Abstract
Ribosomal RNAs are the most abundant and universal noncoding RNAs in living organisms. In eukaryotes, three of the four ribosomal RNAs forming the 40S and 60S subunits are borne by a long polycistronic pre-ribosomal RNA. A complex sequence of processing steps is required to gradually release the mature RNAs from this precursor, concomitant with the assembly of the 79 ribosomal proteins. A large set of trans-acting factors chaperone this process, including small nucleolar ribonucleoparticles. While yeast has been the gold standard for studying the molecular basis of this process, recent technical advances have allowed to further define the mechanisms of ribosome biogenesis in animals and plants. This renewed interest for a long-lasting question has been fueled by the association of several genetic diseases with mutations in genes encoding both ribosomal proteins and ribosome biogenesis factors, and by the perspective of new anticancer treatments targeting the mechanisms of ribosome synthesis. A consensus scheme of pre-ribosomal RNA maturation is emerging from studies in various kinds of eukaryotic organisms. However, major differences between mammalian and yeast pre-ribosomal RNA processing have recently come to light. WIREs RNA 2015, 6:225–242. doi: 10.1002/wrna.1269
INTRODUCTION
The eukaryotic ribosome consists of two subunits formed by the intricate association of 79 ribosomal proteins (RPs) with 4 distinct ribosomal RNAs (rRNAs).1,2 The small subunit (40S) comprises the 18S rRNA assembled to 33 RPs (RPSs), whereas the large subunit (60S) contains the 5S, 5.8S, and 25S/28S rRNAs associated with 46 RPs (RPLs). The production of this huge molecular machine consumes the major part of the cellular energy3 and occupies vast nuclear domains before its final maturation in the cytoplasm.4 Indeed, ribosome biogenesis is an oriented process starting in the nucleolus where three of the rRNAs (18S, 5.8S, and 25S/28S rRNAs) are produced as a long primary transcript originating from the head-to-tail tandem repeats of rDNA (∼150 copies per haploid genome in yeast Saccharomyces cerevisiae and 300–400 in a diploid human cell), which are transcribed by RNA polymerase I (Pol I). In baker's yeast, the rDNA repeat also encodes the 5S rRNA, which is transcribed in the reverse direction. In human cells, the rDNA clusters, called nucleolar organizer regions (NORs), are localized on the short arms of the five acrocentric chromosomes (HSA13–15, HSA21, and HSA22),5,6 while the precursor to the 5S rRNA is synthesized from multiple genes located in close proximity to the nucleolus.7
Within the eukaryotic primary rRNA transcript, the mature 18S, 5.8S, and 25S/28S rRNAs are separated by the internal transcribed spacers 1 (ITS1) and 2 (ITS2) and flanked by the 5′ and 3′ external transcribed spacers (5′-ETS and 3′-ETS). The nascent primary transcripts associate co-transcriptionally with some RPs, numerous pre-ribosomal factors (PRFs), and small nucleolar ribonucleoprotein particles (snoRNPs) to form a series of large RNPs,8 in which pre-rRNA folding and modification take place together with RP assembly. Along this process, the transcribed spacers are sequentially eliminated through a complex series of endonucleolytic and exonucleolytic cleavages. The production pathways of the two ribosomal subunits diverge after cleavage in the ITS1. The maturating pre-60S particles form the so-called granular component of the nucleolus, seen by electron microscopy (EM),4 whereas pre-40S particles are more rapidly exported to the cytoplasm. Proteomic studies have established a highly dynamic pattern in the protein composition of these pre-ribosomes, with gradual association of RPs and association/dissociation of PRFs, either as individual factors or as multiprotein modules.9,10 The major part of this process occurs in the nucleolus, but additional maturation events take place in the nucleoplasm. Pre-ribosomes are then actively exported to the cytoplasm, where they undergo final processing to form the mature ribosomal subunits. Here, we provide an overview of the mechanisms underlying pre-rRNA conversion into mature 18S, 5.8S, and 25/28S rRNAs, as it emerges from the study of unicellular and multicellular eukaryotes. Synthesis and maturation of the 5S rRNA have been reviewed elsewhere.11
DIFFERENT PATHWAYS FOR PRE-rRNA PROCESSING
Maturation of the nascent pre-rRNA begins with the early stages of the production of the 18S rRNA, namely endonucleolytic processing of the 5′-ETS and cleavage within the ITS1 (Figures 1 and 2). In yeast cells, the 18S rRNA is exclusively generated by a series of endonucleolytic cleavages within the 5′-ETS and ITS1 sequences (Figure 1), whereas a combination of endonucleolytic and exonucleolytic processing steps in the ITS1 is involved in mammalian cells (Figure 2). Following cleavage of the ITS1 in the early precursors, the 5′ end of the 5.8S rRNA is tailored through two alternative processing pathways that yield a long and a short form. Elimination of the ITS2 is initiated by endonucleolytic cleavages allowing 3′→5′ and 5′→3′ exonucleases to respectively generate the mature 3′ end of the 5.8S rRNA and the mature 5′ end of the 25S/28S rRNA.
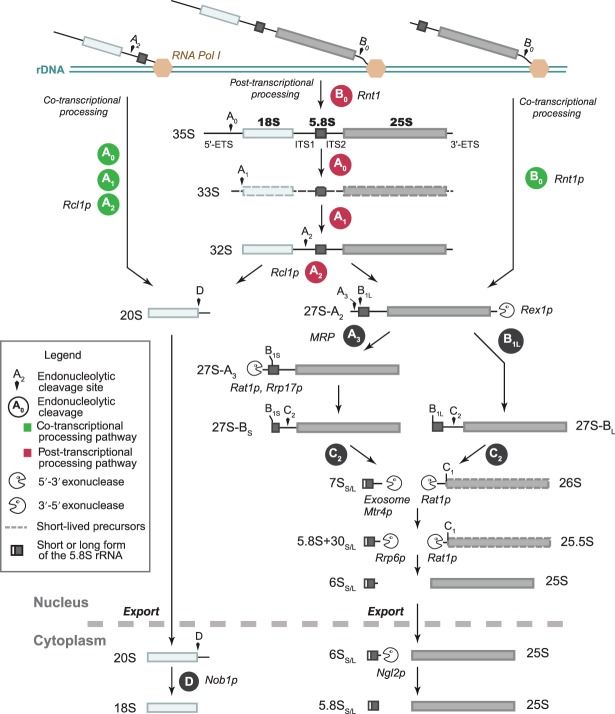
Figure 1.
Pre-ribosomal RNA (rRNA) processing in yeast Saccharomyces cerevisiae. The majority of the nascent transcripts are cleaved co-transcriptionally at sites A0, A1, and A2, yielding the 20S and 27S-A2 pre-rRNAs (green).12 Alternatively, the full-length 35S pre-rRNA is processed post-transcriptionally (red). After elimination of the 5′-ETS and cleavage at site A2, maturation of the 18S rRNA 3′ end from the 20S pre-rRNA requires a single endonucleolytic cleavage step by Nob1p, which takes place in the cytoplasm after nuclear export of the pre-40S particle. Maturation of the large subunit follows two pathways, which yield two versions of the 5.8S rRNA 5′ end. The major pathway produces a short form by endonucleolytic cleavage of the 27S-A2 pre-rRNA at site A3 by RNase MRP and subsequent exonucleolytic processing of the 27S-A3 pre-rRNA by Rat1p and Rrp17p.13 Alternatively, the 27S-A2 pre-rRNA is cleaved at site B1L, yielding a long form of the 5.8S rRNA. Maturation of the 5.8S rRNA 3′ end is completed in the cytoplasm by exonuclease Ngl2p.14
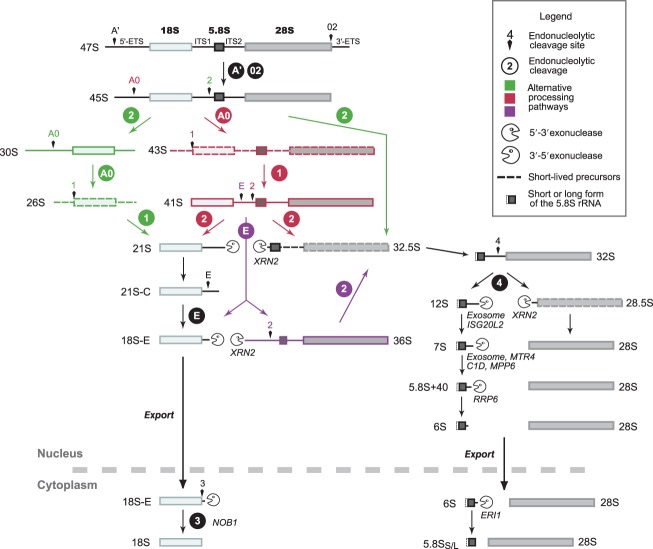
Figure 2.
Pre-ribosomal RNA (rRNA) processing in mammalian cells. The pre-rRNA processing presented here combines data from studies in human and murine cells. The nomenclature refers to human cells15,16; the corresponding nomenclature in mouse is indicated in Figure 3. Alternative cleavage sequences are depicted in different colors. Short-lived precursors are represented with dotted lines. Cleavage of the 45S pre-rRNA can either start in the 5′-ETS (red) or in the ITS1 (green), which defines two pathways. If cleavage of the 5′-ETS occurs first (41S pre-rRNA), subsequent cleavage in the ITS1 takes place either at site 2 or at site E (purple). Initial cleavage at site 2 is the major pathway in HeLa cells, considering the abundance of the 30S pre-rRNA relative to the 41S. In mouse cell lines, the 36S pre-rRNA is readily detected. The endonuclease NOB1 is necessary for maturation of the 3′ end of the 18S-E pre-rRNA in the cytoplasm. Formation of the long and short 5′ ends of the 5.8S rRNA is not fully documented in mammalian cells. The 5.8S rRNA 3′-end maturation pathway primarily involves exonucleases,16–20 but the 7S pre-rRNA was also proposed to result from endonucleolytic cleavage of the 12S pre-rRNA (site 4a in mouse, see Figure 3).21,22 It has not been formally demonstrated that final maturation of the 6S pre-rRNA takes place in the cytoplasm in mammalian cells, but this was shown in Xenopus laevis23 and Saccharomyces cerevisiae.14
The timing of 5′-ETS removal relative to ITS1 cleavage in the primary transcript varies among organisms or cell lines and may also differ from one cell type to another in multicellular organisms.15,24–26 In human cells, early cleavage of the 5′-ETS yields the 41S pre-rRNA, whereas the presence of the 30S pre-rRNA indicates that ITS1 cleavage at site 2 precedes 5′-ETS cleavage (Figure 2). Similarly, ITS1 cleavage at site E prior to the cleavage at site 2 results in the formation of the 36S pre-rRNA, which is commonly observed in mouse cell lines. This kinetic variability, long acknowledged in the case of animals and plants, may also apply to S. cerevisiae, albeit to a lesser extent. While 5′-ETS is cleaved first in the major pathway in yeast, the 23S pre-rRNA, produced by ITS1 direct cleavage at site A3 prior to 5′-ETS, is present at low levels in wild-type yeast cells.27,28 The 22S (A0–A3) and 21S (A1–A3) pre-rRNAs are also detected. These species accumulate in ribosome biogenesis mutant strains, where they are targeted for degradation. They are therefore usually considered as dead-end intermediates. However, these RNAs are strongly co-immunoprecipitated with several components of the processing machinery in wild-type cells.29,30 In addition, the 21S pre-rRNA accumulates in yeast strains bearing mutations in RRP5 or PFA1, in which A2 cleavage is strongly inhibited.31,32 These mutant strains display a growth rate indistinguishable from wild-type cells, suggesting that the 21S intermediate can be productively converted to the mature 18S rRNA in yeast. In higher eukaryotes, this kinetic variability in the early cleavages is often interpreted as the existence of different processing pathways. It is tempting to speculate that it represents a potential source of structural and functional heterogeneity among ribosomes, an idea that requires further experimental support. These alternative pathways may also contribute to the robustness of the maturation process and to its regulation. It is of note that pre-rRNA maturation also includes nonuniversal processing steps in some organisms (discussed in Ref 26).
