Abstract
Background
Stereotactic body radiotherapy (SBRT) has emerged in recent years as a clinically viable treatment option for early-stage non-small-cell lung cancer (NSCLC) patients. Comprehensive assessment of quality of life (QoL) after SBRT is relatively sparse.
Objective
To describe QoL and symptoms in a small, prospective cohort of early-stage NSCLC patients treated with SBRT.
Methods
19 NSCLC patients who were medically unfit for surgery or chose not to undergo surgery were included in the study. All of the patients were treated with SBRT between 2009 and 2013 at a single comprehensive cancer center. Patients completed a baseline assessment of functional and cognitive status, symptoms, psychological distress, and overall QoL. Questionnaires were repeated at 6 and 12 weeks after accrual.
Results
There were no significant differences in all outcomes across the 3 evaluation time points. Overall QoL scores were moderate, and the lowest score was observed for the functional well-being domain. The most severe symptoms at baseline were pain, lack of energy, cough, nervousness, difficulty sleeping, shortness of breath, and worry. Severity scores for pain, lack of energy, and cough increased, whereas nervousness, difficulty sleeping, and worry decreased at the 12 week evaluation.
Limitations
Small sample size and lack of sufficient diversity in the cohort.
Conclusions
QoL scores remained relatively stable across time. Anxiety improved after SBRT, whereas symptoms such as generalized pain, lack of energy, and cough worsened. The findings suggest that SBRT is overall a well-tolerated treatment with no significant decrement in patient-centered outcomes.
About 15%-20% of patients with non-small-cell lung cancer (NSCLC) present with early-stage disease without clinical evidence of lymph node metastasis.1 It is noteworthy that up to 80% of screen-detected lung cancers are detected as stage I disease.2 Surgical resection has been the traditional therapy for these patients, but recently, stereotactic body radiotherapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), has emerged as an alternative for patients who are either medically unfit for surgery or who choose not to undergo surgery. Numerous studies have demonstrated good rates of local control for SBRT in lung cancer.3-6
Despite the increasing use of SBRT as standard of care in early-stage lung cancer, there is limited information about patients' quality of life (QoL) with this treatment modality. For this patient population with substantial comorbid disease, the impact of treatment on QoL is of particular importance. Dutch investigators examined QoL before and after SBRT in 39 patients with lung cancer who received SBRT.7 There was no significant change in QoL scores except for an increase in emotional function after treatment. Respiratory symptoms, including dyspnea, chest pain, and cough were not significantly different. Another study from the Netherlands of 382 patients with lung cancer found no significant difference in quality of life scores after SBRT.8 Likewise, a prospective study of 21 patients from the Cleveland Clinic found no significant change in QoL and shortness of breath scores, despite a decrease in measured diffusion capacity on pulmonary function tests.9
Current published studies on SBRT are limited in their depth of QoL assessment. For example, the EORTC-QLQ-C30 has been used in several studies as the only main QoL instrument,6,7 while another study used only the FACT-L and UCSD shortness of breath questionnaire.9 The inclusion of symptom, social well-being, and functional well-being measures allows for a more in-depth perspective of overall QoL in SBRT patients. The purpose of this prospective study was to describe comprehensive QoL, symptoms and functional status in a cohort of early-stage, non-small-cell lung cancer (NSCLC) patients who were treated with SBRT.
Methods
Patients
Study participants were recruited from the Thoracic Surgery Ambulatory Clinic at a National Cancer Institute (NCI)-designated comprehensive cancer center in Southern California in the United States. Patients included in the study were enrolled in the early-stage project of an NCI-funded program project grant for lung cancer. Between 2009 and 2013, 95 early-stage NSCLC patients (stage I, II, IIIa) were enrolled in the program project grant. Of those patients, 19 diagnosed with early-stage NSCLC who were deemed medically unfit for surgical intervention by thoracic surgeons or who chose not to undergo surgery were included in this analysis.
SBRT technique
Patients were treated using image-guided radiation therapy (IGRT) and intensity-modulated radiation therapy (IMRT) techniques, using either helical tomotherapy (Accuray Inc, Sunnyvale, CA) or RapidArc on the Varian Truebeam STX linear accelerator (Varian Medical Systems, Palo Alto, CA). All of the patients were treated over 5 fractions, with doses ranging between 50 and 60 Gy.
