Abstract
Dopaminergic dysfunction is a putative mechanism underlying HIV-associated neurocognitive disorders. Dopamine transporter (DAT), brain-derived neurotrophic factor (BDNF), and catechol-O-methyltransferase (COMT) have been specifically implicated. We report analyses examining the main effects of functional polymorphisms within dopamine-modulating genes, as well as their interactive effects with disease severity, upon neurocognitive functioning in HIV+ adults.
Method
A total of 184 HIV+ adults were included in the analysis. Three polymorphisms were examined within dopamine-modulating genes: COMT val158met, BDNF val66met, and the DAT 3 variable number tandem repeat. Separate hierarchical regression analyses for five neurocognitive domains (working memory, processing speed, learning, memory, motor) were conducted. Predictor variables were age, ethnicity, gender, education, CD4+ T-cell count, current depression, genotype, and an interaction term capturing genotype and disease severity (CD4).
Results
None of the polymorphisms or HIV disease variables significantly improved the hierarchical regression models. Younger age, higher education, and Caucasian ethnicity were almost invariably associated with better functioning across all five cognitive domains. A trend was noted for current depression as a predictor of motor and learning ability.
Conclusion
This study did not find evidence to support direct or interactive effects of dopamine-related genes and HIV disease severity on neurocognitive functioning.
Keywords: Catechol-O-methyltransferase, Brain-derived neurotrophic factor, Dopamine transporter, HIV-associated neurocognitive disorders, NeuroAIDS, Pharmacogenetic
HIV causes neurocognitive impairment in 40% or more of infected individuals (Cysique, Maruff, & Brew, 2004). The neurocognitive impairment ranges from subtle difficulties with day-to-day tasks to debilitating dementia. Research criteria were updated in 2007 in order to provide a consistent and reliable framework for identifying and studying neurocognitive and functional impairments in HIV+ individuals (Antinori et al., 2007). The term for HIV-associated neurocognitive disorders (HAND) was officially established in that paper and includes the spectrum of neurocognitive disorders seen in those with HIV. Nonetheless, the neuropathogenesis of HAND remains unclear. Neuropathological and neurophysiological studies have been useful in delineating the structures affected by HIV infection. HIV-related neuropathology is particularly prevalent in the basal ganglia and related structures, including the putamen and caudate nuclei (Berger & Nath, 1997; Dal Pan et al., 1992) and substantia nigra (Itoh, Mehraein, & Weis, 2000), as well as the white matter tracts connecting these structures to the frontal lobes (Power et al., 1993). Reduced volume and increased blood–brain barrier permeability in the basal ganglia have also been reported (Aylward et al., 1993; Berger et al., 2000). Functional imaging studies have shown aberrant metabolism of the basal ganglia throughout the course of HIV-associated dementia, the most severe form of HAND (Hinkin et al., 1995; Rottenberg et al., 1996; van Gorp et al., 1992). Chang and colleagues (Chang et al., 2008) used positron emission tomography to show decreased dopamine transporter availability in the putamen of HIV+ individuals with HIV-associated dementia, and this correlated with a variety of neuropsychological measures. In addition, the highest concentrations of viral DNA and RNA have been reported in the basal ganglia and frontal lobes (Fujimura et al., 1997; Fujimura, Khamis, Shapshak, & Goodkin, 2004; Wiley & Nelson, 1990), further implicating the frontostriatal system as particularly vulnerable to infection. Importantly, Kumar et al. (2009) found decreased dopamine (DA) concentrations throughout the basal ganglia in HIV+ brains, and viral RNA levels negatively correlated with dopamine levels (Kumar et al., 2009). Further, in a more recent study, Kumar and colleagues (Kumar, Ownby, Waldrop-Valverde, Fernandez, & Kumar, 2011) found that DA concentrations in postmortem HIV-infected brains were 2–53% lower than HIV-negative controls, and that the substantial decrease (45%) in the substantia nigra was correlated with the lower neuropsychological performance across multiple domains. This sampling of neurophysiological and neuropathological observations are supported by clinical studies of HIV+ individuals demonstrating cognitive, motor, and behavioral deficits suggestive of dopaminergic dysfunction, including the presence of Parkinsonian signs and symptoms (Arendt & von Giesen, 2002; Edelstein & Knight, 1987; Kieburtz, Epstein, Gelbard, & Greenamyre, 1991; Koutsilieri, Sopper, Scheller, ter Meulen, & Riederer, 2002; Navia, Jordan, & Price, 1986). Thus, there is compelling evidence to suggest that dysfunction of DA-modulated neurons within the frontostriatal system of the brain plays a role in HAND (Berger & Arendt, 2000).