These differences in cleavage kinetics may also be linked to the timing of pre-rRNA processing relative to transcription. While detection of the full-length rRNA precursors (35S pre-rRNA in yeast and 47S pre-rRNA in mammals) clearly points to the occurrence of post-transcriptional processing, co-transcriptional cleavage of the pre-rRNA also takes place in yeast, as evidenced from the visualization of rDNA transcription units by EM after chromatin spreading. The so-called Christmas trees display nascent transcripts of variable lengths, proportional to their distance from the promoter region, with terminal globular structures at their 5′ end.33,34 In yeast, these structures were identified as the small subunit (SSU) processome, a large RNP involved in early maturation steps of the small ribosomal subunit.35–37 In actively growing yeast cells, two thirds of these terminal structures disappear from the nascent transcripts when the polymerase reaches the 5′ region of the 25S coding sequence, suggesting that the precursors to the 40S particles are released co-transcriptionally after 5′-ETS removal and ITS1 cleavage at site A236 (Figure 1). In agreement with these observations, high-resolution pulse-chase analyses in yeast suggested that 70–80% of the nascent transcripts are processed co-transcriptionally at site A2,12 which requires the exoribonuclease Rat1p.38 In higher eukaryotes, similar chromatin spreads show no evidence of co-transcriptional release of pre-40S particles.34,36
MATURATION OF THE 18S rRNA
Elimination of the 5′-ETS
Formation of 18S rRNA requires removal of 5′-ETS and processing of ITS1 (Figures 1 and 2). Endonucleolytic cleavage at sites A0/A0 and A1/1 is a coordinated process occurring almost simultaneously at the two positions. In mammalian cells, uncoupling of cleavage at sites A0 and 1 leads to the formation of the 43S and 26S precursors (Figure 2), both of which accumulate upon impairment of ribosome biogenesis.39,40 These cleavages are generated by endoribonucleases, as the spacer fragments corresponding to the intervening sequences were detected in yeast41–43 as in mouse cells.44 At present, the enzymes responsible for the 5′-ETS endonucleolytic cleavages remain unknown. However, a large number of factors are required for processing of the 5′-ETS including the U3, snR30/U17, and U14 snoRNAs, the SSU processome,35–37 and about half of the RPSs40,45 (see below). Sites A0 and 1 are thought to be located close to one another in the 5′-ETS structure, and are always found near binding sites of the U3 snoRNA.46,47 The U3 snoRNA plays a central role in 5′-ETS processing by hybridizing with distant segments of the pre-rRNA and chaperoning RNA folding,26,48–51 which was shown to orientate the order of the early cleavages in Xenopus laevis.26,52 According to secondary structure predictions made for the human and murine 5′-ETS,53,54 sites A0 and 1 flank the stem region of a very large structural domain, the size of which is bigger than that of the 18S rRNA. In yeast, degradation of the 5′-ETS fragments is mostly ensured by the exosome,42,55 whereas it primarily requires the cooperative action of the 5′→3′ exoRNase XRN2 and the exosome in mammalian cells.16,44,56
An additional very early cleavage site in the 5′-ETS, called A′ or 01, was described in animals and plants, but the frequency of this cleavage varies among species and cell types.26,57–59 This site is characterized by an evolutionarily conserved motif (ECM) of 11 nucleotides, which allows recruitment of the U3 snoRNP and of the abundant phosphoprotein nucleolin. Efficient cleavage at A′ was shown to depend on the U3, U14, E1, and E3 snoRNPs,60,61 but recent data indicate that the sole UTP-A complex, recruited upstream of U3 snoRNA, is strictly necessary in human cells.56 Surprisingly, this endonucleolytic cleavage requires the 5′→3′ exonuclease XRN2.56,62 This cleavage appears optional for production of mature rRNAs because further processing of the 5′-ETS at sites A0 and 1 is not precluded by defects in A′ cleavage.56,63 Conversely, affecting cleavage at sites A0 and 1 by depleting RPs does not hamper A′ cleavage.40 This cleavage could facilitate access of the processing machinery for the subsequent maturation steps.
Processing of the ITS1
Recent studies have highlighted major differences in the formation of the 3′ end of the 18S rRNA between yeast and mammals. While this process is accomplished through two endonucleolytic cleavages (sites A2 and D) in yeast (Figure 1), it requires the combined action of both endonucleases and exonucleases in human cells (Figure 2). As in yeast, two endonucleolytic sites have been identified within the human ITS1 so far: site 2 and site E. Usually, cleavage at site 2 occurs first. In human cells, available data suggest that it is located ∼900 nucleotides downstream of the 18S 3′ end.59 Cleavage at site E occurs after nucleotides C5605 and C5608, i.e., 78 and 81 nucleotides after the 18S–ITS1 junction, resulting in the formation of 18S-E pre-rRNA (Figure 3).16 Unlike in yeast, the pre-rRNAs generated by cleavage in the ITS1 are then trimmed by 3′→5′ exonucleolytic activities.16,17,64,65 Removal of around 250 nucleotides at the 3′ end of the 21S pre-rRNA produces the 21S-C intermediate. This exonucleolytic step stops at the boundary of a highly conserved domain of ITS1 in mammals (domain C in Figure 3). Similarly, the 3′ end of the 18S-E precursor is gradually shortened in the nucleus and the cytoplasm.16 The identity of these exonucleases and their role need to be clarified. The exosome has been proposed to ensure the trimming of the 21S pre-rRNA.16,17,65 It was suggested that processing of the 21S RNA by the exosome represents a major pathway for generating the 18S-E pre-rRNA,65 but rapid amplification of cDNA 3′ ends (3′-RACE) analysis of the 18S-E pre-rRNA does not support this hypothesis.16 Other 3′→5′ exonucleases are likely to be involved in the processing of the 18S-E pre-rRNA.16 After nuclear export, the PIN domain-containing protein NOB1 likely cleaves the 18S-E pre-rRNA at site 3, which yields the mature 18S rRNA.16,65 Exonucleolytic processing of the 18S-E pre-rRNA in the cytoplasm could facilitate access of NOB1 to site 3. Alternatively, 3′→5′ trimming could proceed up to the 18S rRNA 3′ end and constitutes an alternative maturation pathway. Interestingly, the 18S-E pre-rRNA is oligouridylated in the cytoplasm, which might stimulate the recruitment of an exonuclease.16 In yeast, the pre-40S particles containing the 20S pre-rRNA are exported to the cytoplasm, where endonucleolytic cleavage by Nob1p forms the mature 18S rRNA.67,68 Recently, it was shown that the final maturation stages of the 40S subunits in yeast involve a ‘translation-like’ cycle, where the cytoplasmic pre-40S particles associate with mature 60S subunits for a proofreading test prior to processing by Nob1p.67,69
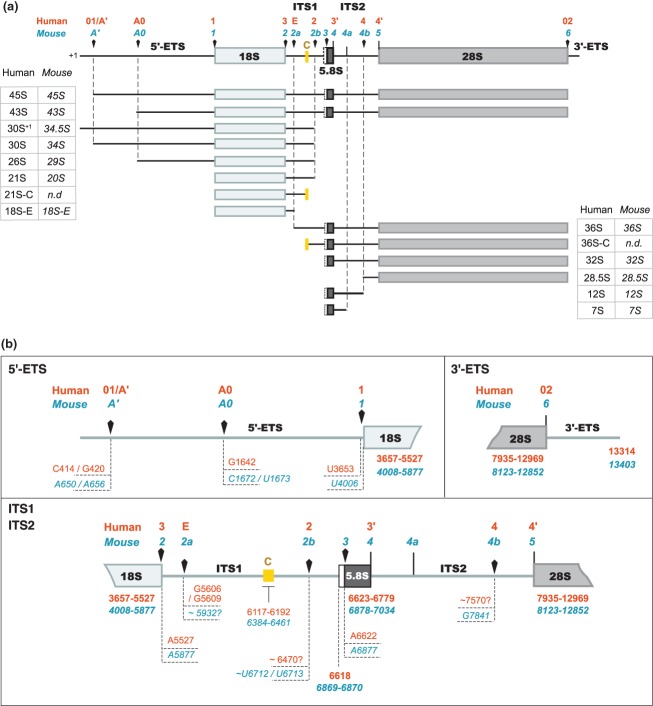
Figure 3.
The different pre-rRNAs detected in human and murine cells and their nomenclature.15,16,24,39,40,44,47,59,66 (a) Definition of the major pre-ribosomal RNAs (rRNAs). Arrowheads indicate the endonucleolytic cleavage sites. The yellow box corresponds to a highly conserved domain in ITS1 among mammals. Its boundaries define the 5′ end of the 36S-C and the 3′ end of the 21S-C, suggesting that exonucleolytic trimming of these extremities is stopped by a secondary structure and/or a protein complex anchored on this region.16 The 36S-C and the 30S+1/34.5S species are examples of precursors that are only detected upon perturbation of ribosome biogenesis. (b) Map of the processing sites in the human (orange) and murine (blue) pre-rRNAs. Arrowheads indicate the endonucleolytic cleavage sites. The nucleotides correspond to the residue located 5′ to the cleavage site. The extremities of the 18S, 5.8S, and 28S rRNAs are indicated in bold letters. The mapping of these sites is partly discussed in the text and was extensively reviewed by Mullineux and Lafontaine.59 Human site E was determined by primer extension and 3′-RACE.16 Localization of site 4b in mouse was mapped by primer extension.44 The human sites equivalent to sites 3 and 4a in mouse have not been formally designated. Details on site 4a are given in the text. The numbering of the nucleotides refers to GenBank sequences U13369.1 (human rDNA) and BK000964.3 (mouse rDNA).
In mammalian cells, the efficiency of cleavage at site 2 is rather insensitive to defects in cleavage at sites A0, 1, and E. It depends on a number of factors including PES1, BOP1, NOL12, and RPs of the large subunit (RPL15 and RPL26).16,65,66 Absence of any of these proteins slows down cleavage at site 2 relative to 5′-ETS processing and affects processing by XRN2, leading to accumulation of 41S and 36S pre-rRNAs (Figure 2). Interestingly, the orthologs of PES1, BOP1, and NOL12 in S. cerevisiae (Nop7p, Erb1p, and Rrp17p) are part of the ‘A3 factors’, which are required for 5′→3′ exonucleolytic tailoring of the 5′ extremity of the 5.8SS rRNA by Rat1p after cleavage at site A3 (see below). These observations draw a strong parallel between site 2 in human and site A3 in yeast. In contrast, cleavage at site E in human requires prior cleavage at sites A0 and 1 and is blocked upon knockdown of RPS18, RPS19, or Bystin/ENP1,40,64 similar to what has been observed in yeast for site A2.70 Therefore, processing sites E and 2 appear to be functionally related to sites A2 and A3 in yeast, respectively. In yeast, Rcl1p, a protein belonging to the RNA cyclase family, has been proposed to be the enzyme responsible for cleavage at site A2.71 RCL1, the human ortholog of Rcl1p, is required for cleaving the ITS1 at site E.65 Cleavage at site A3 in yeast is catalyzed by the RNase MRP, a RNP composed of a structural RNA associated with 10 essential proteins.72 However, knockdown of MRP components was found to have no effect on ITS1 cleavage in human cells.65 Nevertheless, these strong functional analogies allow envisioning a unified nomenclature of the endonucleolytic cleavage sites in eukaryotes.
