Abstract
Objective
To investigate whether a histone deacetylase inhibitor (HDACi) would be effective in an in vitro model for the neurodegenerative disease Friedreich ataxia (FRDA) and to evaluate safety and surrogate markers of efficacy in a phase I clinical trial in patients.
Methods
We used a human FRDA neuronal cell model, derived from patient induced pluripotent stem cells, to determine the efficacy of a 2-aminobenzamide HDACi (109) as a modulator of FXN gene expression and chromatin histone modifications. FRDA patients were dosed in 4 cohorts, ranging from 30mg/day to 240mg/day of the formulated drug product of HDACi 109, RG2833. Patients were monitored for adverse effects as well as for increases in FXN mRNA, frataxin protein, and chromatin modification in blood cells.
Results
In the neuronal cell model, HDACi 109/RG2833 increases FXN mRNA levels and frataxin protein, with concomitant changes in the epigenetic state of the gene. Chromatin signatures indicate that histone H3 lysine 9 is a key residue for gene silencing through methylation and reactivation through acetylation, mediated by the HDACi. Drug treatment in FRDA patients demonstrated increased FXN mRNA and H3 lysine 9 acetylation in peripheral blood mononuclear cells. No safety issues were encountered.
Interpretation
Drug exposure inducing epigenetic changes in neurons in vitro is comparable to the exposure required in patients to see epigenetic changes in circulating lymphoid cells and increases in gene expression. These findings provide a proof of concept for the development of an epigenetic therapy for this fatal neurological disease.
Friedreich ataxia (FRDA; Online Mendelian Inheritance in Man database #229300) is an autosomal recessive inherited degenerative disorder affecting the nervous system and the heart, with a prevalence of approximately 2 to 3 in 100,000 in North America and in Europe.1 This neurological syndrome is characterized by progressive trunk and limb ataxia, dysarthria, instability of fixation, sensory neuropathy, and pyramidal weakness. Signs of hypertrophic cardiomyopathy are found in most patients,2 10% have diabetes, and almost all have systemic carbohydrate metabolism abnormalities.3 At the molecular level, >95% of FRDA patients carry a GAA•TTC trinucleotide repeat expansion in the first intron of the FXN gene,4 leading to heterochromatin-mediated transcriptional repression5–9 and reduction of the essential mitochondrial protein frataxin.4 Frataxin is a component of the protein complex that assembles iron-sulfur clusters in mitochondria.10 Its loss leads to impaired mitochondrial function and altered cellular iron homeostasis.11
One therapeutic approach for FRDA is epigenetic modulation of gene expression at the FXN locus through chromatin acetylation by histone deacetylase (HDAC) inhibition.6 A recent report has shown efficacy of the sirtuin inhibitor nicotinamide at high doses in reactivating the FXN gene in blood from patients in a phase I clinical trial, providing support for this therapeutic approach.12 It has been shown previously that HDAC inhibition leads to increased expression of FXN mRNA in patient lymphoblastoid cell lines and peripheral blood mononuclear cells (PBMCs)6,13–15 treated ex vivo. Although in vivo treatment using transgenic animal models that carry expanded GAA•TTC repeats has corroborated the findings in human blood cells, showing increased FXN mRNA and protein in target tissues13,16,17 and reduced disease-related pathology,17 the question remains whether the human target tissue in FRDA, the neuron, would demonstrate the same molecular pathology and response to treatment with a disease-modifying agent as the surrogate tissue, the PBMC. Derivation of neurons from patient-derived induced pluripotent stem cells (iPSCs) is an important new tool to address this question.18,19
Here we demonstrate that HDAC inhibition in vitro via 10913 (under the development name of RG2833 for the formulated drug product) in FRDA neurons derived from patient iPSCs reverses FXN gene silencing to a degree comparable to that found in previous studies employing human PBMCs and mouse models. 6,13,16,17 In these latter studies, brain penetration and HDAC inhibition were established in vivo. We now report reversal of the heterochromatin state and upregulation of FXN mRNA and frataxin protein in these neuronal cells. We also demonstrate HDAC inhibition and increased H3K9 acetylation in PBMCs and an increase in FXN mRNA in blood from patients treated with RG2833. Importantly, we observe that threshold exposures for FXN gene expression changes in vivo are comparable to those observed in vitro with both patient PBMCs and iPSC-derived neurons, validating these cellular systems as valuable tools for projecting effective doses in vivo.
Materials and Methods
Cell Culture and In Vitro Differentiation
iPSC culture condition, neuronal differentiation, neurosphere, and neuronal culture were described previously.20,21 Generally, experiments were done with neurons at 8-days postdifferentiation, except for the electrophysiology experiments, where the neurons were matured for 7 to 8 weeks.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 10 minutes at ambient temperature and permeabilized/blocked with 10% goat serum/0.1% Triton X-100 detergent for 1 hour at ambient temperature (all in phosphate-buffered saline [PBS]). Primary antibodies were incubated at 4°C overnight or at ambient temperature. After three 5-minute washes, secondary antibodies were incubated at ambient temperature for 1 hour. After 3 more washes, nuclei were stained with DAPI (10μg/ml) at ambient temperature for 15 minutes. All washes and incubations were performed in blocking buffer. Cells were imaged using an Olympus (Tokyo, Japan) IX-70 inverted fluorescent microscope. Primary antibodies included β-III tubulin (Tuj1; Covance, Princeton, NJ; 1:400), microtubule associated protein 2 (MAP2; Millipore, Billerica, MA; 1:500), and neuronal nuclei/FOX3 (NeuN; Millipore, 1:10), and secondary antibodies included antimouse Alexa 488 and antirabbit Alexa 488 (Invitrogen, Carlsbad, CA; both at 1:1,000).
Conventional Polymerase Chain Reaction, Quantitative Reverse Transcriptase Polymerase Chain Reaction, and Microarray Analysis
Polymerase chain reaction (PCR) conditions to measure GAA•TTC repeat length were described previously.21 Quantitative reverse transcriptase PCR (qRT-PCR) was performed using the qScript One-Step SYBR Green qRT-PCR kit from Quanta Biosciences (Gaithersburg, MD) according to the manufacturer’s instructions. qRT-PCR reactions were detected on a PTC-200 thermal cycler with the Chromo4 real-time module (MJ Research, Waltham, MA). Primers to detect FXN mRNA were previously described.6 Primers for neuronal characterization were as follows: MAP2-R1 (5′-CAGGAGTGATGGCAGTAGAC-3′), MAP2-F2 (5′-TTTGGAGAGCATGGGTCAC-3′) for the MAP2 gene, HUC-F1 (5′-GGTTCGGGACAAGATCACAG-3′), and HUC-R1 (5′-CTGAACTGGGTCTGGCATAG-3′) for the ELAVL3 gene. qRT-PCR primers for pluripotency mRNA markers were as previously published.20 Neuronal cell gene expression profiling was performed on Illumina (San Diego, CA) HT12 arrays, and statistical analysis was as described in Ku et al.20
Flow Cytometry and Quantitative Western Analysis
Flow cytometry and quantitative Western analysis were performed as previously described.20 Antibodies used in flow cytometry were the following: Tuj1 (Covance, 1:2500), MAP2 (BD Biosciences, Franklin Lakes, NJ; 1:40), glial fibrillary acidic protein (GFAP; Millipore, 1:20), Tra1–81 (Millipore, 1:500), and SSEA3 (Millipore, 1:500). Secondary antibodies were antimouse Alexa 488 (1:1,000), antirat Alexa 488 (1:1,000), or antirabbit Alexa 647 (1:1,000). The following antibodies were used in quantitative Western analysis: frataxin (MitoSciences, Eugene, OR; 1:1,000), RPL13a (Cell Signaling Technology, Danvers, MA; 1:2,000), and RNA polymerase II (Millipore; 1:2,000). Antibodies against acetylated residues of histone H3 and H4 have been described.22 The following secondary antibodies were all obtained from LI-COR Biosciences (Lincoln, NE) and used at the same dilution (1:5,000): antimouse IR680, antimouse IR800, antirabbit IR680, and antirabbit IR800.
Chromatin Immunoprecipitation
Analyses of histone tail modifications on the FXN gene in neurons, iPSCs, and fibroblasts were performed as previously reported.6,22 Native chromatin immunoprecipitation was performed as follows. PBMCs were resuspended at 8 × 106 cells/ml in ice-cold buffer N (15mM Tris pH 8, 15mM NaCl, 60mM KCl, 250mM sucrose, 5mM MgCl2, 1mM CaCl2, and protease inhibitors); 0.5% NP-40 was added, and cells were incubated on ice for 10 minutes to complete cell lysis. Nuclei were collected by centrifugation at 524 × g for 5 minutes and resuspended in ice-cold buffer N. After determining the nucleic acid content by spectrophotometry, 1U of micrococcal nuclease was added per microgram of DNA and samples were incubated for 10 minutes at 37°C. The reaction was stopped by addition of 10mM ethylenediaminetetraacetic acid (EDTA) and 10mM ethyleneglycoltetraacetic acid. Samples were centrifuged for 5 minutes at 10,000rpm, and supernatant was collected and visualized on an agarose gel to determine the average DNA length. Chromatin (4–5μg) was diluted in chromatin immunoprecipitation (ChIP) low salt buffer (20mM Tris-Cl, pH 8, 140mM NaCl, 2mM EDTA, 0.1% NP-40), 1μg of antibody against acetylated histone H3K9 (Millipore) was added, and samples were incubated at 4°C overnight. The next day, 60μl of protein A agarose bead suspension (Millipore) was added, and samples were incubated for 2 hours at 4°C. Beads were then washed 3 times with ChIP low salt buffer and once with ChIP high salt buffer (same as ChIP low salt buffer but with 0.5 M NaCl). Chelex 100 resin (100μl; 10% solution wt/vol in water; Bio-Rad Laboratories, Hercules, CA) was added to the bead–immunoglobulin G complexes, and samples were heated at 100°C for 10 minutes. Proteinase K was added (100μg/ml), and samples were incubated at 55°C for 30 minutes, followed by heat inactivation at 100°C for 10 minutes. Supernatant was collected, and DNA recovery was analyzed by quantitative PCR using primers specific for the FXN region upstream of the GAA•TTC repeat expansion in intron 1 (see Herman et al6 for primer sequences).