Procedures and assessment tools
Study protocol and procedures were approved by the cancer center's institutional review board. Patients were screened for eligibility by research nurses during a regularly scheduled clinic visit with their oncology providers, and the decision was made to pursue SBRT. All of the patients provided written informed consent for participation in the study, after which they completed a baseline assessment using validated questionnaires that included functional and cognitive status, symptoms, psychological distress, and overall QoL. Questionnaires were repeated at 6 and 12 weeks after accrual. Those timeframes were selected because the incidence of acute treatment-related toxicities are expected to peak at those times. Medical chart audits were conducted to obtain key demographics, and clinical and system resource use characteristics.
Functional status was assessed using several measures. The Index of Activities of Daily Living (ADL) assesses an individual's ability to complete basic self-care skills such as bathing or dressing, using 6 items that are rated on a 3-point Likert scale (range, 1-7; higher score = more independent).10 The Instrumental Activities of Daily Living (IADL) Scale assesses the degree to which an individual can maintain independence at home and in the community, using 7 items rated on a 3-point Likert scale (range, 0-14; higher score =more independent).11,12The Timed Up and Go (TUG) Test is a performance-based measure of physical function administered by the research nurse. The test is measured in seconds and is scored based on the time it takes for a person to stand up from a standard arm chair (approximate seat height, 18 inches), walk a distance of 10 feet, turn, walk back to the chair, and sit down again (0 to 10.09 sec = normal; >10.1 sec = slow)- ).13 The Karnofsky Performance Status (KPS) Scale, a general measure of patient independence in carrying out normal activities, was also used to assess functional status (range, 0-100; higher score = normal functioning).14 Cognitive status was assessed using the Blessed Orientation-Memory-Concentration (BOMC) Test, a measure that consists of 6 items designed to screen for gross cognitive impairment.15,16 Social activities were assessed with the Medical Outcomes Study (MOS) Social Activity Limitations Scale, a four-item scale that assesses the impact of physical and emotional problems on social activities (range, 0-100; higher score = more activity).17 Social support was assessed with the MOS Social Support Survey, a 5-point Likert scale that determines perceived access to material, behavioral, physical, and emotional assistance or advice from others(range, 0-100; higher score = more social support). Only the Emotional/Information and Tangible Subscales were used.17
Symptoms were assessed using the Memorial Symptom Assessment Scale (MSAS), a tool that measures symptom prevalence and severity, as well as the perceived level of distress associated with 32 common cancer symptoms.18 Psychological distress was evaluated using the Distress Thermometer, an efficient method, based on a scale of 0-10 (0 =no distress, 10 = extremely distressed), to monitor emotional distress over the previous week.19 Finally, the Functional Assessment of Cancer Therapy-Lung (FACT-L) and the Functional Assessment of Chronic Illness Therapy-Spirituality Tool (FACIT-Sp-12) were used to assess multidimensional QoL (physical, social/familial, emotional, functional, spiritual well-being, lung cancer symptom index).20-24 The FACT-L is 37-item tool with a reported Cronbach's alpha of 0.89.23
Statistical analysis
Patient data forms and chart audit forms were scanned, audited for accuracy, and transformed into SPSS system files for analysis. Each data form was exported into an SPSS system file for analysis. Before analysis, missing data were imputed for patients who had not died and were too ill to continue the study. The 19 patients treated with SBRT were then selected for further analysis. Descriptive statistics were calculated on all variables, and contingency tables used to examine the association between demographic variables. Means, standard deviations, and medians were computed for key study variables. Because of the small sample size, the nonparamentric Friedman test, an equivalent of a one-way repeated measures analysis of variance (ANOVA), was used to test differences over time (baseline, 6 weeks and 12 weeks).
