Abstract
One of the major challenges in the design of a new class of medical device is ensuring that the device will be usable by a broad range of its intended users. Human Factors Engineering addresses these concerns through direct study of how a user interacts with newly designed devices with unique features. In this study, a novel long duration, low intensity therapeutic ultrasound device is tested by 20 end users representative of the intended user population. Over 90% of users were able to operate the device successfully. The therapeutic ultrasound device was found to be reasonable safe and effective for the intended users, uses, and use environments.
I. Introduction
Therapeutic ultrasound has been used to treat pain associated with musculoskeletal conditions and increase local blood flow in soft tissues for the past 60 years. The skill required to apply the treatment properly, along with the cost and size of the equipment, has confined ultrasound to the practitioner’s office. As a result, treatments are typically applied once or twice a week for 5–15 minutes [1, 2]. These factors have limited the therapeutic potential of ultrasound, which research has shown is more effective when ultrasound is applied more frequently or for longer durations. Recently, long duration, low intensity therapeutic ultrasound has demonstrated an ability to maintain joint cartilage and modify osteoarthritis in animal models [3, 4], as well as treat tendinopathies and heal non-union bone fractures in humans [5]. Advances in piezoelectric technology have enabled a miniaturization of therapeutic ultrasound technology. As a result, it is now possible for an ultrasound therapy system to be wearable and self-applied by an end user e.g. the patient themselves. Under the care of a physician, such a device has been used to relieve muscle spasm [6], treat tendinopathy [7], and reduce pain associated with osteoarthritis [8]. While there is significant potential for such a technology to provide a non-pharmaceutical alternative to pain relief, the device must take into account how a non-professional user will handle the product. These studies, called Human Factors Engineering (HFE), represent a significant portion of designing a new medical device and ensuring it is optimally designed for the intended users and use case scenarios. This work describes an IRB-approved clinical study conducted to ascertain the degree of usability of a new medical device.
A. Therapeutic Device and User Interface
The therapeutic device, called sam® (subsequently referred to as the therapeutic ultrasound device), is a portable, wearable ultrasound device (Figure 1). It is currently available with a prescription in the US. The device is comprised of three components: a power controller, an ultrasound transducer (applicator), and a coupling bandage. The power controller consists of the operator interface, timing circuit and battery for the device. The applicator generates a high frequency signal and converts the electric energy to acoustic driver energy at 98% efficiency [9, 10]. The coupling bandage consists of a non-woven fabric coated with a medical grade adhesive, a plastic interlock that secures the ultrasound transducer to the body, and a reservoir of coupling medium that acts as a conduit for the ultrasound signal. Prior to use, the device must be charged through a micro-USB port. The device is capable of delivering continuous 132 mW/cm2 ultrasound at 3 MHz for treatment intervals up to four hours.
Figure 1.
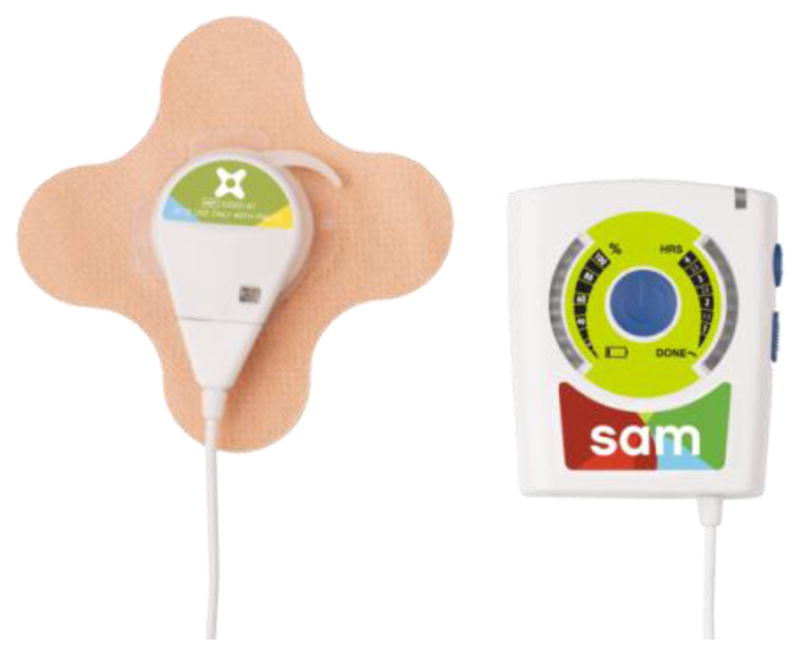
The therapeutic ultrasound system tested. On the left is the ultrasound transducer attached to a bandage. On the right is the power controller, which contains the user interface.
