Abstract
Cellular reprogramming from adult somatic cells into an embryonic cell–like state, termed induced pluripotency, has been achieved in several cell types. However, the ability to reprogram human amniotic epithelial cells (hAECs), an abundant cell source derived from discarded placental tissue, has only recently been investigated. Here we show that not only are hAECs easily reprogrammed into induced pluripotent stem cells (AE-iPSCs), but hAECs reprogram faster and more efficiently than adult and neonatal somatic dermal fibroblasts. Furthermore, AE-iPSCs express higher levels of NANOG and OCT4 compared to human foreskin fibroblast iPSCs (HFF1-iPSCs) and express decreased levels of genes associated with differentiation, including NEUROD1 and SOX17, markers of neuronal differentiation. To elucidate the mechanism behind the higher reprogramming efficiency of hAECs, we analyzed global DNA methylation, global histone acetylation, and the mitochondrial DNA A3243G point mutation. Whereas hAECs show no differences in global histone acetylation or mitochondrial point mutation accumulation compared to adult and neonatal dermal fibroblasts, hAECs demonstrate a decreased global DNA methylation compared to dermal fibroblasts. Likewise, quantitative gene expression analyses show that hAECs endogenously express OCT4, SOX2, KLF4, and c-MYC, all four factors used in cellular reprogramming. Thus, hAECs represent an ideal cell type for testing novel approaches for generating clinically viable iPSCs and offer significant advantages over postnatal cells that more likely may be contaminated by environmental exposures and infectious agents.
Introduction
Induced pluripotent stem cells (iPSCs) have been generated from adult somatic cells in both mouse and human with defined transcription factor transduction (Maherali et al., 2007; Park et al., 2008; Takahashi et al., 2007; Takahashi and Yamanaka, 2006; Yu et al., 2007). The concept of induced pluripotency from somatic cells has gained tremendous attention in both basic and clinical research over the past several years. New technologies to reprogram target cells safely and more efficiently have been developed with stunning speed, from using mRNAs to recombinant proteins (Kim and de Vellis, 2009; Stadtfeld et al., 2008; Warren et al., 2010; Yakubov et al., 2010; Zhou et al., 2009). On the other hand, emerging new findings on iPSCs indicate the importance of target cell selection (Aoi et al., 2008). Overall, the somatic cell type and age of the parental cells are critical factors for generating quality iPSCs. Research data suggest that epithelial cells are easier to reprogram than fibroblasts (Aasen et al., 2008), and the reprogramming efficiency of fetal cells is higher than in adult somatic cells (Carey et al., 2010; Markoulaki et al., 2009). It is speculated that reprogramming efficiency variations are dependent on the epigenetic memory of the parental cells (Hu et al., 2010). Recently, the epigenetic memory in human iPSCs was clearly demonstrated by whole-genome DNA profile analysis using shotgun bisulfate-sequencing methods (Lister et al., 2011). Therefore, an effort to find suitable parental cell sources is one of the essential tasks for future clinical applications of iPSCs.
Human amniotic epithelial cells (hAECs) are derived from the epiblast stage at 8 days after fertilization. Previously, we have shown that hAECs express embryonic stem cell (ESC) markers and have the potential to differentiate into cells of all three germ layers (Miki et al., 2005). Because hAECs are obtained at birth, these cells exhibit intact host DNA that carries little to no environmental or age-acquired DNA damage. Recently amniotic fluid (AF) cells, which share the unique advantages of neonatal cells such as hAECs, were shown to reprogram more efficiently than human foreskin (Galende et al., 2010; Li et al., 2009; Trovato et al., 2009). However, AF cells, which are normally obtained during clinical sampling for fetal health diagnoses, are acquired through an invasive procedure (amniocentesis) and often yield low cell numbers. hAECs can be isolated in relatively large quantities (average±SD, 100.25±81.8×106) from one placenta after delivery, thus requiring no invasive measures to obtain suitable cells (Miki et al., 2005). Two additional advantages to using hAECs obtained from discarded placentae as the parental cell source for iPSCs are: (1) the abundance of placental tissue available worldwide and (2) the lack of an invasive cell procurement technique for isolating hAECs (Miki, 2011). Here, we examined the reprogramming efficiency of hAECs compared to neonatal and adult fibroblasts and investigated the basal reprogramming gene expression, methylation status, histone acetylation status, and mitochondrial DNA damage status of primary hAECs to elucidate the higher cellular reprogramming efficiency of these unique, but abundant cells.
Materials and Methods
hAECs isolation and primary culture
Human placentae were obtained with the approval of the institutional review board (IRB), University of Pittsburgh, after uncomplicated elective cesarean deliveries from healthy mothers. Human AECs were isolated by following the published protocol (Miki et al., 2010). In brief, the amnion layer was mechanically peeled off from the chorion and washed several times with Hanks' balanced salt solution (HBSS) without calcium and magnesium to remove blood. To dissociate hAECs, the amnion membrane was incubated at 37°C with 0.05% trypsin containing 0.53 mM EDTA (Invitrogen). Cells from the first 10-min digestion were discarded to exclude debris. The cells from the second and third 40-min digestions were pooled and washed three times with HBSS. The viability of the hAECs was determined by exclusion of Trypan Blue dye and counted with a hemocytometer.
Human foreskin fibroblast and human ESC culture
Human foreskin fibroblasts (HFFs; American Type Culture Collection, Manassas, VA, USA) and human dermal fibroblasts (ScienCell Research Laboratories, Carlsbad, CA, USA) were purchased and cultured in 10% fetal bovine serum Dulbecco's modified Eagle's medium (FBS DMEM) with 1 mM L-glutamine, 1 mM nonessential amino acids, and 1% penicillin/streptomycin (all from Invitrogen). National Institutes of Health (NIH) Registry-approved WA01 (H1) and WA09 (H9) human (h) hESCs (Wicell, Madison, WI), used for comparisons with induced pluripotent stem cell lines derived in this study, were continually cultured in feeder-free culture conditions on hESC-qualified Matrigel (BD Biosciences) with mTeSR1 medium (STEMCELL Technologies). Medium changes occurred daily, and cells were passaged every 5–6 days using Dispase (STEMCELL Technologies) as per the manufacturer's instructions.