In recent years, there have been numerous reports of polymorphisms within DA-related genes, resulting in measurable differences in neurophysiological and neurocognitive functioning in nonHIV cohorts. Among the most commonly examined is the gene for catechol-O-methyltransferase (COMT). COMT is involved in the metabolism of the catecholamine neurotransmitters within the prefrontal cortex, an area essential for a variety of cognitive abilities. A commonly occurring single nucleotide polymorphism within this gene results in a methionine (Met) → valine (Val) amino acid substitution at codon 158. The resulting COMT in those with the Met allele has lowered enzymatic activity (Lachman et al., 1996) and subsequently greater availability of DA within the prefrontal cortex, with a putative enhancement of tonic DA transmission in the striatum (Bilder, Volavka, Lachman, & Grace, 2004). Homozygosity for the Val allele has been linked to poorer cognitive functioning in persons with schizophrenia (Bilder et al., 2002; Egan et al., 2001; Nolan, Bilder, Lachman, & Volavka, 2004), in their relatives (Rosa et al., 2004), and in unrelated healthy individuals (Malhotra et al., 2002). Neuropsychological studies demonstrate that individuals homozygous for the Met allele perform better on neuropsychological tests of executive functioning and working memory, presumably due to the increased DA availability within the prefrontal cortex (Egan et al., 2001; Goldberg et al., 2003; Mattay et al., 2003). These findings hold particular significance to HIV, as reduced neural processing capacity in working memory networks in those with HIV has recently been shown (Tomasi, Chang, de Castro Caparelli, Telang, & Ernst, 2006). Perhaps more directly relevant to HAND are the genes for dopamine transporter (DAT) and brain-derived neurotrophic factor (BDNF). DAT levels have been associated with perseverative errors on the Wisconsin Card Sorting Test (Hsieh et al., 2010), and DAT availability was also correlated with learning performance, executive functioning, and motor ability (Mozley, Gur, Mozley, & Gur, 2001). In the context of HAND, positron emission tomography (PET) studies have found that decreased levels of DAT were associated with HIV-associated dementia and specific neurocognitive deficits (Chang et al., 2008; Wang et al., 2004). A variable number tandem repeat polymorphism within exon 15 of SLC6A3, the gene for DAT, is associated with DAT expression and, subsequently, availability of DA (VanNess, Owens, & Kilts, 2005). Individuals who are homozygous for the 10-repeat allele have lower DAT availability and binding than those who have one or two alleles of the 9-repeat allele. The latter has been associated with enhanced cortical activation during cognitive tasks (Bertolino et al., 2006; Prata et al., 2009). Further, DAT is most prevalent in the striatum, an area of considerable HIV-related neuropathology (Hinkin et al., 1995; Rottenberg et al., 1996; van Gorp et al., 1992). Thus, alterations in DA availability in the striatum due to the 10-repeat allele may lead to augmentation of HIV-related neurocognitive impairment. BDNF, which plays a regulatory role in the DA and serotonin systems (Guillin et al., 2001; Mossner et al., 2000), has been implicated in reducing the neurotoxic effects of HIV in animal models (Nosheny et al., 2007; Nosheny, Mocchetti, & Bachis, 2005). A single nucleotide polymorphism within the gene for BDNF, resulting in a nonsynonymous Val → Met amino acid substitution at codon 66 has been associated with aberrant intracellular trafficking and secretion of BDNF (Egan et al., 2003). A functional magnetic resonance imaging (fMRI) study (Hariri et al., 2003) found the Met allele to be associated with lower hippocampal activity during encoding and retrieval processes, and decreased hippocampal and prefrontal cortex volumes have been found in a structural imaging study (Pezawas et al., 2004). BDNF has been shown to be neuroprotective against HIV neurotoxicity (Mocchetti, Nosheny, Tanda, Ren, & Meyer, 2007; Nosheny et al., 2007), suggesting that aberrant forms may reduce this shielding capacity. Further, because of the high prevalence of encoding and retrieval deficits among HIV-infected individuals (Woods, Moore, Weber, & Grant, 2009), the importance of examining this BDNF polymorphism in the context of neuroAIDS is clear.
In the current study, we examine the contribution of host genetics to neuropsychological functioning among a cohort of HIV+ individuals. The focus is on genes with demonstrated modulation of DA functioning within the frontostriatal system, and which show evidence of subsequent effects on behavioral and/or physiological phenotypes. These include the genes for BDNF, DAT, and COMT. Herein, we refer to the more familiar gene product rather than using the gene name (e.g., DAT instead of SLC6A3). The polymorphisms examined here have previously been shown to be associated with neurocognitive and neurophysiological endophenotypes as described above and may further explain the neurocognitive difficulties faced by many with HIV. We hypothesized that the COMT 158Met allele, DAT 9-repeat allele, and BDNF 66Val allele would be associated with better neurocognitive functioning in at least one of the domains examined. Further, we hypothesized that an interaction exists between disease severity and genotype, such that those with lesser disease severity (assessed via baseline CD4+ T-cell count) and the alleles described above would have the strongest neurocognitive functioning.
Method
Participants
Data and tissue/fluid samples were collected from HIV+ individuals enrolled in the National NeuroAIDS Tissue Consortium in compliance with Human Subjects regulations stipulated by the University of California–Medical Institutional Review Board. The National NeuroAIDS Tissue Consortium is a multicenter, longitudinal study of well-characterized HIV+ adults (Morgello et al., 2001). Subjects are followed longitudinally until death, and a rapid autopsy is performed to extract tissues for research. The National NeuroAIDS Tissue Consortium consists of four sites within the United States: (a) the National Neurological AIDS Bank located in Los Angeles, CA, (b) the Texas Repository for AIDS Research located in Galveston, TX, (c) the Manhattan HIV Brain Bank located in New York, NY, and (d) the California NeuroAIDS Tissue Network located in San Diego, CA. Upon study entry at one of the four sites, participants are administered a comprehensive battery of psychometric measures in order to determine past and current substance use disorders, psychiatric illness, and neuropsychological functioning. Neurological examinations were also performed along with immunological (CD4+ subsets) and virologic testing (via plasma and, when available, cerebrospinal fluid HIV viral load).
Using established criteria (“Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders,” 1996), neurocognitive diagnosis was reached via consensus agreement between the examining study neurologist and neuropsychologist, with consideration of results from neuromedical examination, neuropsychological assessment, psychiatric/substance use history, laboratory results (e.g., viral load and CD+ T-cell count), neuroimaging (when available), and psychosocial background. Only those individuals who were diagnosed as neurologically normal, or had subsyndromic impairment, mild cognitive/motor disorder, or HIV-associated dementia at the time of study entry were included. Of 1,642 National NeuroAIDS Tissue Consortium participants who had undergone the full baseline evaluation, this left 467 eligible for inclusion. Note that the more recent criteria for HAND (Antinori et al., 2007) were not used, as most of our participants were diagnosed prior to 2007. Strict inclusion criteria were then applied to the remaining participants in order to avoid the potential confound of neurocognitive deficits due to comorbid conditions. Specifically, individuals included in the current study had no history of opportunistic infections affecting the central nervous system (including cryptococcal meningitis, toxoplasmosis, and progressive multifocal leukoencephalopathy), no history of traumatic brain injury with loss of consciousness of greater than 1 hour, no self-reported history of learning disability or other developmental disorders, no past or current bipolar disorder (as determined via structured psychiatric interview, described below), and no other major neurologic syndromes (e.g., epilepsy, multiple sclerosis, Parkinson's disease, or brain tumor, as determined via neuromedical examination) as determined via participant self-report. After removing individuals with these conditions, as well as those for whom genotyping was not reliable, 342 participants remained. Individuals with current dependence on alcohol or drugs were then excluded, as well as those with current cocaine and/or amphetamine abuse (as determined via psychiatric interview, described below). Positive urine screen for cocaine, amphetamines, or opiates was also exclusionary. A total of 199 participants met these strict criteria. Finally, after removing those with missing ethnicity data, the final dataset included 184 participants. A flow chart of participant selection is shown in Figure 1.
