Abstract
We seek to exploit the natural tendency of melanomas and other tumors to convert glucose to lactate as a method for selective intracellular acidification of cancer cells and for potentiating the activity of N-mustard antineoplastic agents. We performed this study to evaluate whether induction of hyperglycemia (26 mM) could enhance the effects of lonidamine (LND, 100 mg/kg; i.p.) on inducing intracellular acidification, bioenergetic decline and potentiation of the activity of melphalan (LPAM) against DB-1 melanoma xenografts in mice. Intracellular pH (pHi), extracellular pH (pHe) and bioenergetics (βNTP/Pi) were reduced by 0.7 units (p<0.001), 0.3 units (p>0.05) and 51.4% (p<0.05), respectively. Therapeutic response to LPAM (7.5 mg/kg; i.v.) + LND (100 mg/kg; i.p.) was reduced by about a factor of 3 under hyperglycemic conditions compared to normoglycemia, producing a growth delay of 7.76 d (tumor doubling time = 5.31 d, cell kill = 64%) compared to LND alone of 1.70 d and LPAM alone of 0.29 d. Under normoglycemic conditions LND plus LPAM produced a growth delay of 17.75 d, corresponding to a cell kill of 90 % at the same doses for each of these agents. The decrease in tumor cell kill under hyperglycemic conditions correlates with an increase in tumor ATP levels resulting from increased glycolytic activity. However, hyperglycemia substantially increases lactic acid production in tumors by a factor of ~6 (p<0.05), but hyperglycemia did not increase the effects of LND on acidification of the tumor most likely because of the strong buffering action of carbon dioxide (the pKa of carbonic acid is 6.4). Therefore, this study demonstrates that addition of glucose during treatment with LND diminishes the activity of this agent.
Keywords: lonidamine, monocarboxylate transport inhibitor, tumor acidification, tumor de-energization, melphalan, magnetic resonance spectroscopy
Introduction
Melanoma is the most aggressive form of skin cancer, accounting for fewer than 2% of all skin cancer cases but for the vast majority of skin cancer deaths (1). Incidence of this disease has been increasing for at least 30 years. Since 2004, cases of melanoma among Caucasians have been increasing by almost 3% per year in both men and women, and projections indicate that by mid-century the incidence of melanoma will exceed that of lung, breast or prostate cancer (2). Agents targeting the V600E BRAF mutation exhibited some initial success, but recurrence was inevitable as seen typically with most metastatic disease (3). Anecdotal reports of remarkable responses to adoptive cell-transfer therapy have appeared (4,5), but these are limited to a very few patients. Immunotherapy with Ipilimumab (Yervoy®) is promising but in a recent study immune-related adverse events occurred in 10 to 15% of patients treated with this agent and treatment with gp100, a peptide vaccine, produced 3% adverse events (6). There were 14 deaths related to these agents (2.1%), and 7 associated with immune-related adverse events (6). Overall, progress toward systemic therapy of melanoma is being made, but it is clear that ultimate success will probably involve a combination of therapeutic approaches. The goal of our laboratory has been to develop a novel combination of methods based on the drug lonidamine (LND) (7) that potentiates tumor response to hyperthermia (8–11), radiation therapy (12) and chemotherapy with N-mustards (13–15) and doxorubicin (16). Such a combined modality approach may ultimately in combination with targeted therapy and immunotherapy contribute to the clinical management of this disease.
Specifically, we want to exploit this natural tendency of tumors as a method to selectively induce intracellular acidification of the tumor even under aerobic conditions and to use this method to enhance tumor response to chemotherapeutic agents. In our initial studies, we tried to acidify tumors by injection of exogenous glucose to induce hyperglycemia at a serum glucose concentration of 26 mM. This approach had minimal effect on the intracellular pH (pHi), since the tumor cell has many mechanisms for neutralizing the lactic acid that is produced including the Na+/H+ exchanger, the Cl−/ HCO3− exchanger, the monocarboxylic acid transporters MCT-1 and MCT-4 (MCT), and mitochondrial oxidation of pyruvate via pyruvate dehydrogenase and the TCA cycle. Wahl et al. found that the Na+/H+ exchanger was minimally expressed and that only MCT-1 & 4 were found in the DB-1 melanoma model (17). The Cl−/ HCO3− exchanger, has been reported to be inactive under acidic conditions, so that its contribution to maintaining pHi homeostasis diminishes as pHi decreases (18). Therefore, inhibition of the MCT and/or mitochondrial pathways is essential to induce intracellular acidification of tumors. Administration of meta-iodobenzylguanidine (MIBG, 30 mg/kg), an inhibitor of site 1 of the mitochondrial electron-transport chain, under hyperglycemic conditions (26 mM serum glucose) reduced tumor pHi and extracellular pH (pHe) and by ~0.4 and ~0.6 unit, respectively, for about 40 min; coincidentally, the nucleoside triphosphate to Pi ratio (βNTP/Pi) decreased ~60% relative to the baseline level (19). The combination of hyperglycemia, MIBG, and the MCT inhibitor α-cyano-4′-hydroxycinnamic acid (CHC) produced a transient decrease in the pHi of about 0.6 units with minimal effect on pHe and a monotonic decrease in βNTP/Pi (20). However, MIBG could not be used in the clinic at these concentrations, and CHC has never been used in the clinic. We therefore switched to LND (100 mg/kg, i.p.), an MCT inhibitor as suggested by previous data (7,19–23) and achieved a pHi of 6.33 ± 0.10 (P < 0.001), pHe of 6.80 ± 0.07 (P > 0.05), and a reduction in the βNTP/Pi ratio of 66.8 ± 5.9% (P < 0.001) in the absence of exogenous glucose (7); hence, LND eliminated the need for induction of hyperglycemia and administration of MIBG, both of which introduce potential sources of toxicity (19,20). Furthermore, the effects of LND on pHi, pHe and βNTP/Pi were selective for the tumor with no effect on skeletal muscle or brain and only a slight transient effect on pHi and βNTP/Pi of the liver (7). We have further demonstrated that pretreatment of DB-1 melanoma xenografts with LND potentiates the activity of melphalan (LPAM, 7.5 mg/kg, cell kill 89.4%) (7).
