Abstract
In contrast to peripheral plasmacytoid dendritic cells (pDC), thymic pDC constitutively express low levels of IFN-α. This leads to induction of ISG in medullary thymocytes, raising the question whether IFN-α may play a role in T cell development. When characterizing further differences between peripheral and thymic pDC, we found that thymic pDC have a phenotype consistent with an “activated signature” including expression of TNF-α and BST2, but no expression of ILT7. Given that BST2 is induced by IFN-α and IFN-α secretion is controlled by interaction between ILT7 and BST2, this regulatory pathway is apparently lost in thymic pDC. Further, we also show that BST2 is expressed constitutively on a subset of medullary thymocytes at the mRNA and protein level reflecting a history of IFN-α transduced signals. The majority of BST2+ thymocytes express CCR5 rendering them prevalent targets for R5-tropic HIV infection. Moreover, BST2+ thymocytes express Foxp3 and CD25, consistent with the phenotype of natural Treg cells, and exert suppressive activity as they impair the proliferation of autologous CD3+ thymocytes. Collectively, our results suggest that low levels of IFN-α secreted by thymic pDC play an important role in the development of natural Treg cells.
Keywords: Interferon-α, ISG, thymus, plasmacytoid dendritic cells, Regulatory T-cells
INTRODUCTION
Plasmacytoid dendritic cells (pDC) are the major source of Interferon-alpha (IFN-α) and play an essential role in anti-viral immunity [1–2]. pDC are located in lymphoid tissues and activated by viral ssRNA or DNA via toll like receptors (TLR) 7 and 9, respectively, to secrete IFN-α [1–4]. pDC are also present in the thymus, but their function in the thymus remains largely unexplained [5]. Thymic pDC are located in the medulla and at the cortico-medullary junction and express co-stimulatory molecules CD40 and CD86 [6]. We have shown that they constitutively produce IFN-α, which is likely triggered by LL-37/DNA complexes, and induces Interferon secondary genes (ISG) in surrounding medullary thymocytes [5]. It has been suggested that thymic pDC play a role in natural T regulatory cell (Treg) development, although their exact function in this process is still unknown [7–8].
Activation of peripheral pDC after TLR7/9 engagement induces a mature immunophenotype [9]. Depending on the cellular localization of TLR9 (early or late endosome) different pathways are activated, leading to secretion of different cytokines [2]. Early endosomal triggering of TLR7/9 activates the phosphatidylinositol-3 kinase (PI3K) pathway, which leads to phosphorylation and nuclear translocation of Interferon Response Factor (IRF)7 and subsequent induction of IFN-α production [2, 10]. On the other hand, when TLR9 triggering occurs in the late endosome the NFκB pathway is predominantly activated leading to TNF-α and IL-6 secretion, and upregulation of co-stimulatory and MHC-Class II molecules [2, 10].
Here we report that thymic pDC have a specific immunophenotype ex vivo which is different from ex vivo peripheral pDC (blood and spleen). Thymic pDC constitutively express IFN-α and TNF-α, but not IL-6, and display cell surface markers consistent with the phenotype of peripheral pDC that have been previously activated, thus show an “IFN-α signature”. We show for the first time that constitutive production of IFN-α and TNF-α by pDC may induce expression of Bone Marrow Stromal Cell Antigen 2 (BST2), also called Tetherin or CD317, in the neighbouring thymocytes. The BST2+ thymocytes are not only enriched in cells with an immunophenotype consistent with natural Treg, but more importantly are functionally equipped to act as suppressor T cells. Collectively, our results suggest that thymic pDC, as a consequence of their activated phenotype, have a role in the development of Treg in the thymus.
RESULTS
Thymic pDC constitutively express IFN-α and TNF-α, but not Interleukin-6
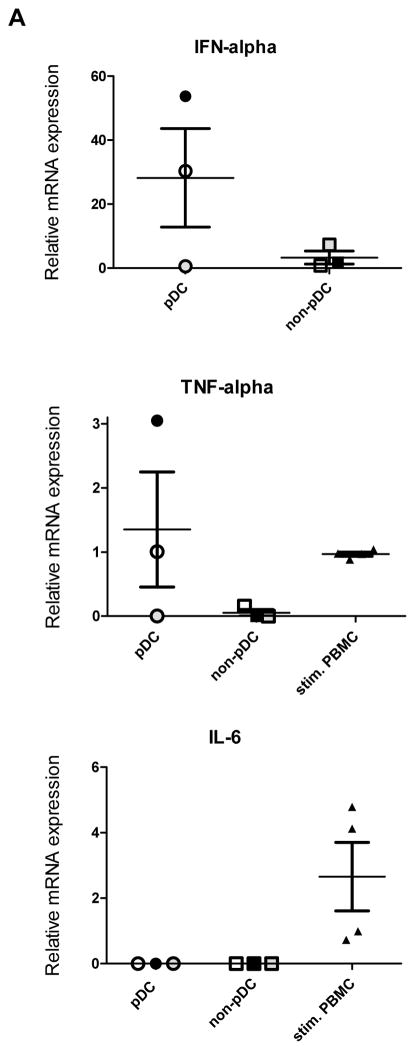
Previously we have shown that human thymic pDC are the likely source of the constitutive production of IFN-α in the thymus as a result of TLR-induced activation [5]. Here we aimed at extending our findings. It has been shown that peripheral blood pDC, in addition to IFN-α, express TNF-α and IL-6 after stimulation with CpG or virus [11]. Hence, we tested whether thymic pDC constitutively express these cytokines as well. Confirming our previously published results [5], we observed IFN-α mRNA expression in 2 out of the 3 sorted pDC samples from 3 different thymus specimens (Figure 1A). The sorting and gating strategy is depicted in Supplemental Figure 1. We have previously shown that the amount of IFN-α produced by thymic pDC constitutively, or after stimulation with CpG or HIV, greatly varies between donors [5, 12]. Further, as shown in Figure 1A, sorted thymic pDC constitutively expressed TNF-α mRNA as well, but surprisingly no IL-6 mRNA. TNF-α mRNA was expressed constitutively in the same 2 out of 3 thymic pDC as IFN-α (Fig. 1A). These results suggest that constitutive expression of TNF-α and IFN-α may be regulated differently than IL-6 in thymic pDC as compared to peripheral pDC, which only express TNF-α, IFN-α and IL-6 mRNA upon activation with CpG or virus [11].