Figure 1.
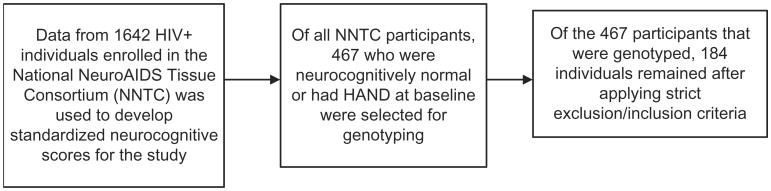
Flow chart of participant selection.
The average age of this final sample was 44.2 years (SD = 8.5), with a range of 21 to 68.6 years. Average education level was 13.5 years (SD = 2.8), with a range of 6 to 20 years of education. Average length of infection was 11.2 years (SD = 5.4), with a range of < 1to 24 years. Average CD4+ T-cell count was 219 (SD = 227). Twenty-three (13.5%) were female. Ethnically, 143 (77.7%) were Caucasian, and 41 (22.3%) were African American. Hispanic ancestry was not considered in classifying ethnicity. A structured interview (Hasin et al., 1996) based on criteria from the Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition (DSM– IV; American Psychiatric Association, 1994) found 29 (17%) to have current major depressive disorder. Based on established criteria (Woods et al., 2004), 52 participants (28.3%) were neurocognitively normal, 51 (27.7%) had subsyndromic HIV-related neurocognitive impairment, 63 (34.2%) had HIV-associated minor cognitive/motor disorder, and 18 (9.8%) had HIV-associated dementia.
Neuropsychological functioning
Neuropsychological functioning was determined at study entry (i.e., baseline) via comprehensive neurocognitive testing conducted by trained neuropsychometrists at each of the four study sites. Tests were selected from a larger battery that addressed the neurocognitive domains most relevant to the genes of interest. Tests were grouped into the following domains (see Table 1): information processing speed, working memory, motor functioning, learning, and memory. Raw test scores were converted to standardized t-scores using all National NeuroAIDS Tissue Consortium cases for whom neuropsychological data were available as a standardization sample (N = 1,642). This was done in order to provide equal weighting to all measures, which would not be possible had external normative data, with disparate normative samples, been used. Functioning within each domain was determined by averaging t-scores for all measures within a domain. Because of a non-normal distribution, Form A of the Trail Making Test and both trials of the Grooved Pegboard were log-transformed. Note that demographic corrections for age, education, gender, and ethnicity (African-American and Caucasian) were not done during the standardization process. Instead, these factors were entered as covariates in the statistical analysis.
Table 1. Neuropsychological measures and domains.
| Neurocognitive domain | Dependent variable | |
|---|---|---|
| I. | Working memory | Letter–Number Sequencing (WAIS–III; Wechsler, 1997) |
| PASAT Trial 1 (Wiens, Fuller, & Crossen, 1997) | ||
| II. | Motor | Grooved Pegboard, dominant and nondominant hand (Klove, 1963) |
| III. | Information processing speed | Digit Symbol (WAIS–III; Wechsler, 1997) |
| Symbol Search (WAIS–III; Wechsler, 1997) | ||
| Trail Making Test–Form A (Battery, 1944) | ||
| IV. | Learning | HVLT–Revised Learning Trials total (Shapiro, Benedict, Schretlen, & Brandt, 1999) |
| BVMT–Revised Learning Trials total (Benedict, Schretlen, Groninger, & Dobraski, 1996) | ||
| V. | Memory | HVLT–Revised Free Recall (Shapiro et al., 1999) |
| BVMT–Revised Free Recall (Benedict et al., 1996) | ||
Note. WAIS–III = Wechsler Adult Intelligence Scale–Third Edition; PASAT = Paced Auditory Serial Addition Test; HVLT = Hopkins Verbal Learning Test; BVMT = Brief Visuospatial Memory Test.
Psychiatric and substance use diagnoses
The Psychiatric Research Interview for Substance and Mental Disorders, or PRISM (Hasin et al., 1996), was administered to participants in order to determine history of substance use disorder, major depressive disorder (MDD), and bipolar disorder. The PRISM is a structured diagnostic interview that yields DSM–IV diagnoses. Participants with past or current bipolar disorder were excluded from the analysis. Participants were classified as “currently depressed” if they met DSM–IV criteria for current major depressive disorder. Participants with current major depressive disorder were included in the analysis, and this designation was entered as a covariate. Current and past abuse or dependence were assessed for alcohol and common drugs of abuse. For the current study, individuals with current alcohol or substance dependence of any kind were excluded from the analysis: Individuals with no current dependence on these substances, or who reported only past dependence on these substances (>12 months prior to study entry), were included. Due to the evidence of additive or synergistic effects of cocaine and methamphetamine on the neuropsychological functioning of HIV+ individuals (Durvasula et al., 2000; Rippeth et al., 2004), those participants meeting criteria for current abuse on these substances were also excluded. Note that for some participants, the PRISM was not administered on the same date as neuropsychological testing. Therefore, only those individuals who underwent the PRISM within 60 days of the neuropsychological assessment were included. As a final measure to control for the effects of drug use in our sample, participants with a positive urine screen for cocaine, amphetamines, and/or opiates were excluded.
