Abstract
Background
Skull base tumors comprise many common benign brain tumors. Treatment has advanced, allowing many survivors to return to work. However, literature is limited about the neuropsychological status of these patients prior to treatment. Literature pertaining to the relationship between neuropsychological functioning and occupational ability prior to surgical intervention is even more limited. The purpose of this analysis was to evaluate the impact of neuropsychological function on work productivity in persons with skull base tumors prior to resection.
Methods
Neuropsychological function and work productivity were assessed in adults newly diagnosed with skull base tumors (n = 45) prior to surgical intervention. Univariate analyses identified potential predictors of work limitations; variables with P < .10 were analyzed using multivariate regression analyses controlled for age, sex, tumor type, and education.
Results
Poorer mental attention and flexibility (MF) and higher depressive symptoms (DS) were significantly associated with poor time management at work (MF: β = -0.59, P = .01; DS: β = 3.42, P < .01; R2 = 0.54). Difficulty meeting physical work demands was significantly associated with poorer visuospatial ability (VA) and higher depressive symptoms (VA: β = −3.30, P = .05; DS: β = 2.29, P < .01; R2 = 0.29). Lower learning and memory scores (LM) and higher depressive symptoms were significantly associated with difficulty meeting mental-interpersonal work demands (LM: β = −3.39, P = .04; DS: β = 3.25, P < .01; R2 = 0.47) and overall health-related loss of work productivity (LM: β = −0.72, P = .05; DS: β = 0.659, P < .001; R2 = 0.43).
Conclusion
Domains of neuropsychological function that predicted work productivity were identified. Future research should examine neuropsychological function, depressive symptoms, and work productivity across the care trajectory from diagnosis through long-term survivorship.
Keywords: cognitive function, neuropsychological function, occupational function, skull base tumors, work ability
Since the 1970s, the incidence of benign brain tumors has been on the rise, due in part to improved technology and ability to detect neoplasms.1,2 According to the Central Brain Tumor Registry of the United States [CBTRUS], ∼142 000 new cases of benign intracranial tumors are diagnosed each year; 48% of all intracranial tumors are benign.1 While there are several different types of benign brain tumors, some of the most common are skull base tumors including adenomas, meningiomas, and Rathke cleft cysts.3–7 The skull base includes the bones that form the bottom of the head and the back of the eye socket.
The focus in brain tumors has centered on the diagnosis and treatment of malignant tumors. There has been growing recognition, however, that benign intracranial tumors, including skull base tumors, can impose significant societal costs related to medical care, case fatality, and lost productivity.1 In contrast to most other brain and nervous system tumors, the diagnosis of a skull base tumor does not automatically qualify an individual for disability under the United States Social Security Act8 and thus may not allow the individual to quit work or qualify for disability. Yet, even benign brain tumors have the ability to cause altered physical and neuropsychological function.9–12 However, the manner in which these alterations affect a person's ability to fulfill normal obligations, such as meeting occupational demands, is unknown. This potential for work disruption in particular has significant societal and personal implications that are not known. The purpose of this analysis was to examine the relationship between neuropsychological function and work productivity in individuals with a skull base tumor prior to any treatment or resection.
Background
Many skull base tumors are considered to be benign. If the tumor does not grow, is seemingly asymptomatic, or produces only minimal symptoms, the recommended treatment may be to continue careful monitoring (watchful waiting) in an attempt to avoid overly aggressive treatment that may expose the patient to more risk than the expected benefit.13,14 The risk/benefit of nonsurgical management of skull base tumors is limited, especially as it impacts daily activities. However, Van Nieuwenhuizen, et al12 found that patients with low-grade meningiomas undergoing the watchful waiting approach had lower psychomotor speed and working memory capacity.
One of the main difficulties in assessing the impact of symptoms on persons' lives is that alterations in neuropsychological function and the accompanying symptoms are common but often “silent” because they can be quite subtle and develop gradually. Alterations in neuropsychological function may not be as readily recognized as physical dysfunction, yet it can still have a large impact on patients' ability to continue societal, familial, and occupational roles. Research has shown an association between impaired neuropsychological function and poor work performance and employability in other patient populations.15–18
There is sparse literature regarding the impact of neuropsychological function on occupational obligations in persons with any type of primary brain tumor. Teixidor, et al19 found preoperatively that patients with a tumor in the language center of the brain scored lower than normal on most measures of verbal working memory. Another study examined patients with tumors in the frontal or temporal lobe and found that more than 90% of their sample displayed impairment in at least one area of cognition.11 These studies examined the impact of malignant brain tumors preoperatively and their effect on neuropsychological functioning, yet altered neuropsychological functioning and its effect on occupational functioning is not reported; thus, the tumor's impact on productivity and quality of life is less well known. Alterations in neuropsychological functioning in patients with skull base brain tumors could affect their job performance and employment status. The purpose of this analysis was to determine neuropsychological functioning of patients with skull base tumors and examine its relation to patients' perceptions of work limitations and their ability to fulfill occupational demands.
