Abstract
Objectives
Cellular fibroepithelial lesions (CFEL) are a heterogeneous group of tumors encompassing cellular fibroadenoma (CFA) and phyllodes tumor (PT). Distinction between the two is challenging on core needle biopsy (CNB). The objective of this study was to evaluate histological features that can help distinguish PT from CFA on CNB.
Methods
Records of all patients diagnosed with CFEL on CNB with follow-up excision between 2002 and 2012 were retrieved. Histopathological stromal features were evaluated on CNB including mitoses per 10 HPF, overgrowth, increased cellularity, fragmentation, adipose tissue infiltration, heterogeneity, subepithelial condensation, and nuclear pleomorphism.
Results
Twenty-seven of 64 (42.2%) were diagnosed as PT (24 BPT, 3 borderline PT) and 37 (57.8%) as CFA on excision. All features except for increased stromal cellularity were statistically significant. The average number of histologic features seen in PT and CFA was 3.9 and 1.4, respectively (OR 7.27; 95% CI: 2.44, 21.69; p= 0.0004). The average mitoses per 10 HPF was 3.0 for PT as compared to 0.8 for CFA (OR 2.14; 95% CI: 1.18, 3.86; p= 0.01).
Conclusions
The presence of mitosis (3 or more) and/or total histologic features of 3 or more on CNB were most helpful features in predicting PT on excision.
Keywords: phyllodes tumor, fibroadenoma, needle cores, biopsies, fibroepithelial lesions
Introduction
Cellular fibroepithelial lesions (CFEL) of the breast are commonly encountered in clinical daily practice. It comprises a heterogeneous group of neoplasms comprised of cellular fibroadenoma (CFA) and phyllodes tumor (PT). The core needle biopsy (CNB) is used as a part of triple approach, along with radiology and clinical examination to make the primary diagnosis on breast lesions. The distinction between CFA and benign phyllodes tumor (BPT) is challenging on CNB due to morphologic overlap in most of those cases. However, it carries a significant impact on clinical management decision. Cellular fibroadenoma behaves in an indolent fashion without significant risk of local recurrence1-3 and may be either clinically monitored or treated by simple surgical removal (enucleation). On the other hand, BPT has an unpredictable biologic behavior and carries a risk of local recurrence without distant metastatic potential.4 The reported rate of local recurrence for BPT is 20% in old literature series.4-6 Therefore, the current standard treatment is surgical excision.
The extent of surgery remains controversial. Most authors believe that BPT should be widely excised to reduce the risk of local recurrence.7-9 These management decisions are mainly based on the reported observations that surgical margins are the single most important predictor of local recurrence, and BPT should be completely excised with adequate margins.5,10-12 However, data from other studies showed that BPT may be followed up if incompletely removed at the first excision, with wide excision only after recurrence.13 Hence, improvement in preoperative diagnostic accuracy is crucial in treatment of patients with cellular FEL on CNB. Furthermore, a substantial proportion of cellular FEL cases were identified as PTs on excision, and consequently, surgical excision has been recommended for complete evaluation of all these lesions.14, 15
Several studies involving CFEL on CNB have been performed in order to identify histological features that can predict BPT on subsequent excision16-19; however, the results are somewhat controversial. Therefore, the purpose of this study is to evaluate several histological features of CFEL on CNB that can help differentiate the two entities and predict BPT on subsequent excision.
Materials and Methods
All patients diagnosed with CFEL on CNB at Mayo Clinic in Rochester, MN were retrieved from the Mayo Clinic anatomic pathology database from January 2002 to December 2012. Since our study focused on evaluating histologic features of indeterminate CFEL on CNB, all patients with clear-cut diagnoses of CFA and BPT on CNB were excluded. All patients without subsequent surgical excision after the initial core biopsies were also excluded from the study. The study was approved by the Mayo Clinic institutional review board (IRB #: 12-006492; 08/13/2012).
Pathology review
Histologic slides of initial core biopsies and surgical excisions were retrieved on all selected cases and reviewed by two breast pathologists (AN and SY) blinded to the original diagnoses on core biopsies and surgical excision specimens. The following histological features were evaluated on both core biopsies and excisional specimens:
Stromal mitoses counted in 10 high power fields (HPF) at 40x microscopic magnification (Figure 1A).
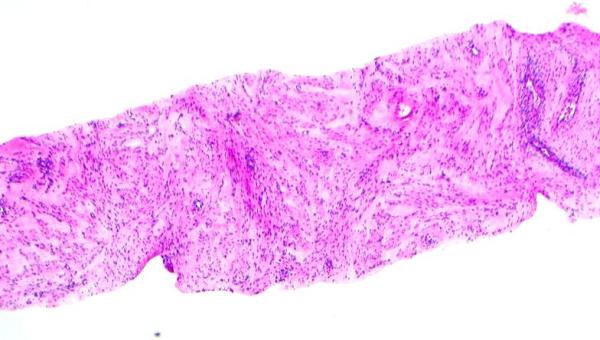
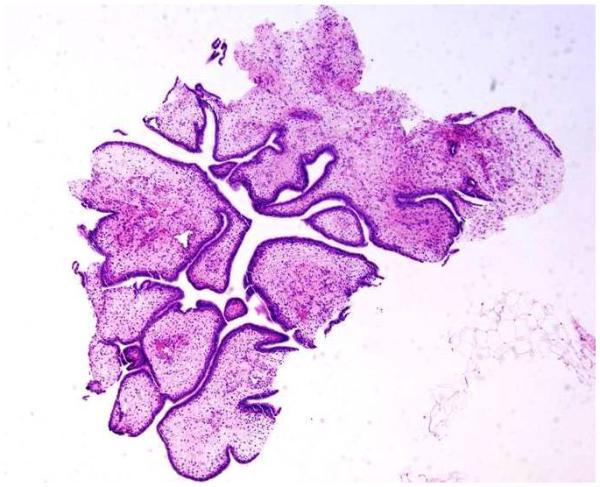
Stromal overgrowth, which was defined as the presence of merely stromal component/absence of epithelial component. For core biopsy specimens, stromal overgrowth was evaluated on 10x (Figure 1B) and for excision specimens on 4x microscopic power magnification (Figure 1C).