Measure of disease severity
CD4+ T-cell count
CD4 count was also used as a proxy of disease severity. While nadir CD4+ T-cell count would have been a preferable measure of disease severity in our analysis, this information was not available for most participants. However, the National NeuroAIDS Tissue Consortium (NNTC) generally focused on recruiting individuals with advanced HIV disease. As such, baseline CD4 count was likely to be near nadir CD4. Based on a small subset of individuals from the National Neurological AIDS BANK consortium site, median nadir CD4 count was 41 (SD = 61.5) whereas median baseline CD4 count was 116 (SD = 242), and the two were highly correlated (r = .913, p < .001).
Tissue processing, DNA extraction, and genotyping
Peripheral blood mononuclear cells and/or frozen tissue samples were shipped to the University of California Los Angeles–Biological Samples Processing Core from the four National NeuroAIDS Tissue Consortium sites for DNA extraction. The Autopure LS nucleic acid purification instrument was used for extracting DNA. Samples were quantified using OD 260/280. Extracted DNA was then genotyped. Prior to genotyping, the samples were checked for concentration by the Quant-iT ds DNA Assay kit from Invitrogen and for quality by agarose gel. DNA amplification was performed on 96- and 384-well polymerase chain reaction (PCR) plates on Applied Biosystems GeneAmp PCR System 9700 thermal cyclers. Single nucleotide polymorphism genotypes (for BDNF and COMT) were determined using the allelic discrimination assay on an Applied Biosystems 7900 Taqman instrument analyzed with SDS2.3 software. DAT genotype was determined using an Applied Biosystems 3730 DNA Analyzer and was analyzed with the Genemapper software. The polymorphisms are listed in Table 2 alongside their respective genes and loci. Data then underwent error checking and data cleaning including control checks, duplicates checks, and checking for Hardy Weinberg equilibrium. Each genotype was evaluated independently according to a number of quality parameters.
Table 2. Gene and polymorphism identifications.
| MAF | ||||
|---|---|---|---|---|
|
|
||||
| Gene or protein name | Polymorphism identification | Gene location | EA | AA |
| COMT | rs4680 | 22q11.2 | .375a | .326a |
| DAT | 3′ UTR 40bp | 5p15.3 | .299b | .181b |
| BDNF | rs6265 | 11p13 | .167a | .065a |
Note. COMT = catechol-O-methyltransferase; DAT = dopamine transporter; BDNF = brain-derived neurotrophic factor; EA = European American; AA = African American; MAF = minor allele frequency.
Minor allele frequencies based on: European American and African American (National Center for Biotechnology Information, 2011).
Minor allele frequencies based on African American and European American samples (Doucette-Stamm, Blakely, Tian, Mockus, & Mao, 1995).
Statistical analysis
Neuropsychological test scores were assessed for normalcy. Separate hierarchical regression analyses were performed for each of the five neurocognitive domains. For each analysis, demographic variables (ethnicity, education, age, and gender), disease severity (CD4+ T-cell count), and current MDD status were entered into the first step. In the second step, genotype and the genotype X disease terms were entered. Ethnicity was entered as either Caucasian or African American. Education and age were entered in years as continuous variables. Due to the low frequency of minor allele homozygotes, DAT and BDNF were coded in a dominant fashion such that heterozygosity or homozygosity for the minor allele was coded the same (Met in the case of BDNF, and the 9-repeat variable number tandem repeat in the case of DAT). The COMT Met allele was coded in an additive manner (i.e., dosage model). Unstandardized betas and semipartial correlation coefficients were calculated for each variable included in the final models. Tolerances were also calculated to determine presence of multicollinearity of predictor variables. Due to missing data (primarily genotyping, CD4, and some neuropsychological measures), none of the regression analyses included all 184 participants.
Results
Five hierarchical regression analyses were conducted. The first sought to determine the best combination of demographic variables, disease severity variable, major depressive disorder, and genotype (COMT, BDNF, and DAT) for predicting working memory functioning t-scores. After forcing in demographic variables, CD4, and MDD in the first block, no additional variance was explained by the genetic markers or the gene X disease severity interaction terms. Only demographic variables, the first block of the hierarchical model, were significant predictors, F(6, 131) = 3.29, p = .005 (Table 3). The adjusted R2 change for this block was .091, indicated that approximately 9% of the variance in working memory functioning was explained by the variables in the first block. Higher education, younger age, and Caucasian ethnicity were significant predictors of working memory functioning.
Table 3. Results of hierarchical regression analysis for working memory functioning.
| Adjusted R2 | R2 change | F change | Significance of F change | ||
|---|---|---|---|---|---|
| Block 1 | .091 | .131 | 3.30 | .005 | |
| Block 2 | .058 | .009 | 3.22 | .969 | |
|
| |||||
| Variables in final block (1) | Partial correlation | Beta | Standard error beta | p-value | Tolerance |
|
| |||||
| Education | .169 | .172 | 0.24 | .05 | .86 |
| Gender | .055 | .053 | 1.62 | .532 | .94 |
| Age | −.176 | −.171 | 0.071 | .043 | .95 |
| Ethnicity | −.228 | −.226 | 1.5 | .008 | .93 |
| MDD | −.095 | −.090 | 1.45 | .278 | .97 |
| CD4 | .078 | .075 | 0.003 | .375 | .93 |
|
| |||||
| Excluded variables | Partial correlation | ||||
|
| |||||
| COMT genotype | .015 | ||||
| BDNF genotype | −.022 | ||||
| DAT genotype | −.070 | ||||
| COMT × CD4 | −.028 | ||||
| BDNF × CD4 | .004 | ||||
| DAT × CD4 | −.072 | ||||
Note. MDD = major depressive disorder; COMT = catechol-O-methyltransferase; DAT = dopamine transporter; BDNF = brain-derived neurotrophic factor.