The purpose of the present study was to determine if the addition of exogenous glucose to mice implanted with DB-1 melanoma can further increase LND-induced tumor acidification by increasing lactate production and if it could increase tumor response to LPAM. Comparative data on normal liver, skeletal muscle and brain have been obtained to determine whether LND has a selective effect on tumors and to delineate possible mechanisms underlying this selectivity. These findings point to the potential utility of N-mustards and LND in the systemic treatment of disseminated melanoma and other malignancies.
Material and Methods
Subjects
Ten tumor-bearing 4–6 weeks old male athymic nude mice (01B74) purchased from the National Cancer Institute (Frederick, MD, USA) were evaluated by localized 31P magnetic resonance spectroscopy (MRS). Mice without tumors were employed in studies of normal tissues, brain pHi (n = 3), surgically exposed liver pHi (n = 3), pHe (n = 3) and skeletal muscle pHi (n = 3), pHe (n = 3), under identical conditions utilized for 31P MRS studies of mice with tumors. Three tumor-bearing mice were included for 1H MRS lactate measurement experiments following LND administration.
Materials
LND and 3-aminopropylphosphonate (3-APP) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The drug (LND; 5 mg) was dissolved in 227 μL of tris/glycine buffer (22.0 mg/mL), vortexed until the solution was clear, and administered i.p. (intraperitoneally) at a dose of 100 mg/kg. The buffer consisted of trizma base (1.2 g) and glycine (5.76 g) in 100 mL sterile water (final pH = 8.3). In addition, 0.2 ml of a 300 mg/ml solution of 3-APP (dissolved in water and pH adjusted to 7) was administered i.p. LPAM was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and was dissolved by solubilization in 5% acid (HCl)/ethanol at 15 mg/ml followed by 10-fold dilution with PBS (1.5 mg/ml) immediately prior to i.v. administration (7.5 mg/kg). LND (100 mg/kg; i.p.) was injected 20 min after the start of the glucose infusion.
Glucose Infusion
A stock solution of D-glucose (2.5 M) was diluted to 0.6 M and delivered through a tail vein catheter (I.V. Catheters FEP, Tyco Healthcare, Tyco International Ltd., Schaffhausen, Switzerland) over a total infusion time of 120 min at a variable rate using a syringe pump (Harvard Apparatus, Holliston, MA, USA) to maintain a blood concentration of 26 mM, using the following protocol: (10ml/hr, 1min; 3ml/hr, 4min; 2.5ml/hr, 2min; 2.0ml/hr, 2min; 1.5ml/hr, 2min; 1.0ml/hr, 2min; 0.5ml/hr, 107min).
Human Melanoma Xenografts development and MR Experiment
Male, 4–6 week old, athymic nude mice were housed in microisolator cages with access to water and autoclaved mouse chow ad libitum. DB-1 melanoma cells were prepared as described in our previous publication (7). One million melanoma cells in 0.10 mL of Hank’s balanced salt solution (Invitrogen/Gibco, Carlsbad, CA, USA) were inoculated subcutaneously into the right thigh of each animal. Melanoma xenografts were allowed to grow until the tumor was ~8 mm in the longest diameter before further study.
For induction of anesthesia, tumor-bearing mice were maintained under 1% isoflurane in oxygen, supplied at 1 L/min. Changes in pHi and pHe in response to LND were monitored by 31P MRS in tumor, brain, skeletal muscle, and exposed liver of nude mice during and following glucose infusion. Tumor, skeletal muscle (hind-leg) and surgically exposed liver were studied by positioning the tissue in a home-built, dual-frequency (1H/31P) slotted-tube resonator (10 mm in diameter). Brain 31P MRS was implemented by placing the animal’s head on top of a 20 mm 31P surface coil in conjunction with a 70 mm quadrature 1H volume coil (T/R). For 31P MRS experiments on liver, a 1-cm incision was made in the abdomen of the anesthetized animal to expose the liver, and the exposed organ was placed inside the resonator. During the liver studies, some surface dehydration was evident in the surgically exposed liver producing some discoloration of the liver surface and small modifications of the 31P spectrum. Both effects of dehydration were eliminated by placement of a parafilm insert filled with saline inside the resonator to maintain hydration of the tissue. For lactate measurements following LND administration, tumors were positioned in a home-built single frequency (1H) slotted tube resonator (inner diameter = 13 mm, outer diameter = 15 mm, depth = 16.5 mm).
MR experiments were performed on a 9.4 T/31 cm horizontal-bore Varian system (Varian Medical Systems, Palo Alto, CA, USA). In vivo 31P MR spectra were acquired with a homemade resonator as described above. Sub-dermal needle electrodes and a rectal thermistor were placed for electrocardiogram and core body temperature monitoring, respectively. The animal’s core body temperature was maintained at 37 ± 1°C by blowing warmed air into the bore of the magnet during a scan with heating controlled by a thermal regulator system. A respiration pillow was placed over the thorax and a pulse-oximeter over the tail to monitor respiration and oxygen saturation, respectively (Model 1025, SA Instruments Inc., Stony Brook, NY, USA). The chemical shifts of Pi and exogenously administered 3-APP were monitored to determine pHi and pHe, respectively (7,24). LND and 3-APP were injected following acquisition of the baseline spectrum through two 26-gauge i.p. catheters inserted into either side of the peritoneum without removing the animal from the magnet. The magnet was shimmed globally until the water line-width of the tumor monitored via the 1H channel reached 60 –70 Hz. A point resolved spectroscopy (PRESS) sequence was used to shim the ISIS (Image Selected In vivo Spectroscopy) tumor voxel of 250–300 mm3 size covering the entire tumor depending on tumor size, to 30–40 Hz line width. Localized 31P MRS was performed on s.c. tumors using the ISIS technique with the following parameters: Hyperbolic Secant-Adiabatic Fast Passage (HS-AFP) slice-selective inversion pulses with 2.5 ms length, 296 scans with a radiofrequency pulse width of 60 μs, corresponding approximately to a 90° flip angle; sweep width, 12 kHz; 512 data points; TR=4 s. A slice-selective, double-frequency, Hadamard-selective, multiple quantum coherence transfer pulse sequence was used to detect lactate and to filter out overlapping lipid signals (25). The acquisition parameters were as follows: sweep width=4 kHz; 2048 data points; TR=8 s; 128 scans.
MR data acquisition, post processing, pH estimation, lactate peak integration and bioenergetic evaluation (βNTP/Pi ratio) were performed as described elsewhere (7).