Materials and Methods
Data for this interim analysis were obtained from a large prospective longitudinal study examining outcomes of patients scheduled to undergo endoscopic assisted microneurosurgery or the use of the expanded endonasal approach for brain tumor resection. Inclusion criteria were recent diagnosis (within 1 month) with a midline brain tumor as determined by MRI, aged 18 years or older, and ability to read and speak English. Patients with a previous history of surgery for brain tumor removal and/or who had a tumor located in the region of the foramen magnum were excluded from the larger parent study. For this analysis, data were gathered from participants diagnosed via surgical pathology with any benign skull base lesion, including pituitary adenomas, meningiomas, and Rathke cleft cysts.
Following Institutional Review Board approval, participants were recruited through the neurosurgery clinic at a Level 1 trauma center, which serves as a major referral source for western Pennsylvania, northern West Virginia, and eastern Ohio. Eligible participants were identified by clinic staff, and permission was obtained by the staff for research personnel to approach the participant. A member of the research team completed a screening enrollment form, explained the study, and obtained written consent. Data were collected via in-person assessments 1–2 days prior to surgical intervention.
Measures
Depressive Symptoms
Study participants completed the Center for Epidemiological Studies - Depression (CESD)-10,20 a widely used, validated, and reliable instrument, especially for studies focusing on depressive symptoms in nonpsychiatric populations. Total CES-D-10 scores range from 0–30, with higher scores indicating higher levels of depressive symptoms.
Profile of Mood States-Short Form (POMS-SF)
The Tension/Anxiety subscale of the POMS-SF21 was administered to assess anxiety. The internal consistency rating for the POMS-SF is 0.76–0.95. The correlation between the subscales and the total score in POMS and POMS-SF was calculated as 0.84. The shortened anxiety subscale of the POMS-SF scores range from 0–20, with higher scores indicating higher levels of anxiety.
Work Functioning
The purpose of the analysis was not to discern the impact of neuropsychological function on return to work but rather to evaluate person's limitations in performing specific tasks within a job. Neuropsychological dysfunction is often a “hidden” limitation, one that may not be readily apparent. However, the ability to perform tasks on the job is heavily dependent upon neuropsychological function, and thus work limitations are a more sensitive indicator of an employee's ability to be productive. Participants completed the Work Limitations Questionnaire (WLQ),22 a 25-item self-report measure of work functioning. The WLQ has shown reliability and validity for use among several different job and chronic health condition groups. Reliability of the WLQ subscales is good, with Cronbach alphas ranging from 0.88 to 0.9. The validity of the WLQ is well established in the literature and ranges from 0.53 to 0.83. The WLQ questionnaire yields 4 subscale scores and a total score. Each WLQ subscale score reflects the percentage of time in the past 2 weeks that the respondent was limited in performing a specific dimension of his or her job (Time, Physical, Mental/ Interpersonal, and Output). The Time Management subscale addresses difficulty meeting a job's time and scheduling demands. The Physical Demands subscale refers to an individual's ability to perform job tasks that involve bodily strength, movement, endurance, coordination, and flexibility. The Mental/Interpersonal domain of the WLQ assesses both the difficulty performing cognitive job tasks or tasks involving the processing of sensory information as well as the problems a person encounters while interacting with people on the job. The Output subscale refers to difficulty meeting demands for quantity, quality, and timeliness of completed work. The WLQ Productivity Loss Score is based on a weighted sum of the 4 subscale scores and indicates the percentage decrement in work output due to health problems. The WLQ Productivity Loss Score expresses the estimated percent differences in output compared with employees who do not have health-related work limitations.
Neuropsychological Assessment
The reliability and validity of each of the neuropsychological tests administered have been well established in the literature. Several domains of neuropsychological function were assessed: learning and memory, attention and mental flexibility, executive function, psychomotor/speed, and language.