The second hierarchical regression model examined the same variables in predicting information processing speed t-scores. Only the initial block predicted a significant amount of variance in this domain, F(6, 145) = 7.9, p < .001. The adjusted R2 was .215 for this initial block, indicating that approximately 22% of the variance in information processing speed was predicted by this model. Unstandardized beta coefficients are shown in Table 4 and indicate that higher education, younger age, and Caucasian ethnicity were significantly associated with better performance.
Table 4. Results of hierarchical regression analysis for information processing speed ability.
| Adjusted R2 | R2 change | F change | Significance of F change | ||
|---|---|---|---|---|---|
| Block 1 | .215 | .246 | 7.90 | <.001 | |
| Block 2 | .213 | .029 | 0.94 | .469 | |
|
| |||||
| Variables in final block (1) | Partial correlation | Beta | Standard error beta | p-value | Tolerance |
|
| |||||
| Education | .337 | .326 | 0.21 | <.001 | .91 |
| Gender | −.016 | −.014 | 1.65 | .85 | .96 |
| Age | −.330 | −.308 | 0.07 | <.001 | .97 |
| Ethnicity | −.207 | −.187 | 1.48 | .012 | .97 |
| MDD | −.125 | −.111 | 1.43 | .130 | .98 |
| CD4 | −.046 | −.042 | 0.003 | .578 | .93 |
|
| |||||
| Excluded variables | Partial correlation | ||||
|
| |||||
| COMT genotype | −.090 | ||||
| BDNF genotype | −.044 | ||||
| DAT genotype | −.024 | ||||
| COMT × CD4 | −.148 | ||||
| BDNF × CD4 | −.035 | ||||
| DAT × CD4 | −.096 | ||||
Note. MDD = major depressive disorder; COMT = catechol-O-methyltransferase; DAT = dopamine transporter; BDNF = brain-derived neurotrophic factor.
The third hierarchical regression examined learning t-score. Only the first block predicted a significant amount of the variance, F(6, 140) = 6.48, p < .001. The adjusted R2 was .184, indicating that the model predicted approximately 18% of variance in learning t-scores. This model and unstandardized beta weights are shown in Table 5 and indicate the higher education, younger age, and Caucasian ethnicity. A trend was evident for MDD (p = .056).
Table 5. Results of hierarchical regression analysis for learning ability.
| Adjusted R2 | R2 change | F change | Significance of F change | ||
|---|---|---|---|---|---|
| Block 1 | .184 | .217 | 6.48 | <.001 | |
| Block 2 | .152 | .004 | 0.12 | .994 | |
|
| |||||
| Variables in final block (1) | Partial correlation | Beta | Standard error beta | p-value | Tolerance |
|
| |||||
| Education | .338 | .333 | 0.228 | <.001 | .91 |
| Gender | −.009 | −.009 | 1.72 | .91 | .96 |
| Age | −.228 | −.232 | 0.074 | .003 | .97 |
| Ethnicity | −.202 | −.185 | 1.55 | .016 | .97 |
| Current MDD | −.161 | −.146 | 1.52 | .056 | .98 |
| CD4 | −.057 | −.059 | 0.003 | .451 | .92 |
|
| |||||
| Excluded variables | Partial correlation | ||||
|
| |||||
| COMT genotype | −.0 | ||||
| BDNF genotype | −.035 | ||||
| DAT genotype | −.022 | ||||
| COMT × CD4 | −.008 | ||||
| BDNF × CD4 | −.019 | ||||
| DAT × CD4 | −.006 | ||||
Note. MDD = major depressive disorder; COMT = catechol-O-methyltransferase; DAT = dopamine transporter; BDNF = brain-derived neurotrophic factor.
The fourth hierarchical regression examined memory t-score. Only the initial block predicted a significant amount of the variance, F(6, 142) = 4.69, p < .001. The adjusted R2 was .130, indicating that the model predicted approximately 13% of variance in memory t-scores. This model along with the unstandardized beta weights is shown in Table 6 and indicates that younger age, higher education, and Caucasian ethnicity are significantly associated with better memory performance.
Table 6. Results of hierarchical regression analysis for memory ability.
| Adjusted R2 | R2 change | F change | Significance of F change | ||
|---|---|---|---|---|---|
| Block 1 | .130 | .165 | 4.69 | <.001 | |
| Block 2 | .108 | .015 | 0.424 | .862 | |
|
| |||||
| Variables in final block (1) | Partial correlation | Beta | Standard error beta | p-value | Tolerance |
|
| |||||
| Education | .208 | .204 | 0.258 | .012 | .91 |
| Gender | .027 | .025 | 1.94 | .751 | .96 |
| Age | −.228 | −.218 | 0.084 | .006 | .96 |
| Ethnicity | −.235 | −.225 | 1.74 | .005 | .97 |
| MDD | −.098 | −.091 | 1.71 | .242 | .98 |
| CD4 | −.095 | −.091 | 0.003 | .255 | .93 |
|
| |||||
| Excluded variables | Partial correlation | ||||
|
| |||||
| COMT genotype | .037 | ||||
| BDNF genotype | −.004 | ||||
| DAT genotype | −.077 | ||||
| COMT × CD4 | .058 | ||||
| BDNF × CD4 | −.057 | ||||
| DAT × CD4 | −.070 | ||||
Note. MDD = major depressive disorder; COMT = catechol-O-methyltransferase; DAT = dopamine transporter; BDNF = brain-derived neurotrophic factor.
The final hierarchical regression model examined motor functioning (Table 7). Only the first block was included in the final model, F(6, 141) = 44.9, p < .001. The adjusted R2 was .137, indicating that the model predicted approximately 14% of variance in motor t-scores. This model along with the unstandardized beta weights is shown in Table 7 and indicates that older age and lower education are significantly associated with poorer motor performance. A trend was evident for current MDD (p = .056).