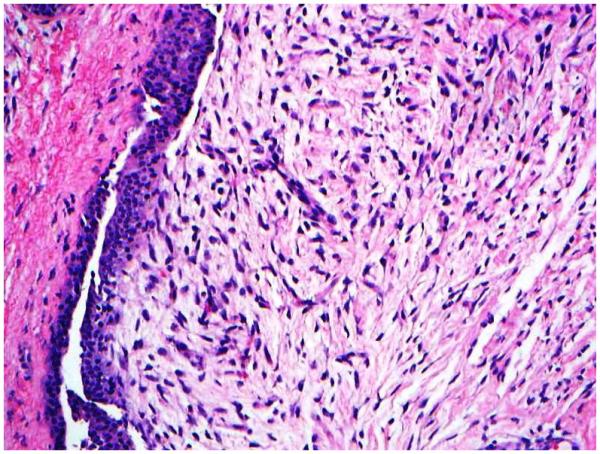
Increased stromal cellularity was categorized as “absent” or “present”. Assessment of stromal cellularity was made on the most cellular areas of the specimen. [0, when the stromal cellularity was not increased; 1, when there is a definitive increase in the density of the stromal cells, in the form of stromal nuclear crowding or overlapping (Figure 1D)].
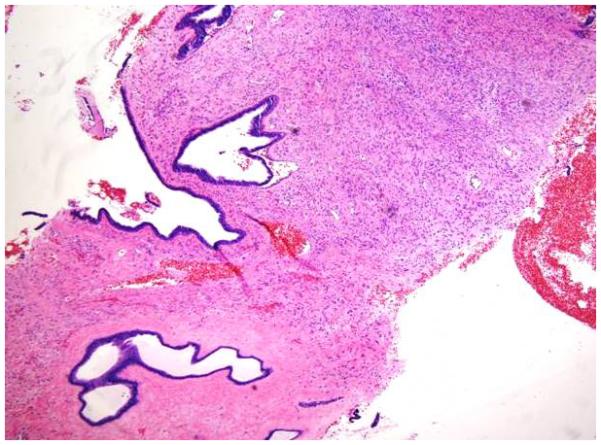
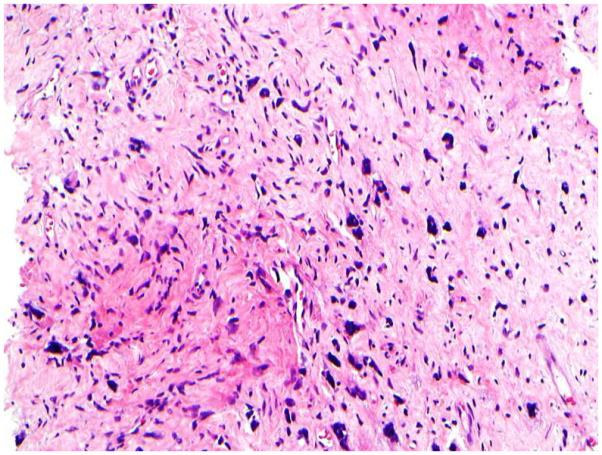
Stromal fragmentation, was defined as detached stromal fragments completely covered by the ductal epithelium (Figure 1E). Stromal fragmentation corresponded to exaggerated intracannalicular growth pattern and leaf like architecture.
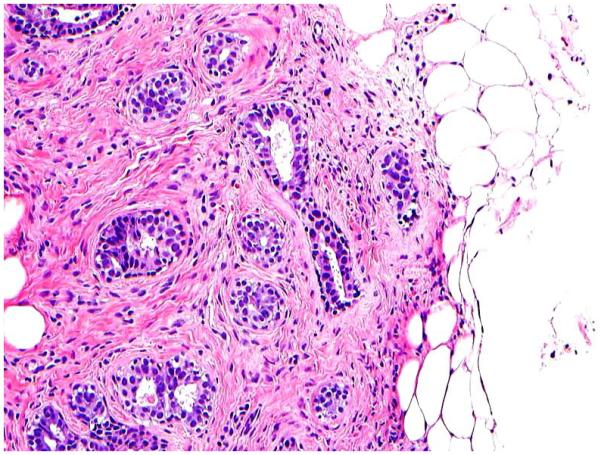
Adipose tissue infiltration or fat entrapment (Figure 1F) was defined as an extension of the stromal cells into the surrounding adipose tissue in an infiltrative pattern. It was considered as a feature of a non-circumscribed lesion.
