Abstract
Generalized social anxiety disorder (gSAD) is associated with impoverished anterior cingulate cortex (ACC) engagement during attentional control. Attentional Control Theory proposes such deficiencies may be offset when demands on resources are increased to execute goals. To test the hypothesis attentional demands affect ACC response 23 patients with gSAD and 24 matched controls performed an fMRI task involving a target letter in a string of identical targets (low load) or a target letter in a mixed letter string (high load) superimposed on fearful, angry, and neutral face distractors. Regardless of load condition, groups were similar in accuracy and reaction time. Under low load gSAD patients showed deficient rostral ACC recruitment to fearful (vs. neutral) distractors. For high load, increased activation to fearful (vs. neutral) distractors was observed in gSAD suggesting a compensatory function. Results remained after controlling for group differences in depression level. Findings indicate perceptual demand modulates ACC in gSAD.
Keywords: social anxiety disorder, brain imaging, fMRI, threat distractors, attentional control
Generalized social anxiety disorder (gSAD) is characterized by pervasive fears of negative evaluation (APA, 2000) and attentional bias toward threat-relevant stimuli (Bögels & Mansell, 2004) making it difficult to ignore such stimuli even in the context of cognitively demanding tasks (Hope, Rapee, Heimberg, & Dombeck, 1990; Mattia, Heimberg, & Hope, 1993; Spector, Pecknold, & Libman, 2003). According to biased competition models of attention, sensory-driven emotional signals compete with task-relevant demands for resources in a limited-capacity processing system (Desimone & Duncan, 1995; Pessoa, McKenna, Gutierrez, & Ungerleider, 2002). What prevails, even if incongruent to cognitive aims, subsequently interacts with emotion-generating regions; therefore, prefrontal areas that modulate attentional deployment such as the anterior cingulate cortex (ACC) (Bush, Luu, & Posner, 2000; Etkin, Egner, & Kalisch, 2011) play a role in emotion generation and regulation (Ochsner, Silvres, & Buhle, 2012). Attentional bias is a proposed causal mechanism in maintaining anxiety that is excessive (e.g., Mathews & MacLeod, 1994; Williams, Watts, MacLeod, & Mathews, 1997); consequently, it is important to elucidate mechanisms associated with bias to threat in gSAD.
When attending to threat faces, gSAD relative to healthy controls (HC), exhibit exaggerated activation in rostral ACC (rACC) (Amir et al., 2005; Blair et al., 2008) and subgenual ACC (Goldin et al., 2009) indicative of aberrant emotion regulation (Etkin et al., 2011); exaggerated dorsal ACC (dACC) (Phan, Fitzgerald, Nathan, & Tancer, 2006) signifying heightened appraisal or reactivity to threat (Etkin et al., 2011); as well as hyper-activation in key limbic emotion regions (e.g., amygdala, anterior insula; Freitas-Ferrari et al., 2010).
Regarding attentional control, we found less rACC engagement in gSAD relative to HC when attention was directed to shapes in a simple task comprising images of face distractors alongside shapes, but no limbic-related group effects (Klumpp, Post, Angstadt, Fitzgerald, & Phan, 2013). Results suggest a failure to optimally resolve emotional interference in gSAD. Similarly, Blair et al. (2012) observed hypo-activation in the dACC in gSAD participants during an attentional control task also without accompanying differential limbic activation. The dACC is involved in conflict monitoring and action initiation to cognitive demands (Botvinick et al., 1999; Bush et al., 2000; Srinivasan et al., 2013). Thus, a lack of dACC engagement in gSAD indicates a deficiency in controlled cognitive processes.
The general finding of impoverished ACC recruitment in gSAD when top-down control is required is consistent with Attentional Control Theory (ACT), in which the failure of anxious individuals to inhibit task-irrelevant stimuli is due to cognitive efficiency deficits (Eysenck, Derakshan, Santos, & Calvo, 2007). Yet, ACT also proposes anxiety-related impairment can be counteracted when a task is particularly challenging to execute, though at the cost of recruiting more resources. For example, in an emotional Stroop task, high-trait anxious individuals have shown greater dACC activity than low-trait anxious individuals during high-conflict incongruent trials, relative to congruent trials (Krug & Carter, 2010). However, Stroop-related dACC recruitment reflects a late-stage selection process (Silton et al., 2010) and attentional bias to threat in anxious individuals is thought to be somewhat involuntary (Mathews & MacLeod, 1994; Williams, Watts, MacLeod, & Mathews, 1997) as evinced by the fact that paradigms commonly employed are low in cognitive load (Freitas-Ferrari et al., 2010).