Table 7. Results of hierarchical regression analysis for motor ability.
| Adjusted R2 | R2 change | F change | Significance of F change | ||
|---|---|---|---|---|---|
| Block 1 | .137 | .173 | 4.9 | <.001 | |
| Block 2 | .142 | .040 | 1.14 | .343 | |
|
| |||||
| Variables in final block (1) | Partial correlation | Beta | Standard error beta | p-value | Tolerance |
|
| |||||
| Education | .183 | .177 | 0.242 | .029 | .92 |
| Gender | −.087 | −.081 | 1.81 | .304 | .96 |
| Age | −.319 | −.312 | 0.079 | <.001 | .96 |
| Ethnicity | −.154 | −.145 | 1.60 | .066 | .96 |
| MDD | −.16 | −.149 | 1.57 | .056 | .98 |
| CD4 | −.038 | −.036 | 0.003 | .65 | .94 |
|
| |||||
| Excluded variables | Partial correlation | ||||
|
| |||||
| COMT genotype | .105 | ||||
| BDNF genotype | −.157 | ||||
| DAT genotype | −.063 | ||||
| COMT × CD4 | .049 | ||||
| BDNF × CD4 | −.092 | ||||
| DAT × CD4 | −.0436 | ||||
Note. MDD = major depressive disorder; COMT = catechol-O-methyltransferase; DAT = dopamine transporter; BDNF = brain-derived neurotrophic factor.
Using the power analysis program PASS (Hintze, 2008), we calculate that we had sufficient power (>80%) to detect an effect of genotype that accounts for as little as 3% of the variation in the neurocognitive domains examined, at a significance level of .05. However, to allow readers to draw their own conclusions as to whether or not the null findings were due to inadequate power, we have included partial correlation coefficients for genotype and Genotype × Disease Severity in Tables 3–7.
Discussion
DA system dysfunction is among the putative mechanisms underlying the neuropathogenesis of HAND (Berger & Arendt, 2000; Chang et al., 2008; Kumar et al., 2009; Kumar et al., 2011). Specific DA factors implicated in HAND neuropathogenesis include DAT, BDNF, and COMT. While specific polymorphisms within these genes have been shown to alter neurocognitive functioning in non-HIV+ populations, this has only recently been considered in the context of HIV. Considering the extensive changes to DA functioning in the brains of individuals with HAND, it is plausible that an additive or perhaps synergistic effect would be observed between genotype and disease severity. In this study, we have examined the effect of functional polymorphisms within these three DA-related genes upon neurocognitive phenotypes in HIV+ adults.
None of the five neurocognitive domains examined were influenced by genotype after accounting for demographic characteristics, disease severity, and current depression. It is unclear whether the lack of significant associations between neurocognitive functioning and the DA gene polymorphisms was due to lack of power, a true null relationship, cohort-specific characteristics (e.g., advanced stage of illness), or some other factor. As described in the Introduction, DAT has been implicated in the pathogenesis of HAND (Chang et al., 2008; Wang et al., 2004), and the polymorphism examined here is associated with DA availability (VanNess et al., 2005) and enhanced cortical activation during working memory tasks (Bertolino et al., 2006), as well as performance on a variety of neurocognitive tests (Mozley et al., 2001). Despite this, neither direct effects of this polymorphism nor interactive effects (with HIV disease severity) on neurocognitive functioning were observed in the current analyses. The role of the BDNF val66met polymorphism appears to be more diffuse, with the absence of specific neurocognitive effects less surprising. The polymorphism examined here has been associated with decreased hippocampal activity during memory processes (Hariri et al., 2003), as well as decreased hippocampal and prefrontal cortex volumes (Pezawas et al., 2004). Coupled with the reported high prevalence of short-term-memory deficits among HIV-infected individuals (Woods et al., 2009), we believed that this marker would be associated with learning and/or memory functioning. However, considering the multifaceted nature of these cognitive domains, including such basic functions as acquisition, encoding, consolidation, retrieval, and recognition, it is plausible that the effect of the BDNF polymorphism would have been detected with a different measure. Finally, the association between the COMT val158met polymorphism and neurocognitive functioning and neuropsychiatric disease is well established (Bilder et al., 2002; Bilder et al., 2004; Egan et al., 2001; Goldberg et al., 2003); however, this polymorphism has only recently been examined for its association with HIV-associated neurocognitive deficits. Bousman et al. (2010) recently reported on the interactive effects of COMT val158met genotype and executive functioning on sexual risk taking in both HIV+ and HIV– individuals (Bousman et al.). While no differences in executive functioning were noted between groups, they did find that among Met allele carriers, those with greater executive functioning deficits reported greater number of sexual partners and other risky sexual practices.
It is also notable that our measure of disease severity, CD4+ T-cell count obtained at study entry, was not associated with severity of neurocognitive impairment. Nadir plasma CD4+ T-cell count would have been the preferred measure of disease severity due to its closer relationship with later neurocognitive dysfunction (Cysique et al., 2010; Cysique, Maruff, & Brew, 2006; Heaton et al., 2010). However, due to the lack of information about nadir CD4 in this cohort, baseline CD4 count was the closest proxy of disease severity. Despite the strong correlation between these two measures in a subsample, it is highly possible that we were unable to adequately capture disease severity.