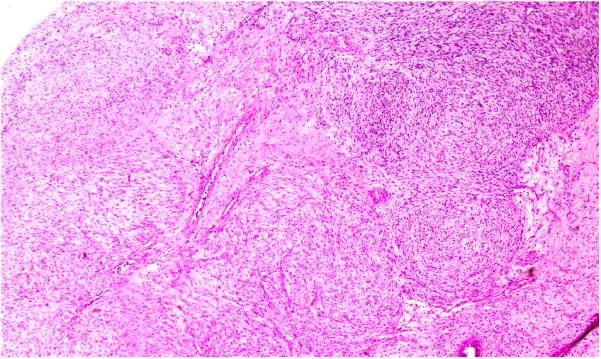
Stromal heterogeneity was defined as the overall heterogeneous appearance of a stromal component. This was demonstrated as a difference in stromal cellularity or atypia in different areas of the same tumor (Figure 1G and 1H). Similarly, the stromal component of an individual case showed hypocellular fibrotic/myxoid appearance (Figure 1G) in one area and hypercellular appearance in another area (Figure 1H).
Subepithelial stromal condensation was defined as an enhancement of stromal density adjacent to or underneath the ductal epithelium (Figure 1I).
Stromal nuclear pleomorphism or cellular atypia [absent, when the stromal cells have small uniform nuclei with evenly distributed chromatin and inconspicuous nucleoli; present, when the stromal cells have variability in nuclear size and shape with nuclear membrane irregularity and conspicuous nucleoli (Figure 1J)].
Figure 1.
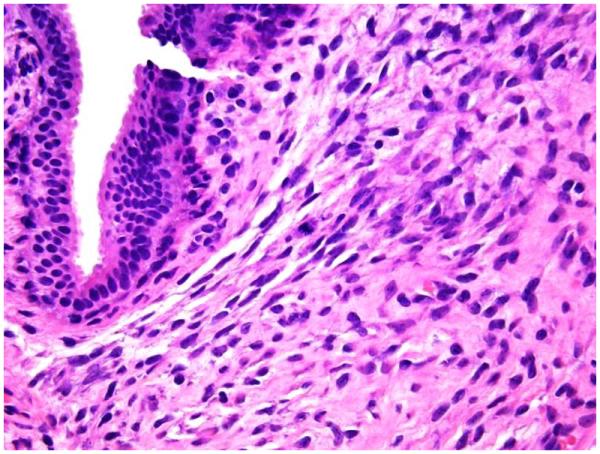
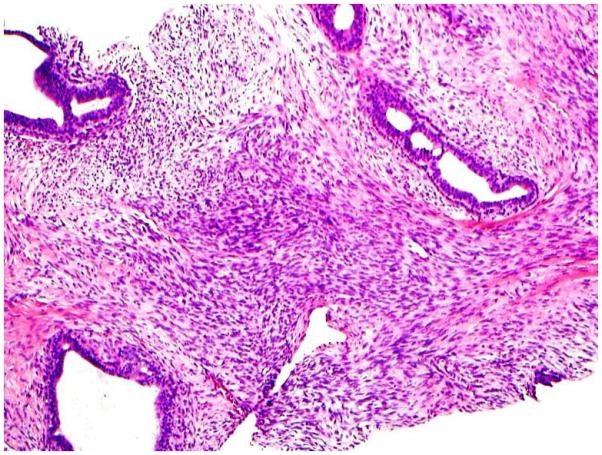
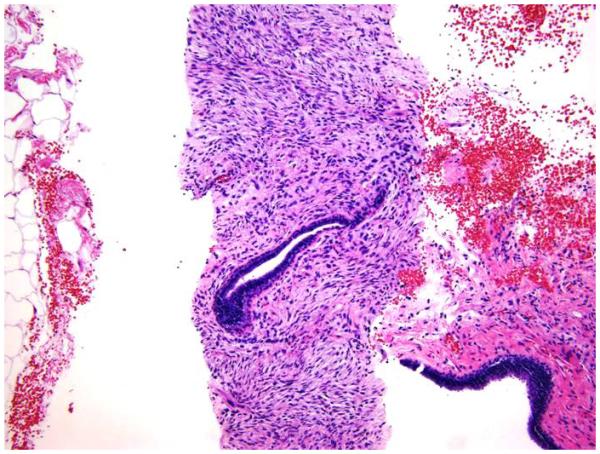
1A: Stromal mitotic activity on 40 x microscopic power magnification (H&E; 40x);
1B: Stromal overgrowth (absence of epithelial component) identified in CNB specimen at 10 x microscopic power magnification (H&E; 10x);
1C: Stromal overgrowth (absence of epithelial component) on excision specimen at 4 x microscopic power magnification H&E; 4x);
1D: Increased stromal cellularity (H&E; 20x);
1E: Stromal fragmentation. Stromal fragments completely lined by epithelial cells (H&E; 4x);
1F: Adipose tissue infiltration (H&E; 4x);
1G: Stromal heterogeneity. Two different areas of the same tumor demonstrate heterogeneity in terms of difference in stromal cellularity and stromal atypia. The stroma is fibrotic (1g) in one area and hypercellular in another area (1h) (H&E; 20x).
1H: Stromal heterogeneity. Two different areas of the same tumor demonstrate heterogeneity in terms of difference in stromal cellularity and stromal atypia. The stroma is fibrotic (1g) in one area and hypercellular in another area (1h) (H&E; 20x).