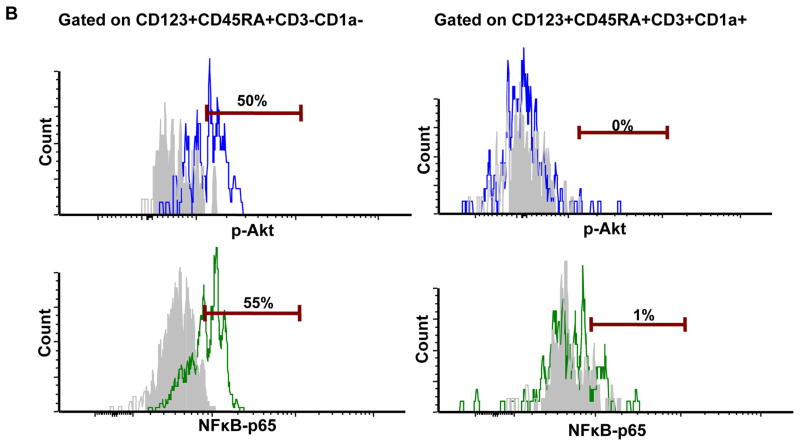
Figure 1. pDC in the thymus are constitutively activated.
A) Taqman RT-PCR was performed in sorted pDC (CD123+CD45RA+CD3−BST2+) for their expression of IFN-α, TNF-α and IL-6 mRNA. Relative expression is normalized to GAPDH expression. The RT-PCR was performed in pDC from three different thymus specimens and each of them was run in duplicate. PHA stimulated peripheral blood mononuclear cells from four different donors served as controls for TNF-α and IL-6 RT-PCR. B) The intracellular expression of p-Akt and NFkB-p65 in thymic pDC was determined by flow cytometry after gating on CD123+CD45RA+CD3−CD1a− (pDC) and CD123+CD45RA+CD3+CD1a+ thymocytes. Filled histograms (gray) represent unstained controls. The percentage indicated is the difference between cells staining with the p-Akt and NFkB-p65 antibodies and their respective controls.
Given the fact that IFN-α and TNF-α are constitutively expressed in thymic pDC, this may suggest that the signalling pathways that drive the induction of these cytokines is activated. It has been described that the PI3K pathway is essential for IFN-α production, while NFκB is essential for TNF-α production [10]. Hence, we measured phosphorylated p-AkT and p-NFκB p65 in thymic pDC by intracellular flow cytometric analysis as described by Guiducci et al. [10]. We observed both p-AKT and p-NFkB p65 expression in thymic pDC, but not in CD3+CD1a+ thymocytes (Figure 1B). The constitutive activation of these pathways in ex vivo thymic pDC lends further support to the continuous expression of IFN-α and TNF-α.
Thymic pDC have an “IFN-alpha signature”
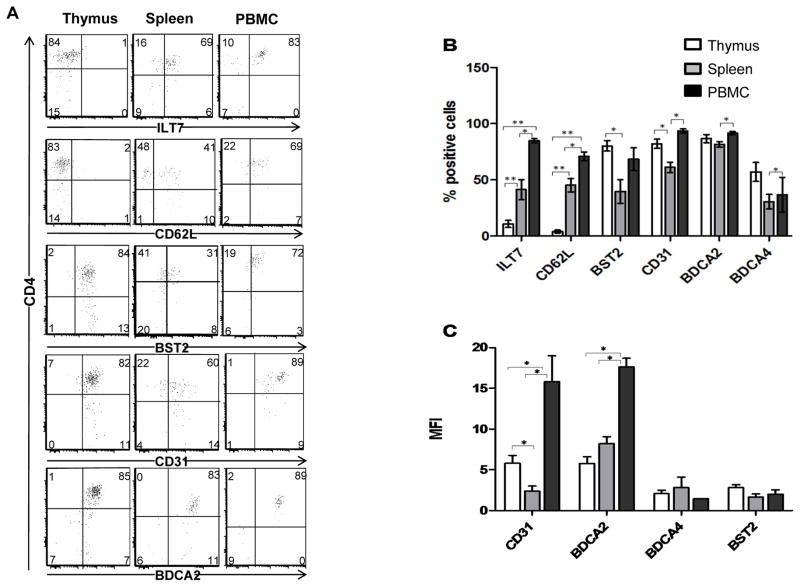
Previously, we showed that the ISG, MxA, was highly expressed in thymic pDC, but not in peripheral pDC [5]. This, together with the notion that IFN-α is constitutively expressed, suggested that thymic pDC respond in a paracrine or autocrine manner to IFN-α. To gain more insight into the consequences of this IFN-α signature, we compared the immunophenotype of thymic pDC and peripheral pDC, including pDC isolated from fetal spleen and blood, using flow cytometric analysis. The gating strategy of pDC is depicted in Supplementary Figure 2. We observed that BST2, which is known to be upregulated by pDC stimulated with IFN-α [13–14], was expressed at a higher percentage of thymic pDC as compared to pDC from either spleen or blood (Figure 2B). Expression of ILT7, which is known to be downregulated by IFN-α, and CD62L which decreases after stimulation with HSV after crosslinking of CD123 [9, 13–14], were hardly expressed on thymic pDC, but in contrast expressed on a high percentage (> 80%) of peripheral pDC and half of the splenic pDC (Figure 2A and 2B). Further, while all pDC irrespective of their origin expressed BDCA2, a C-type lectin shown to be downregulated by TLR ligation [15], the level of BDCA2 expression, i.e. mean fluorescence intensity (MFI), was clearly reduced on thymic pDC as compared to peripheral blood pDC (Figure 2C). Similarly, the MFI of CD31, an immunoglobulin-like receptor involved in regulation of immune responses [16], was higher on peripheral blood pDC as compared to thymic pDC. Collectively, these findings suggest that thymic pDC, but not peripheral pDC, have a phenotype that is reminiscent of an IFN-α signature.
