Abstract
We demonstrate that the effects of lonidamine (LND, 100 mg/kg, i.p.) are similar for a number of xenograft models of human cancer including DB-1 melanoma and HCC1806 breast, BT-474 breast, LNCaP prostate and A2870 ovarian carcinomas. Following treatment with LND, each of these tumors exhibits a rapid decrease in intracellular pH, a small decrease in extracellular pH, a concomitant monotonic decrease in nucleoside triphosphate and increase in inorganic phosphate over a 2–3 hr period. We previously demonstrated that selective intracellular tumor acidification potentiates response of this melanoma model to melphalan (7.5 mg/kg, i.v.), producing an estimated 89% cell kill based on tumor growth delay analysis. We now show that in both DB-1 melanoma and HCC1806 breast carcinoma, LND potentiates response to doxorubicin producing 95% cell kill in DB-1 melanoma at 7.5 mg/kg, i.v. doxorubicin and 98% cell kill at 10.0 mg/kg doxorubicin, and in HCC1806 breast carcinoma producing a 95% cell kill at 12.0 mg/kg doxorubicin. Potentiation of doxorubicin can result from cation trapping of the weakly basic anthracycline. Recent experience with the clinical treatment of melanoma and other forms of human cancer suggests that these diseases will probably not be cured by a single therapeutic procedure other than surgery. A multimodality therapeutic approach will be required. As a potent modulator of tumor response to N-mustards and anthracyclines as well as tumor thermo- and radiosensitivity, LND promises to play an important clinical role in the management and possible complete local control of a number of prevalent forms of human cancer.
Keywords: Lonidamine, Monocarboxylate acid transport inhibitor, tumor acidification, tumor de-energization, Doxorubicin
Introduction
Lonidamine (LND) produces a sustained and substantial selective intracellular acidification of tumors and diminishes their nucleoside triphosphate (NTP) concentration (1–5). This activity of LND has been demonstrated in DB-1 melanoma (4,5) and 9L glioma xenografts in mice and rats, respectively, and in cultured MCF-7 (1,3) and 9L glioma cells (2). We now demonstrate that this effect is of potential clinical utility to the management of a variety of prevalent human carcinomas. We demonstrate this effect in two breast carcinoma lines — the triple-negative HCC1806 subline that lacks estrogen, progesterone and Her2/Neu receptors, and the positive BT-474 line that expresses each of these receptors — and also in a human prostate (LNCaP) and an ovarian carcinoma model (A2780); all tumors were studied as xenografts in nude mice.
We have also recently shown that these properties of LND potentiate the activity of melphalan in DB-1 melanoma xenografts (4). The increase in melphalan activity was attributed to tumor acidification which stabilizes the active intermediate and inhibits glutathione-mediated deactivation of this intermediate. Acid also interferes with DNA repair by inhibiting O6-alkyltransferase (6,7). The same mechanisms are expected to apply to other nitrogen mustard alkylating agents such as cyclophosphamide, chlorambucil and bendamustine. We now show that LND also potentiates the activity of doxorubicin (and potentially other anthracyclines) by different mechanism i.e. cation trapping. We demonstrate this effect in DB-1 melanoma xenografts and in the HCC1806 breast carcinoma model. De-energization of these tumors by LND is expected to further enhance response to anthracyclines and other antineoplastic agents. Overall, LND profoundly and selectively increases the efficacy of anthracyclines and N-mustards.
This manuscript demonstrates that the ability of LND to acidify and de-energize tumors applies to a variety of cancers including several prevalent forms of the disease that are the focus of current clinical research and that this property plays a critical role in potentiating the activity of two important classes of antineoplastic agents. LND represents a class of agents that by themselves have limited antineoplastic activity but which by selectively modifying the biochemical properties of tumors make them more susceptible to chemotherapy and also to hyperthermia (8–12) and radiation (13). Thus, LND and agents like it promise to play a critical role as tumor selective facilitators of multimodality therapy of a variety of cancers.
Materials and Methods
Materials
LND and 3-aminopropylphosphonate (3-APP) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz CA, USA). The drug (LND; 5 mg) was dissolved in 227 μL of tris/glycine buffer (22.0 mg/mL), vortexed until the solution was clear, and administered i.p. (intraperitoneal) at a dose of 100 mg/kg. The buffer consisted of trizma base (1.2 g) and glycine (5.76 g) in 100 mL sterile water (final pH = 8.3). In addition, 0.2 ml of a 300 mg/ml solution of 3-APP (dissolved in water and adjusted to pH 7) was administered i.p. Doxorubicin was purchased from Midwest Veterinary Supply (Burnsville, MN, USA) and administered i.v. (7.5 mg/kg, 10 mg/kg to mice bearing DB-1 melanoma xenografts and 12 mg/kg to mice bearing HCC1806 breast carcinoma xenografts).
Human Melanoma Xenografts in Nude Mice
Male athymic nude mice (01B74) 4–6 weeks of age obtained from the National Cancer Institute, Frederick, MD, USA were housed in microisolator cages with access to water and autoclaved mouse chow ad libitum. DB-1 melanoma cells were early passage human melanoma cells derived from a lymph node biopsy of a human patient with metastatic melanoma that was excised before administration of any treatment by Dr. David Berd (Thomas Jefferson University Hospital, Philadelphia, PA). Cells expressing human melanoma antigens (14) were prepared from the tumor and cryopreserved after the 16th passage. DB-1 melanoma cell preparation and inoculation in male athymic nude mice and tumor volume measurement were performed as described previously (4).
Human Breast Carcinoma Xenografts in Nude Mice
Female athymic nude mice 4–6 weeks of age, purchased from the National Cancer Institute, Frederick, MD, USA, were housed in microisolator cages where they had access to water and autoclaved mouse chow ad libitum. A basal-like human breast carcinoma line, HCC1806, (referred to as triple-negative breast cancer, TNBC) and BT-474, a line that is positive for ER and over-expresses HER2/neu, were implanted into matched cohorts of these mice. Both lines were obtained from ATCC (American Type Culture Collection) and were maintained in DMEM culture medium supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin at 37°C in 5% CO2. Cells in exponential growth phase were harvested; 106 HCC1806 cells suspended in 100 μL culture medium were inoculated subcutaneously into the right flank of the nude mouse. Since the growth of BT-474 is estrogen-dependent, a 60-day-release 17β-estradiol pellet (Innovative Research America, Sarasota, FL) was implanted subcutaneously in the neck region with a 10 gauge precision trocar one week before inoculation of tumor cells. One week later, 107 BT-474 cells suspended in 200 μL of culture medium were inoculated s.c. (subcutaneously) in the right flank of the nude mouse. HCC1806 is a rapidly growing tumor that becomes palpable (3 mm in diameter) about a week after implantation. In contrast, BT-474 grows more slowly, requiring 2–3 weeks to reach palpability.