Treatment with Melphalan and Growth Delay Measurements
When tumors reached the appropriate volume (100 mm3), four cohorts of five age- and weight-matched animals were randomized to the following treatment groups with continuous i.v. infusion of glucose (26 mM) at a variable rate using a syringe pump as described above. Cohort 1 (sham-treated control) was infused intravenously (i.v.) with PBS and given appropriate sham intraperitoneal (i.p.) injections of tris/glycine buffer; cohort 2 was infused i.v. with PBS 40 min after LND administration i.p. (100 mg/kg); cohort 3 was injected i.p. with tris/glycine buffer and infused i.v. with LPAM (7.5 mg/kg delivered in ~10 sec) in PBS; cohort 4 was injected i.p. with LND (100 mg/kg) and after 40 min, LPAM (7.5 mg/kg) was infused i.v. after disconnecting the glucose line that was used for drug delivery for a short period of time.
During the treatment and sham-treatment procedures, all animals were anesthetized with ketamine hydrochloride and acepromazine as described above with additional anesthesia readministered as needed approximately every 45–60 min. Animals were placed on a water pad heater (Gaymar T-Pump, Gaymar Industries, Inc., Orchard Park, NJ, USA) to maintain body temperature during anesthesia.
Tail vein catheters (I.V. Catheters FEP, Tyco Healthcare) filled with heparin (100 USP Units/mL) to prevent blood clotting were placed using a restrainer (MTI Braintree Scientific, Braintree Scientific Inc., Braintree, MA, USA). LPAM was freshly prepared prior to injection. Depending on the treatment group, either tris/glycine or LND (4.5 ul/g) and LPAM or PBS (5.0 ul/g) were injected. Tail vein catheters were removed and animals were allowed to recover in cages.
For the first five days post-treatment, tumor volume and body weight were measured daily with calipers (Scienceware, Bel-Art Products, Wayne, NJ, USA) and scale (Acculab PP401, H & C Weighing Systems, Columbia, MD, USA), respectively. Subsequently, these measurements were repeated every other day. The tumor dimensions were measured in three orthogonal directions, using the equation, V= π (a×b×c)/6, where a, b, and c are the length, width, and depth, respectively, of the tumor.
Chemotherapy studies have been performed on the smallest possible tumor sizes (100 mm3), since the tumor had to be monitored until it reached four times its pretreatment volume and we did not want to go beyond about 400 mm3. On the other hand, the NMR studies had to be performed at a tumor size that produced an optimal spectrum and fit the coils that were available, and we also had to be able to obtain a spatially localized spectrum to avoid contamination of tumor metabolites with exogenous metabolites from muscle. These considerations led to the choice of tumor sizes.
Statistics
ANOVA with Bonferroni and Tukey multiple comparisons were used for statistical analysis (SPSS 16; IBM Corporation; Armonk, NY). The data on pHi, pHe, lactate, βNTP/Pi at time points following LND administration were compared in the presence and absence of glucose infusion. To estimate treatment effects on tumor growth, we conducted a growth delay analysis (26). For each animal, we recorded the time from treatment until the tumor reached a volume of four times the mean volume at the initiation of treatment. We then averaged these times in the (T) treated and (C) control arms. For each animal, we also computed the slope of the log tumor volume curve up to time 0 days (the time of treatment) in the treated animals and time 6 days (when the curves started to become nonlinear) in the controls, and we computed the average slope (B) across both arms of the study. We estimated the proportion of the tumor surviving treatment as exp(−(T–C)×B). We computed confidence intervals by the bootstrap percentile method. We conducted these analyses in R (Version 3.0.2 for Linux; R Foundation for Statistical Computing, Vienna, Austria) (27).
We analyzed the data on acidification and bioenergetics in tumor and normal tissues using mixed models with a random effect for animal and fixed effects for time, treatment arm and a time-by-treatment interaction. We conducted these analyses in SAS Proc Mixed (SAS Version 9.3; SAS Institute; Cary NC).
Results
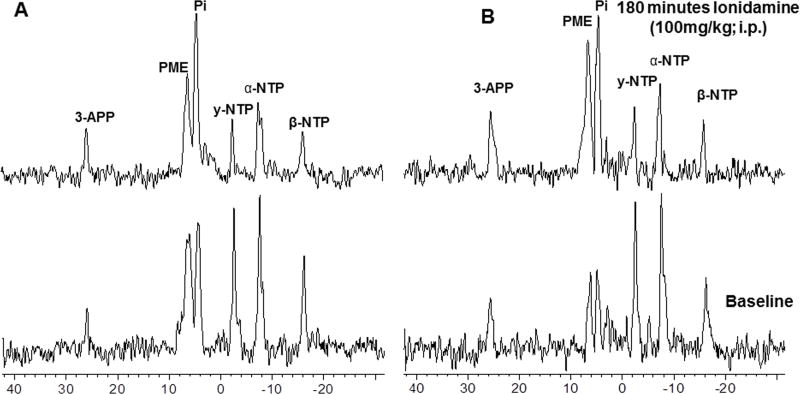
A representative localized 31P MR spectrum of DB-1 human melanoma xenografts prior to and 180 min after administration of LND is displayed in Fig. 1A. Another set of spectra measured prior to and 180 min after LND administration following glucose (26 mM) infusion appears in Fig. 1B. The spectral intensity was maintained throughout the addition of LND and glucose, which were delivered without movement of the animal, so the spectral intensity did not change. The integrated areas under the βNTP and Pi resonances were measured to monitor changes in levels of these metabolites. The integrated intensity of βNTP and Pi decreased by 46% and increased by 104%, respectively, following LND administration in presence of hyperglycemia.
Figure 1.
In vivo localized (Image Selected In vivo Spectroscopy - ISIS) 31Phosphorus magnetic resonance (31P MR) spectra of human melanoma xenograft grown subcutaneously in nude mice (A) without glucose infusion and (B) with glucose (26 mM) infusion. In each set lower spectrum represents baseline and upper represents 180 min following lonidamine (LND; 100 mg/kg, i.p.) administration. Resonance assignments are as follows, 3-APP (3-aminopropylphosphonate); PME (phosphomonoesters); Pi (inorganic phosphate); PDE (phosphodiesters); γ-NTP (γ-nucleoside-triphosphate), α-NTP (α-nucleoside-triphosphate), β-NTP (β-nucleoside-triphosphate). Decrease in β-NTP levels and the corresponding increase in Pi following LND administration (Spectrum B) indicating impaired energy metabolism.