Results
Table 1 shows the sociodemographic and clinical characteristics for the 19 patients who were included in the analysis. The patients were primarily female, college-educated, and married. Mean age was 74 years (range, 59-92 years). All of the patients were treated for stage I NSCLC (1 patient had synchronous primary lung cancers and was treated with surgery for contralateral stage IIA disease and SBRT for stage I disease). Four patients had a previous diagnosis of lung cancer and were diagnosed with a second primary; they had an average of 42 pack-year smoking history. Multiple comorbidities were common, with the most common disorders being cardiovascular, respiratory, and musculoskeletal. Of the 19 patients, 3 chose to not undergo surgical intervention, and 15 had documented pulmonary function test with spirometry and diffusion capacity (DLCO) before SBRT initiation. The median for predicted FEV1 was 66%, and predicted DLCO was 53%.
Table 1. Sociodemographic and clinical characteristics (N = 19).
| Characteristic | Value |
|---|---|
| Mean age, y (SD, range) |
73.95 (9.8, 59-92) |
| Gender, n | |
| Female | 12 |
| Male | 7 |
| Income, n | |
| <$10,000 | 1 |
| $10,001-$30,000 | 2 |
| $30,001-$50,000 | 4 |
| >$50,000 | 8 |
| Prefer not to answer | 4 |
| Education, n | |
| Secondary/high school | 7 |
| College | 12 |
| Marital status, n | |
| Single | 2 |
| Widowed | 4 |
| Married | 9 |
| Divorced | 4 |
| Mean pack-years (SD) | 42.16 (27.5) |
| Disease stage, n | |
| IA | 12 |
| IB | 6 |
| IIA | 1 |
| aComorbidities, n | |
| Cardiovascular | 16 |
| Respiratory | 14 |
| Musculoskeletal | 7 |
| Endocrine | 6 |
| Digestive | 5 |
| Soft tissue/sensory | 5 |
| Genitourinary | 3 |
| Infectious disease | 2 |
| Nervous system | 1 |
| Pretreatment pulmonary function (n = 15) | |
| bFEV1 % predicted (range) | Mean, 77.3 Median, 66 (20-128) |
| cDLCO % predicted (range) | Mean, 57.2 Median, 53 (31-99) |
| Oxygen dependent | |
| Yes | 11 |
| No | 8 |
Percentages will sum to >100 because patients can select more than 1 answer.
Forced expiratory volume in 1 second.
Diffusion capacity.
Overall, patients were moderately functional and able to carry on normal activities with minor signs and symptoms of disease as measured by the KPS. They also reported high scores for both ADLs and IADLs, which suggests that there were no severe problems with basic activities such as bathing and dressing as well as activities such as preparing meals and taking medications. There were no deficits in cognitive status, and patients had no problems completing the TUG exam of physical functioning. Overall, the patients reported high levels of perceived social support for both physical and emotional needs. Scores for social activities were lower, suggesting that physical health and emotional problems were moderately interfering with participation in social activities. There were no significant differences observed across time for all functional, cognitive, and social support variables (Table 2).
Table 2. Functional, cognitive and social status (N = 19).
| Mean | Median | Range | P | |