Human Prostate Carcinoma Xenografts in Nude Mice
4–6 weeks old male 01b74 athymic nude mice purchased from the National Cancer Institute, Frederick, MD, USA were utilized in this study. LNCaP prostate carcinoma cells were obtained from ATCC and were maintained in RPMI 1640 culture medium supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin at 37°C in 5% CO2. Cells in exponential growth phase were harvested; 5×106 cells in a mixture of 75 μl matrigel and 75 μl of RPMI 1640 medium were inoculated s.c. into the right flank of the nude mouse.
Human Ovarian Carcinoma Xenografts in Nude Mice
4–6 weeks old female athymic nude mice purchased from the National Cancer Institute, Frederick, MD, USA were used in the study. A2780 ovarian carcinoma cells were obtained from Sigma Aldrich and were maintained in RPMI 1640 (Sigma Aldrich, St. Louis, MO, USA) culture medium supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin at 37°C in 5% CO2. Cells in exponential growth phase were harvested; 5×106 cells in 0.1 ml of RPMI 1640 medium were inoculated s.c. into the right flank of nude mice.
Identification of Monocarboxylate-1 (MCT-1) in Breast Carcinoma
Western blot analysis was performed based on our published protocol (15) with modifications. Briefly, cells were solubilized in aqueous buffer containing 2% SDS, 62.5 mM Tris, and 20% glycerol at pH 6.8, centrifuged at 1000g for 2 min and boiled. The protein concentration was measured by BCA assay (Bio-Rad Laboratories, Hercules, CA). Fifty μg of cell lysate protein was loaded per lane on a Nu-PAGE gel containing 4–12% Bis-Tris (Invitrogen, Carlsbad, CA). The gel was run at 180 V for 1 hr at room temperature, and the protein was transferred to a PVDF membrane sandwiched between 0.2 μm filter papers (Invitrogen) at 40 V for 1 hr. The membrane was pre-blocked with 10% normal goat serum in PBS overnight at 4°C. The membrane was then incubated with the primary antibodies, MCT-1 goat anti-human polyclonal antibody (Santa Cruz, CA) and monoclonal mouse antiGADPH (a housekeeping protein) antibody (ABCAM, Cambridge, MA) both in 1:200 dilution overnight at 4°C. In the next step, the membrane was incubated with the secondary antibody conjugated with horseradish peroxidase (HRP, Pierce Biotechnology, Inc., Rockford, IL, USA) at 1:5000 dilution for 1 hr at room temperature. Finally, the membrane was incubated with an HRP substrate (Pierce Biotechnology) and was read on a LI-COR Odyssey chemiluminescent detection system (Lincoln, NE).
Magnetic Resonance (MR) experiment for pH measurement and bioenergetic status estimation
Tumor-bearing mice implanted with breast carcinoma [HCC1806 (n=3), BT-474 (n=3)], prostate carcinoma [LNCaP (n=3)], or ovarian carcinoma [A2780 (n=3)] were maintained under 1% isoflurane in 100% oxygen supplied at 1 L/min. Changes in pHi and pHe in response to LND in s.c. tumors of the indicated human cancer type were studied by positioning the tumor in a home-built dual-frequency (1H/31P) slotted-tube resonator (10 mm in diameter). MR experiments were performed with a 9.4 T/31 cm horizontal bore Varian system. In vivo 31P MR spectra (MRS) were acquired with the same home-built dual-frequency (1H/31P) slotted-tube resonator (10 mm in diameter) as mentioned above (4). Sub-dermal needle electrodes and a rectal thermistor were placed for electrocardiogram and core body temperature monitoring, respectively. The animal’s core body temperature was maintained at 37 ± 1°C by blowing warmed air into the bore of the magnet during a scan with heating controlled by a thermal regulator system. A respiration pillow was placed over the thorax to monitor respiration (Model 1025, SA Instruments Inc., Stony Brook, NY, USA). Following acquisition of the baseline spectrum, LND and 3-APP (for measurement of pHe) (4) were injected through two 26-gauge i.p. catheters inserted into either side of the peritoneum without removing the animal from the magnet. The magnet was shimmed globally until the water line-width of the tumor monitored via the 1H channel reached 60 –70 Hz. A point resolved spectroscopy (PRESS) sequence was used to shim the ISIS (Image Selected In vivo Spectroscopy) tumor voxel of 250–300 mm3 size covering the entire tumor depending on tumor size, to 30–40 Hz line width. Localized 31P MRS was performed on s.c. tumors of each of the xenograft using the ISIS technique with following parameters: Hyperbolic Secant-Adiabatic Fast Passage (HS-AFP) slice-selective inversion pulses with 2.5 ms length, 296 scans with a radiofrequency pulse width of 60 μs, corresponding approximately to a 90° flip angle; sweep width, 12 kHz; 512 data points; TR= 4 s.
pHi and pHe were determined from the Henderson-Hasselbalch equation using the chemical shifts of Pi and 3-APP, respectively, referenced to the α-NTP resonances as described previously (4). The ratio of the peak areas of the βNTP and Pi resonances served as an index of tumor bioenergetic status (16). For each animal, the change of βNTP/Pi relative to its baseline value was determined after the administration of LND. We integrated the peak area of each metabolite and normalized to the largest peak. We have chosen to use a simpler and more efficient approach of maintaining constant conditions during the temporal studies and rationing the changes that are seen. Absolute concentration measurements would have been more elegant, and reporting ratios could lead to errors, e.g., if proportionate changes to numerator and denominator occur that do not modify the ratio and hide dramatic changes in multiple metabolites. These issues were discussed by Shungu et al. (17).
Chemotherapy with Doxorubicin of Human Melanoma (DB-1) and Breast Carcinoma (HCC1806) Xenografts
When tumors of human melanoma xenografts (DB-1) and breast carcinoma (HCC1806) reached ~100 mm3 in volume, four cohorts of age- and weight-matched animals from each tumor type were randomized to the following treatment groups: cohort 1 (sham-treated control) was infused intravenously (i.v.) with PBS and administered sham i.p. injections of tris/glycine buffer; cohort 2 was infused i.v. with PBS 40 min after LND administration i.p. (100 mg/kg;); cohort 3 was injected i.p. with tris/glycine buffer and infused i.v. with doxorubicin (7.5 or 10 mg/kg); cohort 4 was injected i.p. with LND (100 mg/kg) and after 40 min, doxorubicin (7.5 or 10 mg/kg) was infused i.v. Values shown are means ± SEM; n = 10 animals for controls, LND, doxorubicin and LND + doxorubicin treated animals. A similar set of experiments was performed on HCC1806 breast carcinoma xenografts treated with a doxorubicin dose of 12 mg/kg. All other conditions maintained the same as cohorts 1–4 described above except for the number of animals in each cohort, which differed as follows: cohort 1 (n=4), cohort 2 (n=4), cohort 3 (n=5) and cohort 4 (n=8). Cohort 2 started with 4 animals; however, 2 animals died on day 8.