Figure 2. pDC in the thymus present an activated “IFN-α signature” phenotype.
A) Multicolor flow cytometry was performed on total thymocytes, fetal splenocytes and PBMC. pDC were gated as CD123+CD45RA+CD3−CD1a−CD4+ cells, and assessed for the expression of: ILT7, CD62L, BST2, CD31, BDCA2 and BDCA4. B) Mean percentages of positive cells ± SD from independent donors analyzed as in A. Thymus, n = 11; spleen, n = 4; PBMC, n = 4. C) Mean fold difference in MFI ± SD that were calculated as: MFI of antigen/MFI of IgG control [35] from: 11 thymus, 4 fetal spleen and 4 PBMC from independent donors analyzed as in A. *p<0.05, **p<0.01 as calculated using the Mann Whitney test.
BST2 is expressed on a subset of thymocytes that resemble mature, medullary T cells
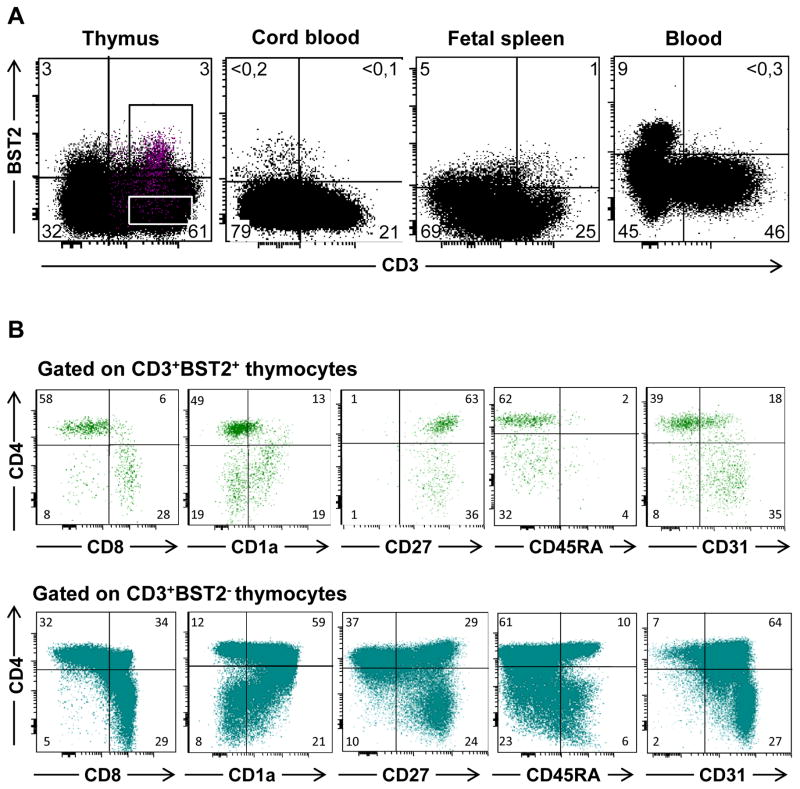
In addition to the IFN-α signature of thymic pDC, we have previously described that medullary thymocytes express the ISG MxA as a result of constitutive secretion of IFN-α [5]. To further assess the result of IFN-α exposure on thymocytes, we examined expression of other ISG in thymocytes. We observed that both ISG15 and BST2 mRNA were present in thymocytes (not shown). As shown in Figure 3A, BST2 was expressed constitutively on the cell surface of both CD3− and CD3+ thymocytes ex vivo. The majority of CD3−BST2+ cells were pDC, but also included a small number of thymic B cells and monocytes (data not shown). In contrast to the subset of CD3+ thymocytes, ≤1% of T lymphocytes in peripheral blood, cord blood or spleen expressed BST2 (Fig. 3A).
Figure 3. BST2 protein is expressed on the surface of medullary thymocytes, but not on T cells from peripheral or cord blood.
Multicolor flow cytometry was performed on thymocytes, peripheral blood, cord blood, and fetal spleen. A) Analysis of BST2 and CD3 expression. B) Thymocytes were analyzed for expression of CD4, CD8, CD1a, CD45RA, CD27 and CD31 after electronic gating on CD3+BST2+ or CD3+BST2− cells. Numbers in the plot indicate percentages of cells in each quadrant in a representative experiment.