|---|---|---|---|---|
| Karnofsky Performance Status Range, 0-100; higher score = more normal functioning | ||||
| Baseline | 82.63 | 80.00 | 50-100 | .924 |
| 6 weeks | 80.00 | 90.00 | 50-100 | |
| 12 weeks | 82.11 | 90.00 | 50-100 | |
| Activities of Daily Living Range, 1-7; higher score = more dependent | ||||
| Baseline | 6.94 | 7.00 | 6-7 | .779 |
| 6 weeks | 6.89 | 7.00 | 6-7 | |
| 12 weeks | 6.95 | 7.00 | 6-7 | |
| Instrumental Activities of Daily Living Range, 0-14; higher score = more independent | ||||
| Baseline | 12.68 | 14.00 | 5-14 | .127 |
| 6 weeks | 12.10 | 13.00 | 7-14 | |
| 12 weeks | 12.00 | 13.00 | 6-14 | |
| Cognition Range, 0-28; ≥11 = cognitive impairment | ||||
| Baseline | .11 | .00 | .00-2.0 | .497 |
| 6 weeks | .10 | .00 | .00-2.0 | |
| 12 weeks | .58 | .00 | .00-7.0 | |
| Timed Up and Go 0-10.09 seconds is normal; ≥10.1 seconds is too slow | ||||
| Baseline | 18.12 | 18.00 | 9-40 | .355 |
| 6 weeks | 18.12 | 13.00 | 9-63 | |
| 12 weeks | 16.20 | 14.00 | 9-32 | |
| Social Activitiesa Range, 0-100; higher score = more activity | ||||
| Baseline | 45.28 | 45.00 | 6-75 | .093 |
| 12 weeks | 39.47 | 37.50 | 25-62 | |
| Social Support Range, 0-100; higher score = more perceived social support | ||||
| Physical | .326 | |||
| Baseline | 79.93 | 100.00 | 6.25-100 | |
| 6 weeks | 73.03 | 87.50 | .00-100 | |
| 12 weeks | 73.68 | 100.00 | .00-100 | |
| Emotional | .161 | |||
| Baseline | 78.78 | 90.62 | 18.75-100 | |
| 6 weeks | 70.07 | 71.87 | 12.5-100 | |
| 12 weeks | 75.66 | 84.37 | 6.25-100 | |
| Total | .084 | |||
| Baseline | 79.16 | 93.75 | 16.67-100 | |
| 6 weeks | 71.05 | 81.25 | 8.33-100 | |
| 12 weeks | 75.00 | 89.58 | 4.17-100 | |
Not assessed at 6 weeks
Mean scores over time for symptoms reported at a score of >1 (mild to moderate severity) at baseline are depicted in Figure 1. These symptoms included generalized pain, lack of energy, cough, nervousness, difficulty sleeping, shortness of breath, and worry. Shortness of breath was the symptom with the highest severity, and the severity scores for this symptom remained relatively constant across the three evaluation time points. Pain, lack of energy, and cough were more severe at the 12-week evaluation, whereas severity for nervousness, difficulty sleeping, and worry decreased over time. Baseline nervousness was significantly more severe than 12-week nervousness (P = .035). Baseline worry was significantly more severe than 6-week worry (P = .008) and 12-week worry (P = .001).
Figure 1.
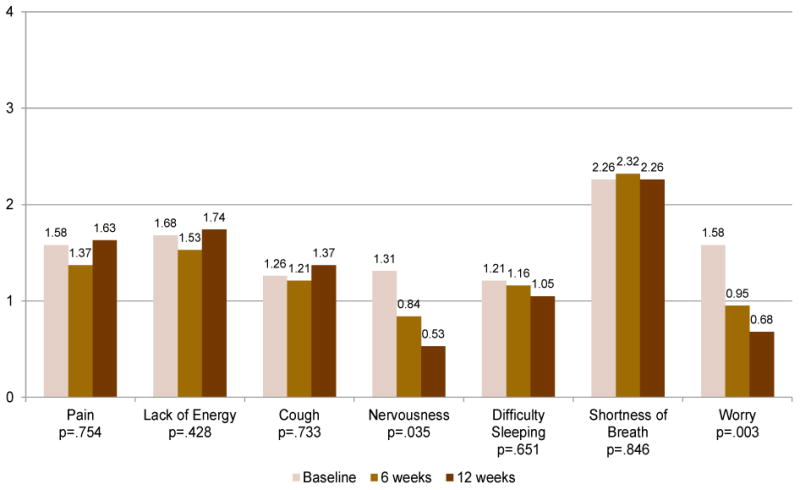
Most Severe Symptoms, N=19 (range=0-4)
Symptom and psychological distress, and QoL findings are shown in Table 3. A decrease in perceived symptom distress was observed over time, although this was not statistically significant. Patients reported mild psychological distress across time. Overall QoL, as measured by the FACT-L, was moderate. QoL subscale scores were moderate, with the exception of the functional domain, which had the lowest score of all the subscales (physical, emotional, social/familial, spiritual domains).