1I: Subepithelial stromal condensation (H&E; 10x);
1J: Stromal cells demonstrate moderate to severe nuclear pleomorphism and hyperchromasia (H&E; 40x)
Final diagnoses of CFA or BPT/borderline PT were made on the excisional specimens based on the previously established histologic criteria including stromal mitoses, stromal overgrowth, stromal cellularity, infiltration into the surrounding adipose tissue, and stromal atypia.18 The evaluation of excision specimens was performed blinded to the histopathological features evaluated on a respective CNB. All histological features evaluated on CNB were then correlated in retrospective manner with the diagnoses on excision specimens to determine which features on CNB would be helpful in predicting the diagnosis of BPT on subsequent excision.
Clinical characteristics
Patient’s clinical records were reviewed to collect demographic and clinical information in a blinded manner, which includes age of the patient, site and size of the lesion, and other information.
Statistical analysis
Patient characteristics and tumor features were summarized with median and interquartile range (or mean and standard deviation, as appropriate) for continuous data, and with frequencies and percentages for categorical data. As a way to incorporate each of seven features into a single summary measure, the total number of features present (among 7) was calculated for each tumor specimen. In calculating the total number of features, each one was given equal weight regardless of univariate significance, so as not to bias this measure based on the limited data available. Data was compared by diagnosis (BPT versus CFA) using two-sample t-tests (age, mitoses, total features) and with chi-square tests (or Fisher’s exact tests, as appropriate) for categorical features. Logistic regression modeling was used for further univariate and multivariable analysis. The c-statistic was reported for each logistic regression model as a measure of predictive accuracy (equivalent to area under the receiver operating curve). Values of 0.5 indicate that the model does no better than chance, and values of 1 indicate perfect predictive accuracy. All analyses were performed using SAS version 9 (Cary, NC). P-values less than 0.05 were considered statistically significant.
Results
Our initial search identified 73 patients (76 cases) with diagnosis of CFEL on CNB. Twelve patients were excluded from the study cohort (5 patients without subsequent excision, 2 patients with hamartoma on excision and 5 patients with no residual CFEL on excision). Finally, a total of 61 patients met our study criteria. There were 64 CNB and excision specimens, as three patients had bilateral disease and underwent two CNBs and subsequent excision of those lesions. The average age of the entire study cohort was 41 years (range 15 – 83 years). Of the 64 specimens, 27 (42.2%) were diagnosed as PT, including 24 BPT and 3 borderline PT. Thirty seven (57.8%) of 64 cases were diagnosed as CFA. The final diagnoses of PT and CFA were based on histologic features on excisional specimens.
Patient’s demographic information and histological characteristics on CNB were tabulated across the excisional diagnoses (Table 1). The mean age of patients diagnosed with PT and CFA groups was 40.7 and 40.6 years, respectively (p=0.98). The mean tumor size in PT and CFA group was 2.9 and 1.8 cm, respectively (p=0.03). The increase in stromal cellularity was seen in 96.3% (26/27) of PT and 86.5% (32/37) of CFA (p=0.39). The stromal cells demonstrated cytologic/nuclear atypia in 74.1% (20/ 27) of PT and 32.4% (12/37) of CFA (p=0.001). Stromal overgrowth was seen in 42.3% (11/27) PT and only 5.4% (2/ 37) CFA (p=0.0004). Twenty four (88.9%) women with PT and 18 women (48.6%) with CFA showed stromal fragmentation (p=0.001). Stromal heterogeneity was observed in 70.4% (19/27) of PT and 24.3% (9/37) of CFA (p=0.0002). Prominent adipose tissue infiltration was seen in 48.1% (13/27) of PT and 16.2% (6/37) of CFA (p=0.006). Subepithelial condensation was prominent in 63% (17/27) of PT and 16.2% (6/37) of CFA (p=0.0001). The average mitotic figures/ 10hpf were 3 and 0.8 in PT and CFA groups, respectively (p<0.0001). In 27 PT, 20 (74.1%) cases had 3 or more mitoses/10hpf, 3 (11.1%) had 1-2 mitoses /10hpf, and 4 (14.8%) had no mitotic activity. Whereas among 37 CFA cases, the majority (59.5%; 22) had no mitotic activity, 11 (29.7%) had 1-2 mitoses/10hpf and only 4 (10.8%) cases had 3 or more mitoses/ 10hpf. The data on mitotic activity was also analyzed based on two categories (0-2 and 3 or more) which showed that 74.1% (20/27) PT had 3 or more mitoses/ 10hpf, and 89.2% (33/37) CFA had 0-2 mitoses/ 10hpf (p=0.0001).
Table 1.
