Abstract
Background
Radial scars (RS) or complex sclerosing lesions (CSL) of the breast are benign radiological and histological entities. Radiologically, they appear as architectural distortion with central radiolucency. These stellate lesions are frequently identified on screening mammography and, with the introduction of population-based screening programs; their incidence has increased to 0.03%–0.09% of all core needle biopsies (CNB). However, they can pose diagnostic difficulty as their radiologic and histologic appearance mimic carcinoma. Because of the high incidence of atypia or associated occult malignancy, the current literature recommendation is excision of all mammographically detected RS/CSL diagnosed on CNB.
Aims and Objective
Our aim was to review all RS/CSL that were previously diagnosed on image-guided CNB from January 1st, 1994 to August 31st, 2013, and assess the pathology from the excisional biopsy to identify cases upstaged to atypia or neoplasm.
Results
There were a total of 113 CNB from 109 women with radial scar without concomitant atypia on CNB diagnosed during that period; five cases were excluded because of concurrent cancer. Average age of these women was 52.9 years (range: 23.0 – 82.0 years). Thirty-five women (38/100 CNB; 38.0%) have follow-up excision. The median size of the excised radial scars is 1.2 cm (range: 0.4 – 3.3 cm). More than two-thirds of excised cases (68.6%; 24/35) were greater than 1.0 cm. The mammographic and ultrasonographic imaging features were listed as architectural distortion in 53.1% (17/32) and hypoechoic nodules with irregular margins in 36.4% (12/33) respectively. Almost all excised cases 91.7%; 33/36) showed radiologic and pathologic concordance, and more than three-quarters (23/29; 79.3%) are designated as Bi-Rads level 4 (suspicious for malignancy). The 38 follow-up excisional biopsies revealed: 2 (5.3%) invasive mammary carcinomas (2 metaplastic carcinomas including adenoid cystic carcinoma); 2 (5.3%) in-situ ductal carcinoma; 1 (2.6%) lobular carcinoma in-situ; 5 (13.2%) atypical lobular hyperplasia; 1 (2.6%) atypical ductal hyperplasia; 22 (57.9%) residual radial scars; and 5 (13.2%) with no residual lesions on follow-up.
Conclusion
Follow-up excisional biopsy is warranted for RS/CSL specifically if they are larger than 1.0 cm with worrisome radiographic images or showed radiologic and pathologic discordance, as approximately 29% (11/38) of these cases will have an upgrade to in-situ or invasive carcinomas or other high risk lesions on follow-up.
Keywords: radial scar, breast cancer, excisional biopsy, core needle biopsy, upstage
INTRODUCTION
Radial Scar (RS) or Complex Sclerosing Lesion (CSL) is a pathological entity characterized by a fibroelastotic core with entrapped ducts. [1] Radiologically it reveals radiolucent central core and radiating spicules, which is indistinguishable from invasive carcinoma mammographically as well as histopathologically. [2, 3] It may be associated with atypical and typical usual epithelial hyperplasia, adenosis, papillomatosis, ductal carcinoma in situ (DCIS) or even invasive carcinoma within or adjacent to RS.[2, 4]
The incidence of RS is reported as 0.03% – 0.07%. [5] The pathogenesis of RS is uncertain. Reaction to an unknown trauma which results in scarring with elastosis or inflammation have been hypothesized.[3] It has been suggested that RS is a premalignant lesion for the development of breast cancer (BC), whereas it has also been proposed that coexistent proliferative epithelial lesions were the underlying causative factors for developing breast carcinoma. [6, 7]
Some groups advocate that all RS diagnosed on a prior CNB should be excised [2, 5, 8–13], whereas others do not support surgical excision. [14–18]
This study was initiated to evaluate the complete spectrum of RS and CSL and to define the clinical, mammographic and histopathologic characteristics in correlation with follow-up excisional biopsies in a single medical center.