Early stages of attention are modulated by load on attentional resources (O’Connor, Fukui, Pinsk, & Kastner, 2002); thus, varying perceptual load in gSAD may capture ACC responses according to ACT. In a study by Bishop et al. (2007), load was manipulated to place varying (high/low) demands on attention resources. Anxiety level and dACC activity to fearful face distractors were inversely related in high-trait anxious individuals but only under low load (with a non-significant trend towards the same finding in rACC). Furthermore, state anxiety positively correlated with amygdala response under low, but not high load. In spider-phobia, phobic individuals showed greater amygdala reactivity to distracting spider images than HC regardless of load but no ACC group effects were found (Straube et al., 2011). These mixed results may be due in part to a subclinical sample (Bishop et al., 2007) and perceptual differences when supplanting fearful faces with spider images (Straube et al., 2011).
To our knowledge the modulation of varied perceptual load on ACC in gSAD is not known despite its potential to expand our understanding of attentional bias mechanisms that may not be detected with behavioral measures (e.g., accuracy, reaction time) particularly when compensatory functions occur. Therefore, we employed a paradigm similar to Bishop et al. (2007). Under low perceptual load, we hypothesized that relative to HC, gSAD would show less rACC recruitment and under high load, greater dACC activation. We also explored whether amygdala and/or anterior insula activation to threat distractors would be greater in gSAD to HC.
Method
Participants
All participants provided written informed consent as approved by the local Institutional Review Board. The gSAD group encompassed 23 individuals (69.6% female) with a mean age of 26.1±6.7 years who met criteria for gSAD as determined by the Structured Clinical Interview for DSM-IV (First, Spitzer, Williams, & Gibbon, 1995). Co-morbidities were specific phobia (n=3), generalized anxiety disorder (n=2), and obsessive-compulsive disorder (n=1). Exclusionary criteria included current or recent (within last 6 months) major depressive disorder or substance abuse. The HC group comprised 24 individuals (54.2% female) with an average age of 25.0±5.6 years. The Liebowitz Social Anxiety Scale, which comprises a total score derived by adding fear and avoidance sub-scores (Liebowitz, 1987), Spielberger State-Trait Anxiety Inventory (Spielberger, 1983) and Beck Depression Inventory (Beck, Steer, & Brown, 1996) were used to evaluate symptom severity, trait anxiety, and depression levels, respectively. Greater symptom severity was evident in the gSAD (M=70.7±15.1) than HC group (M=6.8±5.6), t(44) = 19.4, p<0.001. Similarly, the gSAD group had greater trait anxiety (M=52.3±9.9) and depression (M=11.8±8.1) levels than the HC group (M=26.2±4.5; M=0.8±1.1); t(44)=11.6, p<0.001, t(44)=6.6, p<.001, respectively. The groups were similar in age, years of education, ethnicity, and gender (all ps >0.2). All participants were right-handed and free of major medical or neurologic illness.
Task
During fMRI, participants completed a task modeled on Bishop et al. (2007), which also included angry face distractors, as anger and fear have been shown to differentially perturb emotion processing circuitry (Whalen et al., 2001). Participants viewed a string of six letters superimposed on a task-irrelevant face distractor and had to identify target letters (N or X). In low perceptual load trials, the string was comprised entirely of target letters; under high perceptual load, the string included a single target letter and five non-target letters (H, K, M, W, Z) arranged in randomized order. Distractor faces were from a standardized set of photographs and consisted of fearful, angry, and neutral expressions from 8 different individuals (Ekman & Friesen, 1976). The experiment involved two image acquisition runs, each comprising 12 blocks of 5 trials. A mixed block/event-related design was employed whereby perceptual load (low vs. high) varied across blocks and facial expression (fearful, angry, neutral) varied within blocks on a trial-by-trial basis. Images were presented for 200ms followed by a fixation cross presented for 1800ms; participants were asked to respond by button press as quickly and accurately as possible. Within blocks, trials were separated by a jittered interstimulus interval lasting 2–6s; trials between blocks were separated by 4–8s.