Lentiviral gene transduction
Reprogramming was conducted with a single lentiviral vector, STEMCCA, kindly provided by Dr. Gustavo Mostoslavsky (Boston University) (Sommer et al., 2009), which contains OCT4, SOX2, KLF4, and c-MYC. Approximately 100,000 hAECs or fibroblast cells were infected with lentiviral particles containing STEMCCA in 1 mL of standard hAEC medium (DMEM supplemented with 10% FBS, 10 ng/mL epidermal growth factor, L-glutamine, nonessential amino acids, and penicillin-streptomycin) with 6 μg/mL polybrene. A second transduction was performed with the same conditions after 24 h. On the following day, cells were dissociated with brief trypsinization and reseeded onto human ESC-qualified Matrigel- (BD Bioscience, Franklin Lakes, NJ, USA) coated wells of six-well plates. The culture medium was changed to mTeSR-1 medium (StemCell Technologies, Vancouver CA, USA) on the next day and changed every day thereafter. Colony formations were examined daily by microscopic observations. Nuclear reprogramming was repeated three times using naïve hAECs isolated from three placentae. Six independent reprogramming procedures were performed for each case. After derivation, all iPSCs were cultured in feeder-free conditions on human ESC-qualified Matrigel (BD Biosciences) with mTeSR-1 medium (STEMCELL Technologies). Our iPSC derivation protocol was approved by the University of Pittsburgh Human Stem Cell Research Oversight (hSCRO) committee (hSCRO# ES-07-012-C).
Stem cell marker immunofluorescence
Stem cell marker protein expression was confirmed with immunofluorescence analysis. Colonies on each well were fixed in 4% paraformaldehyde. Immunofluorescence (IF) was performed with the following antibodies; Oct-4, Sox-2, Nanog, SSEA4, and TRA-1-60 (Stem-Lite Kit, Cell Signaling Technologies, Danvers, MA, USA). Negative controls were incubated with appropriate isotype control antibodies and secondary antibodies. Appropriate secondary antibodies for IF were from Invitrogen. Nuclei were visualized with 4′-6-diamidino-2-phenylindole (DAPI) staining.
Stem cell and differentiation marker gene expression
Total RNA was isolated with TRIzol (Invitrogen). Two micrograms of RNA were used per reverse transcriptase reaction performed with ImProm-II Reverse Transcription System (Promega, Madison, WI). Reverse transcriptase (RT) and no-RT reactions were performed identically, except that in no-RT reactions water replaced reverse transcriptase. The TaqMan Human Stem Cell Pluripotency Array (Applied BioSystems, Foster City, CA, USA) was used following the manufacturer's instructions. Gene expression was analyzed using SDS 2.2.2 software (Applied BioSystems). Expression fold changes were calculated using the −ΔCt method and normalized using β-actin as the endogenous control. Gene expression levels for each iPSC line were compared to human ESC gene expression.
Teratoma formation assay
Teratoma formation assay was conducted to test the pluripotency of derived iPSCs at the Transgenic and Molecular Core Facility of Magee-Women's Research Institute. Briefly, teratoma formation was induced by injecting into severe combined immunodeficiency (SCID)-beige mice 3×106 cells at passage 20 after iPSC colonies were established. When tumor size exceeded 20 mm in diameter, animals were sacrificed and the tumor was harvested. Teratomas were fixed overnight in 4% paraformaldehyde (PFA) and subjected it to histological examination using Hematoxylin & Eosin (H&E) staining.
Karyotype analysis of iPSCs
To investigate the genetic stability of AE-iPSCs and HFF-1 iPSCs, the karyotype of iPSCs was determined by standard G-banding procedure at passages 6, 12, 20, and 30. Briefly, cells in single suspension were dropped onto a precleaned glass slide and placed in an oven at 55–65°C for 45 min. Slides were then incubated in 2× standard saline citrate (SSC; 150 mM NaCl, 15 mM trisodium citrate) at 60–65°C for 90 min followed by rinsing thoroughly in 0.9% w/v NaCl at room temperature. Slides were stained in Trypsin-Giemsa (Biomedical Specialties) solution for 4–6 min before transfer to fresh buffer (1× SSC; twice rinsed) and dried by compressed air. Slides were then mounted with glass coverslips and viewed under 100× oil immersion using a Nikon Ti Inverted microscope equipped with an Andor CCD digital camera.
Quantitative real-time RT-PCR analysis
Expression for standard reprogramming genes OCT4, SOX2, KLF4, and c-MYC, was evaluated with quantitative real-time RT-PCR and compared with that of human ESCs. Total RNA samples were isolated from hAECs cultured for 7 days using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. A total of 500 ng RNA served as a template to synthesize cDNA with random hexamers. The quality of cDNA samples was confirmed by means of ethidium bromide staining of samples in a 2% agarose gel. Quantitative mRNA expression was conducted with a TaqMan Gene Expression Assays system, and quantitative real-time PCR was performed using ABI Prism 7900HT (Applied Biosystems, Foster City, CA, USA). Each cDNA template was mixed with PCR master mix and specific primer sets for Oct4 (Hs00742896_s1), Sox2 (Hs01053049_s1), KLF4 (Hs00358836_m1), c-MYC (Hs00905030_m1), POP4 (Hs00198357_m1), and PPIA (Hs99999904_m1) obtained from Applied Biosystems. The relative expression software tool (REST) was used to quantify mRNA expression of each target gene. Prior to the target gene analysis, the stable reference genes POP4 and PPIA were identified from 12 reference genes using statistical algorithms software (geNorm) (Minervini et al., 2009).
Global DNA methylation assay
Global DNA methylation status of human pluripotent stem cells (hPSCs) and their parental cells, naïve AE cells, and human fibroblast cell line cells were evaluated with an enzyme-linked immunosorbent assay– (ELISA) based quantification kit using the manufacturer's instructions (Imprint Methylation DNA Quantification kit, Sigma-Aldrich, St. Louis, MO, USA). Genomic DNA was isolated with AllPrep DNA/RNA Mini Kit (QIAGEN, Valencia, CA, USA). Purified DNA (100 ng) from each sample was tested. Methylated control DNA and DNA binding solution were used as positive control and blank, respectively. The relative methylation levels were shown as percent methylation of the samples relative to that of control DNA by average value of A450 (absorbance at 450 nm) of duplicate determinations.
Mitochondria DNA point mutation assay
A point mutation at A3243G of mitochondria DNA (mtDNA) was evaluated by non-gel-based PCR-restriction fragment analyses employing melting temperature characteristics of the fragments (PCR-RFMT) with previously published primers and the protocol (Jahangir Tafrechi et al., 2007). Briefly, PCR was performed in an end volume of 20 μL, containing 10 μL SYBR Green Mastermix (Applied Biosystems) and 250 nM of both primers. The PCR began with 10 min hot start at 95°C, followed by 42 cycles alternating between 15 sec 95°C and 1 min at 63°C. The amplicons were digested with 5 μL of ApaI (New England BioLabs: R0114S) overnight at 37°C. The melt curves were recorded with an ABI-Prism 7900-HT spectrofluorometric Thermal Cycler (Applied Biosystems) by gradually increasing the temperature over 20 min from 65°C to 90°C.