Bisulfite Sequencing
Genomic DNA was extracted using the QIAamp DNA mini kit (QIAGEN, Valencia, CA), and bisulfite treatment of genomic DNA was performed using the EZ DNA methylation-Gold kit (Zymo Research, Orange, CA), following manufacturer instructions. Bisulfite-converted DNA was amplified using the following primers: for the sequence upstream of the GAA•TTC repeats, 5′-GTTGTGGGGATGAGGAAGATTT TT-3′ and 5′-TAATCCACCTTCCTAAACCTCCCA-3′; for the sequence downstream of the GAA•TTC repeats, primer sequences were described in Al-Mahdawi et al.8 PCR amplicons were cloned into the pCR-Blunt II-TOPO vector (Life Technologies, Grand Island, NY), and 20 clones per amplicon were sequenced.
Electrophysiology
Conventional whole cell patch clamp techniques were used for dissociated cells plated on poly-d-lysine and laminin matrigel–coated glass coverslips. Coverslips containing neuronal iPSCs were placed on the stage of a Nikon (Tokyo, Japan) Eclipse Ti inverted stage microscope for patch clamp recordings. Recordings were made using an Axon 700B Multiclamp amplifier (Axon Instruments, Sunnyvale, CA). Signals were sampled at 10kHz and filtered at 1.6kHz. Whole cell capacitance was fully compensated, and series resistance was compensated 60 to 70%. iPSC-derived neurons were identified based on morphology under bright field differential interference contrast. Coverslips of neuronal iPSCs were transferred to a recording chamber constantly perfused with bath solution consisting of (in millimolars): 140 NaCl, 5 KCl, 0.8 MgCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and 10 glucose (pH 7.4). Recording electrodes (tip resistance of 4–6MΩ) were filled with solution containing (in millimolars): 20 KCl, 100 K-gluconate, 10 HEPES, 4 Mg2+-adenosine triphosphate, 0.3 sodium guanosine triphosphate, and 10 phosphocreatine (pH 7.3). For voltage clamp recordings, cells were clamped at −70mV; Na+ and K+ currents were stimulated by voltage step depolarizations. Command voltages varied from −90 to +50mV in 10mV increments. For current clamp recordings, action potentials (APs) were induced by stimulation steps from −0.2 to +0.5 nA. All recordings were performed at ambient temperature. Offline analysis of data was performed using the Clampfit (pCLAMP 10 software, v10.2 Molecular Devices, Sunnyvale, CA), Excel (Microsoft, Redmond, WA), and Origin (OriginLab, Northampton, MA) programs.
Fluorescence In Situ Hybridization
HEKGFP560GAA cells,23 fibroblasts, and FRDA neurons were plated onto coverslips and fixed in 4% paraformaldehyde in PBS for 30 minutes at room temperature. After fixation, cells were washed twice with PBS and stored overnight in 70% ethanol at 4°C. Cells were then rehydrated in 50% formamide, 2× standard saline citrate (SSC) for 5 minutes at room temperature and hybridized overnight at 37°C in 100μl of a solution containing 10% dextran sulfate, 2mM vanadyl–ribonucleoside complex, 0.2% bovine serum albumin, 100μg yeast tRNA, 2× SSC, 50% formamide, and 1.2μg Cy3-(TTC)10 probe. After hybridization and washing, nuclei were stained in Hoechst 33342 (1:200 dilution) for 30 minutes at ambient temperature. Coverslips were mounted on slides using Prolong Gold Antifade Reagent. Cells were imaged with a ×63 objective on a Zeiss (Oberkochen, Germany) LSM 710 laser scanning confocal microscope.
Formulation of RG2833 for Clinical Studies
Both RG2833 and placebo were produced, packed, and labeled according to Good Manufacturing Practice. RG2833 drug product capsules contained 30mg, 60mg, or 150mg of 109 free-base equivalent. Each capsule contained a blend of RG2833 active pharmaceutical ingredient (109) and Ac-Di-Sol (2% croscarmellose sodium).
Clinical Study Design
A phase I crossover, escalating dose clinical study of orally administered RG2833 was performed in 20 adults with FRDA. The study consisted of 4 cohorts of 5 FRDA patients. Cohort 1 and 2 were open label, with single 30 to 120mg doses. Cohorts 3 and 4 were randomized, double-blind, placebo-controlled crossover studies. Cohort 3 received a single 180mg or placebo dose; Cohort 4 received two 120mg or placebo doses, 4 hours apart. Primary outcome was safety, pharmacokinetic and pharmacodynamic response on HDAC inhibition, and FXN mRNA and frataxin protein levels in patient blood cells. All patients gave written informed consent according to the principles of Good Clinical Practice. The study protocol and consent forms were approved by the Italian Superior Health Institute (protocol No. 31637[11]PRE21–1101; EudraCT No. 2011-000248-12) and by the Ethical Committee of San Luigi Gonzaga University Hospital (protocol No. 10090-25/05/12; file No. 172/2011). An independent data safety monitoring board reviewed major safety assessments and periodically assessed safety and efficacy. Data collection and monitoring, and safety reporting management were performed by independent expert contractors. The academic investigators vouch for the validity of the results.
Patients
Inclusion criteria included age 18 to 55 years inclusive; genotype confirmed for homozygous GAA•TTC triplet expansion at FXN locus, with at least 100 repeats at the shortest allele; left ventricular ejection fraction ≥ 50% documented by echocardiogram or equivalent study within 1 year of day 1; and medically stable condition. Exclusion criteria included chronic or intermittent clinically significant cardiac arrhythmias at any time during the year prior to the screening visit; clinically significant electrocardiogram (ECG) and Holter ECG findings (including clinically significant cardiac arrhythmias) at the screening visit; history of significant allergic reaction or sensitivity; current or history of other clinically significant disease in the past 12 months prior to screening; use of any medication (excluding ibuprofen, acetaminophen, and oral contraceptives) or of any dietary aids or supplements known to modulate drug metabolizing enzymes within 24 hours prior to study drug administration; and treatment with an investigational drug, device, or biological agent within 90 days prior to screening. Patient demographic and clinical characteristics are reported in Table 1. Patients ECG telemetry was monitored for 72 hours starting 12 hours before drug administration, and 12-lead ECG was repeated every 6 hours during the day of drug administration and every 24 hours during the 4 in-hospital days.
TABLE 1.
Demographic and Clinical Characteristics of Study Subjects
| Characteristic | Value |
|---|---|
| Demographics | |
| Age, yra | 30.0±8.1 |
| Sex, No. (%) | |
| M | 9 (40.9) |
| F | 13 (59.1) |
| Disease data | |
| GAA•TTC triplet expansion on shortest allelea | 1,084.8±784.5 |
| Age of onset, yra | 10.7±4.6 |
| FARSb score at screeninga | 59.7±23.2 |
| Cardiac function, ejection fraction %a | 63.0±6.9 |
Data are shown as the mean±standard deviation.
See Beconi et al.45
F=female; FARS=Friedreich Ataxia Rating Scale; M=male.
RG2833 Plasma Drug Concentration Analysis
Plasma samples were thawed, and protein was precipitated by addition of 9 volumes of acetonitrile. Supernatant was then transferred to a new tube containing 9 volumes of an ammonium acetate aqueous buffer. Samples were analyzed by ultra performance liquid chromatography/mass spectrometry using a Waters (Milford, MA) Acquity BEH C18 column with a water/acetonitrile gradient containing formic acid. Polarity of the mass spectrometer was set to positive mode, and the transition from 340.3 to 119.1amu was used to quantify RG2833. Pharmacokinetic parameters for RG2833 and metabolites in plasma were calculated using noncompartmental analysis. Only plasma concentrations greater than the respective lower limit of quantification (LOQ) were used in the pharmacokinetic analysis. The maximum plasma concentration (Cmax) and time to Cmax were taken directly from the data. The elimination rate constant, lz, was calculated as the negative of the slope of the terminal log-linear segment of the plasma concentration–time curve. The slope was determined from a linear regression of the natural logarithm of the terminal plasma concentrations against time; at least 3 terminal plasma concentration time points, beginning with the final concentration greater than LOQ, were selected for the determination of lz, and the regression had to have a coefficient of determination (r2)≥0.9000. The range of data used for each patient was determined by visual inspection of a semilogarithmic plot of concentration versus time. Elimination half-life (t½) was calculated according to the following equation:
Area under the curve (AUC) to the final sample with a concentration greater than LOQ [AUC(0-t)] was calculated using the linear trapezoidal method and extrapolated to infinity [AUC(inf)] using
where Ctf and tf are the final concentration ≥ LOQ and the time at which it occurred.
Clearance (CL/F) and volume of distribution (Vz/F), uncorrected for bioavailability (F), were calculated for RG2833 according to
respectively.