During the treatment and sham-treatment procedures, all animals were anesthetized with ketamine hydrochloride and acepromazine with additional anesthesia being re-administered approximately every 45–60 min to maintain sedation. Animals were placed on a water pad heater (Gaymar T-Pump, Gaymar Industries, Inc., Orchard Park, NJ, USA) to maintain body temperature during anesthesia. Tumor dimensions were measured as well as animal body weight. To prevent blood clotting, tail vein catheters (I.V. Catheters FEP, Tyco Healthcare, Tyco International Ltd., Schaffhausen, Switzerland) filled with heparin (100 USP Units/mL) were placed using a restrainer (MTI Braintree Scientific, Braintree Scientific Inc., Braintree, MA, USA). After treatment, catheters were removed and animals were allowed to recover in cages. For the first five days post-treatment, tumor volume and weight were measured daily with calipers (Scienceware, Bel-Art Products, Wayne, NJ, USA) and scale (Acculab PP-401, H & C Weighing Systems, Columbia, MD, USA), respectively. Afterwards, these measurements were repeated every other day.
Statistics
The pHi, pHe and bioenergetics data at time points following LND administration were compared by the Mann-Whitney test (SPSS 16; IBM Corporation; Armonk, NY, USA). To estimate treatment effects on tumor growth, we conducted a growth delay analysis (18). For each animal, we recorded the time from treatment until the tumor reached a volume of four times the mean volume at the initiation of treatment. We then averaged these times in the (T) treated and (C) control arms. For each animal, we also computed the slope of the log tumor volume curve up to 0 days (the time of treatment) in the treated animals and 6 days (when the curves started to become nonlinear) in the controls (7 days in the HCC1806 controls) using least squares regression. We computed an average slope (B) by taking the mean of the estimated within-animal slopes across both arms of the study. We then estimated the proportion of the tumor surviving treatment as exp (−(T−C)×B). We computed confidence intervals by the bootstrap percentile method, which involves repeating the entire estimation process on repeated re-samples (with replacement) of the animals, in order to estimate the standard errors of the estimates (19). We conducted all analyses in R (Version 3.0.2 for Linux; R Foundation for Statistical Computing).
Results
In vivo 31Phosphorus spectroscopy: pH and bioenergetics
Figure 1 shows 31P MR spectra of DB-1 melanoma, HCC1806 breast and A2780 ovarian carcinoma xenografts in nude mice recorded before and 3 hr after injection of LND (100 mg/kg, i.p.). A prostate carcinoma model, LNCaP, is shown before and 2 hr after LND injection. Each tumor exhibited a significant decrease in βNTP following LND treatment. The average decreases in NTP were 33%, 59%, 68%, 44% and 36% for DB-1, HCC1806, BT-474, A2780 and LNCaP, respectively, relative to their corresponding baseline levels. The corresponding increases in Pi were 211%, 59%, 120%, 175% and 187%, respectively. The representative spectra shown in Figure 1 exhibit approximately these relative changes in NTP and Pi levels. The spectra indicate different levels of NTP without any sign of ADP or AMP. Table 1 summarizes the relative decreases in the bioenergetic status (βNTP/Pi) of the various tumor models (2 hr after LND injection for LNCaP and 3 hr later for the other three tumor types). The decreases in bioenergetic status range between 67 and 78% in the order DB-1 < BT-474 = A2780 < HCC1806< LNCaP. Thus, the extent of bioenergetic decrease is large but varies among the different tumor types.
Figure 1.
In vivo Phosphorus-31 NMR spectra of DB-1 human melanoma xenograft (A), HCC1806 human breast carcinoma xenograft (B), A2780 human ovarian carcinoma xenograft (C) before and 3 hr after administration of 100 mg/kg LND i.p. Spectra of a LNCaP human prostate carcinoma xenograft (D) are shown before and 2 hr after administration of 100 mg/kg LND i.p.
Table 1.
Summary of ΔpHi, ΔpHe and ΔβNTP/Pi of human tumor xenografts measured by 31P MRS following LND treatment.
| Parameter | DB-1 melanoma Xenografts (n = 15) | BT-474 breast cancer xenografts (n = 3) | HCC1806 breast cancer xenografts (n = 3) | A2780 ovarian cancer xenografts (n = 3) | LNCaP prostate cancer xenografts (n = 3) |
|---|---|---|---|---|---|
| ΔpHi | 0.60 ± 0.1 P < 0.05 |
0.44 ± 0.14 P = 0.05 |
0.54 ± 0.23 P < 0.05 |
0.56 ± 0.10 P < 0.05 |
0.47 ± 0.11 P < 0.05 |
| ΔpHe | 0.20 ± 0.07 P < 0.05 |
0.22 ± 0.07 P = 0.05 |
0.28 ± 0.19 P = 0.05 |
0.34 ± 0.23 P = 0.05 |
0.30 ± 0.13 P > 0.05 |
| ΔβNTP/Pi | 66.8 ± 5.7% P < 0.05 |
70.0 ± 0.12% P < 0.05 |
77.0 ± 0.09% P < 0.05 |
70.0 ± 0.22% P < 0.05 |
77.5 ± 0.04% P < 0.05 |
NOTE: The pH values were determined by 31P MR spectroscopy as described in “Materials and Methods” pHi, pHe and βNTP/Pi represent intracellular pH, extracellular pH and bioenergetics, respectively. Time interval was 2 hr for LNCaP prostate cancer xenografts and 3 hr for other tumor types. Data are presented as mean ± S.E.M.
The time course of change of Pi and βNTP is shown in Figure 2 for the DB-1 melanoma model. Pi increased with a small discontinuity at 80 min post-LND injection. βNTP monotonically declined in a biphasic manner, decreasing rapidly in the first 20 min and then slowly diminishing afterwards.
Figure 2.
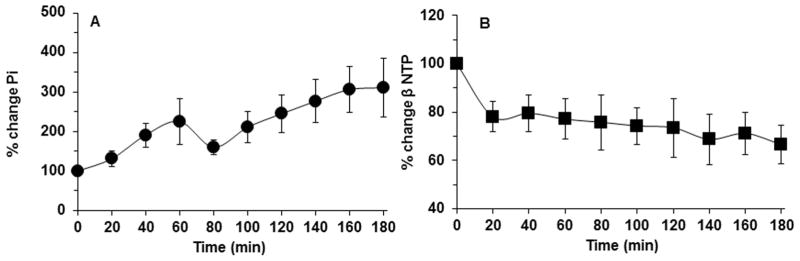
Inorganic Phosphate (Pi) (area within the peak) (A) and βNTP (area within the peak) (B) relative to baseline as a function of time of DB-1 human melanoma xenografts (n=15) in response to LND (100 mg/kg; i.p.) administration at time zero. The values are presented as mean ± S.E.M. When not displayed, S.E.M. values were smaller than the symbol size.