To examine whether exogenous IFN-α can increase the level of BST2 that is constitutively expressed on thymocytes, we cultured total thymocytes for 5 days with 1000U of IFN-α. As shown in Supplemental Figure 3 the percentage of BST2+ cells increased from a mean of 8% on day 0 to a mean of 30% in the presence of IFN-α with mean of 11% BST2+ cells in the thymocytes cultured without IFN-α (mock). Thus, exogenous IFN-α induces BST2 expression on thymocytes above the level of in vivo constitutive BST2 expression. These data are in accordance with studies showing that IFN-α induces BST2 expression on human peripheral blood leukocytes and cell lines [13, 17].
To identify the maturation stage of the CD3+ thymocyte population that expressed BST2, we performed multicolor flow cytometric analysis. We observed that BST2+CD3+ thymocytes consisted mainly of mature CD4+ or CD8+ single positive T cells, and only few CD4+CD8+ double positive immature thymocytes (Fig. 3B, upper panel), indicating that CD3+BST2+ cells have a mature phenotype. This notion is strengthened by the observation that all BST2+CD3+ thymocytes expressed CD27, which is a marker of mature positively selected thymocytes located in the medulla (Fig. 3B). Relative lack of CD1a expression, which is a marker expressed in the cortex where the immature thymocytes are located, further supports their mature phenotype (Fig. 3B). Absence of CD45RA and low CD31 expression on CD3+BST2+ thymocytes, however, suggested that these cells have not yet prepared for emigration out of the thymus [5, 18]. In contrast to CD3+BST2+ cells, the CD3+BST2− cells have a mixed phenotype consisting of both mature and immature thymocytes (Fig. 3B, lower panel).
BST2+CD3+CD4+ thymocytes are enriched in cells with a Treg phenotype
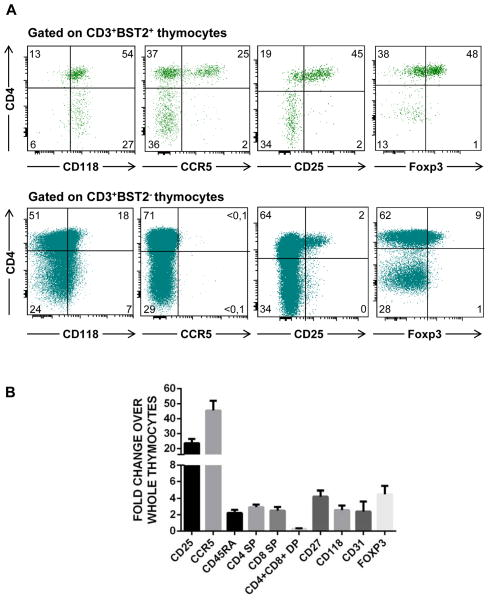
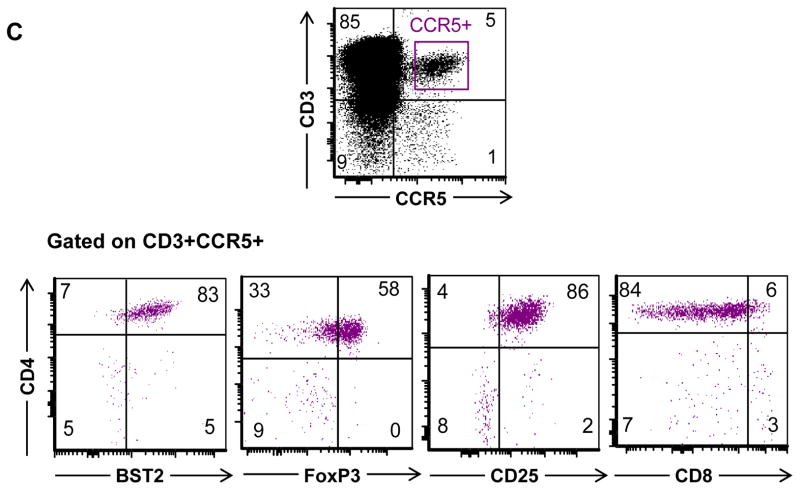
Our data lend support to the notion that pDC interact with a subset of the medulary thymocytes based on the observation that they express the ISG BST2. In line with this we observed that all CD3+BST2+ thymocytes expressed the IFN-α receptor CD118 (Figure 4). As pDC have been shown to play a role in the development of natural Treg in the thymus [7–8], this prompted us to further characterize the BST2 expressing thymocytes using multicolor flow cytometry. We found that Treg cells were greatly enriched among CD3+CD4+BST2+ cells (Fig. 4A, upper panel), but not in CD3+CD4+BST2− cells (Fig. 4A, lower panel), as more than 70% and 55% CD3+CD4+BST2+ cells expressed CD25 and Foxp3, respectively, which are typical markers expressed by Treg cells. Furthermore, we found that a large percentage (>40%) of the CD3+CD4+BST2+ thymocytes expressed CCR5 (Fig. 4A, upper panel) in contrast to none of the CD3+CD4+ thymocytes that lacked BST2 expression (Figure 4A, lower panel). More interestingly >80% of CCR5+ thymocytes expressed BST2 (Figure 4C). These results are in accordance with findings by others that IFN-α may induce CCR5 expression on thymocytes [19]. Interestingly, the large majority of BST2+CCR5+ express CD25+ and Foxp3+ (Figure 4C), suggesting that the thymic Treg that are CCR5+ also express BST2. Moreover, as shown in Figure 4B, the percentages of CD25 and CCR5 are more than 20-fold higher in CD3+CD4+BST2+ thymocytes than in total thymocytes. It is important to note that all Foxp3+ cells were CD25+ (data not shown). Taken together, we conclude that Treg cells are enriched in the CD3+CD4+BST2+ thymocytes and show an increase in expression of CCR5 as compared to the total thymocyte population (Fig. 4B).