The user experience when using the device starts by connecting the applicator to the power controller by use of a cable with a special luer-lock fitting. The user then clips the applicator into the coupling bandage and applies it to the body in the treatment location. The device is operated through an interface on the power controller. There is a large central power button which activates the device. There is a toggle switch to set the treatment time, and there is a lock switch to prevent the treatment time from changing inadvertently during use.
II. Procedures
A. Subject selection and enrollment
Twenty subjects completed this IRB-approved study (Schulman IRB #201307896). This sample size was chosen based on FDA guidance on human factors research [11, 12]. Eligible subjects were 18 years of age or older, with the ability to read, write, and speak English. Individuals were excluded if they had a condition that is contraindicated for ultrasound therapy. Subjects were recruited through flyers, provided informed consent, and enrolled in the study. They would then receive a short (approximately 10 minute) demonstration on how to apply and operate the device.
B. Study Treatments
The therapeutic ultrasound device was the intervention method in the study. This device has an operation mode with one ultrasound transducer and an operation mode with two ultrasound transducers. Subjects were asked to use the device three times within a seven day period, each time for a four-hour treatment duration. For their first treatment, the subjects were asked to use the device with one ultrasound transducer. For the second treatment, the subjects were asked to use the device with two ultrasound transducers. During the third treatment, subjects were free to use the device in either operation mode. After each treatment, subjects were asked to fill out a usability feedback survey.
C. Subject Surveys
Subjects were asked to fill out a 27 question quiz that assessed how the device was used, where it was applied, the ease of use, whether the device was operated successfully, and a discussion of any issues that may have been encountered while wearing the device. The complete survey, along with a summary of subjects’ responses to each question, is shown in Table 1.
Table 1.
Survey Questions and Response Summary
| Question | Summary of Subjects’ Responses |
|---|---|
| (1) Did you use 1 or 2 applicators for today’s treatment? | 43% used 1 applicator. |
| 1A. If you used 2 applicators, did the y-adapter function correctly? (If you used 1 applicator, leave blank) | 92% Yes; 8% No |
| (2) Were you able to connect the applicator to the power controller? | 100% Yes; 0% No |
| (3) Were you able to attach the applicator(s) to the bandage(s)? | 100% Yes; 0% No |
| (4) How difficult was it to attach the applicator(s) to the bandage(s)? | 62% Easy; 33% Somewhat Difficult; 5% Very Difficult |
| (5) Did you attach the applicator to the bandage before or after you applied the bandage to your skin? | 8% After; 92% Before |
| (6) How much ultrasound gel squeezed out when you attached the applicator to the bandage? | 50% A lot; 48% A little; 2% None |
| (7) Did you add additional ultrasound gel to the bandage or to the surface of the applicator? | 2% Yes; 98% No |
| (8) Were you able to successfully apply the bandage to your skin? | 98% Yes; 2% No |
| (9) Were you able to power ‘ON’ the device? | 100% Yes; 0% No |
| (10) Were you able to set your desired treatment duration using the toggle button? | 98% Yes; 2% No |
| (11) Did the applicator(s) function correctly? | 95% Yes; 5% No |
| (12) Did the device function for the entire duration that you programmed? | 90% Yes; 10% No |
| (13) Were you able to power ‘OFF’ the device? | 97% Yes; 3% No |
