Lee and colleagues(25) reported that increased nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) through upregulation of c-Fos increased expression of DC-STAMP. Because IL-6 increases expression of c-Fos and NFATc1, this may explain its capacity to enhance DC-STAMP expression. ATP6v0d2 also was upregulated modestly (1.8-fold) in MVNP OCL precursors. This mostly reflects that NFATc1 can also enhance expression of this fusion molecule.(26) Further, IL-6 enhances p38 mitogen-activated protein kinase (MAPK) signaling in MVNP OCL precursors (data not shown), which may also contribute to the hypermultinuclearity of OCLs formed in marrow cultures from MVNP mice. We previously reported that enhanced p38 MAPK signaling plays a critical role in the increased nuclear number per OCL in OCLs expressing the measles virus nucleocapsid gene.(9)
Marrow stromal cells from p62KI/TIL-6 expressed higher levels of RANKL in response to 1,25-(OH)2D3 than the other mouse marrow stromal cells (Fig. 3A). The RANKL/OPG expression ratio in stromal cells from p62KI/TIL-6 was increased 3.5-fold compared with WT (Fig. 3A). The stromal cells from p62KI/TIL-6 also expressed high levels of TAF12. The expression of TAF12 in stromal cells can result in hyperresponsivity to 1,25-(OH)2D3 and increased VDR transcription because at high levels, TAF12 acts as a coactivator of VDR transcription.(20) Why p62KI/TIL-6 had higher expression of RANKL compared with p62KI stromal cells is not clear. Possibly, p62P394L and IL-6 have additive effects on VDR-TAF12 mediated transcription. These findings may in part explain the enhanced RANKL production present in the marrow microenvironment of pagetic patients.
p62KI/TIL-6 mice did not develop pagetic bone lesions or structural characteristics seen in pagetic patients. They only had increased OCL perimeter scores (Fig. 4A). In contrast, dynamic bone formation variables were similar to those in WT mice (Fig. 4, Table 1). These results suggest IL-6 is not enhancing osteoblast activity. Franchimont and colleagues(27) report that IL-6 can modulate osteoblast proliferation, differentiation, and apoptosis, and supports osteoblast generation. However, as shown in Fig. 6, IL-6 only increased ALP expression in osteoblasts from MVNP mice. These results suggest high levels of IL-6 are not sufficient to induce the enhanced bone formation characteristic of PD.
Interestingly, p62KI osteoblasts had increased expression of the Wnt signaling antagonist, Dkk1, which was further increased by IL-6 (Fig. 6A). Naot and colleagues(28) reported increased expression of Dkk1 in osteoblast cultures from Paget’s patients. The canonical Wnt pathway plays a key role in regulating osteoblast proliferation and differentiation.(29) Tian and colleagues(21) have suggested that the release of Dkk1 from malignant plasma cells in multiple myeloma results in an inhibition of osteoblast proliferation, accentuating the imbalance between bone formation and bone resorption and facilitating local bone loss. In the p62KI/TIL-6 mice, overproduction of Dkk1 in osteoblasts could have a similar effect on bone formation. Possibly increased levels of IL-6 are responsible for the overexpression of Dkk1 in PD and contribute to the development of the lytic phase of PD through further accelerating local bone turnover. These results may explain in part why p62KI/TIL-6 mice did not develop pagetic lesions in vivo.
In summary, these results demonstrate that p62P394L and IL-6 in combination increase OCL formation and activity, but are not sufficient to induce pagetic OCL and bone lesions characteristic of PD in vivo. Further, based on our findings that loss of IL-6 in MVNP mice results in loss of their pagetic phenotype, these results demonstrate that IL-6 is necessary but not sufficient to induce PD. These data further demonstrate that expression of high IL-6 in OCL confers many of characteristics of PD OCL (hyperresponsivity to 1,25-(OH)2D3, increased nuclei/OCL, increased bone resorption) but is not sufficient by itself or in combination with p62P394L to induce PD. Thus, other factors induced by MVNP may also be required to enhance bone formation characteristics of PD, such as coupling factors or osteoblast stimulating factors. Recently, we found that MVNP but not p62P394L increased expression of ephrinB2/EphB4, insulin-like growth factor 1 (IGF1), and semaphorin3A, suggesting MVNP has multiple effects beyond upregulating IL-6 to induce PD (ASBMR 2013 Abstract).(30)
Acknowledgments
This work was supported by R01-AR057308 (to GDR) and R01-AR057310 (to DLG) from NIH-NIAMS, 5P30CA016059 (to JJW) from NIH/NCI Cancer Center support grant, and W81XWH-12-1-0533 (to NK) from Department of Defense. This research project was provided the VCU transgenic/knockout Mouse Shared Resource.
Footnotes
Disclosures GDR is a consultant to Amgen. All other authors state that they no conflicts of interest.