MATURATION OF THE LARGE SUBUNIT rRNAs
Maturation of the 5′ end of the 5.8S rRNA is coordinated with formation of the 3′ end of the 25S/28S rRNA. In yeast, mutation of the 3′-ETS impairs endonucleolytic cleavages both at sites A3 and B1L, indicating that these processing events are somehow coupled.73 A similar phenotype was observed upon loss-of-function of the U8 snoRNA in X. laevis.74 In yeast, the 3′-ETS is cleaved by endonuclease Rnt1p at site B0, which may participate in transcription termination75 (Figure 1). This leaves a ∼20-nucleotide extension that is removed by the 3′→5′ exonuclease Rex1p in the 27S-A precursors.76
Two alternative processing pathways produce 5.8S rRNAs with different 5′ ends. In yeast, the 5′ end of the major form (5.8SS for 5.8S short) results from 5′→3′ exonucleolytic trimming of the 27S-A3 pre-rRNA down to site B1S by two exoribonucleases: Rat1p, the activity of which is stimulated by its co-factor Rai1p, and Rrp17p.13,77–81 Rat1p is recruited within the particles containing the 27SA2 intermediate, prior to A3 cleavage.82 It requires a series of factors referred to as the ‘Nop7p-subcomplex’83 or ‘A3 cluster’,82 which bind to the 3′ end of the 5.8S rRNA, the ITS2, and the 5′ end of the 25S rRNA, and coordinate the processing events occurring at the 5′ and 3′ ends of the 5.8S rRNA.82 The 5′ end of the minor form (5.8SL for 5.8S long) is produced through a direct cleavage at site B1L of the 27SA2 RNA, and probably also the 27SA3, by a yet unidentified endonucleolytic activity.84 Endonucleolytic processing of the ITS2 at site C2 is initiated only after maturation of both the 5′ end of the 5.8S rRNA and 3′ end of the 25S rRNA.85 Cleavage at C2 yields the 7S pre-rRNA, which corresponds to the mature 5.8S rRNA with a ∼140-nucleotide extension at its 3′ end. Production of the mature 3′ end of the 5.8S rRNA from this 7S precursor involves a surprisingly complex succession of exonucleases (Figure 1). First, the nuclear exosome initiates the 3′→5′ processing of the 7S intermediates from site C2.85,86 This process is assisted by the RNA helicase Dob1p/Mtr4p, which probably functions in unwinding the secondary structures or displacing the bound proteins that may otherwise hamper progression of the exosome.42,85,87 Progression of the exosome is stopped ∼30 nucleotides from the 5.8S rRNA 3′ end because of steric hindrance, resulting in the 5.8S + 30 intermediates. The Rrp6p exonuclease in turn further degrades the ITS2 sequence and leaves approximately eight nucleotides, generating the 6S intermediates.85,88 A role of exonucleases Rex1p, Rex2p, and Rex3p in this process was also proposed,89,90 but may rather correspond to a quality control pathway.14 Finally, the Mg2+-dependent exonuclease Ngl2p trims the 3′ end of the 6S pre-rRNA in the cytoplasm, producing the mature 3′ end of the 5.8S rRNA.14,84 Interestingly, the 5′→3′ exonuclease Rat1p90 and its co-factor Rai1p81 are also required for the 3′-end processing of the 5.8S rRNA. These factors may coordinate the 5′- and 3′-end processing of this rRNA. The downstream intermediate of C2 cleavage, the 26S RNA, corresponds to the 25S rRNA with a 5′ extension. Removal of this segment is performed by exonuclease Rat1p.79,91 The Las1p protein is required for the processing events occurring at both ends of the ITS2 sequence.90
This processing scheme, first described for yeast cells, appears to be conserved across evolution, as shown by recent studies in human cells or in Arabidopsis thaliana.17,92 Formation of the 3′ end of the 28S rRNA is a very early event that may participate in transcription termination in mammalian cells.75 60S particles in animals and plants also contain two forms of 5.8S rRNA, suggesting molecular mechanisms similar to those in yeast. In mouse and human cells, XRN2 (yeast Rat1p) processes the downstream products generated at sites 2 and E16 and is required for tailoring the 5′ end of the 32S RNA.44 The 5.8Ss rRNA is lost upon depletion of BOP1 (yeast Erb1p) in mouse cells.65 However, unlike Rat1p depletion, XRN2 knockdown does not strongly affect the ratio of 5.8SL to 5.8SS rRNAs.65,90 Endonucleolytic cleavage of ITS2 (sites 4 in human and 4b in mouse, Figure 3) in the 32S rRNA gives rise to the 12S and 28.5S species, which are precursors to the mature 5.8S and the 28S rRNAs, respectively. The 5′ end of the 28.5S rRNA is then trimmed by XRN2 to form the 28S rRNA.44 Processing of 5.8S rRNA 3′ end from the 12S precursor is a multistep mechanism, as in yeast. Mammalian cells display a shorter precursor, the 7S pre-rRNA.93,94 This species was proposed to result from a second endonucleolytic cleavage within ITS2 (site 4a in mouse, Figure 3).21,22 However, intermediate processing fragments between the 12S and 7S pre-rRNAs accumulate upon depletion of the exosome components, including its catalytic subunits RRP6 and DIS3.16–18 In addition, the nucleolar exonuclease ISG20-L2 was proposed to take part in this process.19 Efficient maturation of the 7S pre-rRNA requires several subunits of the exosome17 and of the exosome-associated factors MTR4, MPP6, and CD1.17,18 Depletion of RRP6 results in very strong accumulation of a smaller intermediate, coined 5.8S + 40, very similar to the 5.8S + 30 species in yeast.17 Finally, a 5.8S rRNA precursor extended of a few nucleotides in 3′ (termed 6S in Figure 2) undergoes final maturation by the 3′→5′ exoribonuclease ERI1, as reported in mouse cells.20 Noticeably, this role of ERI1 is conserved in Schizosaccharomyces pombe and Caenorhabditis elegans.95 Studies in yeast14 and in X. laevis oocytes23 have shown that the very last step of the 5.8S rRNA 3′-end maturation takes place in the cytoplasm. Consistently, ERI1 is a cytoplasmic protein in S. pombe and in C. elegans. ERI1 is not essential for cell viability in S. pombe and in C. elegans, indicating that 60S particles containing 6S rRNA are competent for translation.84 Mice devoid of ERI1 also accumulate functional 60S particles containing 5.8S rRNA with an extended 3′ end, but display embryonic growth defects with less than 10% survival at weaning and die within 2 days postpartum.20 Overall, these studies indicate that the maturation scheme of the large subunit rRNAs in mammals resembles that in yeast, but further studies are necessary to precisely define the relative contribution of the various exoribonucleases and the occurrence of secondary endonucleolytic cleavages.
ROLE OF THE RIBOSOMAL PROTEINS
Exhaustive analyses in yeast and mammalian cells have shown that RPs are required for structural assembly, pre-rRNA processing, and nuclear export of the pre-ribosomal particles.40,45,96–100 For example, only a few RPs are dispensable for production of the mature 18S rRNA, namely Rps12p and Rps25p in yeast,45 and RPS25 in human.40,97 Similar to RPS12 (human) and Rps12p (yeast), human RPL26, unlike its yeast counterpart Rpl26p,101 is essential for 60S subunit production in human cells,102 pointing toward the evolution of RPs' functions.
The precise functions of RPs in pre-rRNA processing and ribosome biogenesis remain elusive. X-ray and cryo-EM structures of mature ribosomes have shown that most RPs share common structural features, in particular globular domains interspersed by poorly structured loops that bind to rRNA or other RPs.103 Studies in prokaryotes and eukaryotes indicate that the interaction network between proteins and RNA, at first somewhat loose and dynamic, gradually tightens during subunit assembly, and eventually forms stable ribosomal subunits.96,99,104,105 It is generally thought that during subunit assembly, RPs act as chaperones assisting the proper folding of the nascent pre-rRNAs. Indeed, in vitro assembly of bacterial ribosomes showed that RPs are required in a hierarchical order.106 A recent study on the Escherichia coli 30S small ribosomal subunit directly demonstrated the guiding role of Rps4 on the correct folding of 16S rRNA helices h3 and h18, allowing in turn the folding of h1 as well as subsequent recruitment of other proteins on the 16S rRNA.107
Depletion of eukaryotic RPs results in blockades at different pre-rRNA processing steps. In human cells, initiation of cleavage at sites A0, 1, and E (but not cleavage at site A′) strictly depends on assembly of half of the small subunit RPs (RPSs).40 This first set of RPSs makes contacts with the 5′ part of the 18S domain (Figure 4). They include the orthologs of the primary and secondary binders that were identified in in vitro assembly of the prokaryotic small subunit, and were indeed found to associate early with pre-ribosomal particles in mammalian cells.109 The second set of RPSs is not required to initiate removal of the 5′-ETS, but for efficient processing of the ITS1 at sites 1, E, or 3 as well as nuclear export.40 Those proteins are incorporated later in pre-ribosomes and structure the 3′ region of the 18S rRNA (Figure 4). Similar results were also found in yeast.45,96 Thus, the 40S structure is sequentially assembled: first, the ‘body’ domain is formed, which contains the 5′ end and central domains of 18S rRNA and primary and secondary binding RPs, followed by the ‘head’ domain, which is composed of the major 3′-end domain of 18S rRNA and secondary/tertiary binding RPs (Figure 4). This in vivo two-stepped, 5′→3′ oriented assembly of the eukaryotic 40S was also observed in prokaryotes in in vitro kinetic studies.104,110,111 Such a strong interdependency between formation of large-scale structural domains and pre-rRNA processing was also recently demonstrated for the large subunit in yeast.100 Joining of RPLs and folding of rRNA are tightly connected and induce a hierarchical assembly of the structural domains in the 60S subunit. Binding of early-acting RPLs to domains corresponding to the 5′ part of the 25S and 5.8S rRNAs stabilizes a pre-ribosomal structure that facilitates A2 and A3 cleavages. These early-acting RPLs form a clamp-like region cupping the convex solvent side of the future 60S subunit, which favors formation of the 27S-B pre-rRNA. Subsequently, stable assembly of middle-acting RPLs allows cleavage of the 27S-B pre-rRNA at the C2 site in ITS2. Binding of middle- and late-acting RPLs to the 3′ region of the mature 25S rRNA sequence is coupled to 7S pre-rRNA processing. Finally, the 5S RNP complex (a structural module comprising the 5S rRNA, Rpl5p, and Rpl11p and recruited in early pre-60S particles) must undergo a semicircular movement to adopt its final position and form the central protuberance of the 60S subunit.112 This step requires correct folding and processing of the 6S precursor to mature 5.8S rRNA, chaperoned by late-acting RPLs.
Figure 4.