Univariate Analysis of clinicopathologic features with final excisional diagnosis (CFA and BPT)
| PT (N=27) |
CFA (N=37) |
Total (N=64) |
p value |
C-
statistic |
|
|---|---|---|---|---|---|
| Age | 0.98071 | 0.51 | |||
| Mean (SD) | 40.7 (15.3) | 40.6 (14.5) | 40.7 (14.7) | ||
| Range | (18.0-69.0) | (15.0-83.0) | (15.0-83.0) | ||
| Tumor size | 0.02931 | 0.70 | |||
| Mean (SD) | 2.9 (2.2) | 1.8 (1.1) | 2.2 (1.7) | ||
| Median | 2.3 | 1.5 | 1.8 | ||
| Q1, Q3 | 1.5, 3.5 | 1.0, 2.2 | 1.1, 2.7 | ||
| Range | (0.8-12.0) | (0.6-5.4) | (0.6-12.0) | ||
| Mitoses per 10hpf | <0.00011 | 0.83 | |||
| Mean (SD) | 3.0 (1.7) | 0.8 (1.3) | 1.8 (1.8) | ||
| Median | 3.0 | 0.0 | 1.0 | ||
| Q1, Q3 | 2.0, 4.0 | 0.0, 1.0 | 0.0, 3.0 | ||
| Range | (0.0-6.0) | (0.0-5.0) | (0.0-6.0) | ||
| Mitoses: categorical | <0.00013 | 0.83 | |||
| 0 (/10 HPF) | 4 (14.8%) | 22 (59.5%) | 26 (40.6%) | ||
| 1-2 (/10 HPF) | 3 (11.1%) | 11 (29.7%) | 14 (21.9%) | ||
| ≥3/10 HPF | 20 (74.1%) | 4 (10.8%) | 24 (37.5%) | ||
| Mitoses (<=2 versus >=3) | <0.00013 | 0.82 | |||
| 0-2 (/10 HPF) | 7 (25.9%) | 33 (89.2%) | 40 (62.5%) | ||
| ≥3 (/10 HPF) | 20 (74.1%) | 4 (10.8%) | 24 (37.5%) | ||
| Stromal Overgrowth | 0.00042 | 0.69 | |||
| Missing | 1 | 0 | 1 | ||
| Absent (0) | 15 (57.7%) | 35 (94.6%) | 50 (79.4%) | ||
| Present (1) | 11 (42.3%) | 2 (5.4%) | 13 (20.6%) | ||
| Increased Stromal cellularity | 0.38773 | 0.55 | |||
| Absent (0) | 1 (3.7%) | 5 (13.5%) | 6 (9.4%) | ||
| Present (1) | 26 (96.3%) | 32 (86.5%) | 58 (90.6%) | ||
| Stromal Fragmentation | 0.00113 | 0.70 | |||
| Absent (0) | 3 (11.1%) | 19 (51.4%) | 22 (34.4%) | ||
| Present (1) | 24 (88.9%) | 18 (48.6%) | 42 (65.6%) | ||
| Infiltration into fat | 0.00582 | 0.66 | |||
| Absent (0) | 14 (51.9%) | 31 (83.8%) | 45 (70.3%) | ||
| Present (1) | 13 (48.1%) | 6 (16.2%) | 19 (29.7%) | ||
| Stromal Heterogeneity | 0.00022 | 0.73 | |||
| Absent (0) | 8 (29.6%) | 28 (75.7%) | 36 (56.3%) | ||
| Present (1) | 19 (70.4%) | 9 (24.3%) | 28 (43.8%) | ||
| Subepithelial Condensation | 0.00012 | 0.73 | |||
| Absent (0) | 10 (37.0%) | 31 (83.8%) | 41 (64.1%) | ||
| Present (1) | 17 (63.0%) | 6 (16.2%) | 23 (35.9%) | ||
| Stromal pleomorphism | 0.00102 | 0.71 | |||
| Absent (0) | 7 (25.9%) | 25 (67.6%) | 32 (50.0%) | ||
| Present (1) | 20 (74.1%) | 12 (32.4%) | 32 (50.0%) | ||
|
Combined histologic features excluding
mitotic activity (of 7 possible from above) |
<0.00011 | 0.94 | |||
| Mean (SD) | 3.9 (1.2) | 1.4 (1.0) | 2.5 (1.6) | ||
| Median | 4.0 | 1.0 | 2.0 | ||
| Q1, Q3 | 3.0, 5.0 | 1.0, 2.0 | 1.0, 4.0 | ||
| Range | (2.0-6.0) | (0.0-4.0) | (0.0-6.0) | ||
|
Combined histologic features excluding
mitotic activity, categorized |
<0.00012 | 0.88 | |||
| 0-2 features | 4 (14.8%) | 33 (89.2%) | 37 (57.8%) | ||
| 3-7 features | 23 (85.2%) | 4 (10.8%) | 27 (42.2%) | ||
Unequal Variance T-Test
Chi-Square
Fisher Exact
Histologic features include: stromal mitotic activity, stromal overgrowth, increased stromal cellularity, stromal fragmentation, infiltration into fat, stromal heterogeneity, subepithelial stromal condensation, and stromal nuclear pleomorphism/cellular atypia
On univariate analysis, except for increased stromal cellularity, all histologic features including stromal mitotic activity of 3 or more /10hpf, stromal overgrowth, stromal fragmentation, adipose tissue infiltration, stromal heterogeneity, subepithelial condensation, and stromal atypia/pleomorphism were seen in a significantly higher proportion of women in the PT group than in the CFA group.
When the total numbers of evaluated histologic features were calculated for each individual case, it was found that PT cases had more features on average compared to CFA. The average number of features seen in PT was 3.9 as compared to 1.4 in CFA (p<0.0001, data shown in Table 1). Among total PT cases (27), 23 (85.2%) had 3-7 features and 4 (14.8%) had 0-2 features. In contrast, of 37 CFA cases, only four (10.8%) had 3-7 features, and 33 (89.2%) had 0-2 features (p<0.0001). In general, the likelihood of PT increases as the total number of features increase. Among those with 0-1 features, none were PT. The percentage with PT increases from 23.5% to 70.0% to 87.5% to 100% for 2, 3, 4, and 5-6 features, respectively (no case had 7 total features). Further, the estimated odds ratio of PT for each additional increase in features is 7.8 (95% CI: 2.8 – 21.4, p<0.0001).