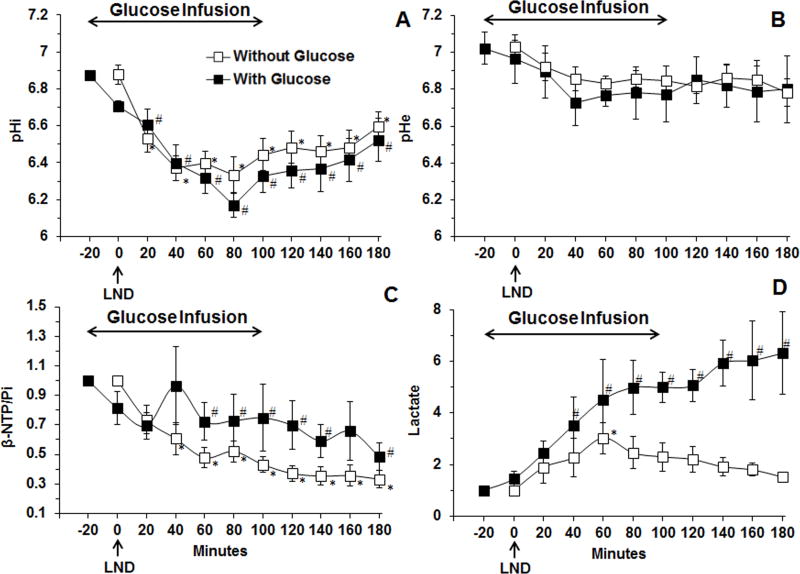
Figure 2 summarizes the quantitative changes in the key metabolic parameters of DB-1 melanoma induced by LND and glucose administration (filled squares). LND produced a monotonic decrease in pHi from 6.87 ± 0.03 to 6.17 ± 0.06 (p < 0.001) (Fig. 2A), a slight non-significant decrease in pHe from 7.02 ± 0.09 to 6.73 ± 0.12 (p > 0.05) (Fig. 2B) and a monotonic decline in bioenergetics ratio (βNTP/Pi) by 51.4 ± 0.09% (p < 0.05) (Fig. 2C) relative to baseline levels; the latter were obtained from 31P MR spectra acquired during the first 20 min of glucose infusion right before LND bolus injection during and up to 100 min for duration of 120 min into human melanoma xenografts. Steady-state tumor lactate increased ~6-fold (p < 0.05) (Fig. 2D) relative to baseline. For comparison, we have provided for each data set the measurements obtained in the absence of glucose infusion (Fig. 2 open squares) (7). The key changes induced by LND were a decrease in pHi to about 6.33 that was reached within 80 min of LND treatment, a monotonic decrease in βNTP/Pi and a 3-fold increase in lactate, which can be attributed primarily to intracellular lactate since pHi decreased much more than pHe. Addition of exogenous glucose produced minimal change in pHi and pHe but increased βNTP/Pi and doubled the level of lactate in the tumor.
Figure 2.
(A) The intracellular pH (pHi,) (n = 10), (B) extracellular pH (pHe) (n = 7) (C) changes of βNTP/Pi (ratio of peak area) relative to baseline (n = 11) (D) changes in tumor lactate, area under the curve was compared to baseline at each time points and was normalized to baseline profile (n=4) as a function of time with infusion of glucose (26 mM) of human melanoma xenografts in response to LND (100 mg/kg; i.p.) administration at time zero after 20 min i.v. infusion of glucose (26 mM) (filled squares). The pHi, pHe, bioenergetics (βNTP/Pi) and lactate data without infusion of glucose (open squares) were adapted from previously published data (7). Baseline measurements for glucose infusion studies were performed at −20 min, up to 100 min for duration of 120 min whereas, baseline without the addition of exogenous and before LND administration were done at 0 min. The values are presented as mean ± S.E.M. When not displayed, S.E.M. values were smaller than the symbol size. *, # statistically significant (p<0.05) relative to starting levels for experiments, without glucose, and with glucose respectively.
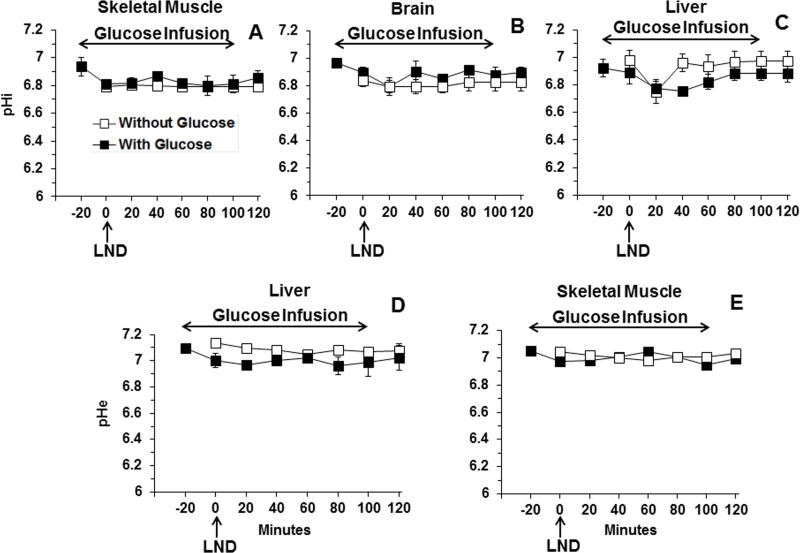
Skeletal muscle (Fig. 3A) and brain (Fig. 3B) exhibited no significant changes in pHi following treatment with LND combined with hyperglycemia; however, liver (Fig. 3C) exhibited a minimal transient change in pHi by 0.17 ± 0.01 pH units (p > 0.05) at 40 min post-LND but no significant change in pHe following addition of exogenous glucose (Fig. 3D). No significant change in pHe was observed in skeletal muscle (Fig. 3E) after LND combined with hyperglycemia. In each data set (pHi, pHe, bioenergetics, lactate), for comparison we have provided the data for studies performed without glucose infusion (7). Overall, there is essentially no change in pHi or pHe of normal tissues due to LND or glucose administration.
Figure 3.