MATERIAL and METHODS
Institutional Board Review from the Mayo Clinic, Rochester, MN, was obtained and approved to perform the study. This is a retrospective analysis of RS (≤ 1.0 cm) and CSL (> 1.0 cm) retrieved from the anatomic pathology at Mayo Clinic database. Study population consisted of patients with a diagnosis of RS or CSL who proceeded onto excisional biopsy at Mayo Clinic, Rochester, MN between January 1st, 1994 – August 31st, 2013. Cases in which the pathological diagnosis in the core biopsy was RS/CSL associated with atypical epithelial hyperplasia, lobular neoplasia, DCIS and malignancy were excluded. Patient’s demographic features such as age, body mass index, menopausal status, age at menarche, history of oophorectomy and/or hysterectomy, age at first live birth, number of births, smoking history, family history of BC, oral contraceptive use, hormonal therapy, and the reason for imaging were retrieved from the hospital records. All core and excisional biopsy specimens as well as radiological images were re-evaluated by two pathologists (BC, AN) and a radiologist (AC) respectively. Radiological evaluation was done using Breast Imaging Reporting and Data System (BI-RADS) score. Radiologic information including ultrasonography, mammography and MRI (magnetic resonance imaging) were captured. Size of the mass was noted from the radiology report. The size of the needle gauge and number of cores obtained during sampling were recorded. Core biopsy and follow-up excisional biopsy specimens were correlated with the diagnosis. Follow-up biopsy was classified as either having residual RS/CSL or associated Fibrocystic Disease (FCD), papillary lesion, atypical epithelial hyperplasia (ductal or lobular), in-situ carcinoma (DCIS or LCIS), invasive carcinoma and its subtype, presence of calcifications, following the histologic criteria developed by Page & Anderson (16).
Characteristics were summarized for each CNB using number and percentage for categorical variables and mean and standard deviation (SD) or median and interquartile range or range (as appropriate) for continuous variables. Three women had CNB performed at more than one site; all other women had a single CNB performed. Each CNB was treated independently for analysis. Characteristics for CNB that proceeded to excision were compared to CNB that had no excision using t-test or Wilcoxon rank sum test for continuous variables, and using chi-square or Fisher’s exact text for categorical variables, as appropriate for the data. All analyses were performed using SAS version 9.3. P-values less than 0.05 were considered statistically significant.
RESULTS
Patients diagnosed as RS/CSL using CNB in Mayo Clinic, Rochester, MN between January 1st, 1994 – August 31st, 2013 were a total of 109 women. Three women were excluded because the excision was done at a site different from the initial core biopsy (2 had invasive carcinoma on the excised site). Two women excluded because they had known invasive carcinoma one week earlier. Two women were excluded because they declined the use of their medical records for research purposes. There were a total of 96 women included in the study with a total of 100 CNB (three women had multiple sites examined). (Table 1) Thirty eight (38.0 %) CNB have follow-up excision (Table 2); whereas 62 (62.0%) cases did not have. Women who had follow-up excisional biopsies have lower BMI (body mass index), and has a higher proportion with history of cigarette smoking compared to those with no excision. Seventy (72.9%) CNB were identified through mammographic screening. Eleven CNB (11.5%) had palpable mass. (Table 1) The median time for surgical excision was 25.5 days; and median days from excision to follow-up were 4.2 years (range 26 days – 10.2 years). The median size of the excised radial scars is 1.2 cm (0.4 – 3.3 cm). (Table 1) More than two-thirds of excised cases (68.6%; 24/35) were greater than 1.0 cm.
Table 1.