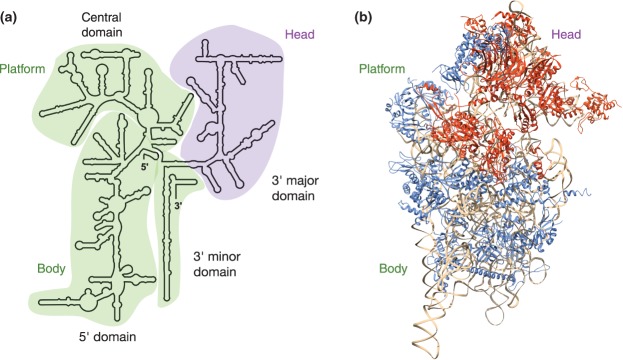
Role of the ribosomal proteins in formation of the 40S subunit. (a) Secondary structure of the human 18S rRNA (http://apollo.chemistry.gatech.edu/RibosomeGallery/). Four topological domains can be distinguished, which form distinct features in the 3D structure: the body and the platform outlined in green, and the head outlined in purple. (b) Two functional groups in RPS proteins. A first class of RPSs in human cells is strictly required for initiating cleavage at sites A0, 1, and E. These ‘initiation RPSs’ or i-RPSs (blue) are associated with the 5′ part of the RNA (body, platform, and back of the head). The ‘progression RPSs’ or p-RPSs (orange) are subsequently required for efficient processing at sites 1, E, or 3; they assemble on or around the head,40 consistent with a delay in the formation of this structure with respect to the body and the platform. Consistent data were found in yeast.45,96 The figure shows the position of these proteins in the structure of the human 40S subunit solved by cryo-electron microscopy (PDB 3J3A).108 The 18S rRNA appears in beige.
FUNCTIONS OF THE trans-ACTING FACTORS
The maturation of pre-ribosomes involves a wealth of trans-acting proteins and snoRNPs that are required at specific stages. The snoRNPs (∼80 in yeast and 200 in human) belong to two families characterized by specific motifs in their RNA component, the C/D or H/ACA boxes.113,114 As described earlier, a subset of snoRNPs, including U3, snR30/U17, U14, and U8, function as RNA chaperones assisting early cleavages of the pre-rRNA. However, most box C/D and box H/ACA RNPs catalyze ribose methylation and uridine isomerization into pseudouridine, respectively, at specific positions of the mature rRNA sequences within the pre-rRNA. Each snoRNA base-pairs with the pre-rRNA at the vicinity of a nucleotide to be modified, thereby guiding the enzymatic modification ensured by one of the core proteins of the snoRNPs: the methyltransferase Nop1p/fibrillarin in box C/D snoRNPs and the pseudouridine synthase Cbf5p/Nap57p/Dyskerin in H/ACA snoRNPs. The modified nucleotides are mostly found in functionally important regions of the ribosome and are necessary for efficient and accurate translation.114,115 Indeed, knockdown of a single snoRNA is sufficient to alter development of zebrafish embryos.116 In addition, a few nucleotides are methylated on the base moiety by methyltransferases Dim1p,117 Bud23p,118 Rrp8p,119 Bmt2p,120 Nop2p, and Rcm1p.121 While several of these methyltransferases are essential for viability in yeast, their enzymatic activity is not always required, suggesting a facultative role of these modifications in ribosome activity.118,122 In the yeast decoding region, a hypermodified uridine residue combines pseudouridylation with base methylation and addition of a 3-amino-3-carboxypropyl group. Absence of these individual modifications was found to delay formation of the 18S rRNA.115
Many of the PRFs belong to recognizable protein families such as AAA ATPases, GTPases, RNA helicases, or kinases.123 The precise molecular function of the vast majority of these factors remains ill defined. RNA helicases involved in ribosome biogenesis are expected to modulate RNA–RNA interactions within the pre-rRNAs or between the pre-rRNAs and snoRNAs. The yeast helicases Dbp4p,124 Rok1p,125 Has1p,126 and Prp43p127 appear to function in the unwinding of snoRNA/pre-rRNA base-pairings, while the release of U8 in mouse requires DDX51.128 The Mtr4p/Dob1p helicase unwinds pre-rRNA secondary structures, thereby facilitating processing or degradation by the exosome.42,87 Helicases may also function as RNPases and displace RNA-bound proteins at specific stages of the maturation process. Several AAA-ATPases were shown in yeast to function in the release of trans-acting factors at specific stages of the maturation pathway. The best characterized example in yeast is Rea1p, a component of pre-60S particles. Rea1p mechanically extracts protein modules from the particles at different stages of the maturation pathway in the nucleus.129,130 Down the line in the pre-60S pathway, another AAA-ATPase, Drg1p, is required to initiate the release of PRFs in the cytoplasm.131 Regarding GTPases, Fun12p promotes joining of the late pre-40S particles to mature 60S subunits in the ‘translation-like’ cycle proposed to proofread these future 40S subunits in yeast cells.69 Recently, the GTPase Nog2p/Nug2p has been suggested to control recruitment of Crm1p-adaptor Nmd3p and regulate nuclear export of pre-60S particles to the cytoplasm.132 As another example, the GTPase Efl1p functions in the release of Tif6p from cytoplasmic pre-60S particles, which allows neo-synthesized 60S particles to engage in translation.133,134 Kinases such as Rio2p or Hrr25p in yeast, RIOK2 or CK1∂/CK1ε in human cells, are required for structural remodeling of the pre-40S particles and release of PRFs in the cytoplasm.135–138 Many PRFs with no predicted enzymatic activity contain putative RNA-binding domains (e.g., GAR, RRM, KH, BRIX, S1, dsRBD, and Zinc finger domains) and/or protein–protein interaction domains (WD40, HEAT, TPR, and HAT). Some of these factors function as protein modules that form before they associate with pre-ribosomes, such as the subcomplexes of the SSU processome: UTP-A,28,139 UTP-B,139–141 UTP-C,139 Mpp10p-Imp3p-Imp4p,142 Bms1p-Rcl1p,143–145 or the pre-60S particle modules Rrp5p-Noc1p-Noc2p,146 Noc4p-Nop14p,29 and Ytm1p-Nop7p-Erb1p.83,147 Finally, an important function of PRFs is also to shield the pre-ribosomal particles from premature interactions with the translation machinery.69
NUCLEOLAR SURVEILLANCE PATHWAYS
Accuracy of pre-RNA processing appears to involve constant monitoring by quality control mechanisms. In the yeast nucleus, improperly processed pre-rRNAs are rapidly targeted by a degradation mechanism known as the nucleolar surveillance pathway.30,148–150 These pre-rRNAs are tagged for degradation through the addition of a short tail consisting of four to five adenosines at their 3′ end by two related complexes, TRAMP4 and TRAMP5. As their names indicate, these complexes contain either the Trf4p or Trf5p homologous proteins belonging to the β-nucleotidyl transferase family, the Air1p/Air2p zinc knuckle RNA-binding proteins and the Mtr4p/Dob1p RNA helicase of the DExD/H family. Trf4p and Trf5p both display a distributive polyadenylation activity in vitro151,152 and are directly responsible for the oligoadenylation of the targeted substrates within the TRAMP complexes. The Air2p protein is an important structural component as it mediates binding of the TRAMP complexes to RNA.153 The Mtr4p/Dob1p helicase appears to bind to oligo-A tails at the 3′ end of the targeted substrates and tightly control their length.87 The 3′→5′ degradation of the oligoadenylated pre-rRNAs is mainly carried out by Rrp6p, although the core exosome might also be involved.152 Mtr4p has been suggested to mediate recruitment of the exosome to the adenylated substrates,152 possibly via its so-called KOW domain.153,154 Although the pre-rRNAs targeted by the nucleolar surveillance pathway are mostly degraded from the 3′ end, some are also attacked from their 5′ end by the Rat1p exonuclease.81
The substrate specificities of the TRAMP4/5 complexes remain unclear at present, but experimental evidence points to partially overlapping functions for these complexes.152,155 Deletion of TRF5 strongly reduces the accumulation of oligoadenylated pre-rRNAs observed in the absence of Rrp6p148,152 and partially restores the accumulation of faulty pre-rRNAs degraded upon loss-of-function of PRFs.30,148,149 Thus, TRAMP5 mainly functions in oligoadenylation of the pre-rRNAs targeted by the nucleolar surveillance pathway. Deletion of TRF4 results in similar phenotypes, although less pronounced, suggesting a redundant function. Interestingly, inactivation of the nucleolar surveillance pathway restores not only the accumulation of the targeted pre-rRNAs30,148,150,152 but also the production of the mature rRNAs in some cases.148 Therefore, in addition to ill-processed pre-rRNAs, the nucleolar surveillance mechanism may also target pre-rRNAs whose processing is only kinetically delayed.
How the nucleolar surveillance pathway in yeast recognizes the defective pre-rRNAs remains unclear. Based on ChIP experiments, it has been proposed that the TRAMP complexes monitor pre-rRNA processing co-transcriptionally.148 Recruitment of the nucleolar surveillance machinery might be mediated by the Nrd1p–Nab3p–Sen1p complex, which interacts with the Spt4p–Spt5p RNA Pol I elongation complex.156 Upon alteration of pre-rRNA processing, the TRAMP and exosome complexes transiently accumulate with pre-rRNAs into a subnucleolar focus called the No-body, which is probably the site for elimination of the targeted pre-rRNAs.150
Similar mechanisms appear to exist in mammalian cells as well. In mouse cells treated with low concentrations of actinomycin D, aberrant pre-rRNAs resulting from abortive elongation by RNA Pol I are polyadenylated by PAPD5, a mammalian ortholog of Trf4p/Trf5p, and are subsequently degraded by the exosome.157 Unlike Trf4p and Trf5p, which require Air1p/Air2p for binding to RNA, PAPD5 possesses its own RNA-binding domain. However, decay of aberrant pre-RNAs in mouse also strongly depends on 5′→3′ exonucleolytic processing by XRN2/Rat1.44 As mentioned above, XRN2 is required for degrading the excised 5′-ETS fragments, as well as for processing the 5′ end of the 36S, 32S, and 28S RNAs.16,44 Therefore, processing by XRN2 follows all the intermediate endonucleolytic cleavages in pre-rRNA processing, namely A′, A0 in the 5′-ETS, E and 2 in the ITS1, and 4 in the ITS2. Similarly, the ‘upstream’ products of these cleavages are trimmed by processive 3′→5′ exoRNases such as the exosome, ERI1, or ISG20-L2.16,17,65 Each endonucleolytic cleavage could thus be envisioned as a quality control step where exoribonucleases would either process or degrade the cleavage products, depending on their conformity for the subsequent maturation step.
CONCLUSION
This overview of pre-rRNA processing shows that despite a deep understanding of these mechanisms, basic elements are still missing in the blueprint of this giant Meccano, like the exact position of some cleavage sites in metazoans or the identity of several endonucleases. But new tools are now available to investigate these questions. For example, high-throughput RNA sequencing can be used to explore pre-rRNA processing and modification on a large scale and to locate maturation factors in the pre-ribosome structure by cross-linking techniques.158–160 Genome-wide siRNA screens17,161 and nucleolus proteomic9,10,162 analyses have provided large sets of functional data and revealed potential maturation factors. Three-dimensional EM has delivered the first structures of pre-ribosomes and will be instrumental to apprehend the structural remodeling of the ribosomal precursors.111,112,130,135,163–166 Recent findings in mammalian cells have revealed significant divergence of the mechanisms of pre-rRNA processing in evolution and sequencing of whole genomes should allow tackling questions such as the co-evolution of rRNAs and their processing machineries. Part of the increased complexity of the ribosome and its synthesis in evolution might be ascribed to additional connections with other cellular processes and to adaptative regulatory responses. In that respect, the discovery of ribosomopathies, a growing set of genetic pathologies or syndromes associated with mutations in genes encoding ribosome biogenesis factors, has shed new light on the link between ribosome biogenesis and cell fate.167 Disorders in pre-rRNA processing, nucleolar organization, and ribosomal subunit accumulation have been detected in cells from patients suffering ribosomopathies, including Diamond–Blackfan anemia,102,168,169 Schwachman–Diamond syndrome,170 aplasia-cutis congenita,171 or trichothiodistrophy.172 Dysregulated ribosome activity is also a common feature in many malignancies. Changes in the number and size of nucleoli have been used as prognostic marker (AgNOR analysis) for aggressive cancers for many years.173 Although an elevated ribosome activity might simply correlate with increased protein synthesis in cancer cells, recent studies rather suggest that increased RNA Pol I-mediated rDNA transcription is a critical step in tumorigenic transformation,174 and new RNA Pol I inhibitors are currently used in clinical trial.175 Modulation of pre-rRNA processing or nucleotide modification (pseudouridylation, ribose or base methylation) could contribute to the making of different ribosomes, under normal or pathological conditions. Hence, the ribose methylation level in rRNAs was recently shown to vary in cancer cells176 or upon p53 loss-of-function.177 Changes in the quality or the quantity of ribosomes may directly alter translational regulation of major regulators of cell growth and differentiation.176–178 Furthermore, recent data support the hypothesis along which ribosomes may display structural and functional heterogeneities in different tissues.179 The tissue-specific phenotypes observed in ribosomopathies call for a deeper understanding of the intricate relationship between ribosome biogenesis and cell regulatory mechanisms. These questions will require further extending the study of pre-rRNA processing from unicellular organisms or isolated cells to multicellular genetic models.