Whole Blood and PBMC FXN qRT-PCR
The levels of FXN transcript were measured with a SYBR green-based qRT-PCR assay. For whole blood analysis, total RNA was isolated from blood collected with PAXgene Blood RNA tubes and processed using PAXgene Blood RNA Kit IVD (QIAGEN). For RG2833 in vitro treated PBMC analysis, PBMCs were isolated from predose patient blood with Ficoll gradient and treated with RG2833 in culture for 24 hours. Cells were then pelleted, and RNA was isolated from the cell pellets using the RNeasy Mini Kit (QIAGEN). cDNA was then synthesized using the RT2 HT First Strand Kit (SABiosciences, Valencia, CA). Twenty-five nanograms of cDNA was combined with RT2 FAST SYBR Green/ROX qPCR master mix, RT2 qPCR primer assay for FXN (PPH05744B, SABiosciences), and RT2 qPCR primer assay for TBP (PPH01091G, SABiosciences) in a 25μl reaction volume. Assays were performed in duplicate using an Mx3005 or Mx3000 instrument and analyzed using MxPro Software (Agilent Technologies, Santa Clara, CA). The amount of FXN mRNA in each sample was determined relative to the predose sample and normalized to the levels of TBP mRNA.
PBMC Isolation, Frataxin Protein, and Deacetylase Assay
PBMCs were isolated from whole blood using BD (Franklin Lakes, NJ) Vacutainer CPT tubes, following manufacturer instructions. Erythrocytes were lysed by treatment with buffer EL (QIAGEN). PBMCs isolated at the clinical site were lysed with lysis buffer (25mM Tris-HCl, pH 8.0, 137mM NaCl, 2.7mM KCl, 1mM MgCl2, 1% IGEPAL CA-630) on ice for 20 minutes. Supernatant was collected after centrifugation, and cell lysate protein concentration was measured with bicinchoninic acid protein assay reagent (Thermo Scientific, Waltham, MA). Frataxin protein was determined with a dipstick assay, as described.15 HDAC deacetylase assay was performed using a 96-well microplate assay by incubating cell lysate containing 0.5mg/ml of protein with 50mM of Fluor de Lys substrate (Enzo Life Sciences, Plymouth Meeting, PA) at ambient temperature for 30 minutes. At the end of the incubation, equal volume of stop solution (10mg/ml trypsin with 2mM trichostatin A) was added, and the mixture was incubated at room temperature for 15 minutes. Fluorescence was then measured at excitation wavelength 360nm and emission wavelength 460nm. The level of deacetylase activity in each dosed PBMC sample was determined as a percentage of the predose sample fluorescence.
Results
A Human Neuronal Model for FRDA
FRDA iPSCs20 were differentiated into neuronal cells in vitro21 using established methods.24 Immunocytochemical analysis revealed positive staining for the neuronal markers Tuj1, MAP2, and NeuN, and quantitative RTPCR showed a decrease in the expression levels of pluripotency-associated mRNAs (OCT4, NANOG, GDF3, TERT, and REX1) and an increase in neuron-specific mRNAs (HUC and MAP2) compared to undifferentiated FRDA iPSCs and neural stem cells (Fig 1). Flow cytometry indicated the presence of a relatively high percentage of Tuj1+ cell population (98%) and a MAP2+ population (95%) and a low percentage of cells expressing the glial-associated marker GFAP. Next, to determine whether our differentiated neuronal cells possessed the electrophysiological properties of typical neurons, we performed whole cell patch clamp recordings. In voltage clamp mode, a series of voltage steps to iPSC-derived neurons evoked large inward currents closely followed by outward currents. These currents resembled the voltage-gated inward Na+ and outward K+ conductances that underlie the generation of APs in typical neurons. In current clamp mode, an injection of a depolarizing current triggered single and, in most cases, multiple APs in unaffected and FRDA neurons. Application of tetrodotoxin (1μM), a voltage-gated Na+ channel antagonist, reversibly blocked AP generation, indicating that iPSC-derived neurons behaved as typical functional neurons (data not shown). No significant differences were found between the unaffected and FRDA neurons after 7 to 8 weeks of differentiation in any of these electrophysiological measures, as previously reported.25 Finally, we performed microarray studies to compare global gene expression of our iPSC-derived neurons to that of unaffected human iPSCs and different fetal tissues. Hierarchical clustering showed that these cells have a transcriptional profile close to that of fetal brain. Together, these data provide evidence that our FRDA iPSCs were differentiated to early Tuj1+/MAP2+ neuronal cells in relatively high purity.
FIGURE 1.
Characterization of induced pluripotent stem cell (iPSC)-derived neuronal cells. (A) Immunohistochemical staining of neuronal markers in early Friedreich ataxia (FRDA) neurons. Staining of β-III tubulin (Tuj1; top 2 rows), MAP2 (third row), and NeuN (bottom row) is shown. Phase contrast, left column; nuclear staining (DAPI), middle column; neuronal markers (Tuj1, MAP2, NeuN), right column. Scale bars=0.1mm. (B) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of mRNA markers of pluripotency (OCT4, NANOG, GDF3, TERT, and REX1) and neuronal differentiation (HUC and MAP2). Samples from iPSCs are shown in white bars, neurospheres (NS) in medium gray bars, and day 8 neurons in dark gray bars. Signals are normalized to GAPDH, and iPSC signals were arbitrarily set to 1. Error bars=standard error of the mean of duplicate qRT-PCR measurements. (C) Population analyses of cells expressing the neuronal markers Tuj1 (first row, left column) and MAP2 (first row, middle column), the glial marker GFAP (first row, right column), and pluripotency markers Tra1–81 (second row, left column) and SSEA3 (second row, right column). Immunoglobulin G (IgG) controls are shown for each marker. Unstained background fluorescence channels (FL2-H or FL3-H) were used to gauge autofluorescence and to determine gating parameters. (D) Neuronal cells derived from unaffected (wild type [WT]) and FRDA iPSCs possess functional characteristics typical of neurons. Top, voltage clamp recordings showing inward and outward conductances in response to voltage steps (−90 to +50mV). Bottom, current clamp recordings of unaffected and FRDA neurons firing multiple action potentials in response to current injection. (E) Unbiased clustering of iPSC-derived normal and FRDA neurons (blue samples) with human (h) iPSCs (6 left samples) and different fetal tissues, based on all detected autosomal transcripts (21,342 probes). Euclidean distance is displayed.
PCR analysis of genomic DNA from both neurospheres and neurons indicated that the expanded GAA•TTC triplet repeats are retained in these FRDA cells (Fig 2). FRDA neurons also retain characteristically repressed levels of FXN mRNA when compared to non-diseased control neurons (Fig 3A) and lower levels of FXN protein. Next, as it has previously been suggested that transcriptional repression is caused by heterochromatin formation along the FXN locus,5,6 we analyzed the chromatin state of the FXN gene in FRDA neurons using quantitative ChIP, as previously described.6 We interrogated 3 locations along the FXN gene (the promoter, a region directly upstream of the GAA•TTC repeats, and a region directly downstream of the GAA•TTC repeats) for the presence of 11 histone post-translational modifications; 8 of them are associated with actively transcribed genes (“Activating,” in Fig 2E) and 3 with repressed chromatin (“Repressing,” in Fig 2E). Except for lysine 14 (H3K14ac) and lysine 36 of histone H3 (H3K36me), the activating marks are under-represented in FRDA cells compared to unaffected cells, as shown by an FRDA/unaffected occupancy ratio of <1. Conversely, the repressing histone marks are higher in FRDA cells. ChIP analysis of the same residues in fibroblasts, iPSCs, and iPSC-derived neurons from the same patient and unaffected individual (see Fig 3B, C) reveals that a similar pattern of histone modifications on FXN is present in the 3 cell types, although the histone mark differences between FRDA and unaffected cells seem to be less pronounced in neuronal cells. The same level of FXN repression is observed in each cell type. Together, these ChIP results indicate heterochromatin formation on the FXN gene near the GAA•TTC repeat expansion in FRDA neurons compared to unaffected neurons, consistent with previous findings.6,8,9 Analysis of DNA methylation on 22 CpG sites upstream and downstream of the GAA•TTC repeats in fibroblasts, iPSCs, and neuronal cells revealed interesting differences among the 3 cell types and between unaffected and FRDA cells, but failed to show any indication that DNA methylation is the determinant of FXN gene silencing (Fig 4).
FIGURE 2.
Molecular phenotypes associated with Friedreich ataxia (FRDA) in induced pluripotent stem cell (iPSC)-derived neuronal cells. (A) GAA•TTC repeat length in iPSCs, neurospheres, and neurons from unaffected and FRDA patients as measured by standard polymerase chain reaction (PCR). (B) Quantitative reverse transcriptase PCR (qRT-PCR) analysis of FXN mRNA expression in unaffected and FRDA neurons. qRT-PCR signals from FXN mRNA were normalized to signals from GAPDH, and the unaffected sample was arbitrarily set to 1. Error bars=standard error of the mean (SEM) of duplicate measurements. (C) Quantitative fluorescence Western analysis of frataxin protein in unaffected and FRDA neurons. The ribosomal protein L13a was used as a loading control (bottom panel). (D) Quantification of fluorescence Western blot from D. Integrated intensity counts for frataxin protein were normalized to counts for RPL13a, and the value for unaffected neurons was arbitrarily set to 1. (E) Quantitative chromatin immunoprecipitation analyses of various histone marks. DNA recovery was measured by quantitative PCR using primers for the promoter region of the FXN gene (prom), a region immediately upstream of the GAA•TTC repeats (UPGAA), and a region immediately downstream of the GAA•TTC repeats (DOWNGAA), and normalized to either GAPDH (“activating” marks), α-satellite DNA (H3K9me2 and H3K9me3), or MYT1 gene (H3K27me3) recovery. Data are plotted as ratio of normalized DNA recovery from FRDA versus unaffected neurons. Error bars=SEM of triplicate measurements.