Clinical and Pathologic characteristics of all CNB included in the study
| No Excision (N=62 CNB, 62 women) |
Excision (any time) (N=38 CNB, 35 women) |
Total (N=100 CNB, 97 women) |
p value | |
|---|---|---|---|---|
| Age at CNB | 0.071 | |||
| Mean (SD) | 54.6 (11.7) | 50.2 (11.8) | 52.9 (11.9) | |
| BMI | 0.031 | |||
| Mean (SD) | 27.0 (6.1) | 24.4 (5.2) | 26.0 (5.9) | |
| Cigarette Smoking | 0.0052 | |||
| N | 56 | 35 | 91 | |
| No | 43 (76.8%) | 18 (51.4%) | 61 (67.0%) | |
| Ex | 1 (1.8%) | 7 (20.0%) | 8 (8.8%) | |
| Yes | 12 (21.4%) | 10 (28.6%) | 22 (24.2%) | |
| Family History of Breast Cancer | 18/51 (35.3%) | 19/35 (54.3%) | 37/86 (43.0%) | 0.083 |
| Age at Menarche | 0.103 | |||
| N | 38 | 34 | 72 | |
| ≤ 12 years | 16 (42.1%) | 8 (23.5%) | 24 (33.3%) | |
| > 12 years | 22 (57.9%) | 26 (76.5%) | 57 (79.2%) | |
| Oral Contraceptive Use | 24/41 (58.5%) | 20/34 (58.8%) | 44/75 (58.7%) | 0.983 |
| Age at First Birth | 0.413 | |||
| N | 33 | 25 | 58 | |
| ≤ 30 years | 28 (84.8%) | 23 (92.0%) | 51 (87.9%) | |
| > 30 years | 5 (15.2%) | 2 (8.0%) | 7 (12.1%) | |
| Number of Births | 0.903 | |||
| N | 53 | 34 | 87 | |
| ≤ 2 | 32 (60.4%) | 21 (61.8%) | 53 (60.9%) | |
| > 2 | 21 (39.6%) | 13 (38.2%) | 34 (39.1%) | |
| Menopause | 0.273 | |||
| N | 61 | 37 | 98 | |
| Post-menopausal | 35 (57.4%) | 17 (45.9%) | 52 (53.1%) | |
| Pre-menopausal | 26 (42.6%) | 20 (54.1%) | 46 (46.9%) | |
| Age at Menopause | 0.671 | |||
| N | 19 | 13 | 32 | |
| Mean (SD) | 44.9 (10.5) | 46.3 (7.3) | 45.5 (9.2) | |
| Hormone Replacement Therapy | 17/51 (33.3%) | 8/35 (22.9%) | 25/86 (29.1%) | 0.293 |
| Hysterectomy | 21/60 (35.0%) | 11/36 (30.6%) | 32/96 (33.3%) | 0.653 |
| Oophorectomy | 12/59 (20.3%) | 4/36 (11.1%) | 16/95 (16.8%) | 0.243 |
| Presentation | 0.052 | |||
| N | 62 | 34 | 96 | |
| Mammographic screening | 51 (82.3%) | 19 (55.9%) | 70 (72.9%) | |
| Palpable mass | 5 (8.1%) | 6 (17.6%) | 11 (11.5%) | |
| Focal breast pain | 1 (1.6%) | 2 (5.9%) | 3 (3.1%) | |
| Screening MRI | 2 (3.2%) | 4 (11.8%) | 6 (6.3%) | |
| Other | 3 (4.8%) | 3 (8.8%) | 6 (6.3%) | |
| Modality of Measurement | ||||
| N | 58 | 35 | 93 | |
| MBI | 0 (0.0%) | 1 (2.9%) | 1 (1.1%) | |
| MRI | 4 (6.9%) | 8 (22.9%) | 12 (12.9%) | |
| Mammogram | 26 (44.8%) | 4 (11.4%) | 30 (32.3%) | |
| Ultrasound | 28 (48.3%) | 22 (62.9%) | 50 (53.8%) | |
| Mammogram Findings | ||||
| N | 57 | 32 | 89 | |
| Architectural distortion + Calcifications | 5 (8.8%) | 7 (21.9%) | 12 (13.5%) | |