References
- 1.Hosking DJ. Paget’s disease of bone. Br Med J (Clin Res Ed) 1981;283:686–8. doi: 10.1136/bmj.283.6293.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, Simon LS. Metabolic consequences of bone turnover in Paget’s disease of bone. Clin Orthop. 1987;217:26–36. [PubMed] [Google Scholar]
- 3.Siris ES, Roodman GD. Paget’s Disease of Bone, Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, Chapter 80. American Society for Bone and Mineral. 2013:659–669. [Google Scholar]
- 4.Roodman GD, Kurihara N, Ohsaki Y, et al. Interleukin 6. A potential autocrine/paracrine factor in Paget’s disease of bone. J Clin Invest. 1992;89:46–52. doi: 10.1172/JCI115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyland JA, Freemont AJ, Sharpe PT. Interleukin-6, IL-6 receptor, and IL-6 nuclear factor gene expression in Paget’s disease. J Bone Miner Res. 1994;1:75–80. doi: 10.1002/jbmr.5650090111. [DOI] [PubMed] [Google Scholar]
- 6.Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115:200–8. doi: 10.1172/JCI24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurihara N, Reddy SV, Menaa C, et al. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–14. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurihara N, Zhou H, Reddy SV, et al. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget’s disease-like bone lesions in mice. J Bone Miner Res. 2006;21:446–55. doi: 10.1359/JBMR.051108. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara N, Hiruma Y, Yamana K, et al. Contributions of the measles virus nucleocapsid gene and the SQSTM1/p62(P392L) mutation to Paget’s disease. Cell Metab. 2011;13:23–34. doi: 10.1016/j.cmet.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundaram K, Shanmugarajan S, Rao DS, et al. Mutant p62P392L stimulation of osteoclast differentiation in Paget’s disease of bone. Endocrinology. 2011;152:4180–9. doi: 10.1210/en.2011-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiruma Y, Kurihara N, Subler MA, et al. A SQSTM1/p62 mutation linked to Paget’s disease increases the osteoclastogenic potential of the bone microenvironment. Hum Mol Genet. 2008;17:3708–19. doi: 10.1093/hmg/ddn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurihara N, Hiruma Y, Zhou H, et al. Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J Clin Invest. 2007;117:133–42. doi: 10.1172/JCI28267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy SV, Scarcez T, Windle JJ, et al. Cloning and characterization of the 5′-flanking region of the mouse tartrate-resistant acid phosphatase gene. J Bone Miner Res. 1993;8:1263–70. doi: 10.1002/jbmr.5650081015. [DOI] [PubMed] [Google Scholar]
- 14.Reddy SV, Hundley JE, Windle JJ, et al. Characterization of the mouse tartrate resistant acid phosphatase (TRAP) gene promoter. J Bone Miner Res. 1995;4:601–6. doi: 10.1002/jbmr.5650100413. [DOI] [PubMed] [Google Scholar]
- 15.Nagy A, Gertsenstein M, Vintersten K, et al. A laboratory manual. 3rd ed CSHL Press; Cold Spring Harbor, NY: 2003. Manipulating the mouse embryo. [Google Scholar]
- 16.Ishizuka H, García-Palacios V, Lu G, et al. ADAM8 enhances osteoclast precursor fusion and osteoclast formation in vitro and in vivo. J Bone Miner Res. 2011;26:169–81. doi: 10.1002/jbmr.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization ofnomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 18.Sudo H, Kodama HA, Amagai Y, et al. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–8. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meloan SN, Puchtler H. Chemical mechanisms of staining methods: von Kossa’s technique. What von Kossa really wrote and a modified reaction for selective demonstration of inorganic phosphate. J Histotechol. 1985;8:11–3. [Google Scholar]
- 20.Kurihara N, Reddy SV, Araki N, et al. Role of TAFII-17, a VDR binding protein, in the increased osteoclast formation in Paget’s disease. J Bone Miner Res. 2004;19:1154–64. doi: 10.1359/JBMR.040312. [DOI] [PubMed] [Google Scholar]
- 21.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 22.Daroszewska A, van’t Hof RJ, Rojas JA, et al. A point mutation in the ubiquitin-associated domain of SQSMT1 is sufficient to cause a Paget’s disease-like disorder in mice. Hum Mol Genet. 2011;20:2734–44. doi: 10.1093/hmg/ddr172. [DOI] [PubMed] [Google Scholar]
- 23.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–51. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagi M, Miyamoto T, Toyama Y, Suda T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J Bone Miner Metab. 2006;24:355–8. doi: 10.1007/s00774-006-0697-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee MS, Kim HS, Yeon JT, et al. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J Immunol. 2009;183:3390–9. doi: 10.4049/jimmunol.0804314. [DOI] [PubMed] [Google Scholar]
- 26.Kim K, Lee SH, Ha Kim J, et al. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol Endocrinol. 2008;22:176–8. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchimont N, Wertz S, Malaise M. Interleukin-6: an osteotropic factor influencing bone formation? Bone. 2005;37:601–6. doi: 10.1016/j.bone.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Naot D, Bava U, Matthews B, et al. Differential gene expression in cultured osteoblasts and bone marrow stromal cells from patients with Paget’s disease of bone. J Bone Miner Res. 2007;22:298–309. doi: 10.1359/jbmr.061108. [DOI] [PubMed] [Google Scholar]
- 29.Gong Y, Slee RB, Fukai N, et al. Osteoporosis-Pseudoglioma Syndrome Collaborative Group. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 30.Kurihara N, Teramachi J, Kitagawa Y, et al. IFGI contributes to the increased bone formation by measles virus nucleocapsid protein expressed by osteoclasts in Paget’s Bone Disease. J Bone Miner Res. 2013;28(sup 1) (Abstract #FR440) [Google Scholar]