Detection of global histone H3 acetylation levels
Histone extraction and measurement of global histone H3 acetylation was performed using the EpiQuik™ global histone H3 acetylation assay kit according to the manufacturer's instructions (Epigentek, NY, USA). Briefly, histone protein was extracted from a total of 10 individual placentae, HDF, HFF1, and hPSCs. Protein concentrations were measured using the Bradford method, and an input of 1 μg of histone proteins was used. Acetylated histone H3 was detected with high-affinity antibodies. Signals were developed using horseradish peroxidase– (HRP) conjugated secondary antibodies and quantified by measuring absorbance at 450 nm. Data analysis was performed according to the manufacturer's instructions. Global acetylation ratios (%) were calculated with reference to provided acetylated histone positive controls.
Statistical analysis
Data are expressed as means±SD. To determine statistical significance, one-way anaysis of variance (ANOVA), two-sample Student's t-test, or Wilcoxon's signed ranked test were used. For the Wilcoxon's signed ranked test, the two-tailed significance level was set at α=0.05. For all statistical analyses, value of p<0.05 was considered to be statistically significant.
Results
hAECs are capable of being reprogrammed into iPSCs
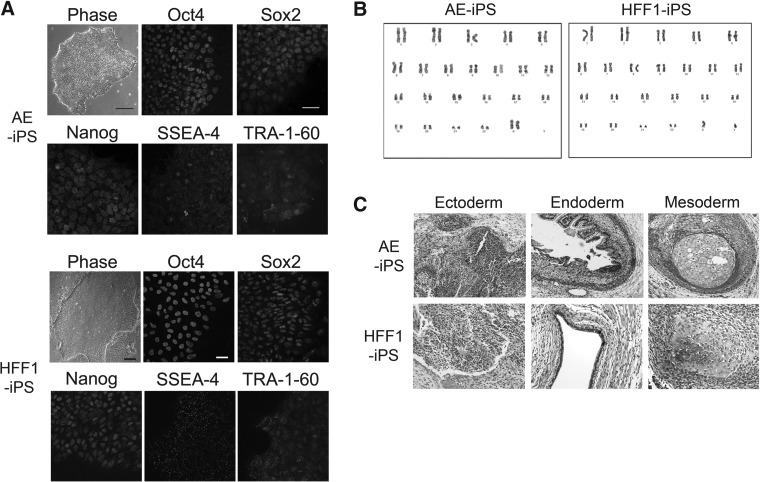
To test reprogramming efficiency of hAECs, naïve AE cells were isolated from placental tissue and reprogrammed with a single lentiviral vector system containing four defined reprogramming factors, OCT4, KLF4, SOX2, and c-MYC (EF1-STEMCCA lentiviral vector, (Sommer et al., 2009). In comparison, HFFs were obtained commercially (HFF1). To validate that each line generated true pluripotent stem cells, both AE-iPSCs and HFF1-iPSCs were probed for pluripotent marker expression by immunofluorescence. AE-iPSCs formed human ESC-like colonies with defined borders and low levels of differentiation within a growing culture (Fig. 1A). AE-iPSCs also expressed pluripotent transcription factors Oct4, Sox2, Nanog, and surface markers SSEA4 and TRA-1-60 (Fig. 1A). Whereas HFF1-iPSCs showed some peripheral differentiation around the colonies, HFF1-iPSCs also expressed the pluripotency markers Oct4, Sox2, Nanog, SSEA4, and TRA-1-60 (Fig. 1A). Pluripotency of each line was also validated by teratoma assay to evaluate each line's ability to differentiate into all three germ layers—endoderm, mesoderm, and ectoderm. Both AE-derived and HFF1-derived iPSCs formed teratomas in immunodeficient mice, with representative tissue from all three germ layers (Fig. 1C). Interestingly AE-iPSCs induced teratoma formation faster than HFF1-derived iPSCs, although the mechanism behind this faster teratoma formation was not explored. Although the tumor growth was faster, cytogenetic analyses demonstrated that AE-iPSCs, like HFF1-iPSCs, maintained a normal karyotype at least up to 30 passages (Fig. 1B).
FIG. 1.
hAECs can be reprogrammed similar to previously published cell lines. hAECs and HFF1 fibroblasts were reprogrammed with the STEMCCA lentiviral cassette containing OCT4, SOX2, KLF4, and c-MYC all on one plasmid vector. (A) Both AE-iPSCs and HFF1-iPSCs grow in colonies similar in appearance to human ESCs and express stem cell markers Oct4, Sox2, Nanog, SSEA4, and TRA-1-60. Scale bars, in phase images 500 μm; in fluorescent images 100 μm. (B) Cytogenic analyses of AE-iPSCs and HFF1-iPSCs after passage 30 in culture indicate that each line is karyotypically normal. (C) Representative histological sections of teratomas from AE-iPSCs and HFF1-iPSCs. For AE-iPSCs: ectoderm, neuroepithelium; endoderm, gastrointestinal epithelium; mesoderm, cartilage/bone. For HFF1-iPSCs: ectoderm, neuroepithelium; endoderm, primitive endoderm; mesoderm, bone.
AECs reprogram faster than neonatal fibroblasts
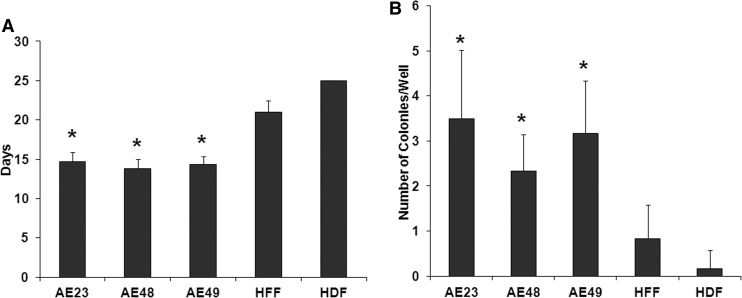
Because we noticed that AE-iPSCs demonstrated noticeably less spontaneous differentiation in our feeder-free culturing environment compared to HFF1-iPSCs (for example, Fig. 1A, phase images), we examined whether hAECs reprogram faster and more efficiently than adult fibroblasts. Naïve AE cells from three placentae and two commercially available human fibroblast lines (neonatal HFFs and adult human dermal fibroblasts, HDF) were reprogrammed. Six independent reprogramming events were performed for each cell line. The timing of first ESC-like iPSC colony with a flat, round shape and a distinct edge was evaluated (Fig. 2). Likewise, the number of iPSC colonies per well was also counted (Fig. 2). Across all cell lines tested, hAECs consistently and significantly formed colonies faster (around day 14) and formed more colonies per well than both neonatal (HFFs) and adult fibroblasts (HDFs) (Fig. 2).