Not surprisingly, education level and age contributed to all of the regression models, whereas ethnicity contributed to all but motor ability. Clearly, these findings lend validation to the use of normative data that stratifies for these demographic characteristics (Heaton, Grant, & Matthews, 1991). Conversely, gender was not a significant predictor for any domain, suggesting that stratifying normative data for this factor may not be worth the trouble. While current MDD was not a significant predictive of any domain, an apparent trend (p = .056) was noted for the motor and learning domains. The relationship between depression and neurocognitive performance has been well established, and some have proposed a common underlying DA system dysfunction as a common underlying cause (Stein, 2008). In addition, there may be a relationship between depression and HAND. As recently reported by our group, current major depressive disorder was associated with markedly increased risk for concurrent HIV-associated dementia (Levine et al., 2009). Others have reported the incidence of major depressive disorder during a 2-year period to be associated with symptomatic HIV disease at baseline (Atkinson et al., 2008), and depression may be associated with accelerated disease progression (Leserman, 2008). Conversely, other researchers examining similar cohorts did not find lifetime or current major depressive disorder to result in diminished neuropsychological test scores as compared to a nondepressed HIV+ group; however, currently depressed individuals reported more complaints of cognitive difficulties in everyday life (Cysique et al., 2007). As such, this relationship requires further examination. Various explanations for a relationship between depression and HIV include a common inflammatory etiology (Dantzer & Kelley, 2007; Raison, Capuron, & Miller, 2006) and slower initiation of treatment and poorer compliance with highly active antiretroviral therapy regimens (Gordillo, del Amo, Soriano, & Gonzalez-Lahoz, 1999; Li et al., 2005).
The current findings should be interpreted with the following caveats. First, we excluded a large number of individuals who are representative of the HIV pandemic in the United States (e.g., substance abusers), thus potentially limiting the study's generalizability. It is well established that injection drug use is a common cause of infection, and infection rates among substance users in general are higher than among nonsubstance users (Buchacz et al., 2005). Despite this, the current study was intended to elucidate the contribution of genetic factors to neurocognitive functioning in those with HIV, and our strict inclusion criteria were intended to control for environmental and endogenous factors that may have confounded interpretation of the results. Secondly, we did not include an HIV-negative group. Inclusion of a seronegative group would have allowed us to more adequately address our hypothesis that a HIV × Genotype interaction exists. We attempted to overcome this by examining the interaction of genotype with disease severity; however, as mentioned earlier, our measure of disease severity was not optimal. Finally, our method of standardizing the neurocognitive test scores involved a very heterogeneous cohort that included individuals with a variety of neurological disorders, substance use, and psychiatric illness. While this cohort may be somewhat representative of NeuroAIDS patients in the United States, some might question the generalizability of findings due to our methods. Indeed, the NNTC, as a tissue resource, has traditionally aimed to recruit individuals in the advanced stage of disease. However, the primary purpose of this preliminary study was to determine the interactive effects of genotype and HIV disease severity on neurocognitive functioning, not to determine degree of impairment relative to an uninfected group.
In summary, this study does not support the hypothesis that DA-related genetic variants interact with HIV disease severity to affect neurocognitive functioning. However, considering the significant limitations of this retrospective analysis and the potential for therapeutic intervention of DA dys-function, further examination of this relationship is warranted. Future studies should include either an HIV-seronegative control group or better measure of disease severity (i.e., nadir CD4). Larger samples will also be preferable as the effects of genetic variation on neurocognitive phenotypes are small. Finally, including an intermediate physiological phenotype, such as homovanillic acid or other DA metabolites, will allow stronger conclusions to be drawn about the relationship between DA-related genetic variation and neurocognitive functioning.
Acknowledgments
This study was funded through the California HIV/AIDS Research Program grant ID06-LA-187, of which Dr Levine was the principal investigator. This study was also made possible through Grants U01-MH08021 and R24-NS38841 (National Neurological AIDS Bank), U01-MH083507 and R24-NS45491 (Texas Repository for AIDS Research), U01-MH083501 and R24-MH59724 (Manhattan HIV Brain Bank), U01-MH083506 and R24-MH59745 (California NeuroAIDS Tissue Network).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt G, von Giesen HJ. Human immunodeficiency virus dementia: Evidence of a subcortical process from studies of fine finger movements. Journal for Neurovirology. 2002;8(Suppl. 2):27–32. doi: 10.1080/13550280290101067. [DOI] [PubMed] [Google Scholar]
- Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, et al. Two-year prospective study of major depressive disorder in HIV-infected men. Journal of Affective Disorders. 2008;108(3):225–234. doi: 10.1016/j.jad.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, et al. Reduced basal ganglia volume in HIV-1-associated dementia: Results from quantitative neuroimaging. Neurology. 1993;43(10):2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Battery AIT. Manual of directions and scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Dobraski M. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment. 1996;8(2):145–153. [Google Scholar]
- Berger JR, Arendt G. HIV dementia: The role of the basal ganglia and dopaminergic systems. Journal of Psychopharmacology. 2000;14(3):214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40(2–3):122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A, Greenberg RN, Andersen AH, Greene RA, Bognar A, et al. Cerebrovascular changes in the basal ganglia with HIV dementia. Neurology. 2000;54(4):921–926. doi: 10.1212/wnl.54.4.921. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. Journal of Neuroscience. 2006;26(15):3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biological Psychiatry. 2002;52(7):701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Atkinson JH, Heaton RK, Grant I, Everall IP, et al. COMT Val158Met polymorphism, executive dysfunction, and sexual risk behavior in the context of HIV infection and methamphetamine dependence. Interdisciplinary Perspectives on Infectious Diseases, 2010. 2010;678648:1–9. doi: 10.1155/2010/678648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19:1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42(2):869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology. 1996;47(5):1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society. 2007;13(1):1–11. doi: 10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, et al. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. 2010;24(7):983–990. doi: 10.1097/QAD.0b013e32833336c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. Journal for Neurovirology. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66(9):1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3. [DOI] [PubMed] [Google Scholar]
- Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, Kumar AJ, et al. Patterns of cerebral atrophy in HIV-1-infected individuals: Results of a quantitative MRI analysis. Neurology. 1992;42(11):2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavavior, and Immunity. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette-Stamm LA, Blakely DJ, Tian J, Mockus S, Mao JI. Population genetic study of the human dopamine transporter gene (DAT1) Genetic Epidemiology. 1995;12(3):303–308. doi: 10.1002/gepi.1370120307. [DOI] [PubMed] [Google Scholar]
- Durvasula RS, Myers HF, Satz P, Miller EN, Morgenstern H, Richardson MA, et al. HIV-1, cocaine, and neuropsychological performance in African American men. Journal of the International Neuropsychological Society. 2000;6(3):322–335. doi: 10.1017/s1355617700633076. [DOI] [PubMed] [Google Scholar]
- Edelstein H, Knight RT. Severe parkinsonism in two AIDS patients taking prochlorperazine. The Lancet. 1987;2(8554):341–342. doi: 10.1016/s0140-6736(87)90937-8. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fujimura RK, Goodkin K, Petito CK, Douyon R, Feaster DJ, Concha M, et al. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;16(3):146–152. doi: 10.1097/00042560-199711010-00002. [DOI] [PubMed] [Google Scholar]
- Fujimura RK, Khamis I, Shapshak P, Goodkin K. Regional quantitative comparison of multi-spliced to unspliced ratios of HIV-1 RNA copy number in infected human brain. Journal of NeuroAIDS. 2004;2(4):45–60. doi: 10.1300/J128v02n04_04. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13(13):1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411(6833):86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability for substance abusers. The American Journal of Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead– Reitan battery: Demographic corrections, research findings, and clinical implications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Hinkin CH, van Gorp WG, Mandelkern MA, Gee M, Satz P, Holston S, et al. Cerebral metabolic change in patients with AIDS: Report of a six-month follow-up using positron-emission tomography. Journal of Neuropsychiatry and Clinical Neurosciences. 1995;7(2):180–187. doi: 10.1176/jnp.7.2.180. [DOI] [PubMed] [Google Scholar]
- Hintze J. PASS 2008 NCSS, LLC. Kaysville, UT: NCSS; 2008. Retrieved from http://www.ncss.com. [Google Scholar]
- Hsieh PC, Yeh TL, Lee IH, Huang HC, Chen PS, Yang YK, et al. Correlation between errors on the Wisconsin Card Sorting Test and the availability of striatal dopamine transporters in healthy volunteers. Journal of Psychiatry and Neuroscience. 2010;35(2):90–94. doi: 10.1503/jpn.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathologica (Berlin) 2000;99(4):376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Kieburtz KD, Epstein LG, Gelbard HA, Greenamyre JT. Excitotoxicity and dopaminergic dysfunction in the acquired immunodeficiency syndrome dementia complex. Therapeutic implications. Archives of Neurology. 1991;48(12):1281–1284. doi: 10.1001/archneur.1991.00530240087028. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster FM, editor. Medical clinics of North America. New York, NY: Saunders; 1963. [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. Journal of Neural Transmission. 2002;109(5–6):767–775. doi: 10.1007/s007020200063. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez J, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, et al. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal for Neurovirology. 2009;15(3):1–18. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: Relationship with neuropsychological performance. Journal for Neurovirology. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70(5):539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, Dandekar S, et al. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobehavioral HIV Medicine. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Margolick JB, Conover CS, Badri S, Riddler SA, Witt MD, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. Journal of Acquired Immune Deficiency Syndromes. 2005;38(3):320–328. [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. The American Journal of Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Nosheny RL, Tanda G, Ren K, Meyer EM. Brain-derived neurotrophic factor prevents human immunodeficiency virus type 1 protein gp120 neurotoxicity in the rat nigrostriatal system. Annals of the New York Academy of Sciences. 2007;1122:144–154. doi: 10.1196/annals.1403.010. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, et al. The National NeuroAIDS Tissue Consortium: A new paradigm in brain banking with an emphasis on infectious disease. Neuropathology and Applied Neurobiology. 2001;27(4):326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Mossner R, Daniel S, Albert D, Heils A, Okladnova O, Schmitt A, et al. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF) Neurochemistry International. 2000;36(3):197–202. doi: 10.1016/s0197-0186(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. The American Journal of Psychiatry. 2001;158(9):1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. [accessed October 12, 2011];dbSNP. 2011 Retrieved from http://www.ncbi.nlm.nih.gov/projects/SNP/
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Annals of Neurology. 1986;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: Differential effects of Val and Met alleles on cognitive stability and flexibility. American Journal of Psychiatry. 2004;161(2):359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Ahmed F, Yakovlev A, Meyer EM, Ren K, Tessarollo L, et al. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. European Journal of Neuroscience. 2007;25(8):2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Mocchetti I, Bachis A. Brain-derived neurotrophic factor as a prototype neuroprotective factor against HIV-1-associated neuronal degeneration. Neurotoxicity Research. 2005;8(1–2):187–198. doi: 10.1007/BF03033829. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. Journal of Neuroscience. 2004;24(45):10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, et al. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: Alterations of the blood–brain barrier. Annals of Neurology. 1993;34(3):339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E, et al. Epistasis between the DAT 3′ UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13600–13605. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, et al. New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. The American Journal of Psychiatry. 2004;161(6):1110–1112. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. Journal of Nuclear Medicine. 1996;37(7):1133–1141. [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test–Revised. Clinical Neuropsychology. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: The role of dopaminergic neurocircuitry. CNS Spectrums. 2008;13(7):561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, de Castro Caparelli E, Telang F, Ernst T. The human immunodeficiency virus reduces network capacity: Acoustic noise effect. Annals of Neurology. 2006;59(2):419–423. doi: 10.1002/ana.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, Paz DK, et al. Cerebral metabolic dysfunction in AIDS: Findings in a sample with and without dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4(3):280–287. doi: 10.1176/jnp.4.3.280. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genetics. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127(11):2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wiens AN, Fuller KH, Crossen JR. Paced Auditory Serial Addition Test: Adult norms and moderator variables. Journal of Clinical and Experimental Neuropsychology. 1997;19(4):473–483. doi: 10.1080/01688639708403737. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Nelson JA. Human immunodeficiency virus: Infection of the nervous system. Current Topics in Microbiology and Immunology. 1990;160:157–172. doi: 10.1007/978-3-642-75267-4_10. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]