Functional imaging
Imaging was performed with blood-oxygen level-dependent (BOLD) sensitive whole-brain fMRI on a 3.0 Tesla GE Signa System (General Electric; Milwaukee, WI) using a standard radio frequency coil. Images were acquired with 30 axial, 5-mm-thick slices using a standard T2*-sensitive gradient echo reverse spiral acquisition sequence (2000ms TR; 25ms TE; 64×64 matrix; 24cm FOV; 77° flip angle). For anatomical localization, a high-resolution, T1-weighted volumetric anatomical scan was acquired. Data were analyzed using the Statistical Parametric Mapping (SPM8) software package (Wellcome Trust Centre for Neuroimaging, London; www.fil.ion.ucl.ac.uk/spm) using standard preprocessing steps. Briefly, images were temporally corrected to account for differences in slice time collection, spatially realigned to the first image of the first run, normalized to a Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm3 voxels, and smoothed with an 8mm isotropic Gaussian kernel.
A general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128s high-pass filter. Blocks of low and high perceptual load were modeled separately based on task-irrelevant face type (fearful, angry, neutral) resulting in six regressors (fearful low, fearful high, angry low, angry high, neutral low, neutral high), the effects of which were estimated for each voxel for each participant and taken to the second level for random effects analysis.
In SPM8, we performed separate 2 (Group: gSAD, HC) × 2 (Face Type: threat, neutral) × 2 (Load: low, high) ANOVAs for fearful (vs. neutral) and angry (vs. neutral) faces. To test hypotheses, anatomically derived regions of interest (ROI) from the Automated Anatomical Labeling (AAL) toolbox based on the atlas of Tzourio-Mazoyer et al. (2002) we re used to examine group effects in ACC, amygdala, and anterior insula. To examine ACC and insula subregions, the rostral ACC was created by taking the AAL ACC below the line z=0 and for dACC, the AAL median cingulate was anterior to y=0. The AAL anterior insula was demarcated as y-axis=0 and forward. Significance was set at p<0.005 uncorrected with a minimum of at least 20 contiguous voxels to strike a balance between Type I and Type II error rates (Lieberman & Cunningham, 2009) and a small volume correction was used to correct for multiple comparisons within ROIs. Additionally, we performed a whole-brain analysis at the p<0.005 uncorrected threshold to examine regions beyond ROI masks. These regions were identified by visual assessment and cross-referenced with the Talairach atlas (Talairach & Tournoux, 1988).
Parameter estimates of peak activation (β weights) were extracted from functionally localized spheres (10-mm diameter) around peak voxels in regions of interest and submitted to post-hoc two-tailed t-tests to clarify the direction of activation and to conduct two-tailed correlations with symptom severity and fear and avoidance measures.
Results
Functional MRI
Fearful (vs. Neutral) Distractors
Regarding ROIs, an ANOVA did not reveal significant group main effects or interactions with group for dACC, amygdala, or anterior insula. A group x load interaction showed anatomically-based rACC activation, however, it was less than 20 contiguous voxels and did not survive small volume correction (i.e., family-wise error, p=0.11). Rather, at the whole-brain level, the interaction was significant for a rostral (pregenual) ACC cluster that abutted the anatomically-based rACC mask [(0, 30, 6), F=12.2, k=57, volume=456 mm3]. Post-hoc t-tests regarding low load showed less rACC activity in the gSAD group, while the HC group demonstrated increased activity for fear relative to neutral distractors. Conversely, under high load, gSAD exhibited increased rACC activity to the HC group for fearful versus neutral distractors (see Figure 1). This pattern of results did not change after controlling for depression level, although the rACC cluster was slightly reduced in size [(0, 30, 6), F=12.0, k=51, volume=408 mm3]. Follow-up analyses using an alternative ‘control’ condition, (e.g., fearful vs. fixation) confirmed that interaction in rACC response [(0, 30, 10), F=14.8, k=69, volume=552 mm3] was in response to fearful faces. There were no relationships between rACC activation and symptom severity or fear or avoidance symptoms.