Table 3. Symptom distress, psychological distress, and QoL (N = 19).
| Mean | Median | Range | P | |
|---|---|---|---|---|
| Global Symptom Distress Range, 0-4; higher score = greater symptom distress | ||||
| Baseline | 1.90 | 1.93 | 0.8-4.0 | .198 |
| 6 weeks | 1.62 | 1.60 | 0.4-3.7 | |
| 12 weeks | 1.69 | 1.60 | .00-1.7 | |
| Psychological Distress Range, 0-10; higher score = greater distress | ||||
| Baseline | 3.47 | 4.00 | 0-7 | .230 |
| 6 weeks | 3.05 | 3.00 | 0-8 | |
| 12 weeks | 2.47 | 2.00 | 0-7 | |
| QoL Higher score = better QoL | ||||
| Type of well-being | .616 | |||
| Physical (range, 0-28) | ||||
| Baseline | 23.02 | 25.67 | 9-28 | |
| 6 weeks | 23.38 | 24.00 | 15-28 | |
| 12 weeks | 22.82 | 25.00 | 14-28 | |
| Emotional (range, 0-24) | .125 | |||
| Baseline | 17.62 | 18.00 | 6-24 | |
| 6 weeks | 19.03 | 20.00 | 6-24 | |
| 12 weeks | 20.10 | 19.20 | 15-24 | |
| Social/familial (range, 0-28) | .430 | |||
| Baseline | 21.84 | 24.00 | 10-28 | |
| 6 weeks | 22.47 | 24.00 | 5-28 | |
| 12 weeks | 22.16 | 24.00 | 7-28 | |
| Functional (range, 0-28) | .211 | |||
| Baseline | 15.95 | 15.00 | 9-23 | |
| 6 weeks | 15.26 | 15.00 | 6-23 | |
| 12 weeks | 16.58 | 18.00 | 8-24 | |
| Spiritual (range, 0-48) | .684 | |||
| Baseline | 34.3 | 35.1 | 18-48 | |
| 6 weeks | 32.2 | 30.6 | 19-48 | |
| 12 weeks | 33.1 | 34.0 | 15-48 | |
| Lung ca symptoms (range, 0-32) | .957 | |||
| Baseline | 25.07 | 27.00 | 11-32 | |
| 6 weeks | 24.83 | 28.28 | 14-32 | |
| 12 weeks | 24.62 | 26.28 | 16-32 | |
| Total FACT-L (range, 0-140) | .692 | |||
| Baseline | 103.49 | 111.03 | 68-133 | |
| 6 weeks | 104.98 | 106.28 | 67-134 | |
| 12 weeks | 106.28 | 108.00 | 68-131 | |
FACT-L, Functional Assessment of Cancer Therapy-Lung; QoL, quality of life
Finally, through medical chart data extraction, health care resource use data were obtained. For the 19 patients, there were no unscheduled admissions or clinic visits observed across the 3 evaluation time points. Supportive care referrals during the 12 week timeframe were made for 12 patients, and the most common referrals were to social work. Only 2 patients had completed advance care planning while on study.
Discussion
To our knowledge, this is one of the first studies that provided a comprehensive assessment of QoL in early-stage lung cancer patients treated with SBRT. Overall, our results suggest that QoL was not seriously impacted in this cohort of early-stage lung cancer patients with extensive comorbid conditions after treatment with SBRT. This finding is in agreement with other QoL studies in SBRT published in recent years, which also observed no clinically significant deteriorations in QoL scores following treatment.7,8,9, 25
The relative stability in QoL that we and others observed is important, given that many patients who are eligible for SBRT have substantial comorbid conditions, and are often elderly and frail. The introduction of SBRT in recent years as a viable treatment option in early-stage lung cancer allows for such patients to undergo curative treatment without compromising their QoL. This treatment modality will become increasingly important with the widespread use of lung cancer screening with chest CT scanning, as an increasing proportion of lung cancers are detected in stage I. Further comparative analysis of QoL in early-stage lung cancer patients treated with surgery versus SBRT may be useful to guide decisions about treatment.
Studies have shown that functional disabilities may influence a patients' perceived QoL.26,27 Although we were not able to determine an association between functional status and QoL in this study, we did observe lower scores for social activities and the functional well-being subscale of the FACT-L. These findings may be explained by our cohort's significant comorbidities. The potential physical limitations resulting from comorbid conditions may have prevented patients from fully participating in social activities, and may have also explained the lower scores in functional well-being observed in this study. Comprehensive assessment of functional status and comorbidities, particularly for elderly patients, may assist with the early identification and management of lung cancer patients who are at risk for experiencing more functional disabilities following treatment.