The intracellular pH (pHi) profile as a function of time of (A) skeletal muscle (n=3), (B) brain (n=3), (C) surgically exposed liver (n=3), and extracellular pH (pHe) profile as a function of time of (D) surgically exposed liver (n=3) and (E) skeletal muscle (n=3) in response to LND (100 mg/kg; i.p.) administration at time zero after 20 min i.v. infusion of glucose (26 mM). The data for experiments without glucose infusion, previously published in (7) and depicted by open squares, are included for direct comparison. Baseline measurements for glucose infusion studies were performed at −20 min, up to 100 min for duration of 120 min whereas, baseline without the addition of exogenous and before LND administration were done at 0 min. The values are presented as mean ± S.E.M. When not displayed, S.E.M. values were smaller than the symbol size.
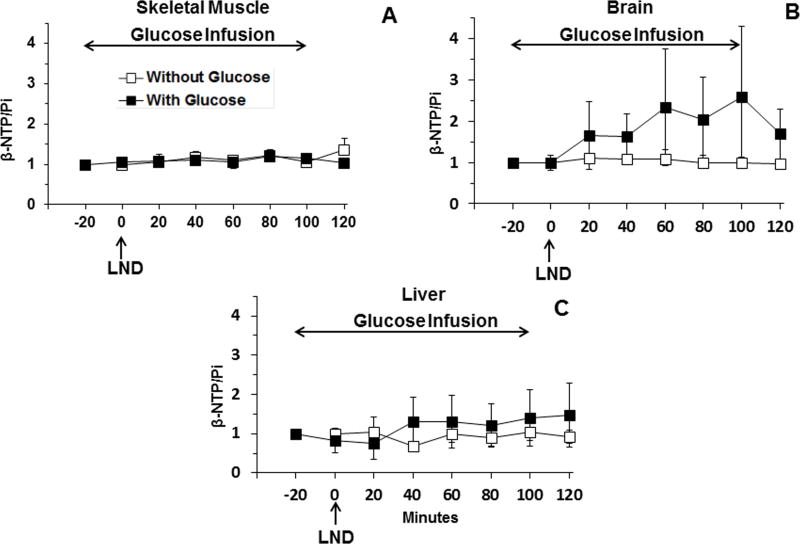
No changes in bioenergetics (βNTP/Pi) were detected in skeletal muscle (p > 0.1) (Fig. 4A) and brain (p > 0.1) (Fig. 4B) for at least 120 min post-LND, and a small non-significant transient decrease was observed in the bioenergetics of liver, 25.5 ± 0.4 % (p > 0.05), at 20 min post-LND (Fig. 4C) administration during glucose infusion. In brain, we observed a trend towards increasing bioenergetic status during glucose infusion, and this could be due to glucose providing additional energy to those areas of the brain where glucose availability might be limiting. Due to the high level of glucose uptake in the brain, availability can become limiting in regions distant from the vascular circulation. Providing an increase in glucose supply can reduce this gradient. In each set, we have provided comparative data (open squares) obtained without glucose infusion, adapted from our previous publication (7).
Figure 4.
(A). The bioenergetics (βNTP/Pi) (ratio of peak area) relative to baseline (n = 3) of profile as a function of time of (A) skeletal muscle (n=3), (B) brain (n=3) and (C) surgically exposed liver (n=3) with and without infusion of exogenous glucose (26 mM) of healthy mice in response to LND (100 mg/kg; i.p.) administration at time zero after 20 min i.v. infusion of glucose. The data for experiments without glucose infusion, previously published in (7) and depicted by open squares, are included for direct comparison. Baseline measurements for glucose infusion studies were performed at −20 min, up to 100 min for duration of 120 min whereas baseline without the addition of exogenous and before LND administration were done at 0 min. The values are presented as mean ± S.E.M. When not displayed, S.E.M. values were smaller than the symbol size.
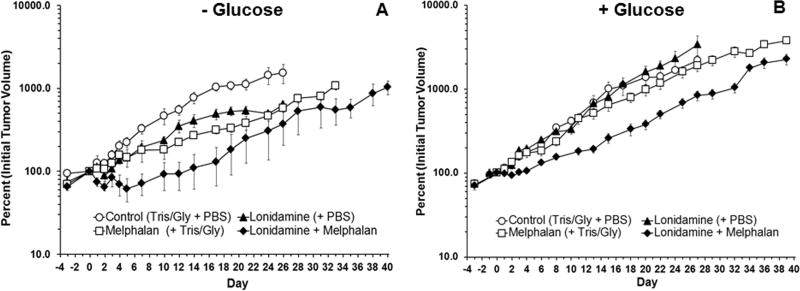
The effects of treatment with LND plus LPAM in the absence (Fig. 5A) and presence (Fig. 5B) of exogenous glucose were evaluated by tumor growth delay experiments. The effect of treatment with LND + LPAM was significantly different from placebo, LND alone and LPAM alone. Tumor growth delay was determined by calculating the time in days between logarithmic regrowth regions of the curves of treated tumors and saline treated controls. Data yielded tumor growth delays of 1.7 d, 0.3 d and 7.8 d for the treatment groups LND alone, LPAM alone and LND + LPAM, respectively, in presence of exogenous glucose (Table 1). By comparison, our previous results without glucose yielded growth delays of 2.0 d, 7.6 d and 17.8 d for the treatment groups LND alone, LPAM alone and LND + LPAM, respectively (Table 1). Tumor doubling times were estimated from the slopes of the log-linear portion of the tumor regrowth curves determined by linear regression analysis using the formula Td = 0.3010/m, where m is the slope and are 9.4 d, 5.8 d and 5.3 d for LND alone, LPAM alone and LND + LPAM, respectively, in presence of exogenous glucose. Growth delays without exogenous glucose are as follows: 6.5 d, 5.9 d and 5.3 d for LND alone, LPAM alone and LND + LPAM, respectively (Table 1). No statistically significant differences were determined in the doubling times of all treatment groups, but significant differences were determined in LPAM treatments, with and without exogenous glucose, and with and without LND (Table 1). The key observations are that in the presence of exogenous glucose, LPAM has limited activity against DB-1 melanoma and that exogenous glucose substantially decreases response of this xenograft to LND + LPAM, decreasing cell kill from one log10 to less than half a log10. With LND + LPAM, maximum treatment response was seen in the absence of glucose.
Figure 5.