FIG. 2.
hAECs reprogram faster and more efficiently than neonatal and adult fibroblasts. (A) Graphical analysis demonstrating the average time (in days) until the first ESC-like colonies appear (n=6, p<0.001. (B) Graphical analysis depicting the average number of ESC-like colonies appearing in each well of a six-well dish (n=6, p<0.01). HFF, human foreskin fibroblasts; HDF, human dermal fibroblasts.
Human stem cell PCR array analysis
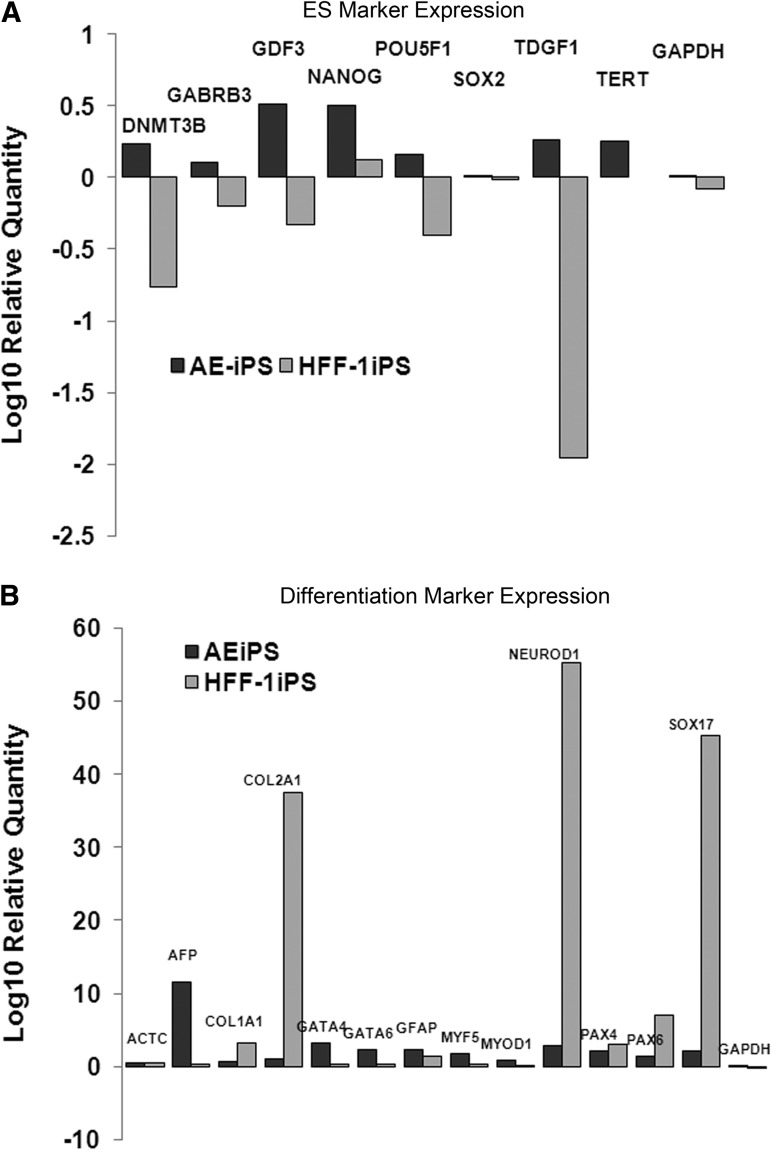
To explore a possible mechanism behind the increase efficiency of cellular reprogramming in hAECs, we next evaluated gene expression profiles in AE-iPSCs and HFF1-iPSCs. Using a commercially available qRT-PCR-based human stem cell pluripotency array, six undifferentiated stem cell marker genes, three pluripotency maintenance genes, 33 stemness correlate genes, and 50 differentiation marker genes expression were investigated in human AE-iPS and and HFF-iPSC and then normalized to human ESCs (H1 and H9). A total of six pluripotent genes (NANOG, POU5F1, TDGF1, DNMT3B, TERT, and GDF3) appeared to eb significant (Fig. 3A). Importantly, compared to HFF1-iPSCs, AE-iPSCs express higher levels of NANOG and POUF5/OCT4 (Fig. 3A), transcription factors critical for the maintenance of pluripotency in ESCs. Interestingly, all six undifferentiated stem cell marker genes, NANOG, POU5F1, TDGF1, DNMT3B, GABRB3, and GDF3, were higher in AE-iPSCs than even in control human ESCs (Fig. 3A).
FIG. 3.
AE-iPSCs express a genetic profile more consistent with human ESCs compared to HFF1-iPSCs. TaqMan Human Stem Cell Pluripotency Arrays were used to evaluate gene expression for pluripotency genes (A) and differentiation genes (B). Expression fold changes were calculated using −ΔΔCt and normalized using β-actin as the endogenous control. Gene expression was then compared to human ESC gene expression and graphed. Graphed results represent average values obtained from six different trials.
We next examined expression of a variety of differentiation markers. Unlike AE-iPSCs, HFF1-iPSCs expressed higher levels of COL2A1, NEUROD1, PAX6, and SOX17 (Fig. 3B). The abundance of these genes may account for the increased prevalence of neuroectoderm and retinal epithelial in HFF1-iPSC-derived teratomas (data not shown). The abundance of alpha-fetoprotein (AFP) in AE-iPSCs (Fig. 3B) is not surprising given the fact that hAECs are derived from placental tissue. The overabundance of neuroectoderm markers in HFF1-iPSCs may indicate that these cells have not been fully reprogrammed compared to AE-iPSCs. Taken together with ESC gene expression profiles, our data suggest that AE-iPSCs are more closely similar in genetic profiles to human ESCs than iPSCs derived from neonatal cells.