Our symptom assessment findings observed an increase at the 12-week evaluation for pain, lack of energy, and cough. A recent study by Videtic and colleagues also found similar changes in several symptoms, including dyspnea and fatigue.9 Although we did not observe an increase in shortness of breath 12 weeks after completion of SBRT, we did observe it at the 6-week evaluations. These findings are in agreement with other published studies, which also observed a gradual increase over time for pulmonary symptoms and fatigue.28 These results may not be entirely attributable to pulmonary changes following SBRT, but also the natural history of chronic and progressive comorbid conditions such as chronic obstructive pulmonary disease, which may have amplified the overall symptom profile. In a cancer population with pre-existing symptoms secondary to significant comorbid conditions, it is a challenge to determine whether symptom changes are attributed to the treatment alone. In addition, we observed significant improvements in baseline scores for emotional symptoms such as nervousness and worry. Similar findings were also reported in a study by van der Voort van Zyp and colleagues, in which an improvement of emotional functioning was observed after SBRT.7 The most likely reason for this observed improvement is that patients might have been anxious about their CT scan result at 3 months, and because the outcome was favorable, their worry scores went down. Our chart audit findings revealed that only 63% of patients in this cohort were referred to supportive care services for management of physical and/or psychological symptoms. Comprehensive symptom assessment and management during and after SBRT may be warranted to better assess for changes in pulmonary function, pain, fatigue, and emotional well-being in this frail patient population.9
Complications, QoL changes, and symptom trajectory associated with SBRT may also be influenced by dose and administration techniques. In general, dose schedules are selected based on factors such as tumor location (peripheral vs central) and occasionally by baseline pulmonary function. Our technique and protocol is comparable to other published QoL studies in SBRT. Future studies assessing the impact of SBRT on QoL should consider the relationship between treatment techniques and long-term complications such as rib fractures.
With overall local control rates exceeding 80%, patients who are treated with SBRT are, in general, not expected to die from their lung cancer. Therefore, emphasis should be on addressing the posttreatment emotional and physical needs of these patients. The need for comprehensive survivorship care has been the subject of numerous reports in the United States, including the Institute of Medicine's seminal report in 2007.29 A primary goal of survivorship care is to improve care coordination for cancer survivors after completion of cancer treatments. For lung cancer survivors who are treated with SBRT, care coordination and communication between oncology specialists and community providers on the management of comorbid conditions and intercurrent illnesses may be of particular importance given that morbidity and mortality will most likely occur secondary to comorbid conditions and not the cancer itself. Compared with surgical resection patients, recurrences for SBRT patients can occur several years after treatment. Recurrences and radiation changes can often be difficult to distinguish initially, so disease surveillance for survivors requires multidisciplinary review and discussion. The use of a comprehensive survivorship care plan can serve as a mechanism to facilitate communication and care coordination across disciplines and specialties so that symptoms, complications, and disease recurrences can be identified and treated in a timely fashion.
The small sample size of this study presented some limitations to the statistical analysis and interpretation of findings. This may have resulted in a lack of power to detect small or moderate differences in QoL, and the findings should be interpreted with caution. The findings may also be somewhat limited by a lack of sufficient diversity in the cohort and may not be generalizable across diverse cultural groups and geographic locations. Finally, the extensive QoL and symptom assessment items may have contributed to response fatigue for patients. Nevertheless, our study contributes to an emerging body of evidence that addresses the QoL outcomes for SBRT in early-stage lung cancer. The QoL findings can be used to aid patients and clinicians in making decisions about treatment, particularly if patients are eligible for both surgical intervention and SBRT.
In conclusion, QoL was not significantly impacted following SBRT, but increases in symptoms such as pain, lack of energy, and cough were observed at 12 weeks. Effective management of comorbid conditions may be of particular importance for this frail patient population.