Conflict of interest:
The authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Klinge S, Voigts-Hoffmann F, Leibundgut M, Ban N. Atomic structures of the eukaryotic ribosome. Trends Biochem Sci. 2012;37:189–198. doi: 10.1016/j.tibs.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Yusupova G, Yusupov M. High-resolution structure of the eukaryotic 80S ribosome. Annu Rev Biochem. 2014;83:467–486. doi: 10.1146/annurev-biochem-060713-035445. [DOI] [PubMed] [Google Scholar]
- 3.Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Verdun D, Roussel P, Thiry M, Sirri V, Lafontaine DL. The nucleolus: structure/function relationship in RNA metabolism. WIREs RNA. 2010;1:415–431. doi: 10.1002/wrna.39. [DOI] [PubMed] [Google Scholar]
- 5.Babu KA, Verma RS. Structural and functional aspects of nucleolar organizer regions (NORs) of human chromosomes. Int Rev Cytol. 1985;94:151–176. doi: 10.1016/s0074-7696(08)60396-4. [DOI] [PubMed] [Google Scholar]
- 6.Worton RG, Sutherland J, Sylvester JE, Willard HF, Bodrug S, Dube I, Duff C, Kean V, Ray PN, Schmickel RD. Human ribosomal RNA genes: orientation of the tandem array and conservation of the 5′ end. Science. 1988;239:64–68. doi: 10.1126/science.3336775. [DOI] [PubMed] [Google Scholar]
- 7.Fedoriw AM, Starmer J, Yee D, Magnuson T. Nucleolar association and transcriptional inhibition through 5S rDNA in mammals. PLoS Genet. 2012;8:e1002468. doi: 10.1371/journal.pgen.1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tschochner H, Hurt E. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol. 2003;13:255–263. doi: 10.1016/s0962-8924(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Y, Boisvert FM, Gregor P, Cobley A, Lamond AI. NOPdb: Nucleolar Proteome Database–2008 update. Nucleic Acids Res. 2009;37:D181–D184. doi: 10.1093/nar/gkn804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coute Y, Burgess JA, Diaz JJ, Chichester C, Lisacek F, Greco A, Sanchez JC. Deciphering the human nucleolar proteome. Mass Spectrom Rev. 2006;25:215–234. doi: 10.1002/mas.20067. [DOI] [PubMed] [Google Scholar]
- 11.Ciganda M, Williams N. Eukaryotic 5S rRNA biogenesis. WIREs RNA. 2011;2:523–533. doi: 10.1002/wrna.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kos M, Tollervey D. Yeast pre-rRNA processing and modification occur cotranscriptionally. Mol Cell. 2010;37:809–820. doi: 10.1016/j.molcel.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger M, Zenklusen D, Ferguson A, Wei KE, El Hage A, Tollervey D, Chait BT, Singer RH, Rout MP. Rrp17p is a eukaryotic exonuclease required for 5′ end processing of pre-60S ribosomal RNA. Mol Cell. 2009;36:768–781. doi: 10.1016/j.molcel.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson E, Tollervey D. The final step in 5.8S rRNA processing is cytoplasmic in Saccharomyces cerevisiae. Mol Cell Biol. 2010;30:976–984. doi: 10.1128/MCB.01359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjiolova KV, Nicoloso M, Mazan S, Hadjiolov AA, Bachellerie JP. Alternative pre-rRNA processing pathways in human cells and their alteration by cycloheximide inhibition of protein synthesis. Eur J Biochem. 1993;212:211–215. doi: 10.1111/j.1432-1033.1993.tb17652.x. [DOI] [PubMed] [Google Scholar]
- 16.Preti M, O'Donohue MF, Montel-Lehry N, Bortolin-Cavaille ML, Choesmel V, Gleizes PE. Gradual processing of the ITS1 from the nucleolus to the cytoplasm during synthesis of the human 18S rRNA. Nucleic Acids Res. 2013;41:4709–4723. doi: 10.1093/nar/gkt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tafforeau L, Zorbas C, Langhendries JL, Mullineux ST, Stamatopoulou V, Mullier R, Wacheul L, Lafontaine DL. The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of pre-rRNA processing factors. Mol Cell. 2013;51:539–551. doi: 10.1016/j.molcel.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Schilders G, van Dijk E, Pruijn GJ. C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res. 2007;35:2564–2572. doi: 10.1093/nar/gkm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coute Y, Kindbeiter K, Belin S, Dieckmann R, Duret L, Bezin L, Sanchez JC, Diaz JJ. ISG20L2, a novel vertebrate nucleolar exoribonuclease involved in ribosome biogenesis. Mol Cell Proteomics. 2008;7:546–559. doi: 10.1074/mcp.M700510-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Ansel KM, Pastor WA, Rath N, Lapan AD, Glasmacher E, Wolf C, Smith LC, Papadopoulou N, Lamperti ED, Tahiliani M. Mouse Eri1 interacts with the ribosome and catalyzes 5.8S rRNA processing. Nat Struct Mol Biol. 2008;15:523–530. doi: 10.1038/nsmb.1417. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy R, Rothblum LI, Subrahmanyam CS, Liu MH, Henning D, Cassidy B, Busch H. The nucleotide sequence of 8S RNA bound to preribosomal RNA of Novikoff hepatoma. The 5′-end of 8S RNA is 5.8S RNA. J Biol Chem. 1983;258:584–589. [PubMed] [Google Scholar]
- 22.Michot B, Joseph N, Mazan S, Bachellerie JP. Evolutionarily conserved structural features in the ITS2 of mammalian pre-rRNAs and potential interactions with the snoRNA U8 detected by comparative analysis of new mouse sequences. Nucleic Acids Res. 1999;27:2271–2282. doi: 10.1093/nar/27.11.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trotta CR, Lund E, Kahan L, Johnson AW, Dahlberg JE. Coordinated nuclear export of 60S ribosomal subunits and NMD3 in vertebrates. EMBO J. 2003;22:2841–2851. doi: 10.1093/emboj/cdg249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman LH, Goldman WE, Goldberg GI, Hebert MB, Schlessinger D. Location of the initial cleavage sites in mouse pre-rRNA. Mol Cell Biol. 1983;3:1501–1510. doi: 10.1128/mcb.3.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savino R, Gerbi SA. In vivo disruption of Xenopus U3 snRNA affects ribosomal RNA processing. EMBO J. 1990;9:2299–2308. doi: 10.1002/j.1460-2075.1990.tb07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerbi SA, Borovjagin AV. Pre-ribosomal RNA processing in multicellular organisms. In: Olson MOJ, editor. The Nucleolus. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 170–198. In:, ed. [Google Scholar]
- 27.Dunbar DA, Wormsley S, Agentis TM, Baserga SJ. Mpp10p, a U3 small nucleolar ribonucleoprotein component required for pre-18S rRNA processing in yeast. Mol Cell Biol. 1997;17:5803–5812. doi: 10.1128/mcb.17.10.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher JE, Baserga SJ. Two-hybrid Mpp10p interaction-defective Imp4 proteins are not interaction defective in vivo but do confer specific pre-rRNA processing defects in Saccharomyces cerevisiae. Nucleic Acids Res. 2004;32:1404–1413. doi: 10.1093/nar/gkh318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn H, Hierlmeier T, Merl J, Jakob S, Aguissa-Toure AH, Milkereit P, Tschochner H. The Noc-domain containing C-terminus of Noc4p mediates both formation of the Noc4p-Nop14p submodule and its incorporation into the SSU processome. PLoS One. 2009;4:e8370. doi: 10.1371/journal.pone.0008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dez C, Dlakic M, Tollervey D. Roles of the HEAT repeat proteins Utp10 and Utp20 in 40S ribosome maturation. RNA. 2007;13:1516–1527. doi: 10.1261/rna.609807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebaron S, Papin C, Capeyrou R, Chen YL, Froment C, Monsarrat B, Caizergues-Ferrer M, Grigoriev M, Henry Y. The ATPase and helicase activities of Prp43p are stimulated by the G-patch protein Pfa1p during yeast ribosome biogenesis. EMBO J. 2009;28:3808–3819. doi: 10.1038/emboj.2009.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torchet C, Jacq C, Hermann-Le Denmat S. Two mutant forms of the S1/TPR-containing protein Rrp5p affect the 18S rRNA synthesis in Saccharomyces cerevisiae. RNA. 1998;4:1636–1652. doi: 10.1017/s1355838298981511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller OL, Jr, Beatty BR. Visualization of nucleolar genes. Science. 1969;164:955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- 34.Mougey EB, O'Reilly M, Osheim Y, Miller OL, Jr, Beyer A, Sollner-Webb B. The terminal balls characteristic of eukaryotic rRNA transcription units in chromatin spreads are rRNA processing complexes. Genes Dev. 1993;7:1609–1619. doi: 10.1101/gad.7.8.1609. [DOI] [PubMed] [Google Scholar]
- 35.Dragon F, Gallagher JE, Compagnone-Post PA, Mitchell BM, Porwancher KA, Wehner KA, Wormsley S, Settlage RE, Shabanowitz J, Osheim Y. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature. 2002;417:967–970. doi: 10.1038/nature00769. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osheim YN, French SL, Keck KM, Champion EA, Spasov K, Dragon F, Baserga SJ, Beyer AL. Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in Saccharomyces cerevisiae. Mol Cell. 2004;16:943–954. doi: 10.1016/j.molcel.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Phipps KR, Charette J, Baserga SJ. The small subunit processome in ribosome biogenesis-progress and prospects. WIREs RNA. 2011;2:1–21. doi: 10.1002/wrna.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Axt K, French SL, Beyer AL, Tollervey D. Kinetic analysis demonstrates a requirement for the Rat1 exonuclease in cotranscriptional pre-rRNA cleavage. PLoS One. 2014;9:e85703. doi: 10.1371/journal.pone.0085703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouquette J, Choesmel V, Gleizes PE. Nuclear export and cytoplasmic processing of precursors to the 40S ribosomal subunits in mammalian cells. EMBO J. 2005;24:2862–2872. doi: 10.1038/sj.emboj.7600752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donohue MF, Choesmel V, Faubladier M, Fichant G, Gleizes PE. Functional dichotomy of ribosomal proteins during the synthesis of mammalian 40S ribosomal subunits. J Cell Biol. 2010;190:853–866. doi: 10.1083/jcb.201005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allmang C, Petfalski E, Podtelejnikov A, Mann M, Tollervey D, Mitchell P. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev. 1999;13:2148–2158. doi: 10.1101/gad.13.16.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Cruz J, Kressler D, Tollervey D, Linder P. Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J. 1998;17:1128–1140. doi: 10.1093/emboj/17.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanchin NI, Goldfarb DS. The exosome subunit Rrp43p is required for the efficient maturation of 5.8S, 18S and 25S rRNA. Nucleic Acids Res. 1999;27:1283–1288. doi: 10.1093/nar/27.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Pestov DG. 5′-End surveillance by Xrn2 acts as a shared mechanism for mammalian pre-rRNA maturation and decay. Nucleic Acids Res. 2011;39:1811–1822. doi: 10.1093/nar/gkq1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira-Cerca S, Poll G, Gleizes PE, Tschochner H, Milkereit P. Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function. Mol Cell. 2005;20:263–275. doi: 10.1016/j.molcel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Borovjagin AV, Gerbi SA. Xenopus U3 snoRNA GAC-Box A′ and Box A sequences play distinct functional roles in rRNA processing. Mol Cell Biol. 2001;21:6210–6221. doi: 10.1128/MCB.21.18.6210-6221.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kent T, Lapik YR, Pestov DG. The 5′ external transcribed spacer in mouse ribosomal RNA contains two cleavage sites. RNA. 2009;15:14–20. doi: 10.1261/rna.1384709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma K, Tollervey D. Base pairing between U3 small nucleolar RNA and the 5′ end of 18S rRNA is required for pre-rRNA processing. Mol Cell Biol. 1999;19:6012–6019. doi: 10.1128/mcb.19.9.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mereau A, Fournier R, Gregoire A, Mougin A, Fabrizio P, Luhrmann R, Branlant C. An in vivo and in vitro structure-function analysis of the Saccharomyces cerevisiae U3A snoRNP: protein-RNA contacts and base-pair interaction with the pre-ribosomal RNA. J Mol Biol. 1997;273:552–571. doi: 10.1006/jmbi.1997.1320. [DOI] [PubMed] [Google Scholar]