Learning and Memory
Auditory Verbal Learning Test
Administration of the Auditory Verbal Learning Test (AVLT)23 includes 5 successive presentations of a list of 15 common words followed by free recall on each trial, an interference trial (presentation and recall of a list of 15 different words), postinterference recall of the words from the original list, and a delayed (30 min) recall. The AVLT test is used to assess immediate and delayed verbal learning and memory, memory acquisition, and retention.
Rey-Osterrieth Figure Test
The Rey-Osterrieth Figure Test (ROCF)24 assesses visual perceptual, skills, spatial organization, constructional ability, and visual memory (immediate and delayed recall). Participants are instructed to carefully copy a figure and then to redraw the figure immediately after the figure is removed and again in ∼30 minutes (delayed recall).
Wechsler Memory Scale III, Logical Memory Subtest
With the Wechsler Memory Scale III (WMS-III), Logical Memory Subtest (LM I-II),25 participants are read 2 short stories out loud and asked to retell the stories from memory immediately and after a 30 minute delay. Story A is read only once, whereas Story B is read twice. Participants are credited for each correctly recalled detail (maximum of 25) and for general themes (maximum Story A = 7, Story B = 8). The WMS-III assesses verbal learning and memory (short- and long-term), logical memory, and retention.
Attention and Mental Flexibility
Wechsler Adult Intelligence Scale III, Digit Symbol Coding Subtest
In the Digit Symbol Coding Subtest,26 participants are presented with a key of symbols paired with numbers under which is a series of rows with randomly ordered numbers. Using the key, participants are instructed to draw the corresponding symbol under each number as fast as they can. The score is determined by the number of symbols correctly drawn within the 120 second time limit. This test assesses psychomotor response speed, visuomotor coordination, and attention.
Trail Making Test
Both Trail Making Tests (TMT A and B)27 consist of 25 circles distributed across a sheet of paper. In Test A, circles are numbered from 1 to 25, and the participant is asked to draw lines connecting the numbers in ascending order. In Test B, the circles include both numbers (1–13) and letters (A-L). Similar to Test A, the participant is asked to connect the circles in ascending order, but with the additional task of alternating between letters and numbers (ie, 1-A-2-B-3-C, etc.). Participants are instructed to draw lines connecting the circles in order as quickly as possible for each test without lifting the pencil from the paper. The TMT scores reflect the total number of seconds it took to complete each trial. The TMT tests assess executive function, mental flexibility, and attention.
Executive Function
Stroop Color Word Test
With the Stroop Color Word Test,28 participants are given a booklet with the colors blue, red, or green listed in random order in 5 columns of 20 colors. They are asked to read the colors out loud, going down each column as fast as they can. The test consists of 3 trials measuring the relative speed of a person's ability to read the names of colors (W), naming colors (C), and naming colors from words of colors printed with an incongruently colored ink. The Stroop Color Word Test is scored by reporting the number of colors read in 45 seconds for each trial and assesses executive functioning through inhibition and cognitive flexibility through interference.
Psychomotor/Speed
Grooved Peg Board Test
The Grooved Peg Board Test (GPT)29 uses a metal board with rows of slotted holes angled in different directions. The task is to insert 25 metal pegs with ridges on the sides into each hole in sequential order. Participants are asked to do the first trial with their dominant hand, and then repeat the task with their nondominant hand. The score is based on the time it takes to fill in all the holes and the number of pegs dropped for each trial; higher scores indicate poorer function. The Grooved Peg Board Test evaluates psychomotor speed, fine motor control, and visual-motor coordination.
Language
Controlled Oral Word Association Test F, A, S and Animal Naming
In the Controlled Oral Word Association Test (COWAT) F, A, S, and Animal Naming30 test of verbal fluency, participants are instructed to orally generate as many words as they can, beginning with the letters F, A, and S, as well as name as many animals as they can in 60 seconds for each trial. The COWAT assesses executive function, auditory attention, short-term memory, cognitive flexibility, and vocabulary. The score is generated based on the total number of words produced in each trial.
Estimated General Intelligence
North American Adult Reading Test
The North American Adult Reading Test (NAART-R)31 requires participants to read and pronounce a list of 61 irregularly spelled words (eg, debt, gauge, leviathan). It provides an excellent estimate of premorbid verbal intelligence, which has been shown to be resistant to the effects of acquired brain damage.