Figure 1.
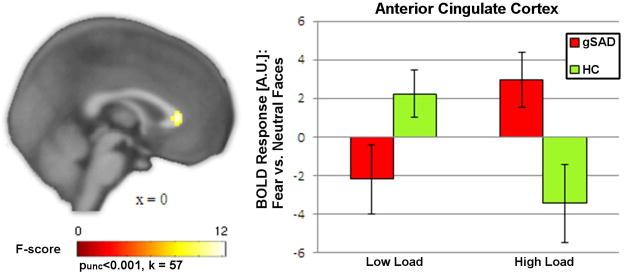
(A) Whole-brain voxel-wise statistical F-map displayed on a canonical brain showing group differences in activation for fearful versus neutral faces as a function of perceptual load (low vs. high) localized to the anterior cingulate cortex (peak voxel at 0, 30, 6, F=12.2). (B) Bar graphs depicting extracted parameter estimates of activation from the ACC ROI within each group showing gSAD exhibited positive ACC activation to fearful versus neutral faces under high but not low perceptual load, whereas HC showed the opposite pattern.
gSAD, Generalized Social Anxiety Disorder; Healthy Control (HC)
Beyond regions of interest, the group x load interaction revealed significant activity in the precuneus [(0, −74, 38), F=12.7, k=117, volume=936 mm3] and medulla [(0, −44, −48), F=11.6, k=29, volume=232 mm3]. Post-hoc t-tests concerning low load showed less precuneus activity in the gSAD than HC group (p<0.002), which was not significant under high load (p=0.12). Also under low perceptual load, the gSAD group exhibited greater activity in the medulla compared to the HC group (p<0.02), which was also not significant in the high-load condition (p=0.15).
Angry (vs. Neutral) Distractors
An ANOVA revealed no significant group main effects or interactions with group for ACC, amygdala, or anterior insula ROIs. Outside a priori regions, we observed a group x load interaction in the midbrain [(6, −32, −6), F=9.84, k=23, volume=184 mm3] such that under low load the gSAD group demonstrated less activity than the HC group (p<0.01).
Findings from all voxel-wide analyses for Angry (vs. Neutral) Distractors and Fearful (vs. Neutral) Distractors can be found in Supplemental Results.
Behavioral Results
Mean accuracy across face type for low load (87.5±26.0) was higher than that for high load (62.6±17.9), t(40)=8.6, p<0.001 demonstrating greater demand on processing resources across all participants. A 2 (Group: gSAD, HC) × 2 (Face Type: fear, neutral) × 2 (Load: low, high) ANOVA showed a significant main effect of load F(3, 37)=26.7, p<0.001 with follow-up comparisons indicating participants were more accurate in both of the low-load conditions compared to high-load conditions (all ps<0.001). No significant effect of group or group x load or face type interaction emerged (all ps >0.05). See Table 1 for accuracy for trial type.
Table 1.
Accuracy and Reaction Times (in milliseconds) by load condition for type of face distractor
| Contrast | gSAD | HC | t | p |
|---|---|---|---|---|
| Accuracy | ||||
| Low load fear | 85.8 ± 30.1 | 88.3 ± 21.5 | −0.3 | 0.8 |
| Low load neutral | 86.5 ± 30.2 | 89.3 ± 23.3 | −0.3 | 0.7 |
| High load fear | 64.4 ± 19.8 | 59.7 ± 18.9 | 0.8 | 0.4 |
| High load neutral | 63.8 ± 18.3 | 62.6 ± 19.0 | 0.2 | 0.9 |
| Reaction Times | ||||
| Low load fear | 1147 ± 216 | 1126 ± 215 | 0.7 | 0.8 |
| Low load neutral | 735 ± 110 | 780 ± 185 | −0.9 | 0.4 |
| High load fear | 726 ± 91 | 755 ± 157 | −0.7 | 0.5 |
| High load neutral | 1161 ± 193 | 1080 ± 246 | 1.2 | 0.3 |
Note: gSAD = generalized social anxiety disorder; HC = healthy control
A similar analysis conducted for reaction time (RT) on accurate trials revealed no main effect for load or face type, but there was a significant load x face type interaction, F(3, 34)=106.1, p<0.001; follow-up comparisons showed participants were slower for both low-load fearful faces and high-load neutral faces compared to both high-load fearful faces and low-load neutral faces (all ps<0.001). There was no significant effect of group or group x load or face type interaction (all ps>0.05). See Table 1 for RT for trial type.