Primary AECs do not show increased elevations in global histone H3 acetylation or decreased accumulation of mitochondrial DNA damage compared to dermal fibroblasts
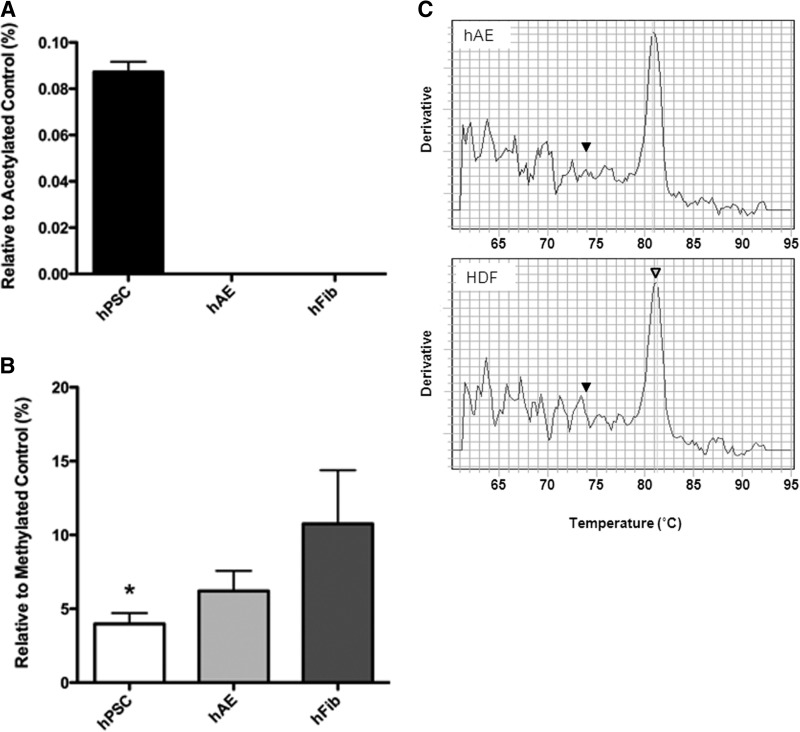
One hallmark of human ESCs is that the genome is in an “open” confirmation, i.e., high levels of histone H3 acetylation and low levels of global DNA methylation (Hattori et al., 2004; Meshorer et al., 2006). To explore whether increased reprogramming efficiency of hAECs is due to elevated histone H3 acetylation levels, we evaluated global histone H3 acetylation in eight separate hAECs, two human fibroblast lines (HFF1 and HDF), and four hPSC lines (H1, H9, AE-iPSC, and HFF1-iPSC). Because all iPSC lines showed similar histone H3 acetylation patterns to hESC lines (data not shown), we compared histone H3 acetylation levels in pluripotent stem cells (pooled data from all pluripotent lines) and compared these results to pooled data from eight different hAEC lines and pooled data from two fibroblast (hFib) lines (HFF1 and HDF). hAECs on average showed similar low levels of global H3 acetylation levels as seen in fibroblasts (unlike high levels of H3 acetylation in hPSCs) (Fig. 4A). Therefore, elevations in global H3 acetylation do not explain why hAECs reprogram faster and more efficiently.
FIG. 4.
Global H3 acetylation and mtDNA damage levels are similar in hAECs compared to HDFs, whereas, global DNA methylation is slightly decreased. (A) Global H3 acetylation levels in hAECs (n=8, eight lines), hFib (n=5, two lines), and hPSCs (n=2). Acetylation levels are presented as the mean±SD relative to positive control. (B) Representative graphical analysis of global DNA methylation levels examined in hAECs (n=11, eight lines), human fibroblasts (n=5, two lines), and pluripotent stem cell lines (hPSCs) (n=10). (C) To determine the presence of mtDNA damage, amplicons from hAECs and HDFs were digested with ApaI. Melt curves were recorded with an ABI-Prism 7900-HT spectrofluorometric Thermal Cycler by gradually increasing the temperature over 20 min from 65°C to 90°C.
We next evaluated global DNA methylation status of hAECs, hFib, and PSCs by ELISA. A total of eight independently isolated hAECs and two human fibroblast lines were tested each in triplicate and compared to pooled data from both hESC lines and iPSC lines (H1, H9, AE-iPSC, and HFF1-iPSC). Global methylation patterns in hAEC-derived iPSC lines and in human fibroblast-derived iPSC lines mirrored patterns observed in H1 and H9 hESC lines (data not shown). As expected, reprogrammed iPSCs demonstrated low levels of global DNA methylation status, similar to human ESCs (data not shown). Compared to human fibroblast samples, hAECs show decreased levels of global DNA methylation, although this decrease was not statistically significant (Fig. 4B).
We next determined whether acquired mitochondrial DNA damage over time influenced hAEC cellular reprogramming. Due to lack of a sophisticated DNA repair system in the mitochondrial compartment, mutation rates of mtDNA are five to 10 times higher than those of nuclear DNA (Muravchick, 2008). Mutations in mtDNA are reported to occur as an age-acquired somatic mutation, possibly due to oxidative stress (Hattori et al., 1991; Sohal and Weindruch, 1996). One of the most common mutations, an A to G substitution at base pair (bp) 3243 in the mitochondrial tRNALeu(UUR) gene (mt3243), has been used to evaluate environmental and age-acquired mtDNA damages (Kang and Hamasaki, 2003). In this experiment, we examined the A3243G mutation by non-gel-based PCR-RFMT using SYBR Green as a reporter (Jahangir Tafrechi et al., 2007) to evaluate mtDNA damage accumulation as a possible cause for decreased iPSC efficiency in adult fibroblasts (HDFs) compared to hAECs. A total of five mtDNA samples from primary hAECs were examined and compared with mtDNA from human adult dermal fibroblasts. Digestion with the restriction enzyme ApaI cleaves only the mutant amplicon. Thus, a unique peak should be observed at 74°C of the SYBR Green fluorescence melting curve of the ApaI-fragmented PCR product, whereas an uncut amplicon from normal mtDNA shows a peak at 80°C. Representative results show there is no recognizable peak at 74°C in both hAEC and HDF samples (Fig. 4C). These data indicate that there is no influence of A3243G mtDNA damage to explain the increased reprogramming efficiency in hAECs.