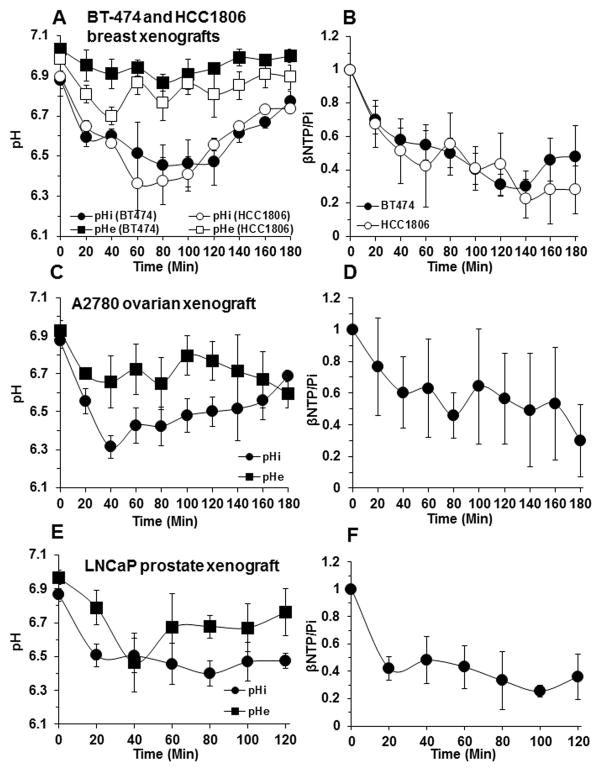
We have previously reported that DB-1 melanoma exhibits a rapid decrease in intracellular pH (pHi) to 6.3, which is sustained for 2 hr and then gradually increases to pre-treatment levels, whereas the extracellular pH (pHe) of this tumor was only slightly decreased from 7.0 to 6.8 (4). The time course of changes in pHi and pHe as well as the βNTP/Pi ratios of the breast, prostate and ovarian tumors is depicted in Figure 3. Table 1 summarizes the net changes in pHi and pHe exhibited by these tumor models over the 2 or 3 hour period following treatment with LND. The pHi of all the tumors decreased by 0.4 – 0.6 pH units within 20 – 40 min of LND administration and then slowly returned to pretreatment levels at rates that differed for the various tumors. For the two breast tumors, the decline of pHi lasted for about 2 hr; for the ovarian tumor, acidification initially decreased rapidly and was then sustained for nearly two hours, whereas for the prostate tumor there was a rapid decline in pHi followed by steady acidification lasting for at least 2 hr. pHe decreased by about 0.2 units for all the tumor types; hence, intracellular acidification far exceeded extracellular acidification.
Figure 3.
In vivo intracellular pH (pHi), extracellular pH (pHe) (A), bioenergetics (βNTP/Pi) (B) of BT-474 and HCC1806 human breast cancer xenografts (n =3) as a function of time after LND (100 mg/kg; i.p.) administration at t=0. Time courses of pHi, pHe (C) and bioenergetics (D) of A2780 human ovarian cancer xenografts (n=3) following treatment with LND (100 mg/kg; i.p.). Corresponding time profiles for pHi, pHe (E) and bioenergetics (F) of LNCaP human prostate cancer xenografts (n=3) post-LND (100 mg/kg; i.p.) administration. The values are presented as mean ± S.E.M.
Our previous studies (4) also showed that the effects of LND on pHi, pHe and the bioenergetic status (βNTP/Pi) of the DB-1 melanoma were tumor-specific with no effect on skeletal muscle and brain and only a slight transient decrease in pHi of the liver at 20 min and a decrease in liver βNTP/Pi 40 min following injection of LND. Since specificity of these spectral parameters for tumor tissue has also been reported by Ben-Yoseph et al. (2) for 9L glioma xenografts in rats, tumor specificity was not confirmed in this study. We don’t feel that specificity will be altered whether the animals are bearing tumors or not, although this might prove to be an area of future study.
Growth Delay: DB-1 melanoma and HCC1806 human breast cancer xenografts
Figure 4 displays growth delay studies of DB-1 melanoma and HCC1806 breast carcinoma treated with 100 mg/kg, i.p. LND and various doses of doxorubicin administered i.v. The melanomas were treated with 7.5 mg/kg doxorubicin and 10.0 mg/kg doxorubicin (Fig. 4A), whereas the breast cancer was treated with 12.0 mg/kg doxorubicin (Fig. 4B). Data yielded tumor growth delays in a representative experiment of 5 d, 5 d and 18 d for LND alone, doxorubicin alone and LND + doxorubicin, respectively, in DB-1 melanoma treated with 7.5 mg/kg and 2 d, 5 d and 29 d, respectively, when treated with 10 mg/kg. In HCC1806 growth delays were 2 d, 7 d and 13 d for LND alone, doxorubicin alone and LND + doxorubicin, respectively. The doubling times were 5.0 d and 3.2 d for DB-1 melanoma and HCC1806 breast carcinoma, respectively. Escalating doses of doxorubicin were used to determine limiting toxicity as assessed by animal weight loss. Significant limiting toxicity was not observed over the drug concentrations used. The logarithm of the percent tumor volume change from the day of treatment is plotted against time after administration of LND and doxorubicin. Separate growth curves are displayed for saline-treated controls, animals treated with LND alone, doxorubicin alone and LND + doxorubicin.
Figure 4.

(A). Growth delay experiments performed on DB-1 human melanoma xenografts in nude mice. Animals were treated on Day 0 as follows: Control (sham i.p. tris/glycine buffer + sham i.v. PBS), LND (100 mg/kg i.p.), doxorubicin (7.5 and 10 mg/kg i.v.), LND + doxorubicin. Values shown are means ± SEM; n = 10 animals for Control, LND, doxorubicin and LND + doxorubicin groups. (B). Growth delay experiments performed on HCC1806 breast tumor xenografts treated with saline (control), LND (100 mg/kg, i.p.), doxorubicin (12 mg/kg, i.v.) and doxorubicin plus LND. Values shown are means ± SEM for n = 4 animals, Control and LND groups; n = 5 animals, doxorubicin and n=8 animals, LND + doxorubicin groups.
Table 2 summarizes tumor growth and response parameters. The surviving fraction for tumors treated with LND alone ranged between 49 and 72%. For doxorubicin alone the surviving fraction ranged between 47 and 23%, varying with the dose of doxorubicin with the largest extent of cell kill corresponding to the largest doxorubicin dose, 12 mg/kg in the HC1806 tumor. The effects of LND + doxorubicin, indicate a 95% cell kill with one dose of 7.5 mg/kg of doxorubicin in melanoma increasing to 98% cell kill at a dose of 10.0 mg/kg, whereas with the breast tumor treated at a still higher dose (12.0 mg/kg) the estimated cell kill was 95% (i.e., same as for the 10.0 mg/kg dose in melanoma with the melanoma being more responsive to the combination). Overall, the combined response to high doses of doxorubicin administered together with the standard dose of LND is quite impressive; nearly two logs of cell kill with a single dose.