| Architectural distortion | 9 (15.8%) | 10 (31.3%) | 19 (21.3%) | |
| Calcifications | 21 (36.8%) | 5 (15.6%) | 26 (29.2%) | |
| Mass | 6 (10.5%) | 0 (0.0%) | 6 (6.7%) | |
| Mass with calcifications | 1 (1.8%) | 0 (0.0%) | 1 (1.1%) | |
| Mass with distortion | 4 (7.0%) | 3 (9.4%) | 7 (7.9%) | |
| Occult | 8 (14.0%) | 6 (18.8%) | 14 (15.7%) | |
| Other | 3 (5.3%) | 1 (3.1%) | 4 (4.5%) | |
| BI-RADS Level | ||||
| N | 1 | 29 | 30 | |
| 3 (probably benign) | 0 (0.0%) | 5 (17.2%) | 5 (16.7%) | |
| 4 (suspicious) | 1 (100.0%) | 23 (79.3%) | 24 (80.0%) | |
| 5 (highly suggestive of malignancy) | 0 (0.0%) | 1 (3.4%) | 1 (3.3%) | |
| Ultrasound Findings | ||||
| N | 37 | 33 | 70 | |
| Hypoechoic area | 4 (10.8%) | 5 (15.2%) | 9 (12.9%) | |
| Hypoechoic area with shadowing | 5 (13.5%) | 7 (21.2%) | 12 (17.1%) | |
| Iso/Hyperechoic | 1 (2.7%) | 0 (0.0%) | 1 (1.4%) | |
| Mass with no shadowing | 13 (35.1%) | 10 (30.3%) | 23 (32.9%) | |
| Mass with shadowing | 6 (16.2%) | 7 (21.2%) | 13 (18.6%) | |
| Occult | 6 (16.2%) | 4 (12.1%) | 10 (14.3%) | |
| Other | 2 (5.4%) | 0 (0.0%) | 2 (2.9%) | |
| MRI Findings | ||||
| N | 6 | 10 | 16 | |
| Enhancing mass | 1 (16.7%) | 7 (70.0%) | 8 (50.0%) | |
| Non-mass enhancement | 3 (50.0%) | 1 (10.0%) | 4 (25.0%) | |
| Occult | 2 (33.3%) | 1 (10.0%) | 3 (18.8%) | |
| Other | 0 (0.0%) | 1 (10.0%) | 1 (6.3%) | |
| MBI Findings | ||||
| N | 2 | 9 | 11 | |
| Mass Moderate | 0 (0.0%) | 1 (11.1%) | 1 (9.1%) | |
| Mass shadowing | 0 (0.0%) | 1 (11.1%) | 1 (9.1%) | |
| Nonmass Marked | 0 (0.0%) | 1 (11.1%) | 1 (9.1%) | |
| Nonmass Mild | 1 (50.0%) | 0 (0.0%) | 1 (9.1%) | |
| Nonmass Moderate | 1 (50.0%) | 2 (22.2%) | 3 (27.3%) | |
| Occult | 0 (0.0%) | 4 (44.4%) | 4 (36.4%) | |
| Laterality | ||||
| N | 0 | 34 | 34 | |
| Left | 0 (0.0%) | 19 (55.9%) | 19 (55.9%) | |
| Right | 0 (0.0%) | 15 (44.1%) | 15 (44.1%) | |
| Size | 0.0434 | |||
| N | 58 | 35 | 93 | |
| Median | 0.9 | 1.2 | 1.1 | |
| Q1, Q3 | 0.5, 1.5 | 0.7, 1.7 | 0.6, 1.6 | |
| CNB Needle Gauge | ||||
| N | 59 | 36 | 95 | <0.0014 |
| 9 | 17 (28.8%) | 3 (8.3%) | 20 (21.1%) | |
| 11 | 22 (37.3%) | 6 (16.7%) | 28 (29.5%) | |
| 14 | 16 (27.1%) | 25 (69.4%) | 41 (43.2%) | |
| 16 | 4 (6.8%) | 2 (5.6%) | 6 (6.3%) | |
| * Gauges 1 and 2 combined with 9 | ||||
| Number of Cores | 0.603 | |||
| N | 57 | 36 | 93 | |
| ≤ 4 | 12 (21.1%) | 6 (16.7%) | 18 (19.4%) | |
| > 4 | 45 (78.9%) | 30 (83.3%) | 75 (80.6%) |
Unequal Variance T-Test
Fisher Exact
Chi-Square
Wilcoxon Rank Sum
Table 2.