| (14) Were you able to remove the device from your body? | 100% Yes; 0% No |
| (15) Did you use the pull tab on the bandage to remove the applicator from the bandage? | 65% Yes; 35% N |
| (16) Overall, were you able to successfully operate the device? | 95% of users were able to operate the device successfully |
| (17) Did the applicator get too hot at any point during your treatment? | The applicator got too hot in 8% of cases; didn’t get too hot in 92% of cases. |
| (18) Did the applicator vibrate at any point during your treatment? | 80% Yes; 20% No |
| (19) Did the applicator fall off at any point during your treatment? | 2% Yes; 98% No |
| (20) Did you have any issues charging the device with the supplied electrical charger? | 0% Yes; 100% No |
| (21) Describe any problems that you experienced with the usability of the device: (If no problems, write “None”) | 63% had comments; 37% had no comments. |
| (22) Did you experience any discomfort from the device or the treatment? | 12% Yes; 88% No |
| (22A). If yes, describe the discomfort you felt: | 3% uncomfortably hot, 5% from vibration of applicator, 3% from skin irritation to bandage |
| (23) Describe the appearance of your skin that was contacting the applicator: normal redness bumps blister other | 33% redness, 67% normal, 0% bumps or blister |
| (24) Overall, do you think the device is easy to use? | 93% Yes; 7% No |
| (25) Did you have a positive experience using this device? | 90% Yes; 10% No |
| (26) Would you use this device again? | 87% Yes; 13% No |
| (27) Compared to traditional ultrasound machines, do you think this device is portable? | 100% said Yes |
III. Results
All twenty subjects completed the study successfully. The complete results, along with the questions, are shown in Table 1. Most importantly, no subjects reported an adverse event or experienced any skin damage from 4-hour treatments with the device. Nearly all subjects were able to successfully operate the device (95%) and thought the device was easy to use (93%) (Figure 2). Additionally, 90% of users had a positive experience overall, and 87% of users would use the device again. The primary constructive feedback related to the device was that the coupling medium was messy, and that there were difficulties with the interlock between the ultrasound transducer and the bandage containing the coupling medium, both in making the connection and in breaking the connection post-use.
Figure 2.
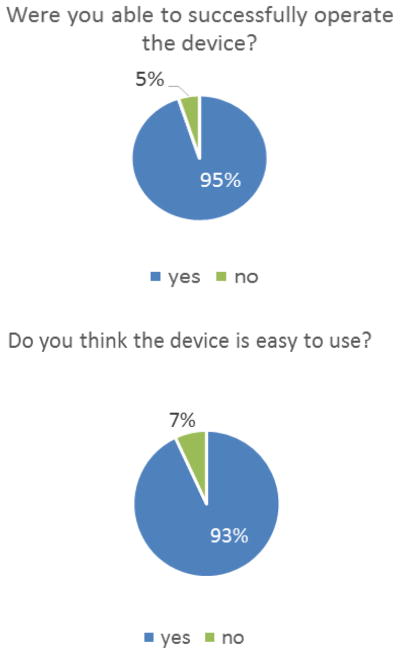
(Top) The fraction of uses where the device was successfully operated. (Bottom) The percentage of responses that felt the device was “easy to use”.
A small fraction of uses (8%) were followed by the subject saying that the applicator got too hot during the treatment, but only a third of them (3%) reported discomfort due to the heat. Other reported causes of discomfort were that the bandage was irritating to the skin (3%) and that the device vibrated too much (5%), which indicates the activation of a safety feature. Following treatment, subjects described their skin as normal in appearance 67% of the time, and having some redness 33% of the time. Slight skin redness can be expected due to the heating of tissue and the increase in local circulation caused by therapeutic ultrasound, and is not considered to be a negative effect.
The sample size of this study, 20 subjects, with each subject using the device three times, means that at a minimum, 95% of all user problems should be found, and the mean percentage is between 98.4% and 99.0% [12]. This demonstrates that the study captured the potential usability issues of this device with a high degree of confidence, and therefore, this feedback is sufficient to assess usability and any residual risk associated with the device.