Table 2.
Estimated surviving fraction as a percent (100× exp (−(T−C)×B), with bootstrap 95% CI), log cell kill method, by experiment and treatment arm.
| Experiment | |||
|---|---|---|---|
|
| |||
| Treatment | DB-1 Melanoma xenografts treated with Doxorubicin (7.5 mg/kg) | DB-1 Melanoma xenografts treated with Doxorubicin (10 mg/kg) | HCC1806 Breast cancer xenografts treated with Doxorubicin (12 mg/kg) |
| Lonidamine | 49 (27, 82) | 72 (45, 121) | 60 (20, 204) |
| Doxorubicin | 47 (23, 83) | 47 (28, 81) | 23 (11, 52) |
| Lonidamine + Doxorubicin | 5 (2, 14) | 2 (1, 10) | 5 (3, 10) |
NOTE: Growth delay experiments performed on DB-1 human melanoma xenografts and HCC1806 breast carcinoma xenografts in nude mice following lonidamine and doxorubicin treatment. Details have been described in figure 4.
Weight loss toxicity
Weight loss served as a surrogate index of overall toxicity. This parameter varied with drug type and dose. For DB-1 melanoma, LND (100 mg/kg, i.p.) had less effect than sham treatment with the saline control, body weight decreasing by 5% immediately after injection and returning to baseline within 5 days. Saline injection produced an 8% decrease in body weight by day 2 and returned to baseline by day 17. Doxorubicin (7.5 mg/kg) decreased body weight by 12% within 4 days of injection and then produced no further change in body weight for at least 32 days. LND plus doxorubicin (7.5 mg/kg) essentially mimicked doxorubicin in its effect on body weight. At the higher dose of doxorubicin (10.0 mg/kg), LND and sham treatment produced negligible effects on body weight and were indistinguishable from each other, but doxorubicin or LND + doxorubicin produced indistinguishable but substantial effects, decreasing body weight by 12–14% within 4 days of treatment and then maintaining stable body weight for about 40 days (data not shown here).
Since there is strong evidence that LND is an inhibitor of the monocarboxylic acid transporter (MCT) (1), it is noteworthy that Wahl et al. (20) have previously demonstrated that DB-1 melanoma expresses two isoforms of this transporter, MCT-1 and MCT-4. Figure 5 demonstrates that HCC1806 and BT-474 tumors both express MCT-1.
Figure 5.
Western blots for monocarboxylate transport inhibitor (MCT-1) expression by BT-474 and HCC1806 human breast carcinoma cell lines.
Discussion
Lonidamine was introduced in 1980 as an antispermatogenic agent (21) and was later found to have chemotherapeutic activity (22) and to sensitize tumors to hyperthermia (8–12) and radiation (13). A number of clinical trials have evaluated its toxicity and efficacy against a variety of malignancies (23–25) and benign prostate hyperplasia (26). LND enhances the activity of various chemotherapeutic agents against various malignancies, but it has not been routinely used in front-line cancer therapy except in Italy. It has not been approved by the FDA, but has been used in a number of clinical trials in the US with FDA sanction (INDs) (26,27). In the clinic, the drug has generally been delivered orally and exhibits limited toxicity to normal tissues.
After evaluating the pharmacokinetics and toxicity of oral and intravenous LND in dogs, Price et al., found that the bioavailability of oral LND may be limited. These authors suggested, therefore, poor bioavailability may limited the utility of oral administration of LND in the clinical and experimental studies of its efficacy and toxicity (28). Moreover, plasma concentrations in dogs receiving 100—800 mg/m2 LND orally were below those capable of sensitizing hyperthermia and chemotherapy in vitro (~2–9 μg/ml). By contrast, plasma concentrations in dogs receiving 400 mg/m2 of LND intravenously were within the range capable of sensitizing hyperthermia and chemotherapy in vitro (~78 μg/ml). Therefore, the authors concluded, it may be preferable to administer LND intravenously when attempting to sensitize hyperthermia and chemotherapy. Furthermore, intravenous administration of LND may circumvent the variable and suboptimal concentrations currently observed in humans (29–31). However, for studies of animal models, i.p. administration is more convenient and also appears to produce uniform response.
About 16% of the patients in a clinical trial of breast carcinoma exhibited myalgia (32). There have been some reports of testicular pain (33), which is not surprising in view of its known antispermatogenic effect. A clinical trial of chronic treatment with LND of patients with benign prostate hyperplasia was terminated because of evidence of liver toxicity as indicated by decreased transaminase activity. However, only six patients out of more than 800 exhibited this effect, and there were no deaths. Preclinical data in mice administered 100 mg/kg i.p. doses of LND demonstrate that this drug selectively affects tumor pHi and bioenergetics with no effect on skeletal muscle (at least not in sedated animals) or brain and only transient small effects on the liver (4). Its selective effect on tumors and limited toxicity to normal organs (when administered orally to humans) as well as its ability to potentiate certain chemotherapeutic agents, hyperthermia and radiation therapy continue to make LND an attractive target for personalized cancer therapy.
It is most likely that inhibiting MCT-1 is a critical step of acidification due to the fact that the Michaelis-Menton constant (Km) for this transporter is an order of magnitude lower than that of other MCT’s (34). Our recent studies indicate that the inhibitory constant (Ki) for inhibition of the MCT’s by LND are approximately the same (unpublished data), meaning that relevant MCT activity should be inhibited at the same concentration of LND and that this blockage of lactate efflux should facilitate acidification. We verify the relevance of this hypothesis by demonstrating the expression of MCT-1 in both of the breast cancer cell lines tested, where MCT-1 transports lactate at concentrations with a Km of approximately 4.5 mM (34).
As noted in the introduction, we have recently demonstrated that selective acidification and de-energization of the DB-1 melanoma with LND (100 mg/kg, i.p.) potentiate the activity of melphalan (7.5 mg/kg) in mouse xenografts with this tumor, producing about an 89% cell kill with one dose of LND + melphalan (7.5 mg/kg, i.v.). This effect was attributed to three effects of tumor acidification: 1) acid-induced stabilization of the aziridinium ion intermediate, 2) acid inhibition of glutathione S-transferase that mediates glutathione deactivation of the aziridinium ion, and 3) acid inhibition of O6-alkyltransferase, the key enzyme involved in repair of DNA damage produced by melphalan (6,7).