Clinical, radiologic, and histologic information about the cases that were upstaged at excision
| Case # | Mammographic findings | Sonographic findings | Size (mm) |
Type of guidance |
No. of cores |
Size of needle (g) |
Vacuum assistance |
Malignancy associated with RS on excision |
|---|---|---|---|---|---|---|---|---|
| 1 | Mass with distortion | Mass with shadowing | 23 | US | 4 | 16 | no | metaplastic adenosquamous carcinoma |
| 3 | Architectural distortion | Mass with no shadowing | 14 | US | 4 | 14 | no | adenoid cystic carcinoma |
| 16 | Architectural distortion | Hypoechoic area | 13 | MRI | 12 | 9 | yes | low grade apocrine DCIS |
| 17 | Architectural distortion with calcifications | Mass with shadowing | 20 | US | 5 | 14 | no | low grade DCIS |
| 4 | Architectural distortion with calcifications | Mass with shadowing | 17 | US | 7 | 14 | no | ALH |
| 5 | Calcifications | NA | 4 | Mam | 4 | 11 | no | ALH |
| 6 | Occult | Mass with shadowing | 10 | US | 6 | 14 | no | ALH |
| 11 | Mass with distortion | Mass with shadowing | 12 | US | 8 | 9 | yes | ALH |
| 12 | Architectural distortion with calcifications | Mass with no shadowing | 27 | US | 8 | 14 | no | ALH |
| 25 | Architectural distortion | Hypoechoic area | 25 | US | 6 | 9 | yes | LCIS |
| 43 | Occult | Mass with no shadowing | 17 | US | 5 | 14 | no | ADH |
US = ultrasound; MRI = magnetic resonance imaging; Mam = mammography; DCIS = ductal carcinoma in situ, ALH = atypical lobular hyperplasia; LCIS = lobular carcinoma in situ; NA = not available
Demographical Analysis of RS/CSL with follow-up excision
Mean age of patients at CNB was 50.2 years (range 23.0–74.0) [Table 1]. Laterality of the radial scar was 15 (44.1%) right breast, and 19 (55.9%) on the left breast [Table 1]. Women with follow-up excision have a statistically significant larger median size of RS (1.2 cm vs. 0.9 cm) than those with no follow-up excision [Table 1]. In addition, women with follow-up excision have larger median CNB needle gauge (median 14.0 vs. 11.0) [Table 1].
Pathological Analysis
All core biopsies consisted of pure RS/CSL except for one with associated flat epithelial atypia. Residual RS/CSL was present in 22 (68.8%; 22/32) follow-up excisional biopsy. There was no residual RS/CSL on five patients (15.6%; 5/32).
Most cases were macroscopic radial scars (75.0%; 24/32) i.e. lesion greater than 5.0 mm. Most cases were surrounded by fat (81.3%; 26/32), and had stellate shape (90.6%; 29/32). Microcalcifications were present in 17 (53.1%; 17/32) radial scars detected on either core or excision.
Fibrocystic disease (FCD) was present in 28 (87.5%) patients with radial scar. Thirteen women (43.3%) had associated papilloma with the radial scar. Twelve women (40.0%) had associated flat epithelial atypia/columnar cell change (one from the carcinoma patients, and three from the high risk lesion patients). Five women (16.7%) had atypical lobular hyperplasia.