- 50.Hughes JM. Functional base-pairing interaction between highly conserved elements of U3 small nucleolar RNA and the small ribosomal subunit RNA. J Mol Biol. 1996;259:645–654. doi: 10.1006/jmbi.1996.0346. [DOI] [PubMed] [Google Scholar]
- 51.Marmier-Gourrier N, Clery A, Schlotter F, Senty-Segault V, Branlant C. A second base pair interaction between U3 small nucleolar RNA and the 5′-ETS region is required for early cleavage of the yeast pre-ribosomal RNA. Nucleic Acids Res. 2011;39:9731–9745. doi: 10.1093/nar/gkr675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borovjagin AV, Gerbi SA. U3 small nucleolar RNA is essential for cleavage at sites 1, 2 and 3 in pre-rRNA and determines which rRNA processing pathway is taken in Xenopus oocytes. J Mol Biol. 1999;286:1347–1363. doi: 10.1006/jmbi.1999.2527. [DOI] [PubMed] [Google Scholar]
- 53.Renalier MH, Mazan S, Joseph N, Michot B, Bachellerie JP. Structure of the 5′-external transcribed spacer of the human ribosomal RNA gene. FEBS Lett. 1989;249:279–284. doi: 10.1016/0014-5793(89)80641-6. [DOI] [PubMed] [Google Scholar]
- 54.Michot B, Bachellerie JP. Secondary structure of the 5′ external transcribed spacer of vertebrate pre-rRNA. Presence of phylogenetically conserved features. Eur J Biochem. 1991;195:601–609. doi: 10.1111/j.1432-1033.1991.tb15743.x. [DOI] [PubMed] [Google Scholar]
- 55.Sloan KE, Schneider C, Watkins NJ. Comparison of the yeast and human nuclear exosome complexes. Biochem Soc Trans. 2012;40:850–855. doi: 10.1042/BST20120061. [DOI] [PubMed] [Google Scholar]
- 56.Sloan KE, Bohnsack MT, Schneider C, Watkins NJ. The roles of SSU processome components and surveillance factors in the initial processing of human ribosomal RNA. RNA. 2014;20:540–550. doi: 10.1261/rna.043471.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kass S, Craig N, Sollner-Webb B. Primary processing of mammalian rRNA involves two adjacent cleavages and is not species specific. Mol Cell Biol. 1987;7:2891–2898. doi: 10.1128/mcb.7.8.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delcasso-Tremousaygue D, Grellet F, Panabieres F, Ananiev ED, Delseny M. Structural and transcriptional characterization of the external spacer of a ribosomal RNA nuclear gene from a higher plant. Eur J Biochem. 1988;172:767–776. doi: 10.1111/j.1432-1033.1988.tb13956.x. [DOI] [PubMed] [Google Scholar]
- 59.Mullineux ST, Lafontaine DL. Mapping the cleavage sites on mammalian pre-rRNAs: where do we stand? Biochimie. 2012;94:1521–1532. doi: 10.1016/j.biochi.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Enright CA, Maxwell ES, Eliceiri GL, Sollner-Webb B. 5′ETS rRNA processing facilitated by four small RNAs: U14, E3, U17, and U3. RNA. 1996;2:1094–1099. [PMC free article] [PubMed] [Google Scholar]
- 61.Sáez-Vasquez J, Caparros-Ruiz D, Barneche F, Echeverría M. A plant snoRNP complex containing snoRNAs, fibrillarin, and nucleolin-like proteins is competent for both rRNA gene binding and pre-rRNA processing in vitro. Mol Cell Biol. 2004;24:7284–7297. doi: 10.1128/MCB.24.16.7284-7297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zakrzewska-Placzek M, Souret FF, Sobczyk GJ, Green PJ, Kufel J. Arabidopsis thaliana XRN2 is required for primary cleavage in the pre-ribosomal RNA. Nucleic Acids Res. 2010;38:4487–4502. doi: 10.1093/nar/gkq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vance VB, Thompson EA, Bowman LH. Transfection of mouse ribosomal DNA into rat cells: faithful transcription and processing. Nucleic Acids Res. 1985;13:7499–7513. doi: 10.1093/nar/13.20.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carron C, O'Donohue MF, Choesmel V, Faubladier M, Gleizes PE. Analysis of two human pre-ribosomal factors, bystin and hTsr1, highlights differences in evolution of ribosome biogenesis between yeast and mammals. Nucleic Acids Res. 2011;39:280–291. doi: 10.1093/nar/gkq734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sloan KE, Mattijssen S, Lebaron S, Tollervey D, Pruijn GJ, Watkins NJ. Both endonucleolytic and exonucleolytic cleavage mediate ITS1 removal during human ribosomal RNA processing. J Cell Biol. 2013;200:577–588. doi: 10.1083/jcb.201207131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lapik YR, Fernandes CJ, Lau LF, Pestov DG. Physical and functional interaction between Pes1 and Bop1 in mammalian ribosome biogenesis. Mol Cell. 2004;15:17–29. doi: 10.1016/j.molcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 67.Lebaron S, Schneider C, van Nues RW, Swiatkowska A, Walsh D, Bottcher B, Granneman S, Watkins NJ, Tollervey D. Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat Struct Mol Biol. 2012;19:744–753. doi: 10.1038/nsmb.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pertschy B, Schneider C, Gnadig M, Schafer T, Tollervey D, Hurt E. RNA helicase Prp43 and its co-factor Pfa1 promote 20 to 18S rRNA processing catalyzed by the endonuclease Nob1. J Biol Chem. 2009;284:35079–35091. doi: 10.1074/jbc.M109.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strunk BS, Novak MN, Young CL, Karbstein K. A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell. 2012;150:111–121. doi: 10.1016/j.cell.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen W, Bucaria J, Band DA, Sutton A, Sternglanz R. Enp1, a yeast protein associated with U3 and U14 snoRNAs, is required for pre-rRNA processing and 40S subunit synthesis. Nucleic Acids Res. 2003;31:690–699. doi: 10.1093/nar/gkg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horn DM, Mason SL, Karbstein K. Rcl1 protein, a novel nuclease for 18S ribosomal RNA production. J Biol Chem. 2011;286:34082–34087. doi: 10.1074/jbc.M111.268649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattijssen S, Welting TJ, Pruijn GJ. RNase MRP and disease. WIREs RNA. 2010;1:102–116. doi: 10.1002/wrna.9. [DOI] [PubMed] [Google Scholar]
- 73.Kufel J, Dichtl B, Tollervey D. Yeast Rnt1p is required for cleavage of the pre-ribosomal RNA in the 3′ ETS but not the 5′ ETS. RNA. 1999;5:909–917. doi: 10.1017/s135583829999026x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peculis BA, Steitz JA. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopus oocyte. Cell. 1993;73:1233–1245. doi: 10.1016/0092-8674(93)90651-6. [DOI] [PubMed] [Google Scholar]
- 75.Nemeth A, Perez-Fernandez J, Merkl P, Hamperl S, Gerber J, Griesenbeck J, Tschochner H. RNA polymerase I termination: where is the end? Biochim Biophys Acta. 1829;2013:306–317. doi: 10.1016/j.bbagrm.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Kempers-Veenstra AE, Oliemans J, Offenberg H, Dekker AF, Piper PW, Planta RJ, Klootwijk J. 3′-End formation of transcripts from the yeast rRNA operon. EMBO J. 1986;5:2703–2710. doi: 10.1002/j.1460-2075.1986.tb04554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henry Y, Wood H, Morrissey JP, Petfalski E, Kearsey S, Tollervey D. The 5′ end of yeast 5.8S rRNA is generated by exonucleases from an upstream cleavage site. EMBO J. 1994;13:2452–2463. doi: 10.1002/j.1460-2075.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson AW. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Hage A, Koper M, Kufel J, Tollervey D. Efficient termination of transcription by RNA polymerase I requires the 5′ exonuclease Rat1 in yeast. Genes Dev. 2008;22:1069–1081. doi: 10.1101/gad.463708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue Y, Bai X, Lee I, Kallstrom G, Ho J, Brown J, Stevens A, Johnson AW. Saccharomyces cerevisiae RAI1 (YGL246c) is homologous to human DOM3Z and encodes a protein that binds the nuclear exoribonuclease Rat1p. Mol Cell Biol. 2000;20:4006–4015. doi: 10.1128/mcb.20.11.4006-4015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang F, Phillips S, Butler JS. Rat1p and Rai1p function with the nuclear exosome in the processing and degradation of rRNA precursors. RNA. 2005;11:1571–1578. doi: 10.1261/rna.2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Granneman S, Petfalski E, Tollervey D. A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease. EMBO J. 2011;30:4006–4019. doi: 10.1038/emboj.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang L, Sahasranaman A, Jakovljevic J, Schleifman E, Woolford JL., Jr Interactions among Ytm1, Erb1, and Nop7 required for assembly of the Nop7-subcomplex in yeast preribosomes. Mol Biol Cell. 2008;19:2844–2856. doi: 10.1091/mbc.E07-12-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faber AW, Vos HR, Vos JC, Raue HA. 5′-End formation of yeast 5.8SL rRNA is an endonucleolytic event. Biochem Biophys Res Commun. 2006;345:796–802. doi: 10.1016/j.bbrc.2006.04.166. [DOI] [PubMed] [Google Scholar]
- 85.Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999;18:5399–5410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell P, Petfalski E, Tollervey D. The 3′ end of yeast 5.8S rRNA is generated by an exonuclease processing mechanism. Genes Dev. 1996;10:502–513. doi: 10.1101/gad.10.4.502. [DOI] [PubMed] [Google Scholar]
- 87.Jia H, Wang X, Anderson JT, Jankowsky E. RNA unwinding by the Trf4/Air2/Mtr4 polyadenylation (TRAMP) complex. Proc Natl Acad Sci U S A. 2012;109:7292–7297. doi: 10.1073/pnas.1201085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Briggs MW, Burkard KT, Butler JS. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8S rRNA 3′ end formation. J Biol Chem. 1998;273:13255–13263. doi: 10.1074/jbc.273.21.13255. [DOI] [PubMed] [Google Scholar]