Growth delay experiments performed on DB-1 human melanoma xenografts in nude mice treated with 7.5 mg/kg; i.v. LPAM (A) without (7) and (B) with i.v. infusion of glucose (26 mM). Mice were treated on Day 0 as follows: Control (sham i.p. tris/glycine buffer + sham i.v. PBS), LND, LPAM, LND + LPAM. In group (A) animals were treated with LPAM 40 min after LND injection at time zero and group (B) animals were treated with LPAM 40 min after LND injection at time zero and 40 min after start of the 120 min glucose infusion. Each tumor was normalized to its own volume on day 0 (the day of treatment). This was done so that variations between the two experimental groups (− Glucose and + Glucose) could be taken into account. This allows each tumor to serve as its own control. As such, day 0 is equal to 100% for each tumor. Values shown are means ± SEM average of n = 4 animals in the control and LND groups (no glucose infusion), n = 3 animals in the LPAM and LND + LPAM groups (no glucose infusion), and n = 5 animals in all cohorts (control, LND, LPAM, LND + LPAM) with glucose infusion. When not shown, error bars are less than symbol size.
Table 1.
Summary of estimated growth delay (T-C), doubling time (Td), and surviving fraction as a percent (100×exp(−(T–C)×B), with bootstrap 95% CI), log cell kill method, by experiment and treatment arm in DB-1 human melanoma xenografts in absence and presence of hyperglycemia.
| Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LND | LPAM | LND+LPAM | ||||||||||
|
| ||||||||||||
| Parameters | Without Glucose | With Glucose | SE | p value | Without Glucose | With Glucose | SE | p value | Without Glucose | With Glucose | SE | p value |
| T-C | 1.99 d | 1.70 d | 2.50 | 0.91 | 7.62 d | 0.29 d | 3.74 | 0.05 | 17.75 d | 7.76 d | 2.82 | < 0.001 |
| Td | 6.50 d | 9.36 d | 100.29 | 0.98 | 5.91 d | 5.83 d | 1.49 | 0.96 | 5.32 d | 5.31 d | 0.38 | 0.98 |
| % Surviving fraction | 81 (46, 158) | 88 (51, 129) | 22 | 0.75 | 41 (13,108) | 97 (38, 192) | 56 | 0.32 | 10 (4, 28) | 36 (16, 77) | 15 | 0.08 |
NOTE: LND: Lonidamine; LPAM: Melphalan; T: median time (in days) required for the treatment group tumors to reach a predetermined size; C: median time (in days) for the control group tumors to reach the same size; B: average slope across both arms of the study; SE: standard error of the difference (27).
Table 1 summarizes growth and response parameters. The surviving fraction for animals treated with LND alone ranges between 81 and 88% in absence and presence of hyperglycemia, respectively. For LPAM alone the surviving fraction ranges between 41 and 97% in absence and presence of hyperglycemia, respectively. The effects of LND + LPAM (7.5 mg/kg), which appear to be approximately additive for the two drugs, indicate that the surviving fraction ranges between 10 and 36% and indicate a 90% and 64% cell kill in absence and presence of hyperglycemia, respectively. Thus, hyperglycemia is decreasing the therapeutic effect of LND + LPAM.
We fit mixed ANOVA models to the normal and tumor tissue data to estimate the effects of time and treatment and their interactions on acidification, bioenergetics and tumor lactate. There was no significant effect of glucose treatment on brain bioenergetics, brain pHi, liver bioenergetics, liver pHe, liver pHi, muscle pHe, or muscle pHi. The βNTP/Pi ratio in liver was significantly higher under glucose treatment and also increased in muscle (estimated increase = 1.61, SE=0.51, p = 0.003). For tumor lactate, there was a significant interaction of time and treatment effect (p = 0.002); lactate levels were lower in the glucose arm prior to t=80 min, and elevated thereafter, roughly doubling the control levels by t=180 min.
Discussion
We have recently shown that treatment of DB-1 melanoma xenografts with LND (100 mg/kg, i.p.) selectively acidifies tumors from pHi = 6.90 ± 0.05 to pHi 6.33 ± 0.10, with a minimal decrease of pHe from 7.00 ± 0.04 to 6.80 ± 0.07, whereas the βNTP/Pi ratio decreased monotonically during a 3 hr period by 66.8 ± 5.7% (7). Pretreatment with LND potentiated the activity of LPAM (7.5 mg/kg, i.v.) producing about one log10 cell kill (i.e., 89.4%) with one dose (7.5 mg/kg, i.v.) (7). The goal of the present study was to determine if the extent of tumor acidification could be increased by infusing high concentrations of glucose into the mouse to induce increased glycolysis, which was expected to increase tumor response to LPAM.
The basis for this assumption was that LPAM is known to be converted to the active aziridinium ion intermediate that is deactivated by hydroxide ion and glutathione. The concentration of the active aziridinium intermediate increases with hydrogen ion concentration. In addition, glutathione transferase which acts as a scavenger to deactivate the aziridinium ion is also inhibited by hydrogen ions as is 6O-alkyltransferase, the key enzyme involved in repair of LPAM-mediated DNA alkylation of guanine bases (28,29). Hence, hydronium ions should have a three-fold effect on enhancing LPAM activity. However, the present experiment has shown that even at 26 mM blood levels of glucose, tumor pHi was not significantly affected, decreasing to 6.17 even though the overall lactate concentration increased six-fold over baseline or twice what it was in the presence of LND alone without exogenous glucose. Since carbonic acid has a pKa of 6.4, this lack of acidification can be attributed to the high buffering capacity of CO2 produced from oxidative phosphorylation and/or introduced via the Cl−/ HCO3− exchanger.
This would imply that LPAM activity should increase slightly following administration of LND at 26 mM blood glucose levels. However, data in Table 1 indicate that rather than increasing tumor response to LPAM, treatment with LPAM under hyperglycemic conditions increased tumor survival from 41% to 97% (i.e., LPAM had essentially no activity against this melanoma model after treatment with LND) and treatment with LND under hyperglycemic conditions increased tumor survival by a factor of 3.6 from 10% to 36%.