Figure 4. BST2+ thymocytes present a phenotype consistent with natural Treg.
A) Multicolor flow cytometry was performed of thymocytes to measure expression of: CD3, BST2, CD4, CD8, CD118, CCR5, CD25, and Foxp3. Shown are the results in a representative experiment after electronic gating on CD3+BST2+ cells as indicated in Figure 3A. B) The relative fold difference in the percentages of the expression of the different markers on CD3+BST2+ thymocytes as compared to total thymocytes for: CD3, BST2, CD4, CD8, CCR5, CD25 (n=9) and FoxP3, CD118, CD31 (n =4). C) Multicolor flow cytometry was performed of thymocytes to measure expression of: CD3, BST2, CD4, CD8, CCR5, CD25, and Foxp3. Shown are the results after electronic gating on CD3+CCR5+ cells. n is the number of specimens.
BST2+CD3+CD4+ thymocytes have suppressor activity
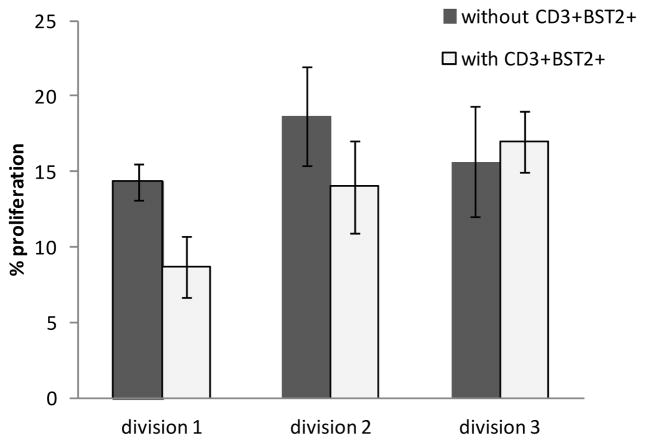
To determine whether the CD3+CD4+BST2+ population, in which the natural Treg population is enriched has suppressive functions we sorted BST2+CD3+ and BST2−CD3+ mature (CD27+) thymocytes to perform a suppression assay. The percentage of CD25+ Treg cells in the sorted BST2+CD3+ was 40–60% as determined by post-sort analysis (data not shown). Our results show that BST2 expressing mature thymocytes suppress proliferation of Violet Tracer labeled mature CD3+BST2− thymocytes that were stimulated with anti-CD3 and anti-CD28 in the presence of IL-2 and IL-4 in three out of three experiments (Figure 5). We observed that proliferation is reduced by 36% (mean value of 3 experiments) in the first cell division when CD3+BST2+ and CD3+BST2− cells were cultured at a 1:1 ratio (Figure 5). We did not observe reduced proliferation at other ratios likely due to the fact that the CD3+BST2+ thymocytes were not a pure, but only an enriched Treg population, and that thymocytes do not show high levels of proliferation [20]. Altogether, our results show that BST2+CD3+ thymocytes are enriched in cells with a natural Treg phenotype, which have suppressive functions.
Figure 5. BST2+CD3+ thymocytes suppress proliferation of autologous BST2negCD3+ thymocytes.
A suppressor assay was performed by co-culture of different ratios of sorted BST2+CD3+ cells with BST2negCD3+ cells, or without BST2+CD3+ cells. BST2negCD3+ cells were labeled with the dye Violet Tracer. Proliferation was induced by anti-CD3, anti-CD28 in the presence of IL-2 and IL-4. After 5 days, cells were stained with antibodies to CD3, BST2, CCR5, CD45RA, CD4, CD25, CD8 and CD27 and flow cytometry was performed to analyze the loss of the Violet Tracer as a measure of proliferation. Results are shown for the co-culture of BST2negCD3+ cells with BST2+CD3+ cells at a one to one ratio as compared to no BST2+CD3+ thymocytes in three experiments performed in cells from three different tissues.
DISCUSSION
Here we show that thymic pDC present an activated signature lacking expression of CD62L and ILT7 (BST2/Tetherin receptor) in combination with low levels of CD31 and BDCA2, which is consistent with their constitutive expression of IFN-α. Another novel finding is that TNF-α is constitutively expressed at the mRNA level in thymic pDC. However we could not detect constitutive expression of TNF-α in peripheral pDC without stimulation (data not shown), consistent with data reported in the literature [11]. We observed that BST2, which is an ISG, is not only expressed on pDC, but also on medullary CD3+CD27+CD4+ thymocytes. Notably, these BST2+ thymocytes are enriched in cells with a Treg phenotype and have suppressive activity. As a subset of these BST2+ thymocytes express CCR5 and virtually all CCR5+ medullary thymocytes are BST2+ (Figure 4C) they are prevalent targets for HIV.
Based on the notion that BST2 expression is regulated by type I IFNs [13] we consider that the expression of BST2 on medullary thymocytes is likely the result of IFN-α secretion by thymic pDC as we reported previously for the ISG, MxA [5]. Other cytokines such as IFN-β may be contributing to BST2 expression in the thymus as it has been reported that exogenous IFN-α as well as IFN-β increased BST2 expression on human peripheral blood pDC [21]. A low level of constitutive IFN-β mRNA expression was found in human thymic epithelial cells [22].