FIGURE 3.
Heterochromatin formation in Friedreich ataxia (FRDA) fibroblasts and induced pluripotent stem (iPS) cells. (A) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of FXN mRNA expression in unaffected and FRDA fibroblasts, iPS cells, and neurons. qRT-PCR signals from FXN mRNA were normalized to signals from GAPDH, and the unaffected sample was arbitrarily set to 1. Error bars=standard error of the mean of triplicate measurements. (B) Quantitative chromatin immunoprecipitation analyses of various histone marks in fibroblasts. DNA recovery was measured by quantitative PCR using primers for the promoter region of the FXN gene (prom), a region immediately upstream of the GAA•TTC repeats (UPGAA), and a region immediately downstream of the GAA•TTC repeats (DOWNGAA), and normalized to either GAPDH (“activating” marks), α-satellite DNA (H3K9me2 and H3K9me3), or MYT1 gene (H3K27me3) recovery. Data are plotted as ratio of normalized DNA recovery from FRDA versus unaffected cells. (C) Same as in B but for histone marks in iPS cells.
FIGURE 4.
DNA methylation analysis upstream and downstream of the GAA•TTC repeats in Friedreich ataxia (FRDA) fibroblasts, induced pluripotent stem (iPS) cells, and neuronal cells. Using bisulfite sequencing, 10 CpG residues upstream (first and second panels from left) and 12 CpG residues downstream (third and fourth panels from left) of the GAA•TTC repeats on the FXN gene were analyzed in unaffected and FRDA fibroblasts (top panels), iPS cells (middle panels), and neuronal cells (bottom panels). Open circles represent unmethylated cytosines; closed circles represent methylated cytosines. Twenty clones per cell type were analyzed.
2-Aminobenzamide HDAC Inhibitors Reverse FXN Gene Silencing in FRDA Neuronal Cells
We previously reported that both FXN mRNA and protein can be upregulated in patient PBMCs, as well as in mouse models, upon treatment with a specific class of HDAC inhibitors, namely 2-aminobenzamides.6,13,16,26 Other chemical inhibitors of the zinc-dependent HDACs, such as the hydroxamic acids toluene sulfonic acid and suberoylanilide hydroxamic acid (SAHA), fail to elicit this effect.6 To demonstrate whether FRDA neurons respond similarly, we assessed the levels of FXN mRNA expression by qRT-PCR after treatment with the HDAC inhibitor (HDACi) 109 (N-[6-(2-aminophenylamino) −6-oxohexyl]-4-methylbenzamide), a compound previously shown to upregulate FXN mRNA and protein levels in both patient lymphocytes13,15 and in the brain in 2 FRDA mouse models.13,17 HDACi 109, which is moderately (3-fold) selective for HDAC3 over the other class I HDACs 1 and 2,13 upregulates FXN mRNA levels in FRDA neurons in a dose-dependent fashion, with a ~2.5-fold increase over vehicle-treated cells at 5μM (Fig 5A), bringing the level of FXN mRNA in these cells to that expected for asymptomatic heterozygous carriers.13,15
FIGURE 5.
Effect of histone deacetylase (HDAC) inhibitors on FXN mRNA levels and frataxin protein in neuronal cells. (A) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of FXN expression in Friedreich ataxia (FRDA) neurons after 24-hour treatment with HDAC inhibitor 109 at varying concentrations. FXN mRNA levels were normalized to 18S RNA. Signals from dimethylsulfoxide (DMSO)-treated samples were arbitrarily set to 1. Error bars=standard error of the mean (SEM) of duplicate experiments. **p < 0.01. (B) qRT-PCR analysis of FXN expression in FRDA neurons after 24-hour treatment with HDAC inhibitors 109, 233, and 966 at 10μM. FXN mRNA levels were normalized to total RNA. Error bars=SEM of triplicate measurements. **p < 0.01. (C) Example of a fluorescence Western blot of FRDA neurons treated with 10μM 109, 233, and 966 for 48 hours. Pol II signal was used as loading control. (D) Average of the quantification of 4 fluorescence Western blots of FRDA neurons (differentiated for 8, 10, and 14 days), treated as in C. Integrated intensity counts for frataxin protein were normalized to counts for Pol II, and the value for DMSO-treated neurons was arbitrarily set to 1.**p < 0.01. (E) qRT-PCR analysis of FXN expression in FRDA neurons after 24-hour treatment with HDAC inhibitors 109, 233, and 966 at 10μM or with a combination of 233 and 966 at 5 or 10μM. FXN mRNA levels were normalized to total RNA. Error bars=SEM of triplicate measurements. *p < 0.05.
Targeting HDAC1, 2, and 3 Is Necessary to Reverse FXN Silencing
To explore the role of HDAC selectivity in FXN gene activation in FRDA neurons, the effects of an HDAC1/2-selective compound (HDACi 233; N-[2-amino-5-(2-thienyl)phenyl]-7-nicotinoylamino-heptanamide; >200-fold selectivity over HDAC3)27 and a highly HDAC3-selective compound (HDACi 966; [E]-N-[2-amino-4-fluorophenyl]-3-[1-cinnamyl-1H-pyrazol-4-yl]acrylamide; ~30-fold selectivity for HDAC3 over HDAC1/2)28 were examined (see Fig 5B). Neither of these latter compounds induced FXN gene expression to the extent observed with HDACi 109. This result was expected for compound 233, as another HDAC1/2-selective compound (N1-[4-aminobiphenyl-3-yl]-N7-phenylheptanediamide) was previously shown to be inactive in terms of FXN upregulation in patient lymphocytes.26 Moreover, quantitative fluorescence Western blotting showed an upregulation of frataxin protein in 109-treated cells but not in 233- or 966-treated cells (see Fig 5C, D). In-cell histone deacetylase assays confirm that each of these molecules is a potent inducer of global histone acetylation (at several residues in histones H3 and H4) in human neuroblastoma cells (data not shown), ruling out the possible trivial explanation that these latter compounds are not active HDAC inhibitors in neuronal cells.
We also tested the combination of 233 and 966 for effects on FXN mRNA levels in neuronal cells; however, this combination was not as effective as compound 109 in achieving increases in mRNA levels (see Fig 5E), suggesting that compound 109 may have a different mechanism of action, such as a longer residence time on its target enzymes (as found for the related compound 10629). These results offer insight into the mechanism of FXN gene reactivation and suggest that only compounds that inhibit the class I HDACs 1, 2, and 3 can induce upregulation of FXN mRNA and frataxin protein.
Lysine 9 of Histone H3 Is a Key Residue in the Regulation of FXN Transcription
To investigate the link between the observed FXN upregulation and HDAC inhibition at pathogenic FXN alleles, we monitored the changes in histone post-translational modifications along the FXN gene in FRDA neurons following compound treatment using quantitative ChIP. Interrogating the same 3 locations along the FXN gene and the same acetylated residues as in Figure 2E, we observed significant changes upon treatment with 109, specifically large increases in occupancy of acetylated H3K9, H4K5, H4K8, and H4K16 (Fig 6A). A similar analysis was conducted in FRDA fibroblasts and iPSCs (Fig 7). In these cell types, 109 has no effect in restoring FXN transcription. Whereas in iPSCs, the increase in the level of acetylation upon 109 treatment is 3- to 4-fold for all residues analyzed, in fibroblasts the acetylation changes are similar to that of neuronal cells with the exception of H3K9. For this residue, the effect of 109 is higher in neuronal cells versus the other 2 cell types. From these data, it is clear that H3K9 acetylation at the FXN gene is related to whether transcriptional changes occur, and acetylation changes are quantitatively greater in cells that show FXN mRNA increases.
FIGURE 6.
Mechanism of action of histone deacetylase inhibitors on the FXN gene. (A) Quantitative chromatin immunoprecipitation (ChIP) analysis of histone acetylation marks in Friedreich ataxia neurons treated with 10μM 109 for 24 hours. DNA recovery for the FXN promoter (prom), a region upstream of the GAA•TTC repeats (UPGAA), and a region downstream of the GAA•TTC repeats (DOWNGAA) is shown. The ratio of DNA recovery for 109-treated versus dimethylsulfoxide (DMSO)-treated cells is plotted. Error bars=standard error of the mean (SEM) of triplicate measurements. (B) Quantitative ChIP analysis of the same histone acetylation marks as in A upon treatment with 109, 233, or 966 at 10μM. The UPGAA region is probed. The ratio of DNA recovery for 109-treated versus DMSO-treated cells is plotted. Error bars=SEM of triplicate measurements. *p < 0.05, **p < 0.01. (C) Quantitative ChIP analysis of H3K9ac/me3 ratio in fibroblasts, induced pluripotent stem (iPS) cells, and neurons. UPGAA region DNA recovery for the H3K9ac/me3 ratio in 109-treated versus DMSO-treated cells is plotted. Error bars=SEM of 2 independent ChIP assays, both quantified in triplicate. *p < 0.05, **p < 0.01.
FIGURE 7.