The reason for this anomalous result is that tumor response to LPAM depends not only on the level of active aziridinium ion, but also on the bioenergetic state of the tumor, which is significantly enhanced under hyperglycemic conditions. Figure 2C shows that the βNTP/Pi ratio of the tumor increases from about 0.4 to 0.6 after 3 hr at 26 mM blood glucose. There is substantial evidence that LPAM activity is subject to multidrug resistance, an energy-driven process associated with formation of glutathione conjugates with this agent (30–32). This could account for the increased resistance of DB-1 melanomas to LPAM under hyperglycemic conditions. The reason for choosing two different tumor sizes for chemotherapy (100 mm3) and NMR studies (~250–300 mm3) were dictated by the need for therapeutic efficacy of chemotherapy and by the need for sensitivity of 31P MRS, respectively. Effects of tumor volume on bioenergetics have been discussed in our previous publications (19,20). In addition, treatment of larger tumors has produced a similar response but limits the duration of monitoring treatment effects.
Shestov et al. (33) have recently monitored ATP production by DB-1 melanoma cells grown on solid microcarrier beads perfused with 26 mM [1,6-13C2]glucose under normoxic conditions in a bioreactor system monitored by 13C MRS. Utilizing bonded cumomer analysis to analyze the kinetics of 13C-isotopomer exchange in terms of flux through specific metabolic pathways, these authors concluded that 54% of the ATP was derived from oxidative metabolism and 46% from glycolytic metabolism. No data are yet available on the effect of LND on the metabolism of these cells. Data presented in the references (7,21–23) support the mechanism of MCT inhibition by LND. As noted above, DB-1 melanoma tumors express both the MCT-1 and MCT-4 isoforms, but MCT-1 has a Km of about 4.5 mM for lactate that is about one order of magnitude lower than that for MCT-4 (34). A number of investigators have reported that LND also decreases levels of NTP in various tumor models including perfused MCF7 breast cancer cells (21,23), 9L glioma perfused cells and xenografts in rats (22), and DB-1 melanoma xenografts (7). Floridi and Lehninger (35) have proposed that LND inhibits the mitochondrial electron transport chain, but the exact site of inhibition remains to be identified. Nath et al. (7) have suggested that because CHC behaves in a similar manner to LND, and CHC has been demonstrated to inhibit both the MCT and the mitochondrial pyruvate carrier (MPC) (36), LND is also inhibiting the MPC. However, this mechanism does not exclude the possibility that inhibition of mitochondrial electron transport is also occurring. Whereas glycolytic metabolism could be inhibited by tumor acidification since phosphofructokinase is known to be subject to acid inhibition, the overall effect of such inhibition on ATP production is likely to be small since overall glycolytic activity is increased six-fold in the presence of hyperglycemia (Fig. 2D). Therefore, it is likely that the mechanism of tumor ATP depletion following LND treatment involves inhibition of mitochondrial activity at the level of the MPC and perhaps also at the level of electron transport. Direct evidence of the effect of LND on mitochondrial metabolism will be presented in the future. Hyperglycemia would augment this ATP production thereby enabling multidrug resistance to LPAM. Therefore, addition of exogenous glucose to the LND treatment protocol with LPAM and should be avoided.
Acknowledgments
NMR experiments were performed at the University of Pennsylvania Small Animal Imaging Facility with technical support of Drs. Stephen Pickup and Weixia Liu. Advice and guidance of Andrew M. Ho, Christina Gustafson and Cory Alvey are acknowledged. Support for this project was provided by NIH grants R01-CA129544 and R01-CA172820.
Abbreviations
- LND
lonidamine
- MRS
Magnetic Resonance Spectroscopy
- MCT
monocarboxylate transporter
- pHi
intracellular pH
- pHe
extracellular pH
- 3-APP
3-aminopropylphosphonate
- PME
phosphomonoester
- Pi
inorganic phosphate
- MIBG
meta-iodobenzylguanidine
- i.p
intraperitoneal
- s.c
subcutaneous
- CHC
α-cyano-4-hydroxycinnamic acid
- LPAM
melphalan
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.American CS. Cancer Facts and Figures-2014. American Cancer Society; Atlanta, GA, USA: 2014. [Google Scholar]
- 2.MacLennan R, Green AC, McLeod GR, Martin NG. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992 Sep 16;84( 18):1427–1432. doi: 10.1093/jnci/84.18.1427. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006 May-Jun;29(3):284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- 5.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006 Oct 6;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nath K, Nelson DS, Ho AM, Lee SC, Darpolor MM, Pickup S, Zhou R, Heitjan DF, Leeper DB, Glickson JD. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013 Jan;26(1):98–105. doi: 10.1002/nbm.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Kim SH, Alfieri A, Young CW, Silvestrini B. Lonidamine: a hyperthermic sensitizer of HeLa cells in culture and of the Meth-A tumor in vivo. Oncology. 1984;41:30–35. doi: 10.1159/000225882. [DOI] [PubMed] [Google Scholar]
- 9.Ning SC, Hahn GM. Combination therapy - Lonidamine, hyperthermia, and chemotherapy against the RIF-1 tumor in vivo. Cancer Research. 1991 Nov;51( 21):5910–5914. [PubMed] [Google Scholar]
- 10.Silvestrini B, Hahn GM, Cioli V, Demartino C. Effects of lonidamine alone or combined with hyperthermia in some experimental cell and tumor systems. British Journal of Cancer. 1983;47(2):221–231. doi: 10.1038/bjc.1983.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teicher BA, Holden SA, Ara G, Menon K. Whole-body hyperthermia and lonidamine as adjuvant therapy to treatment with cisplatin with or without local radiation in mouse bearing the lewis lung-carcinoma. International Journal of Hyperthermia. 1995 Sep-Oct;11(5):637–645. doi: 10.3109/02656739509022496. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Alfieri AA, Kim SH, Young CW. Potentiation of radiation effects on 2 murine tumors by lonidamine. Cancer Research. 1986 Mar;46(3):1120–1123. [PubMed] [Google Scholar]