Analysis
There was a sample size calculation performed for the larger parent study; however, this pilot analysis used a subset of the larger study. Descriptive analyses were conducted to explore the frequency and distribution of the independent and dependent variables. To examine relationships between sociodemographic characteristics, estimated general intelligence, mood, neuropsychological domains, and work limitations in each WLQ subscale and total sum score, 2-tail t tests and Pearson correlations were conducted. Variables that reached statistical significance of P < .10 were included as potential predictor variables in the multiple linear regression model. Separate backward multiple linear regression analyses were conducted for each WLQ subscale and total sum score to model the impact of neuropsychological function on work productivity, controlling for general intelligence, depression, and anxiety scores. All analyses were performed using SPSS version 19.
Results
Approximately 75% of the patients approached agreed to participate in this study. A total of 45 participants were included in this analysis (see Table 1). Almost all participants had more than one symptom complaint prior to resection. More than 60% of participants reported headaches (n = 27), and more than 30% reported visual disturbances (n = 14). Other reported symptoms included endocrinopathies (acromegaly, Cushing's syndrome, and dysmenorrhea), sexual dysfunction (decreased libido, erectile dysfunction, and infertility), and mood disturbances (anxiety and depression). The majority of participants were white females with an average age of 43.1 years (SD = 13.17). The average number of years of formal education was 15.05, equivalent to some college education. Seventy-one percent of all participants' tumors were classified as adenomas with an average tumor volume of 4.27 cc. Categorizing participants' jobs according to the International Standard Classification of Occupations revealed that participants were most likely to be professionals or clerical support workers (40%, n = 18).26
Table 1.
Sociodemographic characteristics of sample (n = 45)
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Years of education | 15.05 (2.75) |
| Age (years) | 43.4 (13.17) |
| Tumor volume(cc)* | 4.27 (7.16) |
| Sex | |
| Male | 17 (37.8%) |
| Female | 28 (62.2%) |
| Race | |
| White | 36 (87.8%) |
| Black | 4 (9.8%) |
| Hispanic | 1 (2.4%) |
| Tumor type | |
| Adenoma | 32 (71.1%) |
| Meningioma | 7 (15.6%) |
| Rathke's cleft cyst | 6 (13.3%) |
| Job classification | |
| Managers | 5 (11.1%) |
| Professionals | 9 (20.0%) |
| Technicians and associate professionals | 5 (11.1%) |
| Clerical support workers | 9 (20.0%) |
| Service and sales workers | 7 (15.6%) |
| Skilled agricultural, forestry, and fishery workers | 1 (2.2%) |
| Craft and related-trades workers | 4 (8.9%) |
| Plant and machine operators | 2 (4.4%) |
| Elementary occupations | 1 (2.2%) |
| Unemployed** | 2 (4.4%) |
*Resultafter removing one outlier.
**The participantquit work immediately before an expanded endonasal approach procedure.
Descriptive statistics for participants' performance on preoperative neuropsychological tests are reported in Table 2 as compared with mean and standard deviations found in population normative data.25,32 Sample mean scores on neuropsychological tests were within one standard deviation of population normative values; thus, it was assumed that the sample's overall neuropsychological status did not significantly differ from that of the general population.
Table 2.
Comparison of sample neuropsychological test scores to normative data
| Cognitive Domain | Neuropsychological Test | Expected Score (SD)* | Sample Mean (SD) |
|---|---|---|---|
| Verbal learning | AVLT learning slope (V-I) | 5.8 (1.7) | 5.6 (1.5) |
| Logical Memory learning slope (WMS-III) | 5.0 (3.0) | 5.2 (2.7) | |
| Verbal memory | AVLT delayed recall | 10.2 (2.8) | 8.3 (2.6) |
| Logical Memory total unit delayed recall (WMS-III) | 24.0 (11.0) | 26.4 (7.1) | |
| Logical memory | Logical Memory total theme delayed recall (WMS-III) | 11.0 (3.0) | 12.1 (2.5) |
| Spatial organization | ROCF copy | 34.4 (2.3) | 34.0 (2.2) |
| Constructional ability | ROCF recall | 23.6 (6.9) | 20.0 (5.6) |
| Visual memory | ROCF delayed recall | 23.1 (6.7) | 19.6 (4.9) |
| Executive function | TMT B time | 64.6 (22.3) | 59.9 (17.7) |
| Stroop color/word T-score | 50 (10) | 47.0 (8.8) | |
| Verbal fluency | COWAT total FAS | 45.1 (11.2) | 39.0 (11.0) |
| COWAT animal naming | 23.0 (4.7) | 19.0 (5.7) | |
| Psychomotor processing speed and visual-motor coordination | Digit Symbol Coding | 75.0 (3.0) | 74.5 (16.6) |
| GPT nondominant hand | 72.9 (15.3) | 84.3 (23.6) |
Abbreviations: AVLT, Auditory Verbal Learning Test; COWAT, Controlled Oral Word Association Test; GPT, Grooved Peg Test; ROCF, Rey-Osterrieth Figure Test; TMT, Trail Making Tests; WMS, Wechsler Memory Scale.