With respect to the predictive accuracy of each measure or feature, the total number of evaluated histologic features performed the best (c-statistic for continuous version: 0.94), followed by mitoses (c-statistic: 0.83). Most of the remaining features performed similarly (c-statistics ranging from 0.66-0.73), with exception of increased stromal cellularity (c-statistic: 0.55) and age (c-statistic 0.51) which had essentially no predictive accuracy. Stromal heterogeneity and subepithelial condensation appears to be the best predictors for phyllodes tumor (c-statistics: 0.73 each), followed by stromal pleomorphism (c-statistics = 0.71). [See Table 1].
A multivariable logistic regression model was estimated, including mitoses and total number of other histologic features (stromal overgrowth, increased stromal cellularity, stromal fragmentation, infiltration into fat, stromal heterogeneity, subepithelial stromal condensation, and stromal nuclear pleomorphism) (Figures 2A and 2B). Each variable was statistically significant (mitoses, p=0.01; total histologic features, p=0.0004). The c-statistic for this model was 0.95, and this indicates excellent discrimination between these two groups based on this model. The odds ratio for PT (as compared to CFA) for mitoses was 2.14 (adjusted for total features, 95% CI: 1.18, 3.86); the odds ratio for total number of histologic features was 7.27 (adjusted for mitoses, 95% CI: 2.44, 21.69). The odds ratio can be interpreted as the multiplicative increase in odds of PT for each 1-unit increase in the predictor. In a separate model that included tumor size, similar results were found, and tumor size was not statistically significant (data not shown).
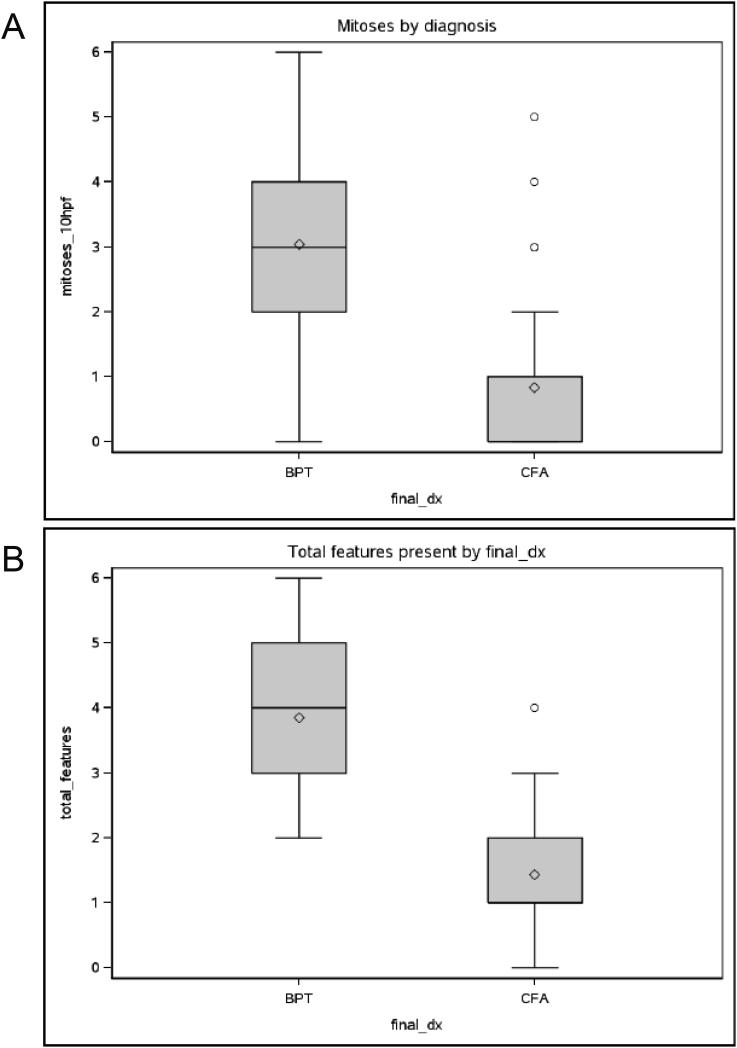
Figure 2.
The boxplots below show the difference in mitoses (2A) and total number of histologic features (2B) between the groups. Note how little they overlap. To interpret the boxplots, the shaded box shows the middle 50% of the data (between the 25th and 75th percentiles), along with the median shown as the horizontal line in the middle of the box – note, that the median and 25th percentile are equal among those with CFA for each of these two measures. The mean is shown by the diamond.
The sensitivity and specificity values for mitotic activity and total number of histologic features were calculated. The sensitivity for detecting PT with mitosis ≥ 3 was 74.1%. Similarly, the sensitivity for detecting PT with total histologic features of 3 or more is 85.2%.
Among those who have CFA, the specificity of mitoses of ≤ 2 is 89.2%, and the specificity of total histologic features of ≤ 2 for CFA is also 89.2%.