In contrast to peripheral blood pDC, we observed that neither thymic pDC nor thymocytes express ILT7, which is the receptor that ligates BST2. Cao et al. reported that BST2/ILT7 interaction is a negative feedback for IFN-α, TNF-α and IL-6 production by peripheral pDC [13]. Hence, constitutive production of IFN-α and TNF-α by pDC in the thymus may be the consequence of the observed lack of ILT7 expression in the thymus. Tavano et al. reasoned in a recent publication that the interaction of BST2 and ILT7 functions as a homeostatic mechanism for pDC rather than a negative feedback loop for activated mature pDC [17]. The contradictory results between the studies of Cao et al. and Tavano et al. may be due in part that Tavano et al. used total PBMC while Cao et al. used pDC isolated by negative selection. Importantly, in both reports data were obtained in vitro hence more studies in vivo or ex vivo are needed to shed light on the implications of BST2/ILT7 interactions. The study we present here was done ex vivo and is more supportive of BST2/ILT7 interaction functioning as a negative feedback loop for the expression of IFN-α and TNF-α. Furthermore, our observation that thymic pDC express only low levels of BDCA2, which also functions as a negative regulator of IFN-α production [23], may contribute to the constitutive production of IFN-α in the thymus.
We found that thymic pDC, in contrast to peripheral blood pDC, do not express CD62L (L-Selectin). As it is known that CD62L is shed from the cell surface upon activation [14, 24], this enforces our observation that thymic pDC have an activated phenotype. Moreover, since CD62L is important for trafficking of pDC to different organs [25], this suggests that thymic pDC may not leave the thymus and remain in the same location, i.e. the thymic medulla. Alternatively, pDC may emigrate from the thymus in a CD62L independent way, but this remains to be investigated.
In line with our results that thymic pDC constitutively express IFN-α is our observation that the pAkt and NFkB pathways are constitutively activated in thymic pDC. It has been reported that TLR-induced IFN-α expression is regulated by the pAkt pathway, while TNF-α and IL-6 are regulated by NFkB [10]. While we detected also constitutive expression of TNF-α in thymic pDC, we did not observe constitutive IL-6 expression. This may suggest that IL-6 expression is regulated differently than TNF-α at least in pDC in the thymus. Notably, lack of IL-6 expression by thymic pDC may be important to promote Treg development in the thymus as IL-6 negatively affects this process at least in the mouse [26]. The presence of receptors of the TNF receptor superfamily, including TNF-R2, on murine Treg has been recently reported to specifically promote Treg development and repertoire [27], indicating that the constitutive production of TNF-α by pDC in the human thymus may also play a role in Treg differentiation. Thymic pDC have been described to play an important role in human Treg development in two different studies, both of which suggest that pDC activated either by CD40 ligand (CD40L) and IL-3 or by thymic stromal lymphoprotein (TSLP) induce Foxp3+ Treg in CD4+CD8+ thymocytes [7–8]. However, the role of IL-6 has not been investigated in these studies. Our results suggest that IFN-α may play a role in Treg development as thymocytes expressing BST2, likely as a result of constitutive expression of IFN-α by pDC, are mature medullary thymocytes enriched in cells with a Treg phenotype and function. It is of interest that in particular low IFN-α levels produced by pDC have been shown to positively contribute to Treg expansion, which was impaired in the presence of high levels of IFN-α [28]. This, together with our data shown here and in our previous study [5] that thymic pDC produce only low levels of IFN-α in the absence of IL-6, reinforce the notion that pDC play an important role in the generation of natural Treg.
It is interesting to note that both BST2 and ISG15, which we found to be constitutively expressed in the thymus, have been previously described to have anti-HIV properties [29–30]. Moreover, we found that a subset of the BST2+ Treg express CCR5, which in the context of HIV infection has been reported to be increased in the thymus [19]. Vice versa, all CCR5+ thymocytes express BST2 and the majority of natural Treg express CCR5 on their surface. This correlation of BST2 expression and CCR5 expression and the results from others showing that IFN-α induced CCR5 in HIV infection [19], suggests that IFN-α may play an important role in inducing CCR5 expression on thymocytes even in the absence of a viral threat.
Interaction of BST2 with HIV induces the expression of pro-inflammatory genes, possibly through activation of NFκB [31]. This suggests that BST2 in addition to being an anti-HIV protein may have a signalling role during T cell development. Our data that BST2 is preferentially expressed on natural Treg cells suggest that BST2 may play a role in their development. As Foxp3 expression requires NFκB activation [32], IFN-α may be contributing to Treg development by inducing BST2 and thereby activation of NFκB [33]. TNF-α has also been described to be important in boosting Treg in the periphery [34], thus TNF-α similar to IFN-α may be favoring selection and proliferation of Treg in the thymus.
Altogether our data suggest that thymic pDC, which have been described to be important in natural Treg development through CD40:CD40L interactions or exposure to TSLP, may additionally support thymic Treg development by secreting low levels of IFN-α and TNF-α.