Descriptive statistics for participants' perception of work limitations (as measured by the WLQ questionnaire) can be found in Table 3. Overall, participants with skull base tumors reported the highest dysfunction in the time management subscale. The percentage of time in the past 2 weeks that participants were limited in performing time management skills was 27.09%. Difficulty meeting mental/interpersonal demands presented the second highest level of dysfunction in work functioning for participants, followed by difficulty meeting physical demands, and finally, difficulty meeting output demands. Calculations of total percent health-related work productivity loss yielded a mean productivity loss of 6.15% in participants with a skull base tumor.
Table 3.
Percent decrement in work functioning
| Work domain | n | Mean (SD) |
|---|---|---|
| Time | 45 | 27.09 (25.84) |
| Physical | 42 | 25.07 (24.89) |
| Mental/Interpersonal | 45 | 26.02 (27.54) |
| Output | 44 | 19.79 (25.18) |
| Total | 41 | 6.15 (5.89) |
Table 4 contains the results of the multiple linear regression analyses examining the relationship between domains of neuropsychological function and work limitations. The first subscale of the WLQ was time management. Greater difficulties with time management were predicted by poorer mental attention and flexibility (β = −0.59, P = .01) as measured by the Digit Symbol Coding task and higher depressive symptoms (β = 3.42, P < .01) as measured by the CES-D (total model R2 = 0.55, P < .01).
Table 4.
Neuropsychological predictors of work limitations
| Domain | Test | β | SE | P |
|---|---|---|---|---|
| Time | R2 = 0.55; Model P < .01 | |||
| Digit Symbol Coding | −0.59 | 0.22 | .01 | |
| Depression (CES-D) | 3.42 | 0.63 | <.01 | |
| Physical | R2 = 0.29; Model P < .01 | |||
| Rey Figure Copy | −3.30 | 1.63 | .05 | |
| Depression (CES-D) | 2.29 | 0.65 | <.01 | |
| Mental/ Interpersonal | R2 = 0.47; Model P < .01 | |||
| AVLT learning delay | −3.39 | 1.56 | .04 | |
| Depression (CES-D) | 3.25 | 0.68 | <.01 | |
| Output* | R2 = 0.07; Model P = .10 | |||
| – | – | – | – | |
| Total Work Loss | R2 = 0.43; model P < .01 | |||
| AVLT learning delay | −0.72 | 0.35 | .05 | |
| Depression (CES-D) | 0.66 | 0.16 | <.01 | |
Abbreviations: AVLT, Auditory Verbal Learning Test; CES-D, Center for Epidemiological Studies – Depression.
*No neuropsychological tests were found to significantly predict difficulty in the Output domain of work functioning.
Difficulty meeting the physical demands of work was predicted by poorer visuospatial ability (β = −3.30 P = .05) as measured by the Rey Figure Copy task and higher depressive symptoms (β = 2.29, P < .01) (total model R2 = 0.29, P < .01). Problems meeting the mental/interpersonal demands of work were associated with poorer verbal learning and memory (β = −3.394, P = .04) as measured the AVLT delay and higher depressive symptoms (β = 3.246, P < .01) (total model R2 = 0.47, P < .01).
There were no significant associations between difficulty meeting output demands and performance on any neuropsychological measures that were administered.
The total percent of health-related loss of work productivity was calculated by a weighted summing of the 4 subscales of the WLQ (Time, Physical, Mental/Interpersonal, and Output). Lower learning and memory scores, specifically lower scores on AVLT learning delay test, significantly predicted total work productivity loss (β = −0.72, P = .05). Higher reports of depressive symptoms were also associated with greater loss of work productivity (β = .66, P < .01) (total model R2 = 0.43, P < .01).
Discussion
Findings from this study revealed that certain neuropsychological tests may predict work limitations in patients with skull base tumors. It is worth noting that, although the sample mean was similar to that of the general population, slight individual differences could not be detected given the small sample size. A multivariate regression analysis revealed that neuropsychological tests were found to predict occupational functioning despite the overwhelming majority of persons included in this study who scored within published population normative values. Difficulties with tasks of mental attention and flexibility and learning and memory, as well as visuospatial dysfunction and higher depressive symptoms, were significantly associated with difficulty in one or more subscales of work life.