IV. Discussion
The device was successfully used by 20 subjects over 60 treatments with no negative effects or adverse events. The overall lessons taken from this study are that the device was usable for a layperson, that the vast majority of user experiences were positive, and the majority of user feedback involved making the applicator interface with the bandage easier to handle. The device was successful in providing treatments, and nearly 9 in 10 subjects who tried the device would use it again. The therapeutic ultrasound device was found to be reasonable safe and effective for the intended users, uses, and use environments. The 95% success rate of usage and the methods for collecting data support this conclusion. Any residual risk that remains would not be reduced by modification of the design of the user interface, and is outweighed by the therapeutic benefit derived from the use of the device. To further increase user convenience, future design work should include researching alternate coupling materials which may reduce the mess associated with ultrasound gel and developing a more robust clip in and breakaway system. User feedback is an invaluable part of the design loop, allowing engineers to improve on products and learn design lessons for the next products. Long duration, low intensity therapeutic ultrasound holds the promise of providing non-pharmaceutical pain relief to patients suffering from a broad range of conditions. This study demonstrates that the wearable ultrasound device is accessible to a general audience, and that the technology will fulfill that promise.
Acknowledgments
This research was supported by NIH Grant #1R43MD008597-01 and the Connecticut Innovations Talent Bridge Program.
The authors wish to acknowledge Vanessa Levine and Kelly Stratton for their contributions in subject interaction and assistance with data collection. The authors also thank Transducer Engneering for valuable insights and assistance with device construction.
References
- 1.Cameron MH. Physical agents in rehabilitation: from research to practice. W.B. Saunders; 1999. [Google Scholar]
- 2.Michlovitz SL, Bellew JW, Nolan TP. Modalities for Therapeutic Intervention. 5. Philadelphia: F.A. Davis Company; 2012. [Google Scholar]
- 3.Loyola-Sánchez A, Richardson J, Beattie KA, Otero-Fuentes C, Adachi JD, MacIntyre NJ. Effect of Low-Intensity Pulsed Ultrasound on the Cartilage Repair in People With Mild to Moderate Knee Osteoarthritis: A Double-Blinded, Randomized, Placebo-Controlled Pilot Study. Archives of physical medicine and rehabilitation. 2012;93:35–42. doi: 10.1016/j.apmr.2011.07.196. [DOI] [PubMed] [Google Scholar]
- 4.Gurkan I, Ranganathan A, Yang X, Horton WE, Todman M, Huckle J, et al. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:724–733. doi: 10.1016/j.joca.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated Healing of Distal Radial Fractures with the Use of Specific, Low-Intensity Ultrasound. A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Study*. The Journal of Bone & Joint Surgery. 1997;79:961–73. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Lewis GK, Langer MD, Henderson CR, Ortiz R. Design and Evaluation of a Wearable Self-Applied Therapeutic Ultrasound Device for Chronic Myofascial Pain. Ultrasound in medicine & biology. 2013;39:1429–1439. doi: 10.1016/j.ultrasmedbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Lewis G, Hernandez L, Lewis GK, Sr, Ortiz R. Wearable long duration ultrasound therapy pilot study in rotator cuff tendinopathy. Proceedings of Meetings on Acoustics. 2013;19 doi: 10.1121/1.4800272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer MD, Levine V, Taggart R, Ortiz R, Hernandez L, Lewis G., Jr Pilot Clinical Studies of Long Duration, Low Intensity Therapeutic Ultrasound for Osteoarthritis. presented at the Northeast Bioengineering Conference; Boston, MA. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis GK, Jr, Olbricht WL. Development of a portable therapeutic and high intensity ultrasound system for military, medical, and research use. Rev Sci Instrum. 2008 Nov;79:114302. doi: 10.1063/1.3020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis GK, Jr, Olbricht WL. Design and characterization of a high-power ultrasound driver with ultralow-output impedance. Rev Sci Instrum. 2009 Nov;80:114704. doi: 10.1063/1.3258207. [DOI] [PubMed] [Google Scholar]
- 11.FDA. Draft Guidance for Industry and Food and Drug Administration Staff-Applying Human Factors and Usability Engineering to Optimize Medical Device Design. 2011 Available: http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm259748.htm.
- 12.Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. 2003 Aug;35:379–83. doi: 10.3758/bf03195514. [DOI] [PubMed] [Google Scholar]