- 89.van Hoof A, Lennertz P, Parker R. Three conserved members of the RNase D family have unique and overlapping functions in the processing of 5S, 5.8S, U4, U5, RNase MRP and RNase P RNAs in yeast. EMBO J. 2000;19:1357–1365. doi: 10.1093/emboj/19.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schillewaert S, Wacheul L, Lhomme F, Lafontaine DL. The evolutionarily conserved protein Las1 is required for pre-rRNA processing at both ends of ITS2. Mol Cell Biol. 2012;32:430–444. doi: 10.1128/MCB.06019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geerlings TH, Vos JC, Raue HA. The final step in the formation of 25S rRNA in Saccharomyces cerevisiae is performed by 5′→3′ exonucleases. RNA. 2000;6:1698–1703. doi: 10.1017/s1355838200001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lange H, Sement FM, Gagliardi D. MTR4, a putative RNA helicase and exosome co-factor, is required for proper rRNA biogenesis and development in Arabidopsis thaliana. Plant J. 2011;68:51–63. doi: 10.1111/j.1365-313X.2011.04675.x. [DOI] [PubMed] [Google Scholar]
- 93.Farrar JE, Nater M, Caywood E, McDevitt MA, Kowalski J, Takemoto CM, Talbot CC, Jr, Meltzer P, Esposito D, Beggs AH. Abnormalities of the large ribosomal subunit protein, Rpl35a, in Diamond-Blackfan anemia. Blood. 2008;112:1582–1592. doi: 10.1182/blood-2008-02-140012. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gazda HT, Sheen MR, Vlachos A, Choesmel V, O'Donohue MF, Schneider H, Darras N, Hasman C, Sieff CA, Newburger PE. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet. 2008;83:769–780. doi: 10.1016/j.ajhg.2008.11.004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gabel HW, Ruvkun G. The exonuclease ERI-1 has a conserved dual role in 5.8S rRNA processing and RNAi. Nat Struct Mol Biol. 2008;15:531–533. doi: 10.1038/nsmb.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferreira-Cerca S, Poll G, Kuhn H, Neueder A, Jakob S, Tschochner H, Milkereit P. Analysis of the in vivo assembly pathway of eukaryotic 40S ribosomal proteins. Mol Cell. 2007;28:446–457. doi: 10.1016/j.molcel.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 97.Robledo S, Idol RA, Crimmins DL, Ladenson JH, Mason PJ, Bessler M. The role of human ribosomal proteins in the maturation of rRNA and ribosome production. RNA. 2008;14:1918–1929. doi: 10.1261/rna.1132008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poll G, Braun T, Jakovljevic J, Neueder A, Jakob S, Woolford JL, Jr, Tschochner H, Milkereit P. rRNA maturation in yeast cells depleted of large ribosomal subunit proteins. PLoS One. 2009;4:e8249. doi: 10.1371/journal.pone.0008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohmayer U, Gamalinda M, Sauert M, Ossowski J, Poll G, Linnemann J, Hierlmeier T, Perez-Fernandez J, Kumcuoglu B, Leger-Silvestre I. Studies on the assembly characteristics of large subunit ribosomal proteins in Scerevisiae. PLoS One. 2013;8:e68412. doi: 10.1371/journal.pone.0068412. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gamalinda M, Ohmayer U, Jakovljevic J, Kumcuoglu B, Woolford J, Mbom B, Lin L, Woolford JL., Jr A hierarchical model for assembly of eukaryotic 60S ribosomal subunit domains. Genes Dev. 2014;28:198–210. doi: 10.1101/gad.228825.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Babiano R, Gamalinda M, Woolford JL, Jr, de la Cruz J. Saccharomyces cerevisiae ribosomal protein L26 is not essential for ribosome assembly and function. Mol Cell Biol. 2012;32:3228–3241. doi: 10.1128/MCB.00539-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gazda HT, Preti M, Sheen MR, O'Donohue MF, Vlachos A, Davies SM, Kattamis A, Doherty L, Landowski M, Buros C. Frameshift mutation in p53 regulator RPL26 is associated with multiple physical abnormalities and a specific pre-ribosomal RNA processing defect in diamond-blackfan anemia. Hum Mutat. 2012;33:1037–1044. doi: 10.1002/humu.22081. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M. One core, two shells: bacterial and eukaryotic ribosomes. Nat Struct Mol Biol. 2012;19:560–567. doi: 10.1038/nsmb.2313. [DOI] [PubMed] [Google Scholar]
- 104.Chen SS, Williamson JR. Characterization of the ribosome biogenesis landscape in Ecoli using quantitative mass spectrometry. J Mol Biol. 2013;425:767–779. doi: 10.1016/j.jmb.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clatterbuck Soper SF, Dator RP, Limbach PA, Woodson SA. In vivo X-ray footprinting of pre-30S ribosomes reveals chaperone-dependent remodeling of late assembly intermediates. Mol Cell. 2013;52:506–516. doi: 10.1016/j.molcel.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nierhaus KH. The assembly of prokaryotic ribosomes. Biochimie. 1991;73:739–755. doi: 10.1016/0300-9084(91)90054-5. [DOI] [PubMed] [Google Scholar]
- 107.Kim H, Abeysirigunawarden SC, Chen K, Mayerle M, Ragunathan K, Luthey-Schulten Z, Ha T, Woodson SA. Protein-guided RNA dynamics during early ribosome assembly. Nature. 2014;506:334–338. doi: 10.1038/nature13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anger AM, Armache JP, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature. 2013;497:80–85. doi: 10.1038/nature12104. [DOI] [PubMed] [Google Scholar]
- 109.Hadjiolov AA. The Nucleolus and Ribosome Biogenesis. New York: Springer-Verlag; 1985. [Google Scholar]
- 110.Adilakshmi T, Bellur DL, Woodson SA. Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature. 2008;455:1268–1272. doi: 10.1038/nature07298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mulder AM, Yoshioka C, Beck AH, Bunner AE, Milligan RA, Potter CS, Carragher B, Williamson JR. Visualizing ribosome biogenesis: parallel assembly pathways for the 30S subunit. Science. 2010;330:673–677. doi: 10.1126/science.1193220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leidig C, Thoms M, Holdermann I, Bradatsch B, Berninghausen O, Bange G, Sinning I, Hurt E, Beckmann R. 60S ribosome biogenesis requires rotation of the 5S ribonucleoprotein particle. Nat Commun. 2014;5:3491. doi: 10.1038/ncomms4491. [DOI] [PubMed] [Google Scholar]
- 113.Kiss T, Fayet E, Jady BE, Richard P, Weber M. Biogenesis and intranuclear trafficking of human box C/D and H/ACA RNPs. Cold Spring Harb Symp Quant Biol. 2006;71:407–417. doi: 10.1101/sqb.2006.71.025. [DOI] [PubMed] [Google Scholar]
- 114.Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. WIREs RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 115.Liang XH, Liu Q, Fournier MJ. Loss of rRNA modifications in the decoding center of the ribosome impairs translation and strongly delays pre-rRNA processing. RNA. 2009;15:1716–1728. doi: 10.1261/rna.1724409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Higa-Nakamine S, Suzuki T, Uechi T, Chakraborty A, Nakajima Y, Nakamura M, Hirano N, Kenmochi N. Loss of ribosomal RNA modification causes developmental defects in zebrafish. Nucleic Acids Res. 2012;40:391–398. doi: 10.1093/nar/gkr700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lafontaine D, Delcour J, Glasser AL, Desgres J, Vandenhaute J. The DIM1 gene responsible for the conserved m6(2)Am6(2)A dimethylation in the 3′-terminal loop of 18S rRNA is essential in yeast. J Mol Biol. 1994;241:492–497. doi: 10.1006/jmbi.1994.1525. [DOI] [PubMed] [Google Scholar]
- 118.White J, Li Z, Sardana R, Bujnicki JM, Marcotte EM, Johnson AW. Bud23 methylates G1575 of 18S rRNA and is required for efficient nuclear export of pre-40S subunits. Mol Cell Biol. 2008;28:3151–3161. doi: 10.1128/MCB.01674-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peifer C, Sharma S, Watzinger P, Lamberth S, Kotter P, Entian KD. Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Res. 2013;41:1151–1163. doi: 10.1093/nar/gks1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharma S, Watzinger P, Kotter P, Entian KD. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41:5428–5443. doi: 10.1093/nar/gkt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharma S, Yang J, Watzinger P, Kotter P, Entian KD. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–9076. doi: 10.1093/nar/gkt679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lafontaine DL, Preiss T, Tollervey D. Yeast 18S rRNA dimethylase Dim1p: a quality control mechanism in ribosome synthesis? Mol Cell Biol. 1998;18:2360–2370. doi: 10.1128/mcb.18.4.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 1803;2010:673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 124.Kos M, Tollervey D. The putative RNA helicase Dbp4p is required for release of the U14 snoRNA from preribosomes in Saccharomyces cerevisiae. Mol Cell. 2005;20:53–64. doi: 10.1016/j.molcel.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 125.Bohnsack MT, Kos M, Tollervey D. Quantitative analysis of snoRNA association with pre-ribosomes and release of snR30 by Rok1 helicase. EMBO Rep. 2008;9:1230–1236. doi: 10.1038/embor.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liang XH, Fournier MJ. The helicase Has1p is required for snoRNA release from pre-rRNA. Mol Cell Biol. 2006;26:7437–7450. doi: 10.1128/MCB.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bohnsack MT, Martin R, Granneman S, Ruprecht M, Schleiff E, Tollervey D. Prp43 bound at different sites on the pre-rRNA performs distinct functions in ribosome synthesis. Mol Cell. 2009;36:583–592. doi: 10.1016/j.molcel.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Srivastava L, Lapik YR, Wang M, Pestov DG. Mammalian DEAD box protein Ddx51 acts in 3′ end maturation of 28S rRNA by promoting the release of U8 snoRNA. Mol Cell Biol. 2010;30:2947–2956. doi: 10.1128/MCB.00226-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bassler J, Kallas M, Pertschy B, Ulbrich C, Thoms M, Hurt E. The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Mol Cell. 2010;38:712–721. doi: 10.1016/j.molcel.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ulbrich C, Diepholz M, Bassler J, Kressler D, Pertschy B, Galani K, Bottcher B, Hurt E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell. 2009;138:911–922. doi: 10.1016/j.cell.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 131.Pertschy B, Saveanu C, Zisser G, Lebreton A, Tengg M, Jacquier A, Liebminger E, Nobis B, Kappel L, van der Klei I. Cytoplasmic recycling of 60S preribosomal factors depends on the AAA protein Drg1. Mol Cell Biol. 2007;27:6581–6592. doi: 10.1128/MCB.00668-07. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsuo Y, Granneman S, Thoms M, Manikas RG, Tollervey D, Hurt E. Coupled GTPase and remodelling ATPase activities form a checkpoint for ribosome export. Nature. 2014;505:112–116. doi: 10.1038/nature12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Menne TF, Goyenechea B, Sanchez-Puig N, Wong CC, Tonkin LM, Ancliff PJ, Brost RL, Costanzo M, Boone C, Warren AJ. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat Genet. 2007;39:486–495. doi: 10.1038/ng1994. [DOI] [PubMed] [Google Scholar]
- 134.Finch AJ, Hilcenko C, Basse N, Drynan LF, Goyenechea B, Menne TF, Gonzalez Fernandez A, Simpson P, D'Santos CS, Arends MJ. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes Dev. 2011;25:917–929. doi: 10.1101/gad.623011. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schäfer T, Maco B, Petfalski E, Tollervey D, Bottcher B, Aebi U, Hurt E. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature. 2006;441:651–655. doi: 10.1038/nature04840. [DOI] [PubMed] [Google Scholar]