Subscales of work life represent various aspects of work productivity. Time management indicates the ability to handle scheduling, organizing, and prioritizing tasks to accomplish a goal. Measuring physical demands provides insight into whether a person is able to coordinate movement and has the strength and endurance to accomplish a task. Lower scores on the mental/interpersonal demands subscale indicate that a person has deficits in cognitively processing information and working with others in the occupational setting to accomplish a task. Finally, limitations in the output demands subscale indicate that a person's productivity is at risk, either from decreased quantity and/or quality of work. Neuropsychological functions that affect these areas of occupational productivity are described in the following sections.
Specifically, the Digit Symbol Coding Test (a measure of attention and mental flexibility) was useful for predicting difficulty with time management at work. Poorer performance on the Rey Figure Copy task, a measure of visuospatial ability, was a predictor for difficulties managing the physical demands associated with work. Poorer performance on the AVLT learning delay test predicted difficulty meeting the mental and interpersonal demands of work. As other studies have shown,33,34 reports of greater number of depressive symptoms were highly correlated with difficulties meeting the time management, physical, and mental/interpersonal demands of work as well as the overall percent of health-related work productivity loss.
The Digit Symbol Coding test was developed to assess attention and mental flexibility under time pressure. The time management subscale of occupational functioning includes the ability to meet demands for quantity, quality, and timeliness of work completed; thus, it is not surprising that the results of this study found the Digit Symbol Coding test to be a predictor of time management in an occupational setting. The Rey Figure Copy Task is a test used to assess visuospatial ability and motor functioning. In this study, the Rey Figure Copy Task was found to be a predictor of difficulties meeting the physical demands of a person's occupation. The results of this study, which found that the AVLT predicted ability to meet mental/interpersonal demands, have been shown in other studies,33,34 although in different populations such as patients who had experienced a traumatic brain injury and patients with primary brain tumors. In relation to interpersonal demands, a study of patients with traumatic brain injury reported that the AVLT predicted difficulty with social adaptation.33 The association between AVLT and mental, or cognitive, impairment was also found in a study examining patients with primary brain tumors prior to intervention.34 Consistent with findings from other studies, neuropsychological tests that assess visuospatial ability and psychomotor skills may be useful for predicting work dysfunction.
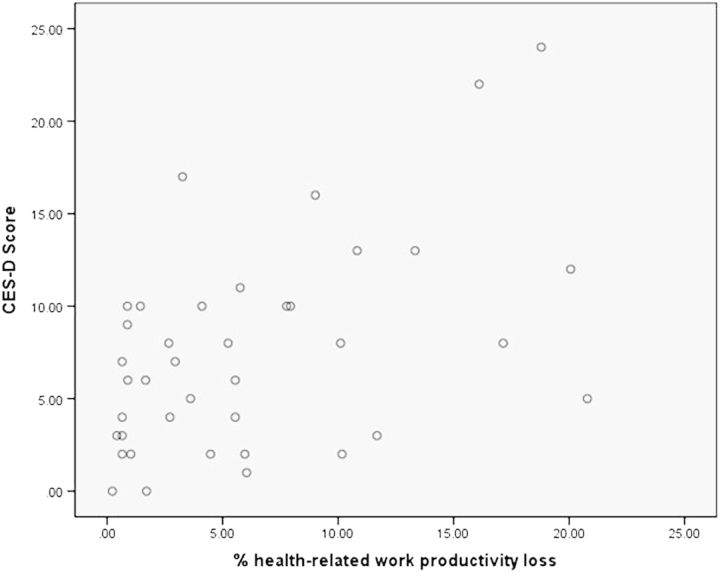
Previous studies have been conducted examining the influence of depression on work productivity. These studies, however, relied on the classification of patients as “depressed” or “nondepressed.” The current study correlated their measure of self-reported depressive symptoms on the CES-D, although interestingly, very few of these patients actually met the criteria for being at risk for clinical depression. Past research in patients with pituitary adenomas has shown that these individuals have a higher incidence of mood disorders, including depression, than the general population.35–37 Examining the scatter plot of CES-D scores, as it correlates with occupational difficulty (Fig. 1), revealed that many of the participants with skull base tumors showed decrements in neuropsychological functioning and difficulty completing occupational tasks beginning with a CES-D score of 10.
Fig. 1.