METHODS
Cell cultures
Fetal tissues were obtained from UCLA CFAR Gene and Cellular Therapy Core laboratory; adult peripheral blood mononuclear cells (PBMC) from the UCLA CFAR Virology Core laboratory; normal human postnatal thymus specimens from children undergoing corrective cardiac surgery at UCLA. Thymocytes were prepared and cultured, as previously described, in serum-free medium consisting of IMDM (Omega Scientific) supplemented with delipidated BSA (Sigma-Aldrich) at 1100 μg/ml, transferrin (Sigma-Aldrich) at 85 μg/ml, 2 mM glutamine, and penicillin/streptomycin at 25 U/25 μg/ml [12]. Thymocytes were cultured in serum-free medium as pellet cultures at 1–2×107 cells/ml in round-bottom tissue culture tubes at 37°C and 5% CO2. To examine the effect of exogenous IFN-α on BST2 expression on thymocytes, the cells were cultured for 5 days in serum-free medium in the presence or absence of 1000U/ml of exogenous IFN-α (InVitrogen).
Flow cytometry and Cell sorting
For cell surface staining 1×106 cells were stained with antibodies to CD123, BDCA2, BDCA4 (Miltenyi), CD3, CD45RA, ILT7, BST2, CD62L, CD31, CD25, CD1a, CD8 (eBioscience), CD4 and CD27 (Becton Dickenson, BD), CD118 (PBL Medical Laboratories), CCR5 (BD Pharmingen), or their control IgG, conjugated with FITC, PE, PerCP-Cy5.5, PE-Cy7, APC, APC-Alexa750, Pacific Blue, 605 eFluor or 650 eFluor (Nano-crystals). Cells were then fixed in 1% paraformaldehyde (PF), washed with Nano Crystal (NC) buffer (eBiosciences), and incubated for 20 min at 4°C. Cells were washed again in NC buffer and resuspended in NC buffer before acquisition on a High Throughput (HT) LSRII flow cytometer (BD). To calculate the mean fold difference in the Mean fluorescence intensity (MFI) (Geometric mean), the MFI of the cell surface antigen divided by the MFI of the IgG control was used [35].
For combined cell surface and intracellular staining with the antibody to Foxp3 (eBioscience) cells were first surface stained, then fixed with Fix/Perm buffer (eBioscience) for 30 minutes at 4°C and washed two times with the Fix/Perm buffer. After resuspending the cells in 25μl of Human AB serum Foxp3 antibody or rat IgG2a was added for 30 minutes at 4°C. Cells were then washed with NC buffer and acquired on HT LSRII flow cytometer (BD).
For combined cell surface and intracellular staining to detect phosphorylated Akt and NFkB 1×106 cells were cells were first surface stained with the desired antibodies, then fixed with 1% paraformaldehyde (PF) for 10 min and permeabilized with 0.2% Tween-20 for 15 min. at 37°C. After washing with 0.5% PBS, 0.5% BSA in NC buffer, the cells were stained intracellularly with p-Akt or NFkB-p65/RelA antibody (BD) for 30 min at room temperature, washed with 0.5% PBS, 0.5% BSA in NC buffer and acquired on HT LSRII flow cytometer.
To obtain purified populations of pDC and BST2+CD3+ and BST2negCD3+ cells, thymocytes were washed in serum-free medium. 100μl of HAB was added to pelleted cells and the cells were stained with antibodies to BST2, CD3, CD123 and CD45RA in a total volume of 500μl serum free medium and incubated for 20min at 4°C, washed, resuspended in 1.5ml serum free medium followed by cell sorting of CD123+CD45RA+ (pDC) and BST2+CD3+ and BST2negCD3+ thymocytes using a FACSAria II cell sorter (BD).
Real-time quantitative PCR (Taqman)
RNA was extracted from sorted cells with TriReagent (Trizol) (Invitrogen), as previously described [5]. Taqman gene expression assays were used to measure gene expression (Applied Biosystems). Real-time PCR measurements were done in duplicate for analysis of TNF-α, IFN-α and IL-6 mRNA levels following manufacturer’s instructions. 96 well plates were run on an ABI 7300 and analyzed with the 7300 system software. Values of TNF-α, IFN-α and IL-6 mRNA were normalized for total RNA content by dividing by GAPDH mRNA.
Treg assays
Sorted BST2+CD3+ thymocytes, which are enriched in natural Treg, were tested for their suppressive effect on the proliferation of autologous BST2negCD3+ thymocytes labeled with Violet Tracer (Invitrogen) in round bottom 96 well plates (Falcon) coated with T3 antibody (Coulter) at 1.25 μg/ml. Briefly 1×106 sorted BST2negCD3+ thymocytes were stained with 1μl of 5nM Violet Tracer (Invitrogen) at 37°C for 20 min., washed in 5ml of serum free medium, and 50μl at 5×105 cells/ml was added to each well. BST2+CD3+ Treg at 5×105cells/ml were serially 1:2 diluted and 50μl of the diluted cells was added to the Violet Tracer labeled BST2negCD3+ thymocytes. 100μl of serum free medium containing IL-2 (20u/ml), IL-4 (20ng/ml) and anti-CD28 (10ng/ml) was added to a total volume of 200μl/well and the cells were cultured for 5 days at 37°C. at which time the cells were washed and run on an HT LSRII to determine the amount of proliferation of Violet Tracer labeled cells.
Statistics
Two sided Wilcoxon-Mann-Whitney test was used to compare mean total expression of pDC markers and to compare mean fluorescence intensity of the pDC markers.
Supplementary Material
Acknowledgments
We thank the cardiac surgery team and the staff of the Translational Pathology Core laboratory at UCLA for providing us with the thymus specimens. The UCLA Virology Core and UCLA Gene and Cellular Therapy Core, supported by the UCLA Center for AIDS Research (CFAR) for providing us with peripheral blood specimens and fetal lymphoid tissues, and the staff of the UCLA CFAR Flow Cytometry Core for their assistance with the multicolor flow cytometry. We are grateful for the help of Robert Furler for his critical review of the manuscript, Otto Haller and George Kochs for the antibody to MxA, and Joshua Craft for his excellent technical assistance.