Discussion
Individuals with gSAD, relative to HC, exhibited less rostral (i.e., pregenual) ACC (rACC) activity under low perceptual load for fearful (vs. neutral) face distractors. In a previous study involving high- and low-trait anxious individuals, greater anxiety was associated with less activation in the low-load condition in prefrontal regions that included dorsal ACC (dACC) with a non-significant trend in rACC (Bishop et al., 2007). In contrast, our findings revealed a discrete group effect in rACC at the whole-brain level that was adjacent to our anatomy-based rACC mask, reflecting nuances in the way in which ACC is parceled. We observed a similar outcome in an earlier study (Klumpp et al., 2013) though there deficient rACC engagement in gSAD was not limited to fear, but extended to emotional face distractors in general. In that study task-relevant and irrelevant stimuli were presented alongside each other and for a longer duration (i.e., 4s vs. 200ms). Due to the ease in executing that simple task, it could be considered a low perceptual load condition; however, methodological differences preclude direct comparison between studies, especially as the task employed here tapped into earlier stages of attention, whereas the previous study included a longer stimulus presentation duration involving later, more voluntary, stages of attention.
Importantly, under high perceptual load, gSAD patients showed enhanced rACC recruitment to fearful distractors compared to HC, suggesting a compensatory function according to ACT (Eysenck et al., 2007). In support, gSAD patients performed similarly to HC in terms of accuracy and reaction time for low and high perceptual load, indicating rACC engagement under high load in gSAD may have played a compensatory role in maintaining intact task performance. Given the increase in task difficulty in the high- relative to low-load condition and role dACC plays in monitoring conflict and initiating action in the face of cognitive demands (Botvinick et al., 1999; Bush et al., 2000; Srinivasan et al., 2013), we hypothesized greater dACC activation in gSAD than HC. However, results showed the predicted pattern of differential activation in the rACC. Potentially, the offsetting of emotion regulation deficits under high load reflects chronic compensatory recruitment of top-down control to regulate excessive reactivity to threat-relevant cues when demands on attentional resources amplified. In the context of control models of emotion, high load may be the more optimal regulation condition in gSAD, as threat distractors would be expected to be less extensively processed in this condition (Ochsner et al., 2012). We did not observe an association between rACC activation and gSAD symptoms, suggesting the modulation of load on rACC may be a general abnormality that may extend to other anxiety disorders in which aberrant rACC activity has been shown during emotional interference (e.g., post-traumatic stress disorder, specific phobia; Britton, Gold, Deckersbach, & Rauch, 2009; Offringa et al., 2013).
ACC results were limited to task-irrelevant fearful, but not angry, faces which were included to evaluate the influence of threat content. Namely, angry expressions signify immediate threat from the person directed toward the viewer (Biehl et al., 1997; Ewbank et al., 2009; Whalen, 1998), whereas the source of threat is more ambiguous for fearful faces (Ewbank et al., 2009; Whalen, 1998). In this paradigm, the less direct threat signal conveyed by fear was more effective in detecting group differences.
Outside ACC findings, fearful (vs. neutral) face distractors under low perceptual load revealed less precuneus and greater medulla activation in gSAD than HC. Also under low load, but for angry (vs. neutral) face distractors, gSAD exhibited less midbrain activation than HC. We did not have hypotheses regarding these regions and hesitate to interpret these preliminary findings. No group differences were observed for high perceptual load.