Primary AECs express key genes for iPSC derivation
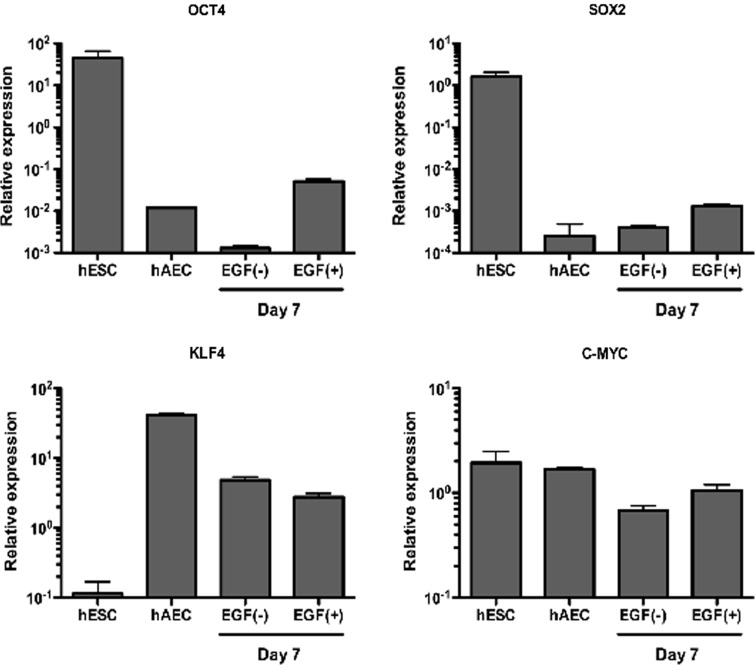
While global DNA methylation may be a root cause for the increased efficiency of hAECs (though the result is inconclusive), global H3 acetylation and A3243G mtDNA damage do not explain why hAECs are easily reprogrammable compared to fibroblasts. Previously, we have shown that some hAECs possess ESC-like characteristics (Miki et al., 2005). Using quantitative real-time RT-PCR, the basal expression level of all four defined transcription factors used in iPSC reprogramming, OCT4, SOX2, KLF4, and c-MYC in hAECs, were compared with expression in human ESC lines (H1 and H9). Total RNA was isolated from hAE cell culture at day 0 and day 7 with or without epidermal growth factor (EGF) (100 ng/mL) and was isolated from undifferentiated human ESCs. Because the quantitative analysis comparing different cell types is difficult, we used reference genes (POP4 and PPIA) that were selected based on the method described in our previous publication (Minervini et al., 2009). Previously, OCT4 and SOX2 expression was detected by a conventional RT-PCR method (Miki et al., 2005). Here, carefully designed quantitative analysis showed relative OCT4 expression was 0.43% and 1.86% of hESCs at day 0 and day 7, respectively (Fig. 5). SOX2 expression was less than 0.01% in both day 0 and day 7 samples (n=5) (Fig. 5). On the other hand, two of defined reprogramming factors, KLF4 and c-MYC, were expressed at comparable levels with hESCs (Fig. 5). On the other hand, HFFs lack expression of transcription factors involved in iPSC reprogramming (data not shown and Kim et al.,. 2011). These findings suggest that the basal levels of KLF4 and c-MYC might represent a root cause explaining why hAECs reprogram faster and more efficiently than human fibroblasts.
FIG. 5.
Comparative gene expression analyses indicate that hAECs express low levels of the four key reprogramming factors, OCT4, SOX2, KLF4, and c-MYC. Comparative gene expression analysis utilizing qPCR data of four reprogramming factor genes (OCT4, SOX2, KLF4, c-MYC) in hESCs (n=7) and hAECs (n=5 each) were performed. Relative quantitation of target gene expression for each sample was determined using the equation 2−ΔΔCt, where glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference gene. EGF, epidermal growth factor.
We previously reported that OCT4 expression and cell proliferation were increased under a culture condition containing EGF (Miki et al., 2005). Human AECs were isolated and cultured for 7 days with or without EGF (100 ng/mL). Under EGF-containing culture conditions, endogenous OCT4 gene expression was increased (Fig. 5). These data indicate that although endogenous OCT4 gene expression was relatively low in untreated hAECs, OCT4 expression could be upregulated simply by activating the EGF/TGFβ pathway through EGF treatment (Fig. 5). Similar results are observed with SOX2 (Fig. 5). Regardless of the level of expression, primary hAECs express all of the key genes for iPSC derivation—OCT4, SOX2, KLF4, and c-MYC in the presence of EGF treatment. These findings suggest that hAECs are “primed” for iPSC reprogramming and thus explain why hAECs reprogram faster and more efficiently than hFib.
Discussion
One of the major advantages of iPSC studies is the possibility of generating patient-specific or disease-specific PSCs that could possibly be used to treat or further understand a number of human disorders. Several studies have shown proof-of-principle of using induced iPSCs for therapies of various genetic (Hanna et al., 2007), malignant, and degenerative diseases (Dimos et al., 2008; Ebert et al., 2009; Soldner et al., 2009). However, low reprogramming efficiency extends the lead time to generate patient-specific iPSCs and/or prohibits establishing a biobank to provide immunotype-matched iPSCs for clinically based research. The low reprogramming efficiencies observed in typical iPSC line derivations also result in labor-intensive processes and high costs to generate each iPSC line, which ultimately delays research in generating disease specific iPSCs for further biomedical analyses.
In addition, although the results using animal disease models highlight the great promise for iPSCs, transplanting iPSCs or differentiated iPSCs into humans carries a high risk. Due to ectopic gene expression, particularly uncontrolled c-MYC, upregulation of certain pluripotent genes leads to tumor development in about 20% of chimeric mice generated from iPSCs within a 2- to 10-month time frame (Okita et al., 2007). The random integration of the reprogramming genes could also induce tumorigenesis by activating other oncogenic factors. To avoid genetic modification, current research has been targeted at deriving iPSCs using plasmids, adenoviruses, proteins, miRNAs, RNA, or chemicals. However, these approaches further reduce reprogramming efficiency.
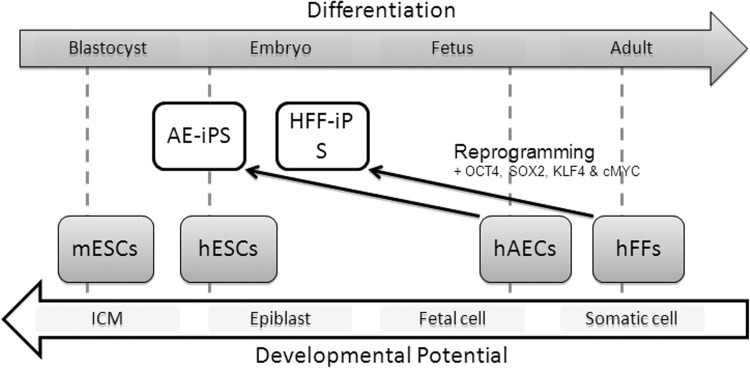
A recently reported study using a live imaging system indicated that the low reprogramming efficiency was due to the difficulty of maintaining the reprogrammed state (Araki et al., 2009; Chan et al., 2009). These data indicate that in addition to the essential defined factors there are secondary or supportive factors that maintain the reprogrammed state. Here, we investigated the expression of several stem cell markers in both hAECs and HFF cells. We identified some of the defined reprogramming genes are already expressed in naïve hAECs. Unlike ectopic expression, the basal endogenous expression of these genes is considered more stable. This gene expression profile also indicates that the promoter/enhancer regions of these genes are actively open. Most importantly, the downstream genes, which could play supportive roles to maintain reprogrammed pluripotency, might be also expressed. Because hAECs endogenously express certain pluripotent factors, this unique cell type might be an excellent candidate for exploring novel techniques that induce cellular reprogramming without the use of genetic manipulation (Fig. 6).