Effect of 109 on H3 and H4 acetylated residues in Friedreich ataxia (FRDA) fibroblasts and IPSCs. (A) quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of FXN mRNA expression in fibroblasts, induced pluripotent stem cells (iPSCs), and neurons treated with either 0.1% dimethylsulfoxide (DMSO) or 10μM 109. qRT-PCR signals from FXN mRNA were normalized to total RNA, and the DMSO-treated sample was arbitrarily set to 1. Error bars=standard error of the mean (SEM) of triplicate measurements. (B) Quantitative chromatin immunoprecipitation analysis of histone acetylation marks in FRDA fibroblasts treated with 10μM 109 for 24 hours. DNA recovery for the FXN promoter (prom), a region upstream of the GAA•TTC repeats (UPGAA), and a region downstream of the GAA•TTC repeats (DOWNGAA) is shown. The ratio of DNA recovery for 109-treated versus DMSO-treated cells is plotted. Error bars=SEM of triplicate measurements. (C) Same as in B for FRDA iPSCs.
To address whether this is an indication of the mechanism of action of 2-aminobenzamide HDAC inhibitors in reversing FXN gene silencing, we compared the effect of 109 on histone acetylation upstream of the GAA•TTC repeats with that of compounds 233 and 966 in FRDA neuronal cells (see Fig 6B). Based on the observed global activity of each of these compounds in neuroblastoma cells (data not shown), we expect to see global increases in histone acetylation in the neuronal cells; however, the ChIP experiments clearly show differences in the ability of the compounds to induce histone acetylation (see Fig 6B) and transcription (see Fig 5B) at the FXN locus. Increases in H3K9 and H4K8 acetylation are a signature of HDAC inhibitors that increase FXN mRNA level compared to compounds that are ineffective. These differences between compounds that show differential activity on the FXN gene could be due to specific HDAC complexes at pathogenic FXN alleles, where only compound 109 has the requisite activity for gene activation, or to mechanism of action (see Discussion). A similar ChIP analysis in PBMCs from blood of 2 FRDA patients also showed the important contribution of H3K9ac and H4K8ac in FXN derepression (Fig 8). We note that although the same trends are observed with each compound in different patients, the absolute magnitudes of effects of the compounds on FXN histone acetylation differ between patients. Similarly, the quantitative response to drug for increases in FXN mRNA can differ between individuals in the patient population,15 pointing to epigenetic differences within the human population.
FIGURE 8.
Quantitative chromatin immunoprecipitation analysis of H3 and H4 histone acetylation marks in peripheral blood mononuclear cells from 2 Friedreich ataxia patients upon treatment with 109, 233, or 966 at 10μM. The region upstream of the GAA•TTC repeats is probed. The ratio of DNA recovery for 109-treated versus dimethylsulfoxide-treated cells is plotted. Error bars=standard error of the mean of triplicate measurements. *p < 0.05, **p < 0.01.
To further support the idea that lysine 9 of histone H3 is a key residue in the regulation of FXN transcription, we measured how the ratio of acetylation versus trimethylation for this residue (H3K9ac/me3) changes upon treatment with 109 in fibroblasts, iPSCs, and neurons. Although the ratio of H3K9ac/me3 increases upon treatment with 109 in each of the 3 cell types (see Fig 6C), the magnitude of this effect is highest in neuronal cells, the only cell type among the 3 in which HDACi treatment can reverse FXN silencing. We argue that the balance between acetylation and methylation of H3K9 on FXN determines the transcriptional status of the gene. It is likely that increases in H4K8 acetylation may also contribute to FXN gene activation (see Fig 6B).
Increasing FXN Transcription in iPSC-Derived Neurons Does Not Promote GAA Repeat Instability and Does Not Induce RNA Foci
We next addressed 2 concerns about the use of therapeutics that increase endogenous FXN transcription and mRNA levels in FRDA. The first is the observation that triplet repeat instability increases with transcriptional activity.30–33 Higher levels of transcription of the FXN gene could possibly increase GAA•TTC repeat length in affected tissues and worsen the disease. In addressing this issue, we found that the increase in FXN transcription observed upon treatment of FRDA neuronal cells with HDACi 109 had no effect on repeat instability over the course of 12 days, a time frame sufficient to detect repeat instability in FRDA iPS cells (Fig 9).21 The second concern relates to a possible toxic effect of the expanded triplet-repeat-containing transcript, as in other trinucleotide expansion diseases such as myotonic dystrophy type 1 and fragile X-associated tremor/ataxia syndrome.34 Two lines of evidence argue against this possibility: (1) we found that expanded intron 1 RNA is present in extremely low levels, in agreement with previous findings 35; and (2) we failed to detect GAA repeat RNA–containing foci in FRDA fibroblasts and iPSC-derived neurons with fluorescence in situ hybridization, even after FXN upregulation with HDACi. Such foci can be observed with an artificial reporter construct, showing that our methods are capable of detecting GAA-repeat RNA foci. These observations as well as the recent report of prolonged treatment with HDACi in a mouse model of FRDA17 without negative effects suggest that HDACi treatment should not exacerbate the disease.
FIGURE 9.
Histone deacetylase inhibitor 109 does not induce repeat instability in Friedreich ataxia (FRDA) neurons, and GAA-containing RNA does not form foci in FRDA cells. (A) Quantitative reverse transcriptase polymerase chain reaction analysis of FXN transcript in FRDA neurons treated with either dimethylsulfoxide (DMSO) or 109 at 5μM. Treatment was performed for 24 hours at days 7, 10, 13, and 16 after neuronal induction, and cells were collected for analysis at day 19. FXN mRNA levels were normalized relative to 18S rRNA levels. (B) GAA•TTC repeat length in FRDA fibroblasts, neurospheres, and neurons treated with either DMSO or 5μM 109 as described in A. (C) DAPI-stained nuclei from unaffected (left) and FRDA (right) fibroblasts hybridized with a Cy3-labeled TTC probe. (D) DAPI-stained nuclei from FRDA neurons treated with either DMSO or 10μM 109 and hybridized as in C. (E) DAPI-stained nuclei from cell line GFP_ (GAA•TTC)560 in the absence (−Tet, left panel) and presence (+Tet, right panel) of tetracycline. This cell line expresses green fluorescent protein with an artificial intron that contains 560 GAA•TTC repeats, under the control of a tetracycline-inducible cytomegalovirus promoter. One bright spot is visible in the presence of tetracycline, demonstrating that our Cy3-(TTC)10 probe can detect RNA foci. Scale bars=2μM.
Phase I Clinical Trial in FRDA with 109/RG2833
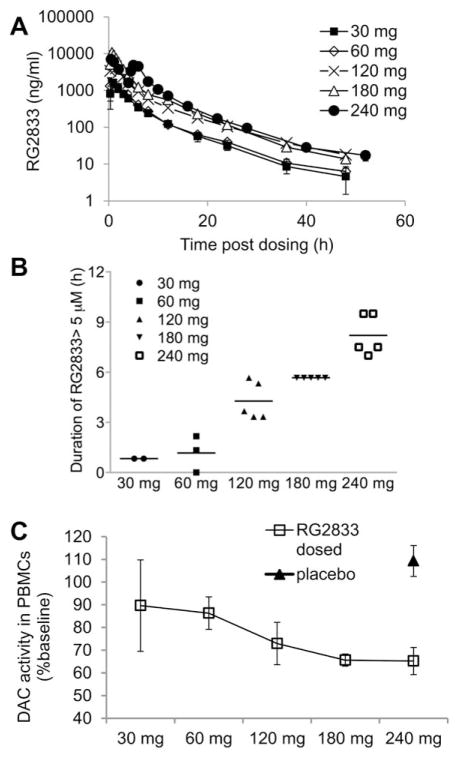
HDACi 109 was evaluated in preclinical toxicology, pharmacology, and safety studies consistent with supporting the use of this compound in FRDA patients. Approval was obtained from the Italian Health Ministry after review by the European Medicines Agency for an ascending dose crossover study in adults with FRDA. A phase I clinical study to evaluate the safety, pharmacokinetics, and pharmacodynamics of orally administered RG2833 (solid dose form of 109) was conducted with appropriate informed consent and ethical committee approval as described in Materials and Methods. The study consisted of 4 cohorts with 5 FRDA patients in each cohort (patient characteristics are summarized in Table 1). Cohort 1 and 2 were open label, wherein Cohort 1, 2 patients received a single 30mg dose and 3 patients received a single 60mg dose. In Cohort 2, all 5 patients received a single 120mg dose. Cohorts 3 and 4 were randomized, double-blind, placebo-controlled crossover studies. Based on the pharmacokinetics and safety data from the first 2 cohorts, each patient in Cohort 3 was administered a single dose of 180mg RG2833 and a single dose of placebo. Each patient in Cohort 4 received 2 doses of 120mg RG2833, administered 4 hours apart, and 2 doses of placebo, administered 4 hours apart. The 2 treatments of drug or placebo for a given patient in Cohort 3 or 4 were separated by 29 days and administered in random order. All 20 enrolled patients completed the study. RG2833 was well tolerated with no limiting toxicities reported. One patient suffered from 2 asymptomatic episodes of sinus tachycardia, which occurred >8 hours after dosing, neither of which was considered to be drug related because the Cmax of RG2833 is ≤2 hours. All the other side effects have been considered unrelated to the study drug. The independent data safety monitoring board identified no safety signals after reviewing the safety, clinical laboratory, vital signs, physical and neurological examinations, and cardiology data from both 72-hour telemetry and 12-lead ECGs (data not shown).