Limitations
We did not observe group effects in dACC, amygdala, or anterior insula. Though the literature on exaggerated reactivity during attentional control in anxiety is mixed, our acquisition parameters, task design, and relatively small sample may have limited power to detect group differences in limbic/paralimbic reactivity to threat as a function of perceptual load. Moreover, the anatomy-based rACC finding was not significant after applying small volume correction indicating results may reflect Type I error. In addition, in Bishop et al. (2007), state, not trait, anxiety level was shown to positively correspond to amygdala activity under low perceptual load. Therefore, temporal anxiety levels, not measured here, may be a better measure of limbic response to threat distractors in this paradigm. Our gSAD group had higher levels of general anxiety and depression than the HC group; therefore, any findings related to these effects cannot be ruled out, though our pattern of results was maintained when controlling for depression level. Lastly, results in individuals with gSAD may not generalize to other anxious populations.
Conclusions
Despite limitations, these preliminary findings suggest gSAD patients differentially modulate rACC during attentional control over task-irrelevant fearful faces relative to healthy volunteers. Specifically, when demands on attentional resources are low, impoverished rACC recruitment occurs. Once demands are high, rACC is engaged, indicating a compensatory response in gSAD to threat distractors. Findings further implicate ACC dysfunction in gSAD in the context of emotional conflict.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Institute of Mental Health (MH076198 to KLP and MH093679 to HK).
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text revision. [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological psychiatry. 2005;57(9):975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Biehl M, Matsumoto D, Ekman P, Hearn V, Heider K, Kudoh T, Ton V. Matsumoto and Ekman’s Japanese and Caucasian facial expressions of emotion (JACFEE): Reliability data and cross-national differences. Journal of Nonverbal Behavior. 1997;21(1):3–21. [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: Effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, Devido J, Otero M, Pine DS. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry. 2012;72:476–482. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. The American journal of psychiatry. 2008;165(9):1193. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: Hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24:827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Britton JC, Gold AL, Deckersbach T, Rauch SL. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depression and anxiety. 2009;26(9):796–805. doi: 10.1002/da.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, Calder AJ. Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage. 2009;44(3):1144–1151. doi: 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, Crippa JAS. Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuropsychopharmacology & Biological Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66(2):170. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope DA, Rapee RM, Heimberg RG, Dombeck MJ. Representations of the self in social phobia: Vulnerability to social threat. Cognitive Therapy and Research. 1990;14:177–189. [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biology of Mood & Anxiety Disorders. 2013;45:83–91. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug MK, Carter CS. Adding fear to conflict: A general purpose cognitive control network is modulated by trait anxiety. Cognitive, Affective & Behavioral Neuroscience. 2010;10:357–371. doi: 10.3758/CABN.10.3.357. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR. Liebowitz social anxiety scale. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual review of psychology. 1994;45(1):25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mattia JI, Heimberg RG, Hope DA. The revised Stroop color-naming task in social phobias. Behaviour Research and Therapy. 1993;31:305–313. doi: 10.1016/0005-7967(93)90029-t. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature neuroscience. 2002;5(11):1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offringa R, Brohawn KH, Staples LK, Dubois SJ, Hughes KC, Pfaff DL, Shin LM. Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biology of mood & anxiety disorders. 2013;3(1):10. doi: 10.1186/2045-5380-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, Miller GA. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. 2010;50(3):1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector IP, Pecknold JC, Libman E. Selective attentional bias related to the noticeability aspect of anxiety symptoms in generalized social phobia. Journal of Anxiety Disorders. 2003;17:517–531. doi: 10.1016/s0887-6185(02)00232-3. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Srinivasan L, Asaad WF, Ginat DT, Gale JT, Dougherty DD, Williams ZM, Eskandar EN. Action Initiation in the Human Dorsal Anterior Cingulate Cortex. PloS one. 2013;8:e55247. doi: 10.1371/journal.pone.0055247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Lipka J, Sauer A, Mothes-Lasch M, Miltner WH. Amygdala activation to threat under attentional load in individuals with anxiety disorder. Biol Mood Anxiety Disord. 2011;1:12. doi: 10.1186/2045-5380-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-Dimensional proportional system: an approach to cerebral imaging. Thieme; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current directions in psychological science. 1998;7(6):177–188. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Williams JM, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. 2. Chichester, U.K: John Wiley & Sons; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