The upgrade to cancer (in-situ ductal carcinoma/DCIS and invasive carcinoma) at excision was 10.5% (4/38), while the upgrade to high risk lesion (lobular carcinoma in-situ/LCIS; atypical lobular hyperplasia; atypical ductal hyperplasia) at excision was 18.4% (7/38) (Table 2). Two women (5.3%) had invasive carcinoma (metaplastic and adenoid cystic carcinoma) [Figure 1] and another two (5.3%) had in-situ ductal carcinoma (DCIS) [Figure 2] on follow-up excisional biopsies. One woman (2.6%) had lobular carcinoma in situ (LCIS), one (2.6%) had atypical ductal hyperplasia, and 5 patients (13.2%) had isolated atypical lobular hyperplasia.
Figure 1.
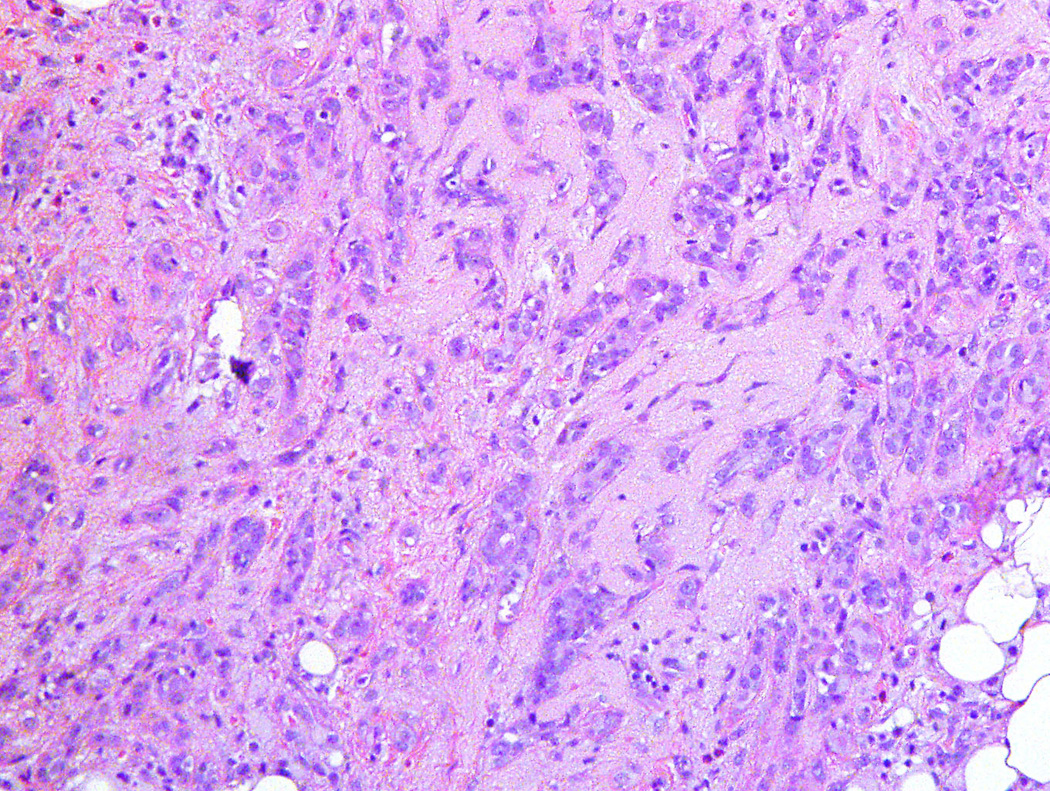
Medium-power magnification of low grade metaplastic adenosquamous carcinoma with infiltrating glands showing squamous differentiation (H&E stain; 40×)
Figure 2.