Pharmacokinetics of RG2833 was evaluated by analyzing drug levels in the plasma of treated patients. An elimination half-life of 6 to 10 hours among the dose groups was observed (Fig 10A and Table 2). Metabolites of RG2833 were identified in patient serum. The major metabolite was a benzimidazole, which showed AUC exposure ratio between 160 and 250% of the parent compound in all dose groups (not shown). The benzimidazole had considerably longer elimination half-life than the parent compound, with t½ ranging from 35 to 42 hours. Other metabolites were products of amide hydrolysis, including o-phenylenediamine (OPD), acetylated OPD, and acids, as well as the glucuronide of RG2833 (data not shown).
FIGURE 10.
Pharmacokinetics of RG2833 in plasma after oral administration and inhibition of deacetylase (DAC) activity in peripheral blood mononuclear cells (PBMCs). (A) Mean plasma concentrations of RG2833 for each cohort of patients as measured by liquidchromatography coupled with tandem mass spectrometry in the first 48 or 52 hours postdosing. Error bars=standard error of the mean (SEM). (B) Duration of plasma concentration of RG2833 over 5μM for each patient in each dose group. (C) Mean of maximal suppression of deacetylase activity in PBMC extracts from patients in each dose group. Deacetylase activity in the cell lysate was quantified by deacetylation of the Fluor-de-Lys (Enzo Life Sciences) substrate. Error bars=SEM. Baseline is the deacetylase activity in the predose sample of each patient.
TABLE 2.
Summary of Pharmacokinetic Parameters for RG2833 after Oral Administration of RG2833 to Adult Friedreich Ataxia Patients
| Parameter | Cohort 1 | Cohort 2, 120mg | Cohort 3, 180mg | Cohort 4, 240mg | |
|---|---|---|---|---|---|
| 30mg | 60mg | ||||
| Cmax, ng/ml | 1,975±106 (2) | 2,563±1,049 (3) | 6,474±3,711 (5) | 10,930±2,431 (5) | 7,154±2,034 (5) |
| Tmax, h | 0.67 (2) [0.67–0.67] | 0.67 (3) [0.67–2.00] | 1.00 (5) [0.33–1.48] | 0.67 (5) [0.65–1.00] | 0.50 (5) [0.47–0.93] |
| AUC (0–t), h × ng/ml | 7,695±1,095 (2) | 9,631±2,487 (3) | 21,689±6,624 (5) | 34,903±5,447 (5) | 39,344±9,476 (5) |
| AUC (inf), h × ng/ml | 7,758±1,166 (2) | 9,715±2,470 (3) | 21,973±6,470 (5) | 35,058±5,475 (5) | 39,569±9,576 (5) |
| λz, 1/h | 0.0897±0.0306 (2) | 0.0854±0.0208 (3) | 0.0739±0.0223 (5) | 0.1016±0.0225 (5) | 0.0844±0.0191 (5) |
| t½, h | 8.20±2.80 (2) | 8.43±1.93 (3) | 10.1±3.16 (5) | 7.07±1.43 (5) | 8.56±1.93 (5) |
| CL/F | |||||
| l/h | 3.91±0.59 (2) | 6.43±1.51 (3) | 5.80±1.46 (5) | 5.24±0.89 (5) | 6.39±1.71 (5) |
| l/h/kg | 0.061±0.016 (2) | 0.087±0.011 (3) | 0.085±0.026 (5) | 0.099±0.018 (5) | 0.119±0.032 (5) |
| Vz/F | |||||
| L | 45.1±8.84 (2) | 79.0±31.3 (3) | 89.7±48.4 (5) | 52.8±10.7 (5) | 75.5±6.1 (5) |
| l/kg | 0.69±0.059 (2) | 1.04±0.164 (3) | 1.32±0.825 (5) | 1.00±0.234 (5) | 1.41±0.143 (5) |
Mean±standard deviation (No.) except Tmax, for which the median (No.) [range] is reported. All pharmacokinetic calculations were performed using SAS for Windows (SAS Institute, Cary, NC).
λz = elimination rate constant; AUC = area under the curve; Cmax = maximum plasma concentration; inf = extrapolated to infinity; t½ = elimination half-life; Tmax = time to maximum; CL = clearance; Vz = volume of distribution; F = bioavailability.
Based on in vitro efficacy data for FXN mRNA induction in primary lymphocytes13,15 and neuronal cells (see Fig 5A), we estimate that a 5μM plasma concentration of RG2833, sustained for at least 6 to 8 hours, would result in similar increases of FXN expression in circulating PBMCs, and such levels were obtained in Cohorts 3 and 4 (see Fig 10B). Using a deacetylase assay, up to 50% inhibition of total HDAC activity was detected in PBMCs from subjects of the 3 highest dose groups and an average of 35% inhibition was observed at the 180mg and 240mg doses (see Fig 10C). (Note that this assay reflects inhibition of total deacetylase activity, not just the activity contributed by HDACs 1, 2, and 3.)
Next we assessed whether oral administration of RG2833 resulted in an increase in FXN mRNA in blood from patients. qRT-PCR demonstrated an upregulation of FXN mRNA levels in patients from the 3 highest dose groups, with an average induction of 1.5- to 1.6-fold observed within 24 hours postdosing, and a correlation (R2=0.417) was observed between the maximal FXN induction and RG2833 exposure in plasma (represented by AUC[inf ]) for each patient (Fig 11). We also find a good correlation between increases in FXN mRNA in individual patients in Cohorts 3 and 4 and maximum inhibition of deacetylase activity measured in the same patient’s PBMCs (R2=0.636), providing evidence for the mechanism of action of the compounds as HDAC inhibitors. For each patient in Cohort 4, we plotted FXN mRNA levels relative to their predose level (as a function of time after dosing), and compared these results to the placebo response for the same individuals. Four of 5 patients in Cohort 4 showed evident drug-induced FXN upregulation when their response to the drug was compared over time with their placebo response. PBMCs isolated from the predose blood sample from the fifth patient treated with 10μM of RG2833 in vitro for 24 hours failed to show FXN mRNA induction, indicating this subject may be a nonresponder at the time of the trial (data not shown).
FIGURE 11.
FXN mRNA, H3K9 acetylation, and frataxin protein response in adult Friedreich ataxia patients after oral administration of RG2833. (A) Mean fold-change of FXN mRNA in whole blood for each cohort upon dosing. FXN mRNA was quantified using quantitative reverse transcriptase polymerase chain reaction on RNA extracted from blood and plotted relative to the predose sample. The expression level of TBP was used to normalize each sample. (B) Relationship between individual patient maximum fold-change of FXN mRNA in whole blood and RG2833 plasma area under the curve extrapolated to infinity (AUC[inf]). (C) Relationship between maximum fold changes in FXN mRNA in individual patients in Cohorts 3 and 4 and maximal deacetylase (DAC) activity in peripheral blood mononuclear cells (PBMCs). (D) FXN mRNA levels in response to dosing in Cohort 4 crossover study. Data for both dosed and placebo samples are plotted for each patient. FXN mRNA levels were normalized by TBP gene expression. Error bars=standard deviation (SD) of duplicate measurements. *p < 0.05, **p < 0.01. (E) Comparison of dosed versus placebo H3K9 acetylation response in Cohort 4 PBMCs. Error bars=standard error of the mean of triplicate measurements. *p < 0.05, **p < 0.01. (F) Frataxin protein levels in PBMCs from Cohort 4 patients were measured with a dipstick assay, as described.15 Data for both dosed and placebo samples are plotted for each patient. Error bars=SD of duplicate measurements. **p < 0.01. ^For Patient B, the 28-hour and 52-hour data points are missing due to technical problems.
As a downstream signaling event indicating HDAC inhibition, we used native ChIP assays to monitor H3K9 acetylation at the upstream GAA•TTC repeat site of the FXN locus in PBMCs. We observed increased H3K9 acetylation in the same 4 of 5 patients in this cohort (see Fig 11E). Although the same trends are observed with compound 109 in different patients, the absolute magnitudes of effects of the compounds on histone acetylation differ between patients. Similarly, the quantitative response in terms of increases in FXN mRNA differs between individuals in the patient population. This variability could be due to differences in GAA repeat numbers within the FRDA patients, but the current sample size is too small to draw quantitative conclusions. The effects of drug treatment on both FXN mRNA levels and histone acetylation in PBMCs are transitory (see Fig 11), as would be expected from the half-life of the compound in serum (see Fig 10A). We also analyzed the frataxin protein levels in the PBMCs. No induction was seen in the first 2 cohorts, whereas 1 patient in the 180mg group (not shown) and 2 patients in the 240mg group showed ~1.4-fold increases at a single time point (between 8 and 12 hours postdosing; see Fig 11F). Although encouraging, the transient increase in FXN mRNA would not be expected to significantly add to total frataxin protein in this single dose study. A 50% increase in frataxin protein would require a sustained 2-fold increase in FXN mRNA equivalent to the 50-hour half-life of the protein. 36 Moreover, given the half-life of HDACi 109 in serum (see Fig 10A), a multiple dosing regimen would be required to see sustained FXN mRNA resulting in a measurable increase in frataxin protein.
Discussion
In this work, we evaluated the effect of an HDACi on frataxin expression in PBMCs in a clinical trial and correlated the effect within a disease-relevant cell type using iPSC-derived neurons. This approach can potentially be used to test other therapeutics for FRDA and for neurological disorders in general.37 Furthermore, because we assayed cellular responses at a relatively early stage of neuronal differentiation, we are able to exploit the mitotically active nature of differentiated neural stem cells and scale our model system as needed to accommodate pilot studies (or potentially larger throughput screening studies) while maintaining relative population homogeneity. Although protocols for the derivation of sensory neurons from iPSCs have been published,38–40 they do not yield sufficiently pure populations of cells for biochemical analyses.