Discussion
Cellular fibroepithelial lesions on CNB are commonly encountered entities in everyday practice, and differential diagnosis includes CFA or PT. Distinction between the two is often challenging due to significant morphological overlap and lack of consensus on objective criteria and clear cut-off values. In other words, there are no uniform or standardized distinguishing features that discriminate between CFA and PT on CNB. Most of the previous studies had focused on identifying individual histologic features for differentiation between the two entities. Similarly, our data highlights important histological features on CNB that can help predict PT on subsequent excision. On univariate analysis, except for increased stromal cellularity, all other histological features evaluated including stromal mitoses ≥ 3 /10hpf, stromal overgrowth, stromal fragmentation, adipose tissue infiltration, stromal heterogeneity, subepithelial condensation, and stromal nuclear pleomorphism were statistically significant features that discriminate PT from CFA.
Jara-Lazaro et al.20 showed that moderate-to-severe stromal cellularity, stromal overgrowth, moderate nuclear atypia, stromal mitoses of ≥ 2/ 10hpf and ill-defined lesional borders on CNB were exclusive to the diagnosis of PT on excision. Lee et al.21 reported that an increase in stromal cellularity of at least 50% greater than typical fibroadenoma, stromal overgrowth, fragmentation, and adipose tissue infiltration were significantly more common in CNB of phyllodes tumors. Similar results were reported in another study as well.15 Gould et al. have found additional interesting features that are more associated with phyllodes tumor on excision after a diagnosis of fibroepithelial neoplasm in CNB: larger tumor diameter (average 4.0 cm; p < 0.002) and increased hyperechoic mass density (p < 0.001) on preoperative imaging, in addition to Hispanic ethnicity.22
In agreement with other studies15, 20, we found that the presence of stromal overgrowth evaluated at 10x magnification was a useful feature. In comparison to other studies15, 20, we found that increased stromal cellularity was not a helpful feature (seen in 96.3% and 86.5% of PT and CFA, respectively). This difference could be related to our lower threshold for increased stromal cellularity.
Most of the individual histologic features assessed in this study were seen in both PT and CFA groups. Therefore, the use of a combination of histologic features would be desirable and more helpful. Our data highlights an important aspect of our review that the total number of histologic features seen in an individual case of PT is significantly more than in those with CFA regardless of which particular features were seen. The vast majority of PT cases (85.2%) demonstrated a combination of > 3 histologic features. In contrast, the majority of CFA cases (89.2%) showed < 3 histologic features. In our cohort, an average number of histologic features seen in PT were 3.9 (SD – 1.2), whereas it is 1.4 in CFA (SD – 1.0). In other words, there is very little overlap in the number of features seen in PT and CFA groups. This is statistically significant (p <0.0001) and helpful in real practice, as it provides clear cut number of histologic features that are in favor of PT.
Our data supports that the presence of any three or more evaluated histologic features (stromal overgrowth, increased stromal cellularity, stromal fragmentation, infiltration into fat, stromal heterogeneity, subepithelial stromal condensation, and stromal nuclear pleomorphism) on CNB favors PT over CFA on multivariate analysis. The likelihood or probability of BPT increases with every 1-unit increase in the number of histologic features assessed on CNB. In particular, the constellation of stromal heterogeneity, subepithelial condensation and stromal pleomorphism appears to be the best predictors for PT (table 1).
Furthermore, our data showed that the stromal mitotic activity of ≥ 3 by itself was very helpful in discriminating PT based on a multivariable logistic regression model. Both mitotic activity (p=0.01) and the total number of histologic features (p=0.0004) were statistically significant. The patient’s clinical characteristics including age and tumor size were not helpful in distinguishing PT and CFA on univariate (data shown in Table 1), and multivariate analysis respectively (date not shown). In contrast to previous studies, we showed that patient’s age as well as tumor size is not a useful clinical parameter in the distinction of PT.7,22-24
In conclusion, there are two important significant findings in the current study that are in favor of the diagnosis of PT on follow-up excision. The finding of prominent mitotic activity (≥3/ per 10 HPF) is by itself adequate to favor PT over CFA based on multivariate analysis. On the other hand, if there is no stromal mitotic activity, the constellation of other (at least three) histologic features (stromal overgrowth, increased stromal cellularity, stromal fragmentation, infiltration into fat, stromal heterogeneity, subepithelial stromal condensation, and stromal nuclear pleomorphism) in a CNB are more predictive of PT than CFA.
Acknowledgements
We acknowledge the editorial assistance of Victoria L. Jackson, MLIS (Academic and Research Support, Mayo Clinic, Jacksonville, FL).
Footnotes
Conflict of Interest Statement: All authors disclose that there is neither financial interest nor significant affiliation with any entity.