We have now shown that the same level of antineoplastic activity as melphalan can be achieved with the same dose of LND combined with doxorubicin, but that an even greater response, about 98% cell kill, can be achieved with the LND + doxorubicin combination using a higher but still tolerable dose of the anthracycline. One could consider administering multiple doses of this combination, but the number of doses would be limited by the potential cardiotoxicity of doxorubicin. The latter effect could be mitigated by using a liposomal formulation of doxorubicin in the form of Doxil. An even more effective approach would be to use ThermoDox®, which delivers doxorubicin in thermally sensitive liposomes that disintegrate at 40.5 – 42°C and which can be administered with mild hyperthermia to the immediate vicinity of the tumor (8–11). Both the thermal and the chemotherapeutic effects of this combination should be potentiated by LND. Delivery of doxorubicin to tumors selectively heated to 43°C by High Intensity Focused Ultrasound (HIFU) under MRI guidance and non-invasive thermometry has recently been implemented by Staruch et al. in rabbits with implanted VX2 tumors (35). Furthermore, a clinical trial with ThermoDox® has demonstrated the absence of any cardiotoxic effect of doxorubicin when delivered by this liposomal agent (36). Given the known ability of LND to sensitize tumors to radiation therapy (13,37,38), one could readily envisage a combined thermochemotherapy procedure followed by fractionated radiation therapy to treat melanoma. Since hyperthermia typically produces 3–4 logs of cell kill (39,40), and we have demonstrated about 2 logs of cell kill with doxorubicin, only about 4 more logs of cell kill would be required from radiation therapy (or from another therapeutic agent such as immunotherapy) to achieve total local control or cure.
A critical obstacle to implementing this strategy in human melanoma patients is that neither melphalan nor doxorubicin is utilized in the systemic therapy of melanoma. Melphalan is, however, utilized in treatment of melanoma in transit (i.e., isolated to one of the limbs) by hyperthermic isolated limb perfusion (HILP) (41). This procedure includes HCl in the perfusion fluid to activate melphalan in the perfused limb by the mechanisms noted above. However, HCl is toxic to normal muscle. Cantor et al. (42) have demonstrated that acidification of the perfusate could be achieved by infusing high-dose glucose, which is converted to lactate in the tumor, and adding meta-iodo-benzylguanidine (MIBG) to inhibit oxidative metabolism of pyruvate, which would diminish lactic acid accumulation in the tumor (5,43). A growth delay of 51 days was achieved in a rat model of HILP treated with this procedure, but it required therapeutic concentrations of MIBG, which are not sanctioned by the FDA (42). A simpler approach would be to use LND together with melphalan in the HILP procedure.
A still simpler strategy is to implement these methods in other cancers in which systemic chemotherapy with drugs such as doxorubicin and nitrogen mustards like melphalan or cyclophosphamide could be used. For this reason, we have initiated exploratory studies with two models of human breast carcinoma, one model of human prostate carcinoma and one of human ovarian carcinoma. Each of these models exhibited LND-induced acidification and depletion of NTP that are essential to implementation of this strategy, and in one of these models (HCC1806) 95% cells kill was achieved with a single dose of LND plus doxorubicin. There should be less resistance to use of this approach in breast, ovarian and prostate (44) carcinoma. Li et al. have recently developed targeted liposomes for simultaneous delivery of lonidamine and epirubicin (another anthracycline) to tumors (45).
In the case of early-stage breast carcinoma, there is another problem with implementing such a procedure — the availability of an alternative successful procedure, breast conserving surgery. While this procedure has produced a large proportion of apparent cures and has become popular because of its limited morbidity and rapid recovery time, it is far from perfect. A more recent study indicates a significantly lower 8-yr local failure rate of 3-4% and improved 8-yr rates of overall survival (86-87%) as well as cause-specific survival (94-95%) (46). Thus, the efficacy of breast-conserving surgery is improving because of the improved radiation therapy (RT) procedure, but since our method would utilize the same RT procedures, it too should improve. In addition, surgery is painful and sometimes deformative, requiring subsequent cosmetic surgery. Availability of a non-surgical but equally effective procedure could offer a reasonable alternative to women who now undergo breast-conserving surgery. Therefore, development of such a method based on LND or drugs like it that selectively sensitize tumors to chemotherapy, hyperthermia and radiation therapy remains a worthwhile objective.
Gerweck’s laboratory first postulated that the pH gradient across the plasma membrane of tumors plays a critical role in the intracellular concentration and the activity of various chemotherapeutic agents (47). They noted that since most tumors exhibited a slightly more acidic pHe than pHi (i.e., pHi>pHe) and since most (but not all) cancer drugs entered the tumor cell by free diffusion, this would favor the uptake of weak acids compared to weak bases. Weak acids would be converted to the protonated carboxyl form in the extracellular milieu, and would more readily diffuse across the plasma membrane. But once they entered the cell, they would ionize and be retained in the cell, whereas weak bases would be converted to the charged protonated ammonium form outside cell, which would be incapable of diffusing into the cell. They demonstrated this effect by extracellular acidification of tumor cells with exogenous glucose. This led to increased activity of the weak acid chlorambucil and decreased uptake of the weak base doxorubicin.
However, LND reverses the normal pH gradient across the tumor plasma membrane, decreasing pHi much more than pHe. This should have the reverse effect of glucose and should increase uptake of doxorubicin, which has a pKa of ~8.5. The free basic form of doxorubicin will diffuse into the cell and will experience cation trapping as a result of protonation driven by the increased intracellular acidification. However, cation trapping could lead to accumulation of this drug in lysosomes that tend to have an even more acidic pH (~5) than the predominantly cytosolic pHi of the tumor (which is buffered at about 6.4 by carbonic acid). However, since the activity of doxorubicin depends on accumulation of the drug in the nucleus, it was not immediately obvious that LND would greatly potentiate the activity of doxorubicin. Our data clearly demonstrate that it does.
Acknowledgments
NMR experiments were performed at the University of Pennsylvania Small Animal Imaging Facility with advice and guidance of Dr. Stephen Pickup and his assistant, Weixia Liu. Assistance in tumor model development and Western blot was provided by Drs. Ting Liu and Luca Dag, respectively, in Dr. Rong Zhou’s laboratory. Andrew M. Ho, Christina Gustafson and Cory Alvey are acknowledged for their help to perform this study. Support for this project was provided by NIH grants R01-CA129544 and R01-CA172820.