We demonstrated that neurons of FRDA patient genetic background respond to treatment with the HDACi 109, effecting significant changes in histone acetylation near the FXN locus. In turn, this chromatin remodeling coincides with the upregulation of FXN mRNA and protein. Analysis of iPSC-derived neurons also provided insight on the mechanism by which FXN upregulation is achieved. We demonstrated that combined HDAC1, 2, and 3 inhibition is required to counteract the epigenetic changes induced by the GAA•TTC repeat expansion and that H3K9 is a key histone residue whose acetylation/methylation regulates FXN expression. We previously reported that 2-aminobenzamides differed from HDAC inhibitors that failed to activate FXN gene expression in terms of their inhibitory mechanism, where the active molecules inhibit their target enzymes through a slow-on/slow-off mechanism. 29 It is likely that the 4- and 5-substituted 2-aminobenzamides have this property only for their selected HDAC target enzyme and this can account for their lack of effectiveness in reactivating the FXN gene. HDAC inhibitors that fail to activate FXN gene expression, such as SAHA, also target the class I HDACs but differ from the active molecules in that they exhibit rapid-on/rapid-off inhibition mechanisms.29 Alternatively, reversal of epigenetic silencing could be achieved only by the unique action of 109 on the specific complexes residing at the FXN locus. In addition, iPSC-derived neurons allowed us to evaluate potential liabilities41,42 of upregulating FXN transcription in cells bearing expanded repeats by demonstrating repeat stability and lack of RNA nuclear foci in 109 treated cells.
All of our previous studies with 2-aminobenzamide HDACi were in patient PBMCs or lymphoblast cell lines (reviewed in Soragni et al43), which are cell types that are not affected in the human disease. The current study examines the efficacy of this compound class in a cell type that is actually affected in the human disease, namely neuronal cells. The important and unprecedented finding from these studies is that drug exposure inducing epigenetic changes in neurons in vitro is comparable to the exposure required to see epigenetic changes and increases in gene expression in circulating lymphoid cells in FRDA patients. These findings provide a proof of concept that patient-derived neuronal cells can be a quantitative screening tool for the development of an epigenetic therapy for this fatal neurological disease.
In line with the results from the iPSC-derived neuronal cells, we detected dose-dependent suppression of deacetylase activity in the PBMCs from drug-treated subjects, along with induction of histone acetylation and FXN mRNA upregulation. Maximal deacetylase inhibition and FXN upregulation were achieved when plasma RG2833 concentration exceeded target exposure of 5μM, an efficacious drug level predicted by the iPSC-derived neuronal cell model. Importantly, we detected a good correlation between increase in FXN transcript and inhibition of deacetylase activity, providing evidence that the mechanism of action of RG2833/109 is through deacetylation. Although only a small and inconstant frataxin protein modulation was observed in the clinical trial, the single dose treatment only allowed a transient increase in mRNA expression, so it is reasonable to predict that protein upregulation will follow the more sustained gene expression increase that can be expected in a repeat dose study.
Although our results are encouraging, 109/RG2833 suffers from liabilities for chronic use as FRDA therapeutics: namely, less than optimal brain penetration (0.15 brain to blood ratio), and conversion of the active molecule into inactive, potentially toxic metabolic products (benzimidazole and products of amidolysis) that are poorly eliminated in vivo.44,45 In other studies (not shown), we find that the benzimidazole inhibits the human ether-a-go-go related gene (hERG) with an IC50 of 0.92μM in the automated patch clamp assay, which puts RG2833 in the high-risk category for inducing QTc prolongation, based on a published study.46 It is likely the risk factor would increase in a multiple dose regime because of benzimidazole accumulation due to the slow elimination of this metabolite. Thus, it is unlikely that RG2833 will be taken forward to later phase clinical trials; however, compounds related to 109/RG2833 from subsequent medicinal chemistry campaigns have eliminated these metabolic liabilities and have favorable brain penetration (>0.7 brain/plasma ratio).44 These molecules are candidates for future clinical studies in FRDA.
While this article was in preparation, a recent study showed the potential benefits of the use of epigenetic modulation to treat FRDA.12 These authors showed that the sirtuin inhibitor nicotinamide is effective in restoring FXN transcription in peripheral lymphocytes isolated from blood of orally dosed patients. Although the drug seems to have only minor side effects, the doses used in this trial (up to 8g per day) were considerably higher than the suggested daily dose for this supplement, and this is a concern for the long-term use of such drug.47 In contrast, benzamide HDAC inhibitors are effective in the low micromolar range, and the next generation of compounds holds the promise of efficacy in the high nanomolar range, with possibly fewer liabilities for long-term use.
Our neuronal cell model can also be used to identify and investigate new biomarkers common to the neuronal cell lineage and lymphocytes that can aid future clinical trials. Long-term studies on large cohorts of FRDA patients are identifying clinical, functional, and quality of life measures, as well as biomarkers of FRDA progression that can be used as outcome measures in phase II/III clinical trials.48
In conclusion, this study provides proof of principle that an orally dosed class I HDACi can increase both FXN mRNA and acetylation of a key residue in the blood of FRDA patients. The correlation of drug exposure response in iPSC-derived neuronal cells and PBMC of treated patients confirms the relevance of the neuronal cells as a model of the neurodegenerative disease FRDA and establishes a general path for drug development in neurological disorders. Although other potential treatments for neurodegenerative diseases have been tested in human iPSC-derived neurons (as examples, see Ebert et al,49 Egawa et al,50 and Cooper et al51), and iPSC-derived cells have also been used in high-throughput screens,52,53 this work for the first time bridges treatment evaluation in disease-specific patient neurons and drug exposure in a clinical trial.
Acknowledgments
This work was supported by Repligen Corporation as well as by grants from the NIH National Institutes for Neurological Disorders and Stroke (R01 NS062856 to J.M.G.); the California Institute for Regenerative Medicine (RB3-05022 to J.M.G., J.F.L.); GoFAR, European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS), Friedreich’s Ataxia Research Alliance (FARA), and the Muscular Dystrophy Association (to Repligen Corporation); and FARA (J.M.G.). J.D. was supported by a stem cell fellowship from the Scripps Research Institute, and P.P. was supported by a Spanish Ministry of Education postdoctoral fellowship.
We thank I. Singec (Sanford Burnham Institute) for guidance with neuronal differentiation; M. Napierala (University of Alabama, Birmingham) for the gift of GAAGFP cells; E. Midlam, S. Carreiro, and K. Jauregui for support of clinical and regulatory operations; Drs H. Plasterer, A. Marolewski, B. Xia, R. Ramos-Zaya, and V. Jacques for development of methods and materials used in these studies (Repligen Corporation); and FRDA patients and their families for their participation and support, without which this study could not have been accomplished.
Footnotes
Authorship
E.S., S.K., E.C., J.D., and P.P. designed and performed in vitro experiments with human iPSCs, fibroblasts, and neuronal cells. E.S. performed ChIP experiments with clinical study PBMCs. W.M. performed HDAC, qRT-PCR, and frataxin protein measurements on patient samples. D.J. designed and monitored the clinical trial. M.O. managed clinical trial operations. M.I., S.D., M.C., F.L., A.P., and L.D. performed the clinical trial. M.R. treated patient PBMCs with drug ex vivo. J.C.M. and A.M. performed electrophysiology experiments. K.N. and J.F.L. performed and interpreted microarray study data. M.P. served on the data safety monitoring board, provided valuable insight and guidance throughout the project, and supervised M.R. J.R.R. designed and supervised preclinical studies and clinical pharmacodynamic measures. J.M.G. conceived and supervised studies. J.R.R., E.S., W.M., M.P., and J.M.G. wrote the manuscript. E.S., W.M., and M.I. contributed equally to this work. D.J.: employment, BioMarin Pharmaceuticals. M.P.: data safety monitoring board, grant, Repligen Corporation; patent, methods for diagnosing FRDA (licensee, Athena Diagnostics). J.M.G.: US patent applications 20070219244, 20110021562, 20130210918 (licensees, Repligen/BioMarin). J.M.G. serves as a paid consultant to Repligen Corporation. The terms of this arrangement are managed by the Scripps Research Institute. Intellectual property has been licensed by the Scripps Research Institute to Repligen Corporation, and the Scripps Research Institute and J.M.G. have a financial interest in this technology.