Grant support: This work was supported by Grants from the National Institutes of Health (AI 080564 (to CU and BB), AI 080564-S (to CU), AI 52002 (to CU), UCLA CFAR grant AI 28697, UCLA Cancer Center Support grant CA-16042, NIH Training grants T32-AR-053463 and T32-CA-009120 (to ME) and an Idea Award (ID08-LA-053) from the University of California HIV/AIDS Research Program (to CU). Dr. Epeldegui contributed to the experimental design, performed the experiments, interpreted the data and wrote the paper. Dr. Blom contributed to the experimental design, interpretation of the data and writing of the paper. Dr. Uittenbogaart contributed to the experimental design, interpretation of the data and writing of the paper.
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflicts of interest.
References
- 1.Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89:843–855. doi: 10.1016/j.biochi.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao M, Liu YJ. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell. 2013;4:40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briere F, Bendriss-Vermare N, Delale T, Burg S, Corbet C, Rissoan MC, Chaperot L, Plumas J, Jacob MC, Trinchieri G, Bates EE. Origin and filiation of human plasmacytoid dendritic cells. Hum Immunol. 2002;63:1081–1093. doi: 10.1016/s0198-8859(02)00746-2. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87:609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colantonio AD, Epeldegui M, Jesiak M, Jachimowski L, Blom B, Uittenbogaart CH. IFN-alpha is constitutively expressed in the human thymus, but not in peripheral lymphoid organs. PLoS One. 2011;6:e24252. doi: 10.1371/journal.pone.0024252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Res PC, Couwenberg F, Vyth-Dreese FA, Spits H. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–2657. [PubMed] [Google Scholar]
- 7.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010 doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 8.Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster P, Donhauser N, Pritschet K, Ries M, Haupt S, Kittan NA, Korn K, Schmidt B. Co-ordinated regulation of plasmacytoid dendritic cell surface receptors upon stimulation with herpes simplex virus type 1. Immunology. 2010;129:234–247. doi: 10.1111/j.1365-2567.2009.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 12.Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J Immunol. 2004;173:7269–7276. doi: 10.4049/jimmunol.173.12.7269. [DOI] [PubMed] [Google Scholar]
- 13.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanning SL, George TC, Feng D, Feldman SB, Megjugorac NJ, Izaguirre AG, Fitzgerald-Bocarsly P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, Wu J, Liu S, Han X, Lu J, Shi Y, Wang J, Lu L, Cao X. TLR9/TLR7-triggered downregulation of BDCA2 expression on human plasmacytoid dendritic cells from healthy individuals and lupus patients. Clin Immunol. 2008;129:40–48. doi: 10.1016/j.clim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist’s guide to CD31 function in T-cells. J Cell Sci. 2013;126:2343–2352. doi: 10.1242/jcs.124099. [DOI] [PubMed] [Google Scholar]
- 17.Tavano B, Galao RP, Graham DR, Neil SJ, Aquino VN, Fuchs D, Boasso A. Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1. 24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood leukocytes. J Immunol. 2013;190:2622–2630. doi: 10.4049/jimmunol.1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Stoddart CA, Keir ME, McCune JM. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog. 2010;6:e1000766. doi: 10.1371/journal.ppat.1000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uittenbogaart CH, Anisman DJ, Zack JA, Economides A, Schmid I, Hays EF. Effects of cytokines on HIV-1 production by thymocytes. Thymus. 1994;23:155–175. [PubMed] [Google Scholar]
- 21.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1. 24/tetherin in humans. Proc Natl Acad Sci U S A. 2011;108:13688–13693. doi: 10.1073/pnas.1101684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cufi P, Dragin N, Ruhlmann N, Weiss JM, Fadel E, Serraf A, Berrih-Aknin S, Le Panse R. Central role of interferon-beta in thymic events leading to myasthenia gravis. J Autoimmun. 2014;52:44–52. doi: 10.1016/j.jaut.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro JM, Briscoe DM, Tedder TF. Differential regulation of leucocyte L-selectin (CD62L) expression in normal lymphoid and inflamed extralymphoid tissues. J Clin Pathol. 1996;49:721–727. doi: 10.1136/jcp.49.9.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 26.Paiva RS, Lino AC, Bergman ML, Caramalho I, Sousa AE, Zelenay S, Demengeot J. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc Natl Acad Sci U S A. 2013;110:6494–6499. doi: 10.1073/pnas.1221955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, Farrar MA. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, Durand I, Le Mercier I, Bajard A, Bachelot T, Puisieux A, Puisieux I, Blay JY, Menetrier-Caux C, Caux C, Bendriss-Vermare N. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesh S, Bieniasz PD. Mechanism of HIV-1 virion entrapment by tetherin. PLoS Pathog. 2013;9:e1003483. doi: 10.1371/journal.ppat.1003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincetic A, Kuang Z, Seo EJ, Leis J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol. 2010;84:4725–4736. doi: 10.1128/JVI.02478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, Hayashi H, Sugano S. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- 32.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe. 2012;12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilate AM, Lafaille JJ. Can TNF-alpha boost regulatory T cells? J Clin Invest. 2010;120:4190–4192. doi: 10.1172/JCI45262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terstappen LW, Hollander Z, Meiners H, Loken MR. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990;48:138–148. doi: 10.1002/jlb.48.2.138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.