FIG. 6.
hAECs represent an ideal cell source for iPSC reprogramming. This graphic shows that hAECs lie closer to undifferentiated ESCs in a differentiation spectrum compared to neonatal HFFs. Furthermore, hAECs generate iPSCs that are more similar to human ESCs than iPSCs derived from HFFs.
While global histone H3 acetylation levels were not significantly higher in hAECs compared to HFFs, global DNA methylation in naïve hAECs was lower than that of neonatal and adult fibroblasts. Although the epigenetic status of hAECs was not as hypomethylated as PSCs, the less hypomethylated status could represent one reason why hAECs reprogram faster and more efficiently than adult and neonatal fibroblasts.
Although it was not investigated in this study, the epithelial nature of hAECs may be another reason for the high reprogramming efficiency. Previous work has shown that the reprogramming efficiency of epithelial cells is better than that of fibroblasts (Aasen et al., 2008). Using a mouse secondary iPSC system and microarray analysis, Samavarchi-Tehrani et al. (2010) demonstrated that a mesenchymal-to-epithelial transition (MET) event occurs during the initial reprogramming phase (approximately day 5) (Samavarchi-Tehrani et al., 2010). This finding could explain why hAE-derived ESC-like colonies appeared at earlier time points than neonatal and adult fibroblast-derived colonies. By using AECs, the initial MET phase could be skipped from the conventional reprogramming process. Recently, some reports have demonstrated that AF cells may also have similar advantages to hAECs as an ideal cell source for iPSC generation (Galende et al., 2010; Li et al., 2009; Trovato et al., 2009). Although AF cells do share some of the unique advantages of placenta-derived cells, such as hAECs, AF cells possess a mesenchymal origin and thus may require a MET transition during the early stages of reprogramming, whereas hAECs do not.
The concept of patient-specific iPSCs has been proposed as one of the advantages of human iPSCs. However, it is much more practical to establish a Biobank that stores human stem cells with all human leukocyte antigen (HLA) haplotypes, rather than generate patient-specific stem cells on a case-by-case basis. The epigenetic memory of parental cells and accumulated DNA damage over a lifetime in donor somatic cells are likely two potential risks and strategic disadvantages for generating patient specific iPSCs. In fact, a recent report by Pratama et al. described the suitability for primary and cultured hAECs for cell-based therapies (Pratama et al., 2011). An hAEC-iPSC Biobank would be able to provide immunotype-matched stem cells from healthy and younger parental cell sources that would most likely have a low accumulation of environmental-induced DNA damage. hAEC-iPSCs also may lack a true epigenetic memory due to their endogenous expression of a number of stem cell markers (Fig. 6). These cells could thus be used for therapeutic purposes for a wide range of patients with various disorders. Taylor et al. first estimated the feasibility of the stem cell banking system in 2005. They concluded that 10 ESC lines could provide a complete three loci match for 37.7% of recipients and a beneficial match for 67.4% of the United Kingdom population (Taylor et al., 2005). Nakatsuji et al. estimated that a cell bank size of only 30 iPSC lines would be able to find the HLA-A, HLA-B, and HLA-DR haplotypes matches in 82.2% of the Japanese population (Nakatsuji et al., 2008). Because of the diversity of the United States population, the required number of cell lines might need to be increased. However, these estimates suggest it is still feasible to construct HLA-haplotype iPSC banks, and hAECs represent an ideal cell type because they are an abundant cell source easily obtained from discarded placentae and are rapidly and efficiently reprogrammed.
In conclusion, hAECs are more rapidly and efficiently converted into iPSCs using the standard Yamanaka cocktail of reprogramming factors than adult and neonatal fibroblasts. Furthermore, hAEC-iPSCs are far more similar to hESCs than HFF1-derived iPSCs (Fig. 6), not only in gene expression, but also in function because hAEC-iPSCs are metabolically similar to hESCs and exhibit similar DNA repair mechanisms to hESCs (Momcilovic et al., 2010; Varum et al., 2011). Taken together, hAECs represent a superior cell source for investigating novel conditions that generate more clinically relevant iPSCs and thus may serve as an ideal cell source for establishing an iPSC Biobank to provide clinically relevant immunotype-matched iPSCs for future cell replacement therapies.
Acknowledgments
The authors would like to thank Stacie Oliver for her assistance with karyotyping and Carrie Redinger for her assistance with stem cell culture. AE-iPSCs derived in this paper have been used in two published manuscripts (Momcilovic et al., 2010; Varum et al., 2011).
Author Disclosure Statement
The authors state that there are no conflicts of interest and they have received no payment for preparation of this manuscript.