- 136.Ferreira-Cerca S, Sagar V, Schafer T, Diop M, Wesseling AM, Lu H, Chai E, Hurt E, LaRonde-LeBlanc N. ATPase-dependent role of the atypical kinase Rio2 on the evolving pre-40S ribosomal subunit. Nat Struct Mol Biol. 2012;19:1316–1323. doi: 10.1038/nsmb.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zemp I, Wild T, O'Donohue MF, Wandrey F, Widmann B, Gleizes PE, Kutay U. Distinct cytoplasmic maturation steps of 40S ribosomal subunit precursors require hRio2. J Cell Biol. 2009;185:1167–1180. doi: 10.1083/jcb.200904048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zemp I, Wandrey F, Rao S, Ashiono C, Wyler E, Montellese C, Kutay U. CK1δ and CK1{var} are components of human 40S subunit precursors required for cytoplasmic 40S maturation. J Cell Sci. 2014;127:1242–1253. doi: 10.1242/jcs.138719. [DOI] [PubMed] [Google Scholar]
- 139.Krogan NJ, Peng WT, Cagney G, Robinson MD, Haw R, Zhong G, Guo X, Zhang X, Canadien V, Richards DP. High-definition macromolecular composition of yeast RNA-processing complexes. Mol Cell. 2004;13:225–239. doi: 10.1016/s1097-2765(04)00003-6. et al. [DOI] [PubMed] [Google Scholar]
- 140.Dosil M, Bustelo XR. Functional characterization of Pwp2, a WD family protein essential for the assembly of the 90S pre-ribosomal particle. J Biol Chem. 2004;279:37385–37397. doi: 10.1074/jbc.M404909200. [DOI] [PubMed] [Google Scholar]
- 141.Grandi P, Rybin V, Bassler J, Petfalski E, Strauss D, Marzioch M, Schafer T, Kuster B, Tschochner H, Tollervey D. 90S pre-ribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol Cell. 2002;10:105–115. doi: 10.1016/s1097-2765(02)00579-8. et al. [DOI] [PubMed] [Google Scholar]
- 142.Wehner KA, Gallagher JE, Baserga SJ. Components of an interdependent unit within the SSU processome regulate and mediate its activity. Mol Cell Biol. 2002;22:7258–7267. doi: 10.1128/MCB.22.20.7258-7267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Billy E, Wegierski T, Nasr F, Filipowicz W. Rcl1p, the yeast protein similar to the RNA 3′-phosphate cyclase, associates with U3 snoRNP and is required for 18S rRNA biogenesis. EMBO J. 2000;19:2115–2126. doi: 10.1093/emboj/19.9.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wegierski T, Billy E, Nasr F, Filipowicz W. Bms1p, a G-domain-containing protein, associates with Rcl1p and is required for 18S rRNA biogenesis in yeast. RNA. 2001;7:1254–1267. doi: 10.1017/s1355838201012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karbstein K, Jonas S, Doudna JA. An essential GTPase promotes assembly of preribosomal RNA processing complexes. Mol Cell. 2005;20:633–643. doi: 10.1016/j.molcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 146.Hierlmeier T, Merl J, Sauert M, Perez-Fernandez J, Schultz P, Bruckmann A, Hamperl S, Ohmayer U, Rachel R, Jacob A. Rrp5p, Noc1p and Noc2p form a protein module which is part of early large ribosomal subunit precursors in Scerevisiae. Nucleic Acids Res. 2013;41:1191–1210. doi: 10.1093/nar/gks1056. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miles TD, Jakovljevic J, Horsey EW, Harnpicharnchai P, Tang L, Woolford JL., Jr Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Mol Cell Biol. 2005;25:10419–10432. doi: 10.1128/MCB.25.23.10419-10432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wery M, Ruidant S, Schillewaert S, Lepore N, Lafontaine DL. The nuclear poly(A) polymerase and exosome cofactor Trf5 is recruited cotranscriptionally to nucleolar surveillance. RNA. 2009;15:406–419. doi: 10.1261/rna.1402709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Figaro S, Wacheul L, Schillewaert S, Graille M, Huvelle E, Mongeard R, Zorbas C, Lafontaine DL, Heurgue-Hamard V. Trm112 is required for Bud23-mediated methylation of the 18S rRNA at position G1575. Mol Cell Biol. 2012;32:2254–2267. doi: 10.1128/MCB.06623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dez C, Houseley J, Tollervey D. Surveillance of nuclear-restricted pre-ribosomes within a subnucleolar region of Saccharomyces cerevisiae. EMBO J. 2006;25:1534–1546. doi: 10.1038/sj.emboj.7601035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–724. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 152.Houseley J, Tollervey D. Yeast Trf5p is a nuclear poly(A) polymerase. EMBO Rep. 2006;7:205–211. doi: 10.1038/sj.embor.7400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holub P, Lalakova J, Cerna H, Pasulka J, Sarazova M, Hrazdilova K, Arce MS, Hobor F, Stefl R, Vanacova S. Air2p is critical for the assembly and RNA-binding of the TRAMP complex and the KOW domain of Mtr4p is crucial for exosome activation. Nucleic Acids Res. 2012;40:5679–5693. doi: 10.1093/nar/gks223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weir JR, Bonneau F, Hentschel J, Conti E. Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proc Natl Acad Sci U S A. 2010;107:12139–12144. doi: 10.1073/pnas.1004953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Castano IB, Heath-Pagliuso S, Sadoff BU, Fitzhugh DJ, Christman MF. A novel family of TRF (DNA topoisomerase I-related function) genes required for proper nuclear segregation. Nucleic Acids Res. 1996;24:2404–2410. doi: 10.1093/nar/24.12.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lepore N, Lafontaine DL. A functional interface at the rDNA connects rRNA synthesis, pre-rRNA processing and nucleolar surveillance in budding yeast. PLoS One. 2011;6:e24962. doi: 10.1371/journal.pone.0024962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shcherbik N, Wang M, Lapik YR, Srivastava L, Pestov DG. Polyadenylation and degradation of incomplete RNA polymerase I transcripts in mammalian cells. EMBO Rep. 2010;11:106–111. doi: 10.1038/embor.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Granneman S, Kudla G, Petfalski E, Tollervey D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc Natl Acad Sci U S A. 2009;106:9613–9618. doi: 10.1073/pnas.0901997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Granneman S, Petfalski E, Swiatkowska A, Tollervey D. Cracking pre-40S ribosomal subunit structure by systematic analyses of RNA-protein cross-linking. EMBO J. 2010;29:2026–2036. doi: 10.1038/emboj.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kudla G, Granneman S, Hahn D, Beggs JD, Tollervey D. Cross-linking, ligation, and sequencing of hybrids reveals RNA-RNA interactions in yeast. Proc Natl Acad Sci U S A. 2011;108:10010–10015. doi: 10.1073/pnas.1017386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wild T, Horvath P, Wyler E, Widmann B, Badertscher L, Zemp I, Kozak K, Csucs G, Lund E, Kutay U. A protein inventory of human ribosome biogenesis reveals an essential function of exportin 5 in 60S subunit export. PLoS Biol. 2010;8:e1000522. doi: 10.1371/journal.pbio.1000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012;13:R108. doi: 10.1186/gb-2012-13-11-r108. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bradatsch B, Leidig C, Granneman S, Gnädig M, Tollervey D, Böttcher B, Beckmann R, Hurt E. Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel. Nat Struct Mol Biol. 2012;19:1234–1241. doi: 10.1038/nsmb.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Greber BJ, Boehringer D, Montellese C, Ban N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat Struct Mol Biol. 2012;19:1228–1233. doi: 10.1038/nsmb.2425. [DOI] [PubMed] [Google Scholar]
- 165.Nissan TA, Galani K, Maco B, Tollervey D, Aebi U, Hurt E. A pre-ribosome with a tadpole-like structure functions in ATP-dependent maturation of 60S subunits. Mol Cell. 2004;15:295–301. doi: 10.1016/j.molcel.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 166.Sengupta J, Bussiere C, Pallesen J, West M, Johnson AW, Frank J. Characterization of the nuclear export adaptor protein Nmd3 in association with the 60S ribosomal subunit. J Cell Biol. 189:1079–1086. doi: 10.1083/jcb.201001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Armistead J, Triggs-Raine B. Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett. 2010;588:1491–1500. doi: 10.1016/j.febslet.2014.03.024. 2014. [DOI] [PubMed] [Google Scholar]
- 168.Ellis SR, Gleizes P-E. Diamond Blackfan anemia: ribosomal proteins going rogue. Semin Hematol. 2011;48:89–96. doi: 10.1053/j.seminhematol.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 169.Landowski M, O'Donohue MF, Buros C, Ghazvinian R, Montel-Lehry N, Vlachos A, Sieff CA, Newburger PE, Niewiadomska E, Matysiak M. Novel deletion of RPL15 identified by array-comparative genomic hybridization in Diamond-Blackfan anemia. Hum Genet. 2013;132:1265–1274. doi: 10.1007/s00439-013-1326-z. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wong CC, Traynor D, Basse N, Kay RR, Warren AJ. Defective ribosome assembly in Shwachman-Diamond syndrome. Blood. 2011;118:4305–4312. doi: 10.1182/blood-2011-06-353938. [DOI] [PubMed] [Google Scholar]
- 171.Marneros AG. BMS1 is mutated in aplasia cutis congenita. PLoS Genet. 2013;9:e1003573. doi: 10.1371/journal.pgen.1003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nonnekens J, Perez-Fernandez J, Theil AF, Gadal O, Bonnart C, Giglia-Mari G. Mutations in TFIIH causing trichothiodystrophy are responsible for defects in ribosomal RNA production and processing. Hum Mol Genet. 2013;22:2881–2893. doi: 10.1093/hmg/ddt143. [DOI] [PubMed] [Google Scholar]
- 173.Derenzini M, Montanaro L, Trere D. What the nucleolus says to a tumour pathologist. Histopathology. 2009;54:753–762. doi: 10.1111/j.1365-2559.2008.03168.x. [DOI] [PubMed] [Google Scholar]
- 174.Ruggero D. Revisiting the nucleolus: from marker to dynamic integrator of cancer signaling. Sci Signal. 2012;5:pe38. doi: 10.1126/scisignal.2003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D, Anderes K. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Belin S, Beghin A, Solano-Gonzalez E, Bezin L, Brunet-Manquat S, Textoris J, Prats AC, Mertani HC, Dumontet C, Diaz JJ. Dysregulation of ribosome biogenesis and translational capacity is associated with tumor progression of human breast cancer cells. PLoS One. 2009;4:e7147. doi: 10.1371/journal.pone.0007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marcel V, Ghayad SE, Belin S, Therizols G, Morel A-P, Solano-Gonzàlez E, Vendrell JA, Hacot S, Mertani HC, Albaret MA. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell. 2013;24:318–330. doi: 10.1016/j.ccr.2013.08.013. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barna M, Pusic A, Zollo O, Costa M, Kondrashov N, Rego E, Rao PH, Ruggero D. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456:971–975. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, Barna M. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145:383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]