Abbreviations
- LND
Lonidamine
- MRS
Magnetic Resonance Spectroscopy
- MCT
Monocarboxylate Transporter
- pHi
Intracellular pH
- pHe
Extracellular pH
- 3-APP
3-Aminopropylphosphonate
- PME
Phosphomonoester
- Pi
Inorganic Phosphate
- MIBG
Meta-iodobenzylguanidine
- i.p
intraperitoneal
- CHC
α-cyano-4-hydroxycinnamic acid
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Ben-Horin H, Tassini M, Vivi A, Navon G, Kaplan O. Mechanism of action of the antineoplastic drug lonidamine: 31P and 13C nuclear magnetic resonance studies. Cancer Res. 1995 Jul 1;55(13):2814–2821. [PubMed] [Google Scholar]
- 2.Ben-Yoseph O, Lyons JC, Song CW, Ross BD. Mechanism of action of lonidamine in the 9L brain tumor model involves inhibition of lactate efflux and intracellular acidification. J Neurooncol. 1998 Jan;36(2):149–157. doi: 10.1023/a:1005819604858. [DOI] [PubMed] [Google Scholar]
- 3.Mardor Y, Kaplan O, Sterin M, Ruiz-Cabello J, Ash E, Roth Y, Ringel I, Cohen JS. Noninvasive real-time monitoring of intracellular cancer cell metabolism and response to lonidamine treatment using diffusion weighted proton magnetic resonance spectroscopy. Cancer Res. 2000 Sep 15;60(18):5179–5186. [PubMed] [Google Scholar]
- 4.Nath K, Nelson DS, Ho AM, Lee SC, Darpolor MM, Pickup S, Zhou R, Heitjan DF, Leeper DB, Glickson JD. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013 Jan;26(1):98–105. doi: 10.1002/nbm.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou R, Bansal N, Leeper DB, Pickup S, Glickson JD. Enhancement of hyperglycemia-induced acidification of human melanoma xenografts with inhibitors of respiration and ion transport. Acad Radiol. 2001 Jul;8(7):571–582. doi: 10.1016/S1076-6332(03)80681-5. [DOI] [PubMed] [Google Scholar]
- 6.Kuin A, Aalders M, Lamfers M, van Zuidam DJ, Essers M, Beijnen JH, Smets LA. Potentiation of anti-cancer drug activity at low intratumoral pH induced by the mitochondrial inhibitor m-iodobenzylguanidine (MIBG) and its analogue benzylguanidine (BG) Br J Cancer. 1999 Feb;79(5–6):793–801. doi: 10.1038/sj.bjc.6690127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong P, Lee C, Tannock IF. Reduction of intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin Cancer Res. 2005 May 1;11(9):3553–3557. doi: 10.1158/1078-0432.CCR-04-2472. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim SH, Alfieri A, Young CW, Silvestrini B. Lonidamine: a hyperthermic sensitizer of HeLa cells in culture and of the Meth-A tumor in vivo. Oncology. 1984;41:30–35. doi: 10.1159/000225882. [DOI] [PubMed] [Google Scholar]
- 9.Ning SC, Hahn GM. Combination therapy: lonidamine, hyperthermia, and chemotherapy against the RIF-1 tumor in vivo. Cancer Research. 1991 Nov;51(21):5910–5914. [PubMed] [Google Scholar]
- 10.Silvestrini B, Hahn GM, Cioli V, Demartino C. Effects of lonidamine alone or combined with hyperthermia in some experimental cell and tumor systems. British Journal of Cancer. 1983;47(2):221–231. doi: 10.1038/bjc.1983.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teicher BA, Holden SA, Ara G, Menon K. Whole-body hyperthermia and lonidamine as adjuvant therapy to treatment with cisplatin with or without local radiation in mouse bearing the lewis lung-carcinoma. International Journal of Hyperthermia. 1995 Sep-Oct;11(5):637–645. doi: 10.3109/02656739509022496. [DOI] [PubMed] [Google Scholar]
- 12.Coss RA, Storck CW, Wells TC, Kulp KA, Wahl M, Leeper DB. Thermal sensitisation by lonidamine of human melanoma cells grown at low extracellular pH. Int J Hyperthermia. 2014 Feb;30(1):75–78. doi: 10.3109/02656736.2013.858832. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Alfieri AA, Kim SH, Young CW. Potentiation of radiation effects on 2 murine tumors by lonidamine. Cancer Research. 1986 Mar;46(3):1120–1123. [PubMed] [Google Scholar]
- 14.Hill LLKR, Jaworsky C, Murphy G, McCue P, Berd D. Growth and metastasis of fresh human melanoma tissue in mice with severe combined immunodeficiency. Cancer Res. 1991 Sep 15;51(18):4937–4941. [PubMed] [Google Scholar]
- 15.Li ZJ, Qiao H, Lebherz C, Choi SR, Zhou XY, Gao GP, Kung HF, Rader DJ, Wilson JM, Glickson JD, Zhou R. Creatine kinase, a magnetic resonance-detectable marker gene for quantification of liver-directed gene transfer. Human Gene Therapy. 2005 Dec;16(12):1429–1438. doi: 10.1089/hum.2005.16.1429. [DOI] [PubMed] [Google Scholar]
- 16.Rofstad EK, DeMuth P, Fenton BM, Sutherland RM. 31P nuclear magnetic resonance spectroscopy studies of tumor energy metabolism and its relationship to intracapillary oxyhemoglobin saturation status and tumor hypoxia. Cancer Res. 1988 Oct 1;48(19):5440–5446. [PubMed] [Google Scholar]
- 17.Shungu DC, Bhujwalla ZM, Li SJ, Rose LM, Wehrle JP, Glickson JD. Determination of absolute phosphate metabolite concentrations in RIF-1 tumors in vivo by 31P-1H-2H NMR spectroscopy using water as an internal intensity reference. Magn Reson Med. 1992 Nov;28(1):105–121. doi: 10.1002/mrm.1910280111. [DOI] [PubMed] [Google Scholar]
- 18.Corbett TH, Valeriote FA. Rodent models in experimental chemotherapy. In: Kallman RF, editor. The use of rodent tumors in experimental cancer therapy: conclusions and recommendations. Pergamon; New York: 1987. pp. 233–247. [Google Scholar]
- 19.Efron BTR. Bootstrap Method for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statistical Science. 1986;1(1):54–77. [Google Scholar]
- 20.Wahl ML, Owen JA, Burd R, Herlands RA, Nogami SS, Rodeck U, Berd D, Leeper DB, Owen CS. Regulation of intracellular pH in human melanoma: Potential therapeutic implications. Molecular Cancer Therapeutics. 2002 Jun;1(8):617–628. [PubMed] [Google Scholar]
- 21.Cioli V, Bellocci B, Putzolu S, Malorni W, Demartino C. Anti-spermogenic activity of lonidamine (AF-1890) in rabbit. Ultramicroscopy. 1980;5(3):418–418. [Google Scholar]
- 22.Pacilio G, Carteni G, Biglietto M, Decesare M. Lonidamine alone and in combination with other chemotherapeutic-agents in the treatment of cancer-patients. Oncology. 1984;41:108–112. doi: 10.1159/000225897. [DOI] [PubMed] [Google Scholar]
- 23.Amadori D, Frassineti GL, De Matteis A, Mustacchi G, Santoro A, Cariello S, Ferrari M, Nascimben O, Nanni O, Lombardi A, Scarpi E, Zoli W. Modulating effect of lonidamine on response to doxorubicin in metastatic breast cancer patients: Results from a multicenter prospective randomized trial. Breast Cancer Research and Treatment. 1998 Jun;49(3):209–217. doi: 10.1023/a:1006063412726. [DOI] [PubMed] [Google Scholar]