Percent of health-related work productivity loss as a function of CES-D score. A scatterplot was generated to visualize each participant's percent of health-related work productivity loss as measured on the WLQ (X-axis) as a function of individual CES-D scores, which are a measure of depressive symptomatology (Y-axis). There appears to be a trend towards increased percent of work-productivity loss with higher levels of depressive symptoms.
The clinical implications of this study include recognizing that patients with skull base tumors have the potential to experience altered neuropsychological function that may limit their ability to meet the demands of their occupation in one or more areas of work life. It is worth noting that 95% of participants remained employed (n = 34) and that 5% (n = 2) quit work immediately prior to surgery. Thus, although individuals may remain employed, assessment of individuals may be important to detect underperformance in particular areas of work functioning.
The potential for work limitations may be predictable using the individual's performance on specific neuropsychological tests. Specifically, difficulty on tests of attention and mental flexibility, learning and memory, visuospatial ability, and scoring higher than a 10 on the CES-D may indicate that the individual is more likely to experience work limitations. Careful screening of patients with skull base tumors may be able to help better identify those patients who are at particular risk for work limitations in order to intervene and ameliorate the distress or limitations they experience.
This study has shown that higher levels of depressive symptoms are consistently correlated with a decline in the ability to meet occupational demands. Brief screening tools for depressive symptoms are already implemented in many physician practices. Clinicians should recognize that the presence of depressive symptoms may predict occupational difficulties and should be prepared to discuss this with patients. Patients who undergo neuropsychological testing and exhibit difficulty in the visuospatial, attention and mental flexibility, or learning and memory domains should also be screened for difficulty meeting occupational demands. Any patients experiencing occupational dysfunction should then be referred for supportive or rehabilitative services in order to maintain the highest level of functioning possible.
The research implications of this study include recognizing that even mild reporting of depressive symptoms on the CES-D may help to identify patients at risk for difficulty meeting occupational demands. Findings from this study also raise questions as to whether or not patients with benign skull base tumors are able to maintain optimal occupational functioning. Further research should follow these patients longitudinally to assess the relationships between changes in neuropsychological and occupational functioning over time.
Limitations
The sample size was relatively small and largely homogenous in terms of race. A more diverse population may help verify whether these findings hold true for racially diverse groups and for all ages. This study also lacked a control group, so it is unclear how individuals with skull base tumors might differ in comparison with a group of healthy controls without a symptomatic skull base tumor. Patients’ work status is self-reported, as is their functioning at work. Thus, patients' perceptions of their occupational functioning may differ significantly from their employers' perceptions or from objective measures of occupational functioning. Finally, a larger sample may help to detect any differences in occupational or neuropsychological dysfunction related to tumor type.
Funding
National Institute of Health, National Institute of Nursing Research, Patient and Health Care System Outcomes Following EEA (1 R01 NR011044-01), Bethany D. Nugent, Jason Weimer, Chienwen J. Choi, Paula R. Sherwood.
National Institute of Health, National Institute of Nursing Research, Predoctoral Fellowship in Interdisciplinary Training of Nursing Scientists in Cancer Survivorship Research (T32 NR011972), Bethany D. Nugent.
Acknowledgments
Portions of this work were presented in a podium presentation at the Oncology Nursing Society (ONS) Connections Conference, Phoenix, Arizona, November 9, 2012.
Conflict of interest statement: None declared.
References
- 1.(CBTRUS) CBTR of the US. CBTRUS incidence rates adjusted using the Year 2000 United States standard population. 2000. Available at: www.cbtrus.org . Accessed March 12, 2013. [Google Scholar]
- 2.Radhakrishnan K, Mokri B, Parisi JE, O'Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37(1):67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 3.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of Intracranial Meningioma. Neurosurgery. 2005;57(6):1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 4.Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–9. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 5.Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro Oncol. 1999;1(1):14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–8. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 7.Voelker JL, Campbell RL, Muller J. Clinical, radiographic, and pathological features of symptomatic Rathke's cleft cysts. J Neurosurg. 1991;74:535–544. doi: 10.3171/jns.1991.74.4.0535. [DOI] [PubMed] [Google Scholar]
- 8.Social Security Administration. Disability Evaluation Under Social Security. 2014. Available at: http://www.ssa.gov/disability/professionals/bluebook/11.00-Neurological-Adult.htm. Accessed March 6, 2013.