- 13.Jahde E, Glusenkamp KH, Klunder I, Hulser DF, Tietze LF, Rajewsky MF. Hydrogen ion-mediated enhancement of cytotoxicity of bis-chloroethylating drugs in rat mammary carcinoma cells in vitro. Cancer Res. 1989 Jun 1;49(11):2965–2972. [PubMed] [Google Scholar]
- 14.Jahde E, Glusenkamp KH, Rajewsky MF. Nigericin enhances mafosfamide cytotoxicity at low extracellular pH. Cancer Chemother Pharmacol. 1991;27(6):440–444. doi: 10.1007/BF00685157. [DOI] [PubMed] [Google Scholar]
- 15.Jahde E, Roszinski S, Volk T, Glusenkamp KH, Wiedemann G, Rajewsky MF. Metabolic response of AH13r rat tumours to cyclophosphamide as monitored by pO2 and pH semi-microelectrodes. Eur J Cancer. 1992;29A(1):116–122. doi: 10.1016/0959-8049(93)90587-6. [DOI] [PubMed] [Google Scholar]
- 16.Nath K, Nelson DS, Heitjan DF, Leeper DB, Zhou R, Glickson JD. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015 Mar;28(3):281–90. doi: 10.1002/nbm.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl ML, Owen JA, Burd R, Herlands RA, Nogami SS, Rodeck U, Berd D, Leeper DB, Owen CS. Regulation of intracellular pH in human melanoma: Potential therapeutic implications. Molecular Cancer Therapeutics. 2002 Jun;1(8):617–628. [PubMed] [Google Scholar]
- 18.Cassel D, Scharf O, Rotman M, Cragoe EJ, Jr, Katz M. Characterization of Na+-linked and Na+-independent Cl−/ HCO3− exchange systems in Chinese hamster lung fibroblasts. J Biol Chem. 1988 May 5;263(13):6122–6127. [PubMed] [Google Scholar]
- 19.Zhou R, Bansal N, Leeper DB, Glickson JD. Intracellular acidification of human melanoma xenografts by the respiratory inhibitor m-iodobenzylguanidine plus hyperglycemia: a 31P magnetic resonance spectroscopy study. Cancer Res. 2000 Jul 1;60(13):3532–3536. [PubMed] [Google Scholar]
- 20.Zhou R, Bansal N, Leeper DB, Pickup S, Glickson JD. Enhancement of hyperglycemia-induced acidification of human melanoma xenografts with inhibitors of respiration and ion transport. Acad Radiol. 2001 Jul;8(7):571–582. doi: 10.1016/S1076-6332(03)80681-5. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Horin H, Tassini M, Vivi A, Navon G, Kaplan O. Mechanism of action of the antineoplastic drug lonidamine: 31P and 13C nuclear magnetic resonance studies. Cancer Res. 1995 Jul 1;55(13):2814–2821. [PubMed] [Google Scholar]
- 22.Ben-Yoseph O, Lyons JC, Song CW, Ross BD. Mechanism of action of lonidamine in the 9L brain tumor model involves inhibition of lactate efflux and intracellular acidification. J Neurooncol. 1998 Jan;36(2):149–157. doi: 10.1023/a:1005819604858. [DOI] [PubMed] [Google Scholar]
- 23.Mardor Y, Kaplan O, Sterin M, Ruiz-Cabello J, Ash E, Roth Y, Ringel I, Cohen JS. Noninvasive real-time monitoring of intracellular cancer cell metabolism and response to lonidamine treatment using diffusion weighted proton magnetic resonance spectroscopy. Cancer Res. 2000 Sep 15;60(18):5179–5186. [PubMed] [Google Scholar]
- 24.Gillies RJ, Liu Z, Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994 Jul;267(1 Pt 1):C195–203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- 25.Pickup S, Lee SC, Mancuso A, Glickson JD. Lactate imaging with Hadamard-encoded slice-selective multiple quantum coherence chemical-shift imaging. Magnetic Resonance in Medicine. 2008 Aug;60(2):299–305. doi: 10.1002/mrm.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett TH, Valeriote FA. Rodent models in experimental chemotherapy. In: Kallman RF, editor. The use of rodent tumors in experimental cancer therapy: conclusions and recommendations. Pergamon; New York: 1987. pp. 233–247. [Google Scholar]
- 27.Efron BTR. Bootstrap Method for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statistical Science. 1986;1(1):54–77. [Google Scholar]
- 28.Skarsgard LD, Skwarchuk MW, Vinczan A, Kristl J, Chaplin DJ. The cytotoxicity of melphalan and its relationship to pH, hypoxia and drug uptake. Anticancer Res. 1995 Jan-Feb;15(1):219–223. [PubMed] [Google Scholar]
- 29.Wong P, Lee C, Tannock IF. Reduction of intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin Cancer Res. 2005 May 1;11(9):3553–3557. doi: 10.1158/1078-0432.CCR-04-2472. [DOI] [PubMed] [Google Scholar]
- 30.Anderson CP, Reynolds CP. Synergistic cytotoxicity of buthionine sulfoximine (BSO) and intensive melphalan (L-PAM) for neuroblastoma cell lines established at relapse after myeloablative therapy. Bone Marrow Transplant. 2002 Aug;30( 3):135–140. doi: 10.1038/sj.bmt.1703605. [DOI] [PubMed] [Google Scholar]
- 31.Barnouin K, Leier I, Jedlitschky G, Pourtier-Manzanedo A, Konig J, Lehmann WD, Keppler D. Multidrug resistance protein-mediated transport of chlorambucil and melphalan conjugated to glutathione. Br J Cancer. 1998;77(2):201–209. doi: 10.1038/bjc.1998.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larrivee B, Averill DA. Melphalan resistance and photoaffinity labelling of P-glycoprotein in multidrug-resistant Chinese hamster ovary cells: reversal of resistance by cyclosporin A and hyperthermia. Biochem Pharmacol. 1999 Jul 15;58( 2):291–302. doi: 10.1016/s0006-2952(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 33.Shestov AA, Mancuso A, Leeper DB, Glickson JD. Metabolic Network Analysis of DB1 Melanoma Cells: How much energy is derived from aerobic glycolysis? Adv Exp Med Biol. 2013;765:265–271. doi: 10.1007/978-1-4614-4989-8_37. [DOI] [PubMed] [Google Scholar]
- 34.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999 Oct 15;343(Pt 2):281–299. [PMC free article] [PubMed] [Google Scholar]
- 35.Floridi A, Lehninger AL. Action of the antitumor and antispermatogenic agent lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Arch Biochem Biophys. 1983 Oct 1;226(1):73–83. doi: 10.1016/0003-9861(83)90272-2. [DOI] [PubMed] [Google Scholar]
- 36.Halestrap AP. Mitochondrial Pyruvate Carrier - Kinetics and Specificity for Substrates and Inhibitors. Biochemical Journal. 1975;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]