Abstract
Osteoarthritis is one of the leading causes of disability in the aging population. Long duration, low intensity therapeutic ultrasound has had promising impact in animal models to slow the progression of the disease and provide joint relief. Two pilot studies were conducted using a novel, wearable platform for delivering ultrasound to evaluate the potential clinical benefits of ultrasound therapy on knee osteoarthritis. There was a pain reduction effect from using ultrasound, as high as fifty two percent in one study. As well, initial data demonstrates that mobility may be increased for patients experiencing mild to moderate arthritis of the knee.
Keywords: wearable ultrasound, bioregenration, therapeutic ultrasound, long duration ultrasound, osteoarthritis
I. Introduction
Arthritis (most commonly osteoarthritis) is experienced by approximately 50 million Americans. As the aging population expands, the costs associated with treating osteoarthritis increase significantly. In a study of medical spending, the U.S. Centers for Disease Control and Prevention found that the cost of treating arthritis in the U.S. rose from $65 to $81 Billion[1] between 1997 and 2003. Including lost wages due to disability and other indirect costs, the total economic impact of arthritis in 2003 was $128 billion[1]. Osteoarthritis (OA) affects the smooth fibrous connective tissue known as articular cartilage, which is essential for smooth motion and cushioning of the joint. This tissue wears away as the disease progresses, causing inflammation of the synovial membrane, the lining that surrounds the knee joint and contains the synovial fluid. OA can form in any joint but is more common in weight bearing joints such as the knee and hip, and results in joint space narrowing, severe pain and loss of mobility [2, 3]. More than 40% of individuals over 65 have symptomatic OA and reduced mobility due to the degenerative disease [4, 5].
Low Intensity Therapeutic Ultrasound (LITUS) has only recently been evaluated in the last decade as a treatment for diverse musculoskeletal disorders and there is considerably less published literature regarding the clinical efficacy of LITUS[6-9]. This new approach to ultrasound application increases many of the benefits of therapeutic ultrasound regimens, and it may be applied for longer durations without danger of causing pain or thermal damage. In “best practice”, therapeutic ultrasound is applied at 1–4 W/cm2 in 15-20 minute treatment sessions weekly. LITUS is being used at 30-1000 mW/cm2 (an order of magnitude less in intensity) for 4-8 hrs in the research setting. The United States Food and Drug Administration has approved LITUS levels below 132 mW/cm2 for long duration use [10-14].
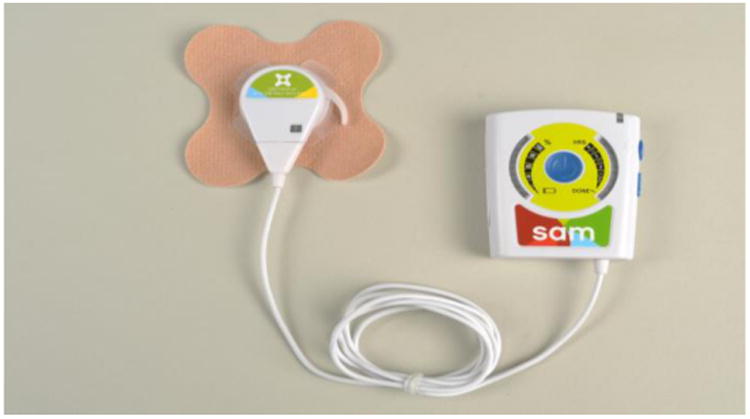
This research was conducted using a battery-powered, wearable LITUS device (Figure 1). A coin-sized transducer applicator is attached to the skin with an ultrasound coupling bandage and delivers diverging beam ultrasound into the joint space. The objective of this study was to determine the effectiveness of daily, low intensity, long duration therapeutic ultrasound treatment in reducing pain in subjects with mild to moderate osteoarthritis of the knee.
Figure 1. LITUS device with bandage.
II. Procedure
Two IRB approved pilot studies were conducted. In the first study, subjects used the treatment for a period of 12 to 60 days on knee OA at various stages. The subjects used the device daily for four hours. The subjects reported their pain scores on a 0-10 Visual Analogue Scale (VAS).
The second study was a placebo controlled, double-blind clinical trial. Subjects were enrolled if they had mild to moderate knee OA, were between 35-80 years, reported a frequent pain score of 3 to 7 on the VAS during the week preceding enrollment, and are deemed appropriate to participate by the study physician.
This study duration was 6 weeks. During the first two weeks of the study, subjects wore an actigraph that tracked their mobility and had a memory function for the input of daily pain scores. Subjects were asked to record pain scores in the morning, afternoon, and evening. After two weeks, the subjects received a wearable ultrasound device, randomized into active devices and placebo devices. They were instructed in its use, and asked to apply the device in the morning, after entering their initial pain score. Subjects wore the device for four hours, and were asked not to alter their daily routine. Subjects continued to wear the actigraph and use the device daily for four hours for four weeks. At the end of the study, subjects returned the device and the actigraph.
The data was analyzed to evaluate whether or not there was a change in pain and mobility as recorded by the actigraph. The user pain data was plotted over the two weeks of baseline, and then each two weeks of treatment data (weeks 3-4 and 5-6) were analyzed as a group. For mobility, the total counts recorded while awake was collected and analyzed in the same manner, averaging the data over two week clusters. This was done to minimize the effect of any outliers in the data set.