- 9.Kangas M, Tate RL, Williams JR, Smee RI. The effects of radiotherapy on psychosocial and cognitive functioning in adults with a primary brain tumor: a prospective evaluation. Neuro Oncol. 2012;14(12):1485–502. doi: 10.1093/neuonc/nos244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello AB, Osborne JW. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract Assessment, Res Eval. 2005;10(7):1–9. [Google Scholar]
- 11.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment. Neurosurgery. 2000;47(2):324–334. doi: 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Van Nieuwenhuizen D, Ambachtsheer N, Heimans JJ, Reijneveld JC, Peerdeman SM, Klein M. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J Neurooncol. 2013;113(3):433–40. doi: 10.1007/s11060-013-1132-4. [DOI] [PubMed] [Google Scholar]
- 13.Acoustic Neuroma Association. Treatment Options. 2014. Available at: http://anausa.org. Accessed February 20, 2014.
- 14.White ML, Doherty GM. Multiple endocrine neoplasia. Surg Oncol Clin N Am. 2008;17:439–459. doi: 10.1016/j.soc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Heaton RK, Chelune GJ, Lehman RA. Using neuropsychological and personality tests to assess the likelihood of patient employment. J Nerv Ment Dis. 1978;166(6):408–416. doi: 10.1097/00005053-197806000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kibby MY, Schmitter-Edgecombe M, Long CJ. Ecological validity of neuropsychological tests: Focus on the California Verbal Learning Test and Wisconsin Card Sorting Test. Arch Clin Neuropsychol. 1998;13(6):523–534. [PubMed] [Google Scholar]
- 17.McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. 2000;45(3):175–84. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 18.Rabkin JG McElhiney M, Ferrando SJ, Van Gorp W, Lin SH. Predictors of employment of men with HIV/AIDS: a longitudinal study. Psychosom Med. 2004;66(1):72–78. doi: 10.1097/01.psy.0000108083.43147.6d. [DOI] [PubMed] [Google Scholar]
- 19.Teixidor P, Gatignol P, Leroy M, Masuet-Aumatell C, Capelle L, Duffau H. Assessment of verbal working memory before and after surgery for low-grade glioma. J Neurooncol. 2007;81(3):305–13. doi: 10.1007/s11060-006-9233-y. [DOI] [PubMed] [Google Scholar]
- 20.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 21.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 22.Lerner D, Amick BC, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Med Care. 2001;39(1):72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 24.Rey A, Osterrieth P. Translations of excerpts from Andre Rey's psychological examination of traumatic encephalopathy and P. A. Osterrieth's The Complex Figure Copy Test. Clin Neuropsychol. 1993;7(1):4–21. [Google Scholar]
- 25.Wechsler D. Wechsler Memory Scale: Third Edition Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 27.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–4. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 28.Golden CJ. Stroop Color and Word Test. Lutz: PAR/Psychological Assessment Resources; 2002. [Google Scholar]
- 29.Trites R. Instruction Manual for the Grooved Peg Board Test. Lafayette: Lafayette Instrument Company, Inc.; 1989. [Google Scholar]
- 30.Benton AL, Hamsher KD. Controlled Oral Word Association Test, multilingual aphasia examination. Iowa City: AJA Associates; 1989. [Google Scholar]
- 31.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 32.Mitrushina M. Handbook of Normative Data for Neuropsychological Assessment. 2nd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 33.Ross SR, Millis SR, Rosenthal M. Neuropsychological prediction of psychosocial outcome after traumatic brain injury. Appl Neuropsychol. 1997;4(3):165–170. doi: 10.1207/s15324826an0403_4. [DOI] [PubMed] [Google Scholar]
- 34.Scotland JL, Whittle IR, Deary IJ. Cognitive functioning in newly presenting patients with supratentorial intracranial tumors: is there a role for inspection time? Neuro Oncol. 2012;14(3):360–7. doi: 10.1093/neuonc/nor222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitzner M. Apathy and pituitary disease: it has nothing to do with depression. J Neuropsychiatry Clin Neurosci. 2005;17(2):159–66. doi: 10.1176/jnp.17.2.159. [DOI] [PubMed] [Google Scholar]
- 36.Korali Z, Wittchen HU, Pfister H, Höfler M, Oefelein W, Stalla GK. Are patients with pituitary adenomas at an increased risk of mental disorders? Acta Psychiatr Scand. 2003;107(1):60–8. doi: 10.1034/j.1600-0447.2003.02383.x. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MD, Woodburn CJ, Vance ML. Quality of life in patients with a pituitary adenoma. Pituitary. 2003;6(2):81–7. doi: 10.1023/b:pitu.0000004798.27230.ed. [DOI] [PubMed] [Google Scholar]