Table 4. Resource use and advance care planning (N = 19).
| Resource/planning | n (%) |
|---|---|
| Supportive care referralsa | |
| Social work | 4 (33.3) |
| Pain/palliative care | 3 (25.0) |
| Psychology | 2 (16.7) |
| Pulmonary rehabilitation | 2 (16.7) |
| Nutrition | 1 (8.3) |
| Advance care planning | |
| Yes | 2 (10.5) |
| No | 17 (89.4) |
Percentages will sum to >100 because patients can select more than 1 answer.
Acknowledgments
The authors thank Gwen Uman, PhD, for biostatistical support. The contents of this research are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
Funding/sponsorship: This research was supported by grant 5 P01 CA136396-02 (PI: Ferrell) from the National Cancer Institute.
Footnotes
Disclosures: none of the authors have any conflicts of interest or financial disclosures to report.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975-2011. [Accessed October 16, 2014]; http://seer.cancer.gov/csr/1975_2011/. Updated September 10, 2014.
- 2.International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haasbeek CJ, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I non-small-cell lung cancer in patients aged > or =75 years: outcomes after stereotactic radiotherapy. Cancer. 2010;116:406–414. doi: 10.1002/cncr.24759. [DOI] [PubMed] [Google Scholar]
- 5.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 6.Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage I non-small-cell lung cancer – mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Van der Voort van Zyp NC, Prevost JB, van der Holt B, et al. Quality of life after stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2010;77:31–37. doi: 10.1016/j.ijrobp.2009.04.080. [DOI] [PubMed] [Google Scholar]
- 8.Lagerwaard FJ, Aaronson NK, Gundy CM, Haasbeek CJ, Slotman BJ, Senan S. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol. 2012;7:1148–1154. doi: 10.1097/JTO.0b013e318252cfef. [DOI] [PubMed] [Google Scholar]
- 9.Videtic GM, Reddy CA, Sorenson L. A prospective study of quality of life including fatigue and pulmonary function after stereotactic body radiotherapy for medically inoperable early-stage lung cancer. Support Care Cancer. 2013;21:211–218. doi: 10.1007/s00520-012-1513-9. [DOI] [PubMed] [Google Scholar]
- 10.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 11.George LK, Fillenbaum GG. OARS methodology. A decade of experience in geriatric assessment. J Am Geriatr Soc. 1985;33:607–615. doi: 10.1111/j.1532-5415.1985.tb06317.x. [DOI] [PubMed] [Google Scholar]
- 12.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 13.Podsiadlo D, Richardson S. The timed “Up & Go:” a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 14.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psych. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 16.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psych Neurol. 1995;8:238–242. doi: 10.1177/089198879500800408. [DOI] [PubMed] [Google Scholar]
- 17.Stewart A, Ware J. Measuring function and well-being: the Medical Outcomes Study approach. Durham: Duke University Press; 1992. [Google Scholar]
- 18.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 19.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55:215–224. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eton DT, Cella D, Yount SE, Davis KM. Validation of the Functional Assessment of Cancer Therapy – Lung Symptom Index-12 (FLSI-12) Lung Cancer. 2007;57:339–347. doi: 10.1016/j.lungcan.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the Functional Assessment of Chronic Illness Therapy – Spiritual Well-Being Scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55:285–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 23.Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 24.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncl. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 25.Widder J, Postmus D, Ubbels JF, Wiegman EM, Langendijk JA. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for Inoperable early-stage lung cancer. Int J Rad Oncol Biol Phys. 2011;81:e291–e297. doi: 10.1016/j.ijrobp.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Economou D, Hurria A, Grant M. Integrating a cancer-specific geriatric assessment into survivorship care. Clin J Oncol Nurs. 2012;16:E78–85. doi: 10.1188/12.CJON.E78-E83. [DOI] [PubMed] [Google Scholar]
- 27.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 28.Langendijk JA, Aaronson NK, de Jong JMA, et al. Quality of life after curative radiotherapy in stage I non-small-cell lung cancer. Int J Rad Oncol Biol Phys. 2002;53:847–853. doi: 10.1016/s0360-3016(02)02847-x. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt ME, Greenfield S, Stovall E. From cancer patient to cancer survivors: lost in transition. Washington, DC: National Academies Press; 2006. [Google Scholar]