III. Results
In the first study, which was not placebo controlled, all twelve subjects had active devices. Subjects reported a decrease in pain between 2 and 4 points on the VAS. Retrospective analysis indicated that this was a 52% decrease in pain, which was statistically significant (p<0.05). These pain decreases are similar to reductions observed when using ultrasound to treat chronic muscle pain and shoulder pain [14-16].
In the second study, seven subjects were enrolled in the pilot phase, with three subjects assigned to the placebo group, and four subjects assigned to the active group. Six subjects successfully wore the device for 6 weeks, applying the treatment for four hours each morning, and charging the device overnight. One subject reported minor skin irritation due to the bandage adhesive, and discontinued treatment after 4 weeks. No adverse effects due to the ultrasound therapy were observed.
While a wide range of results were seen for the pain scores reported, there was an overall reduction of pain in both the active and the placebo groups. The average pain score decrease seen in the active group was 1.3±1.5, while the placebo group observed an average decrease of 1.5±1.9. These differences are statistically similar. One difficulty in the data analysis is that pain scores were not constantly reported by some subjects. For the mobility data, three out of the four subjects in the active group demonstrated a 20% increase in daily activity or more after 6 weeks of daily ultrasound therapy. During analysis, mobility for each subject was normalized by their baseline scores, to control for different progressions of the disease and lifestyles. For the active group, daily activity while receiving treatment was 115±25% of what it was prior to receiving ultrasound therapy. For the placebo group, daily activity during inactive treatment was 105±10% of what it was during their baseline period.
IV. Conclusions
The results of this study revealed that the wearable LITUS device was usable for arthritic subjects on a daily basis as part of their regular routine. Additionally, pain reduction was observed, though the limited sample size and lack of placebo controls in one study make differences difficult to substantiate. In the placebo controlled study, three out of four subjects that received active LITUS increased daily mobility more than 20% over four weeks of treatment. Interestingly, while pain reduction was seen in the placebo cases as well as the treatment case, mobility increases were limited to real treatment. To further prove the clinical relevance of these results, a larger clinical trial is being conducted to demonstrate if the effects observed in this trial will be statistically significant when tested with a fully powered study.
Contributor Information
Matthew D Langer, Email: mlanger@zetroz.com, ZetrOZ, Inc., Trumbull, CT, USA.
Vanessa Levine, ZetrOZ, Inc., Trumbull, CT, USA.
Rebecca Taggart, ZetrOZ, Inc., Trumbull, CT, USA.
George K Lewis, ZetrOZ, Inc., Trumbull, CT, USA.
Lyndon Hernandez, Department of Gastroenterology, Medical College of Wisconsin, Milwaukee, WI.
Ralph Ortiz, Medical Pain Consultants, Freeville, NY.
References
- 1.Billingsley J. U.S. Arthritis numbers, costs soearing. Health Day News. 2007 ed. [Google Scholar]
- 2.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Annals of the Rheumatic Diseases. 2001 Feb 1;60:91–97. doi: 10.1136/ard.60.2.91. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons D, Travell J, Simons L. Myofascial pain and dysfunction: The trigger point manual. 2nd. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 4.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987 Aug;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966 Jan;25:1–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Qin L, Fok P, Lu H, Shi S, Leng Y, Leung K. Low intensity pulsed ultrasound increases the matrix hardness of the healing tissues at bone–tendon insertion—a partial patellectomy model in rabbits. Clinical biomechanics (Bristol, Avon) 2006;21:387–394. doi: 10.1016/j.clinbiomech.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Dijkman B, Bhandari M, Sprague S. Low-intensity pulsed ultrasound: Nonunions. Indian Journal of Orthopaedics. 2009;43:141–148. doi: 10.4103/0019-5413.50848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della Rocca G. The science of ultrasound therapy for fracture healing. Indian Journal of Orthopaedics. 2009;43:121–126. doi: 10.4103/0019-5413.50845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pounder NM, Harrison Aj. Low intensity pulsed ultrasound for fracture healing: A review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48:330–338. doi: 10.1016/j.ultras.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.FDA. US FDA 510(k) ultrasound device approvals K063728, K081075, and K970131. 2008 ed. [Google Scholar]
- 11.AIUM. Bioeffects considerations for the safety of diagnostic ultrasound. Journal of Ultrasound Medicine. 1988;7:S1–S38. [PubMed] [Google Scholar]
- 12.AIUM. Bioeffects and safety of diagnostic ultrasound. Laurel, MD: American Institute of Ultrasound in Medicine; 1993. [Google Scholar]
- 13.Fowlkes JB, Holland CK. Mechanical bioeffects from diagnostic ultrasound: AIUM consensus statements. American Institute of Ultrasound in Medicine. Journal of Ultrasound in Medicine. 2000 Feb 1;19:69–72. doi: 10.7863/jum.2000.19.2.69. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis G, Jr, Langer M, Henderson H, Ortiz R. Mobile Pain Therapy: Design and Clinical Evaluation of a Wearable Long- Duration. 2012 in review. [Google Scholar]
- 15.Lewis G, Jr, Guarino S, Ortiz R. Wearable long-duration ultrasound treatment of chronic trapezius myalgia. The journal of pain : official journal of the American Pain Society. 2012;13:s94. [Google Scholar]
- 16.Lewis G, Hernandez L, Lewis GK, Sr, Ortiz R. Wearable long duration ultrasound therapy pilot study in rotator cuff tendinopathy. Proceedings of Meetings on Acoustics. 2013;19 doi: 10.1121/1.4800272. [DOI] [PMC free article] [PubMed] [Google Scholar]