References
- 1.Pandolfo M. Friedreich ataxia. Arch Neurol. 2008;65:1296–1303. doi: 10.1001/archneur.65.10.1296. [DOI] [PubMed] [Google Scholar]
- 2.Lynch DR, Farmer JM, Wilson RB. Mortality in Friedreich’s Ataxia. Tex Heart Inst J. 2007;34:502–503. author reply 503–504. [PMC free article] [PubMed] [Google Scholar]
- 3.Cnop M, Igoillo-Esteve M, Rai M, et al. Central role and mechanisms of beta-cell dysfunction and death in Friedreich ataxia-associated diabetes. Ann Neurol. 2012;72:971–982. doi: 10.1002/ana.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campuzano V, Montermini L, Molto MD, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 5.Saveliev A, Everett C, Sharpe T, et al. DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nature. 2003;422:909–913. doi: 10.1038/nature01596. [DOI] [PubMed] [Google Scholar]
- 6.Herman D, Jenssen K, Burnett R, et al. Histone deacetylase inhibitors reverse gene silencing in Friedreich’s ataxia. Nat Chem Biol. 2006;2:551–558. doi: 10.1038/nchembio815. [DOI] [PubMed] [Google Scholar]
- 7.Greene E, Mahishi L, Entezam A, et al. Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia. Nucl Acids Res. 2007;35:3383–3390. doi: 10.1093/nar/gkm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mahdawi S, Pinto RM, Ismail O, et al. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum Mol Genet. 2008;17:735–746. doi: 10.1093/hmg/ddm346. [DOI] [PubMed] [Google Scholar]
- 9.Chan PK, Torres R, Yandim C, et al. Heterochromatinization induced by GAA-repeat hyperexpansion in Friedreich’s ataxia can be reduced upon HDAC inhibition by vitamin B3. Hum Mol Genet. 2013;22:2662–2675. doi: 10.1093/hmg/ddt115. [DOI] [PubMed] [Google Scholar]
- 10.Pastore A, Puccio H. Frataxin: a protein in search for a function. J Neurochem. 2013;126(suppl 1):43–52. doi: 10.1111/jnc.12220. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Cabo P, Palau F. Mitochondrial pathophysiology in Friedreich’s ataxia. J Neurochem. 2013;126(suppl 1):53–64. doi: 10.1111/jnc.12303. [DOI] [PubMed] [Google Scholar]
- 12.Libri V, Yandim C, Athanasopoulos S, et al. Epigenetic and neurological effects and safety of high-dose nicotinamide in patients with Friedreich’s ataxia: an exploratory, open-label, dose-escalation study. Lancet. 2014;384:504–513. doi: 10.1016/S0140-6736(14)60382-2. [DOI] [PubMed] [Google Scholar]
- 13.Rai M, Soragni E, Chou CJ, et al. Two new pimelic diphenylamide HDAC inhibitors induce sustained frataxin upregulation in cells from Friedreich’s ataxia patients and in a mouse model. PLoS One. 2010;5:e8825. doi: 10.1371/journal.pone.0008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppola G, Burnett R, Perlman S, et al. A gene expression phenotype in lymphocytes from Friedreich ataxia patients. Ann Neurol. 2011;70:790–804. doi: 10.1002/ana.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plasterer HL, Deutsch EC, Belmonte M, et al. Development of frataxin gene expression measures for the evaluation of experimental treatments in Friedreich’s ataxia. PLoS One. 2013;8:e63958. doi: 10.1371/journal.pone.0063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rai M, Soragni E, Jenssen K, et al. HDAC inhibitors correct frataxin deficiency in a Friedreich ataxia mouse model. PLoS ONE. 2008;3:e1958. doi: 10.1371/journal.pone.0001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandi C, Pinto RM, Al-Mahdawi S, et al. Prolonged treatment with pimelic o-aminobenzamide HDAC inhibitors ameliorates the disease phenotype of a Friedreich ataxia mouse model. Neurobiol Dis. 2011;42:496–505. doi: 10.1016/j.nbd.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet. 2011;20:R109–R115. doi: 10.1093/hmg/ddr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol. 2012;13:713–726. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
- 20.Ku S, Soragni E, Campau E, et al. Friedreich’s ataxia induced pluripotent stem cells model intergenerational GAATTC triplet repeat instability. Cell Stem Cell. 2010;7:631–637. doi: 10.1016/j.stem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Campau E, Soragni E, et al. Role of mismatch repair enzymes in GAA. TTC triplet-repeat expansion in Friedreich ataxia induced pluripotent stem cells. J Biol Chem. 2012;287:29861–29872. doi: 10.1074/jbc.M112.391961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soragni E, Xu C, Cooper A, et al. Evaluation of histone deacetylase inhibitors as therapeutics for neurodegenerative diseases. Methods Mol Biol. 2011;793:495–508. doi: 10.1007/978-1-61779-328-8_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soragni E, Herman D, Dent SY, et al. Long intronic GAA*TTC repeats induce epigenetic changes and reporter gene silencing in a molecular model of Friedreich ataxia. Nucleic Acids Res. 2008;36:6056–6065. doi: 10.1093/nar/gkn604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dottori M, Pera MF. Neural differentiation of human embryonic stem cells. Methods Mol Biol. 2008;438:19–30. doi: 10.1007/978-1-59745-133-8_3. [DOI] [PubMed] [Google Scholar]
- 25.Hick A, Wattenhofer-Donze M, Chintawar S, et al. Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich’s ataxia. Dis Model Mech. 2013;6:608–621. doi: 10.1242/dmm.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C, Soragni E, Chou CJ, et al. Chemical probes identify a role for histone deacetylase 3 in Friedreich’s ataxia gene silencing. Chem Biol. 2009;16:980–989. doi: 10.1016/j.chembiol.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia H, Pallos J, Jacques V, et al. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington’s disease. Neurobiol Dis. 2012;46:351–361. doi: 10.1016/j.nbd.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malvaez M, McQuown SC, Rogge GA, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou CJ, Herman D, Gottesfeld JM. Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases. J Biol Chem. 2008;283:35402–35409. doi: 10.1074/jbc.M807045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y, Dion V, Wilson JH. Transcription promotes contraction of CAG repeat tracts in human cells. Nat Struct Mol Biol. 2006;13:179–180. doi: 10.1038/nsmb1042. [DOI] [PubMed] [Google Scholar]
- 31.Ditch S, Sammarco MC, Banerjee A, Grabczyk E. Progressive GAA. TTC repeat expansion in human cell lines. PLoS Genet. 2009;5:e1000704. doi: 10.1371/journal.pgen.1000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goula AV, Stys A, Chan JP, et al. Transcription elongation and tissue-specific somatic CAG instability. PLoS Genet. 2012;8:e1003051. doi: 10.1371/journal.pgen.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamori M, Pearson CE, Thornton CA. Bidirectional transcription stimulates expansion and contraction of expanded (CTG)*(CAG) repeats. Hum Mol Genet. 2011;20:580–588. doi: 10.1093/hmg/ddq501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoghbi HY, Orr HT. Trinucleotide repeat disorders. Ann Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 35.Bidichandani SI, Ashizawa T, Patel PI. The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet. 1998;62:111–121. doi: 10.1086/301680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Besse EK, Ha D, et al. Iron-dependent regulation of frataxin expression: implications for treatment of Friedreich ataxia. Hum Mol Genet. 2008;17:2265–2273. doi: 10.1093/hmg/ddn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiang L, Fujita R, Abeliovich A. Remodeling neurodegeneration: somatic cell reprogramming-based models of adult neurological disorders. Neuron. 2013;78:957–969. doi: 10.1016/j.neuron.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers SM, Qi Y, Mica Y, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eigentler A, Boesch S, Schneider R, et al. Induced pluripotent stem cells from Friedreich ataxia patients fail to upregulate frataxin during in vitro differentiation to peripheral sensory neurons. Stem Cells Dev. 2013;22:3271–3282. doi: 10.1089/scd.2013.0126. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein AF, Gasnier E, Furling D. Gain of RNA function in pathological cases: focus on myotonic dystrophy. Biochimie. 2011;93:2006–2012. doi: 10.1016/j.biochi.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Soragni E, Xu C, Plasterer HL, et al. Rationale for the development of 2-aminobenzamide histone deacetylase inhibitors as therapeutics for Friedreich ataxia. J Child Neurol. 2012;27:1164–1173. doi: 10.1177/0883073812448533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C, Soragni E, Jacques V, et al. Improved histone deacetylase inhibitors as therapeutics for the neurodegenerative disease Friedreich’s ataxia: a new synthetic route. Pharmaceuticals. 2011;4:1578–1590. doi: 10.3390/ph4121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beconi M, Aziz O, Matthews K, et al. Oral administration of the pimelic diphenylamide HDAC inhibitor HDACi 4b is unsuitable for chronic inhibition of HDAC activity in the CNS in vivo. PLoS One. 2012;7:e44498. doi: 10.1371/journal.pone.0044498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao X, Anderson DL, Ross SA, et al. Predicting QT prolongation in humans during early drug development using hERG inhibition and an anaesthetized guinea-pig model. Br J Pharmacol. 2008;154:1446–1456. doi: 10.1038/bjp.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynch DR, Fischbeck KH. Nicotinamide in Friedreich’s ataxia: useful or not? Lancet. 2014;384:474–475. doi: 10.1016/S0140-6736(14)60573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burk K, Schulz SR, Schulz JB. Monitoring progression in Friedreich ataxia (FRDA): the use of clinical scales. J Neurochem. 2013;126(suppl 1):118–124. doi: 10.1111/jnc.12318. [DOI] [PubMed] [Google Scholar]
- 49.Ebert AD, Yu J, Rose FF, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2008;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egawa N, Kitaoka S, Tsukita K, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 51.Cooper O, Seo H, Andrabi S, et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med. 2012;4:141ra190. doi: 10.1126/scitranslmed.3003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee G, Ramirez CN, Kim H, et al. Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol. 2012;30:1244–1248. doi: 10.1038/nbt.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu X, Lei Y, Luo J, et al. Prevention of beta-amyloid induced toxicity in human iPS cell-derived neurons by inhibition of cyclin-dependent kinases and associated cell cycle events. Stem Cell Res. 2013;10:213–227. doi: 10.1016/j.scr.2012.11.005. [DOI] [PubMed] [Google Scholar]