- 24.Buccheri G, Ferrigno D. A randomised trial of MACC chemotherapy with or without lonidamine in advanced non-small cell lung cancer. Cuneo Lung Cancer Study Group (CuLCaSG) European Journal of Cancer. 1994;30A(10):1424–1431. doi: 10.1016/0959-8049(94)00286-e. [DOI] [PubMed] [Google Scholar]
- 25.Robins HI, Longo WL, Steeves RA, Cohen JD, Schmitt CL, Neville AJ, Okeefe S, Lagoni R, Riggs C. Adjunctive therapy (whole-body hyperthermia versus lonidamine) to total-body irradiation for the treatment of favorable B-cell neoplasms - A report of 2 pilot clinical-trials and laboratory investigations. International Journal of Radiation Oncology Biology Physics. 1990 Apr;18(4):909–920. doi: 10.1016/0360-3016(90)90416-h. [DOI] [PubMed] [Google Scholar]
- 26.Roehrborn CG. The development of lonidamine for benign prostatic hyperplasia and other. Rev Urol. 2005;7( Suppl 7):S12–20. [PMC free article] [PubMed] [Google Scholar]
- 27.Robins HI, Longo WL, Lagoni RK, Neville AJ, Hugander A, Schmitt CL, Riggs C. Phase-I trial of lonidamine with whole-body hyperthermia in advanced cancer. Cancer Research. 1988 Nov;48(22):6587–6592. [PubMed] [Google Scholar]
- 28.Price GS, Page RL, Riviere JE, Cline JM, Thrall DE. Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemotherapy and Pharmacology. 1996 Jun;38(2):129–135. doi: 10.1007/s002800050460. [DOI] [PubMed] [Google Scholar]
- 29.DeAngelis LM, Currie VE, Kim JH, Krol G, O’Hehir MA, Farag FM, Young CW, Posner JB. The combined use of radiation therapy and lonidamine in the treatment of brain metastases. J Neurooncol. 1989 Sep;7(3):241–247. doi: 10.1007/BF00172917. [DOI] [PubMed] [Google Scholar]
- 30.Weinerman BH, Eisenhauer EA, Besner JG, Coppin CM, Stewart D, Band PR. Phase II study of lonidamine in patients with metastatic renal cell carcinoma: a National Cancer Institute of Canada Clinical Trials Group Study. Cancer Treatment Reports. 1986 Jun;70(6):751–754. [PubMed] [Google Scholar]
- 31.Young CW, Currie VE, Kim JH, Ohehir MA, Farag FM, Kinahan JE. Phase-I and clinical pharmacologic evaluation of lonidamine in patients with advanced cancer. Oncology. 1984;41:60–65. doi: 10.1159/000225888. [DOI] [PubMed] [Google Scholar]
- 32.Mansi JL, Degraeff A, Newell DR, Glaholm J, Button D, Leach MO, Payne G, Smith IE. A Phase-II clinical and pharmacokinetic study of lonidamine in patients with advanced breast-cancer. British Journal of Cancer. 1991 Sep;64(3):593–597. doi: 10.1038/bjc.1991.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Cosimo S, Ferretti G, Papaldo P, Carlini P, Fabi A, Cognetti F. Lonidamine: Efficacy and safety in clinical trials for the treatment of solid tumors. Drugs of Today. 2003 Mar;39(3):157–173. doi: 10.1358/dot.2003.39.3.799451. [DOI] [PubMed] [Google Scholar]
- 34.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012 Jan;64(1):1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 35.Staruch RM, Ganguly M, Tannock IF, Hynynen K, Chopra R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. International Journal of Hyperthermia. 2012;28(8):776–787. doi: 10.3109/02656736.2012.736670. [DOI] [PubMed] [Google Scholar]
- 36.Hong CW, Libutti SK, Wood BJ. Liposomal doxorubicin plus radiofrequency ablation for complete necrosis of a hepatocellular carcinoma. Current Oncology. 2013 Jun;20(3):E274–E277. doi: 10.3747/co.20.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Alfieri A, Kim SH, Young CW, Silvestrini B. Radiosensitization of Meth-A fibrosarcoma in mice by Lonidamine. Oncology. 1984;41:36–38. doi: 10.1159/000225883. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Kim SH, He SQ, Alfieri AA, Young CW. Potentiation of radiation effects on multicellular tumor spheroids (MTS) of HeLa cells by lonidamine. International Journal of Radiation Oncology Biology Physics. 1989 May;16(5):1277–1280. doi: 10.1016/0360-3016(89)90298-8. [DOI] [PubMed] [Google Scholar]
- 39.Bezabeh T, Evelhoch JL, Thompson P, Sloop DJ, Ackerman JJH. Therapeutic efficacy as predicted by quantitative assessment of murine RIF-1 tumour pH and phosphorous metabolite response during hyperthermia: an in vivo 31P NMR study. International Journal of Hyperthermia. 2004 Jun;20(4):335–357. doi: 10.1080/0265673042000196469. [DOI] [PubMed] [Google Scholar]
- 40.Spees WM, Evelhoch JL, Thompson PA, Sloop DJ, Ackerman JJ. Defining the pHi-hyperthermia sensitivity relationship for the RIF-1 tumor in vivo: A 31P MR spectroscopy study. Radiation Research. 2005 Jul;164(1):86–99. doi: 10.1667/rr3390. [DOI] [PubMed] [Google Scholar]
- 41.Fraker DL, Alexander HR, Andrich M, Rosenberg SA. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: Results of a tumor necrosis factor dose-escalation study. Journal of Clinical Oncology. 1996 Feb;14(2):479–489. doi: 10.1200/JCO.1996.14.2.479. [DOI] [PubMed] [Google Scholar]
- 42.Canter RJ, Zhou R, Kesmodel SB, Zhang YW, Heitjan DF, Glickson JD, Leeper DB, Fraker DL. Metaiodobenzylguanidine and hyperglycemia augment tumor response to isolated limb perfusion in a rodent model of human melanoma. Annals of Surgical Oncology. 2004 Mar;11(3):265–273. doi: 10.1245/aso.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Zhou R, Bansal N, Leeper DB, Glickson JD. Intracellular acidification of human melanoma xenografts by the respiratory inhibitor m-iodobenzylguanidine plus hyperglycemia: a 31P magnetic resonance spectroscopy study. Cancer Res. 2000 Jul 1;60(13):3532–3536. [PubMed] [Google Scholar]
- 44.Montanari M, Fabbri F, Rondini E, Frassineti GL, Mattioli R, Carloni S, Scarpi E, Zoli W, Amadori D, Cruciani G. Phase II trial of non-pegylated liposomal doxorubicin and low-dose prednisone in second-line chemotherapy for hormone-refractory prostate cancer. Tumori. 2012 Nov-Dec;98(6):696–701. doi: 10.1177/030089161209800604. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Zhang CX, Wang XX, Zhang L, Ma X, Zhou J, Ju RJ, Li XY, Zhao WY, Lu WL. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials. 2013 Apr;34(13):3366–3380. doi: 10.1016/j.biomaterials.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 46.Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008 Jan 20;26(3):386–391. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 47.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Molecular Cancer Therapeutics. 2006 May;5(5):1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]