Study Funding: None
References
- 1.Fekete P, Petrek J, Majmudar B, et al. Fibroadenoma with stromal cellularity. A clinicopathologic study of 21 patients. Arch Pathol Lab Med. 1987;111:427–432. [PubMed] [Google Scholar]
- 2.Remadi S, Ismail A, Karpuz V, et al. Cellular (Juvenile) fibroadenoma of the breast. A clinico-pathologic and immunohistochemical study of 7 cases. Ann Pathol. 1994;14:392–397. [PubMed] [Google Scholar]
- 3.Pike AM, Oberman HA. Juvenile (cellular) adenofibroma. A clinicopathologic study. Am J Surg Pathol. 1985;9:730–736. doi: 10.1097/00000478-198510000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Barth RJ., Jr Histologic features predict local recurrence after breast conserving therapy of phyllodes tumours. Breast Cancer Res Treat. 1999;57:291–295. doi: 10.1023/a:1006260225618. [DOI] [PubMed] [Google Scholar]
- 5.Hajdu SI, Espinosa MH, Robbins GF. Recurrent cystosarcoma phyllodes: a clinicopathologic study of 32 cases. Cancer. 1976;38:1502–1506. doi: 10.1002/1097-0142(197609)38:3<1402::aid-cncr2820380346>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Pietruszuka M, Barnes L. Cystosarcoma phyllodes. Cancer. 1978;41:1974–1983. doi: 10.1002/1097-0142(197805)41:5<1974::aid-cncr2820410543>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Reinfuss M, Mituś J, Duda K, et al. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77:910–916. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.de Roos WK, Kaye P, Dent DM. Factors leading to local recurrence or death after surgical resection of phyllodes tumours of the breast. Br J Surg. 1999;86:396–399. doi: 10.1046/j.1365-2168.1999.01035.x. [DOI] [PubMed] [Google Scholar]
- 9.Mangi AA, Smith BL, Gadd MA, et al. Surgical management of phyllodes tumors. Arch Surg. 1999;134:487–4892. doi: 10.1001/archsurg.134.5.487. discussion 92-93. [DOI] [PubMed] [Google Scholar]
- 10.Zurrida S, Bartoli C, Galimberti V, et al. Which therapy for unexpected phyllodes tumour of the breast. Eur J Cancer. 1992;28:654–657. doi: 10.1016/s0959-8049(05)80119-4. [DOI] [PubMed] [Google Scholar]
- 11.Tan PH, Jayabaskar T, Chuah KL, et al. Phyllodes tumours of the breast: the role of pathologic parameters. Am J Clin Pathol. 2005;123:529–540. doi: 10.1309/U6DV-BFM8-1MLJ-C1FN. [DOI] [PubMed] [Google Scholar]
- 12.Kleer CG, Giordano TJ, Braun T, et al. Pathologic, immunohistochemical, and molecular features of benign and malignant phyllodes tumors of the breast. Mod Pathol. 2001;14:185–190. doi: 10.1038/modpathol.3880282. [DOI] [PubMed] [Google Scholar]
- 13.Tan EY, Tan PH, Yong WS, et al. Recurrent phyllodes tumours of the breast: pathological features and clinical implications. ANZ J Surg. 2006 Jun;76(6):476–80. doi: 10.1111/j.1445-2197.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs TW, Connolly JL, Schnitt SJ. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol. 202;26:1095–110. doi: 10.1097/00000478-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs TW, Chen YY, Guinee DG, et al. Fibroepithelial lesions with cellular stroma on breast core needle biopsy: are there predictors of outcome on surgical excision? Am J Clin Pathol. 2005;124:342–354. doi: 10.1309/5N2C-4N5X-CB8X-W8JL. [DOI] [PubMed] [Google Scholar]
- 16.Morgan JM, Douglas-Jones AG, Gupta SK. Analysis of histological features in needle core biopsy of breast useful in preoperative distinction between fibroadenoma and phyllodes tumour. Histopathology. 2010;56:489–500. doi: 10.1111/j.1365-2559.2010.03514.x. [DOI] [PubMed] [Google Scholar]
- 17.Yohe S, Yeh IT. “Missed” diagnoses of phyllodes tumor on breast biopsy: Pathologic clues to its recognition. Int J Surg Pathol. 2008;16:137–142. doi: 10.1177/1066896907311378. [DOI] [PubMed] [Google Scholar]
- 18.Komenaka IK, El-Tamer M, Pile-Spellman E, et al. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Arch Surg. 2003;138:987–990. doi: 10.1001/archsurg.138.9.987. [DOI] [PubMed] [Google Scholar]
- 19.Tsang AK, Chan SK, Lam CC, et al. Phyllodes tumours of the breast – differentiating features in core needle biopsy. Histopathology. 2011;59:600–608. doi: 10.1111/j.1365-2559.2011.03939.x. [DOI] [PubMed] [Google Scholar]
- 20.Jara-Lazaro AR, Akhilesh M, Thike AA, et al. Predictors of phyllodes tumours on core biopsy specimens of fibroepithelial neoplasms. Histopathology. 2010;57:220–232. doi: 10.1111/j.1365-2559.2010.03607.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee AH, Hodi Z, Ellis IO, et al. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology. 2007;51:336–344. doi: 10.1111/j.1365-2559.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 22.Gould DJ, Salmans JA, Lassinger BK, et al. Factors associated with phyllodes tumor of the breast after core needle biopsy identifies fibroepithelial neoplasm. J Surg Res. 2012;178:299–303. doi: 10.1016/j.jss.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 23.Ward ST, Jewkes AJ, Jones BG, et al. The sensitivity of needle core biopsy in combination with other investigations for the diagnosis of phyllodes tumor of the breast. Int J Surg. 2012;10:527–531. doi: 10.1016/j.ijsu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Foxcroft LM, Evans EB, Porter AJ. Difficulties in the pre-operative diagnosis of phyllodes tumours of the breast: a study of 84 cases. Breast. 2002;16:27–37. doi: 10.1016/j.breast.2006.05.004. [DOI] [PubMed] [Google Scholar]