References
- Aasen T. Raya A. Barrero M.J. Garreta E. Consiglio A. Gonzalez F. Vassena R. Bilic J. Pekarik V. Tiscornia G., et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Aoi T. Yae K. Nakagawa M. Ichisaka T. Okita K. Takahashi K. Chiba T. Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- Araki R. Jincho Y. Hoki Y. Nakamura M. Tamura C. Ando S. Kasama Y. Abe M. Conversion of ancestral fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;28:213–220. doi: 10.1002/stem.282. [DOI] [PubMed] [Google Scholar]
- Carey B.W. Markoulaki S. Beard C. Hanna J. Jaenisch R. Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat. Methods. 2010;7:56–59. doi: 10.1038/nmeth.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E.M. Ratanasirintrawoot S. Park I.H. Manos P.D. Loh Y.H. Huo H. Miller J.D. Hartung O. Rho J. Ince T.A., et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat. Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- Dimos J.T. Rodolfa K.T. Niakan K.K. Weisenthal L.M. Mitsumoto H. Chung W. Croft G.F. Saphier G. Leibel R. Goland R., et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Ebert A.D. Yu J. Rose F.F., Jr. Mattis V.B. Lorson C.L. Thomson J.A. Svendsen C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galende E. Karakikes I. Edelmann L. Desnick R.J. Kerenyi T. Khoueiry G. Lafferty J. McGinn J.T. Brodman M. Fuster V., et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell. Reprogram. 2010;12:117–125. doi: 10.1089/cell.2009.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J. Wernig M. Markoulaki S. Sun C.W. Meissner A. Cassady J.P. Beard C. Brambrink T. Wu L.C. Townes T.M., et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Hattori K. Tanaka M. Sugiyama S. Obayashi T. Ito T. Satake T. Hanaki Y. Asai J. Nagano M. Ozawa T. Age-dependent increase in deleted mitochondrial DNA in the human heart: possible contributory factor to presbycardia. Am. Heart J. 1991;121:1735–1742. doi: 10.1016/0002-8703(91)90020-i. [DOI] [PubMed] [Google Scholar]
- Hattori N. Nishino K. Ko Y.G. Hattori N. Ohgane J. Tanaka S. Shiota K. Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J. Biol. Chem. 2004;279:17063–17069. doi: 10.1074/jbc.M309002200. [DOI] [PubMed] [Google Scholar]
- Hu Q. Friedrich A.M. Johnson L.V. Clegg D. O. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 2010;28:1981–1991. doi: 10.1002/stem.531. [DOI] [PubMed] [Google Scholar]
- Jahangir Tafrechi R.S. van de Rijke F.M. Allallou A. Larsson C. Sloos W.C. van de Sande M. Wahlby C. Janssen G.M. Raap A.K. Single-cell A3243G mitochondrial DNA mutation load assays for segregation analysis. J. Histochem. Cytochem. 2007;55:1159–1166. doi: 10.1369/jhc.7A7282.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. Hamasaki N. Mitochondrial oxidative stress and mitochondrial DNA. Clin. Chem. Lab. Med. 2003;41:1281–1288. doi: 10.1515/CCLM.2003.195. [DOI] [PubMed] [Google Scholar]
- Kim S.U. de Vellis J. Stem cell-based cell therapy in neurological diseases: A review. J. Neurosci. Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- Kim S.Y. Kim M.J. Jung H. Kim W.K. Kwon S.O. Son M.J. Jang I.S. Choi J.S. Park S.G. Park B.C., et al. Comparative proteomic analysis of human somatic cells, induced pluripotent stem cells, and embryonic stem cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0243. Aug 29. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Li C. Zhou J. Shi G. Ma Y. Yang Y. Gu J. Yu H. Jin S. Wei Z. Chen F., et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum. Mol. Genet. 2009;18:4340–4349. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- Lister R. Pelizzola M. Kida Y.S. Hawkins R.D. Nery J.R. Hon G. Antosiewicz-Bourget J. O'Malley R. Castanon R. Klugman S., et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N. Sridharan R. Xie W. Utikal J. Eminli S. Arnold K. Stadtfeld M. Yachechko R. Tchieu J. Jaenisch R., et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Markoulaki S. Hanna J. Beard C. Carey B.W. Cheng A.W. Lengner C.J. Dausman J.A. Fu D. Gao Q. Wu S., et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nat. Biotechnol. 2009;27:169–171. doi: 10.1038/nbt.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E. Yellajoshula D. George E. Scambler P. J. Brown D.T. Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res. Ther. 2011;2:25. doi: 10.1186/scrt66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T. Lehmann T. Cai H. Stolz D.B. Strom S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- Miki T. Marongiu F. Dorko K. Ellis E.C. Strom S.C. Curr. Protoc. Stem Cell Biol. Wiley Interscience; 2010. Isolation of amniotic epithelial stem cells. Chapter 1, Unit 1E 3. [DOI] [PubMed] [Google Scholar]
- Minervini C.F. Izumi M. Miki T. Effect of culture conditions on reference genes expression in placenta-derived stem cells. Int. J. Stem Cells. 2009;2:69–75. doi: 10.15283/ijsc.2009.2.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic O. Knobloch L. Fornsaglio J. Varum S. Easley C. Schatten G. DNA damage responses in human induced pluripotent stem cells and embryonic stem cells. PLoS One. 2010;5:e13410. doi: 10.1371/journal.pone.0013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravchick S. Clinical implications of mitochondrial disease. Adv. Drug Deliv. Rev. 2008;60:1553–1560. doi: 10.1016/j.addr.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N. Nakajima F. Tokunaga K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- Okita K. Ichisaka T. Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Park I.H. Zhao R. West J.A. Yabuuchi A. Huo H. Ince T.A. Lerou P.H. Lensch M.W. Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pratama G. Vaghjiani V. Tee J.Y. Liu Y.H. Chan J. Tan C. Murthi P. Gargett C. Manuelpillai U. Changes in culture expanded human amniotic epithelial cells: implications for potential therapeutic applications. PLoS One. 2011;6:e26136. doi: 10.1371/journal.pone.0026136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P. Golipour A. David L. Sung H.K. Beyer T.A. Datti A. Woltjen K. Nagy A. Wrana J.L. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Sohal R.S. Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F. Hockemeyer D. Beard C. Gao Q. Bell G.W. Cook E.G. Hargus G. Blak A. Cooper O. Mitalipova M., et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C.A. Stadtfeld M. Murphy G.J. Hochedlinger K. Kotton D.N. Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M. Nagaya M. Utikal J. Weir G. Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Taylor C.J. Bolton E.M. Pocock S. Sharples L.D. Pedersen R.A. Bradley J.A. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- Trovato L. De Fazio R. Annunziata M. Sdei S. Favaro E. Ponti R. Marozio L. Ghigo E. Benedetto C. Granata R. Pluripotent stem cells isolated from human amniotic fluid and differentiation into pancreatic beta-cells. J. Endocrinol. Invest. 2009;32:873–876. doi: 10.1007/BF03345764. [DOI] [PubMed] [Google Scholar]
- Varum S. Rodrigues A.S. Moura M.B. Momcilovic O. Easley C.A.t. Ramalho-Santos J. Van Houten B. Schatten G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L. Manos P.D. Ahfeldt T. Loh Y.H. Li H. Lau F. Ebina W. Mandal P. K. Smith Z.D. Meissner A., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov E. Rechavi G. Rozenblatt S. Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010;394:189–193. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- Yu J. Vodyanik M. A. Smuga-Otto K. Antosiewicz-Bourget J. Frane J.L. Tian S. Nie J. Jonsdottir G.A. Ruotti V. Stewart R., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhou H. Wu S. Joo J. Y. Zhu S. Han D. W. Lin T. Trauger S. Bien G. Yao S. Zhu Y., et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]