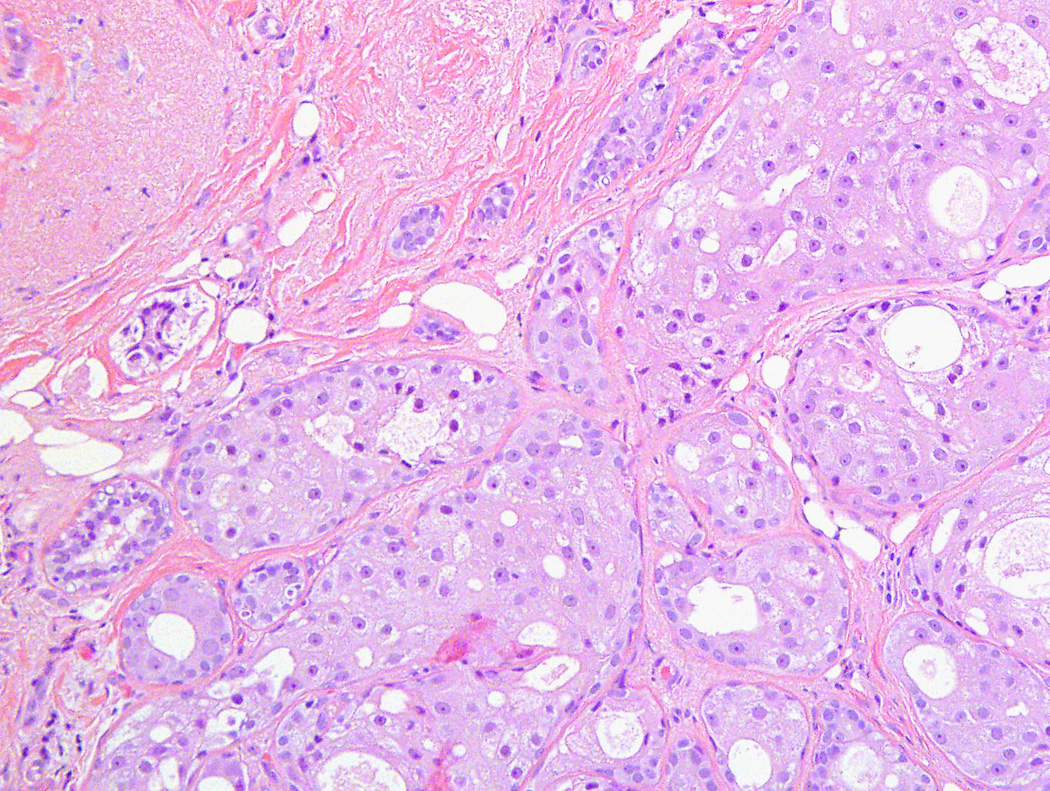
Medium-power magnification of low-grade apocrine ductal carcinoma in situ showing several duct units with solid proliferation of atypical neoplastic cells with apocrine differentiation (H&E stain; 40×)
Radiological Analysis
There was radiologic concordance in 33 cases (91.7%) and discordance in only three cases (8.3%). The average size of the RS detected by mammographic imaging was 1.30 cm (range: 0.40 – 3.30 cm). Mean lesion size for cases in which high-risk lesion detected was 1.39 cm (range 0.4 – 2.7 cm), and for malignancy was 1.45 cm (range 1.20 – 1.90 cm). Almost half (51.4%; 36/70) of women have a mass with or without shadowing on ultrasound imaging. The mammographic and ultrasonographic imaging features were listed as architectural distortion in 53.1% (17/32) and hypoechoic nodules with irregular margins in 36.4% (12/33) respectively. Almost all excised cases (91.7%; 33/36) showed radiologic and pathologic concordance, and that 79.3% (23/29) are designated as Bi-Rads level 4 (suspicious for malignancy).
DISCUSSION
Our study has shown that the upstaging rate to a high risk lesion was 29.0% (11/38) with in-situ and invasive ductal carcinoma in 10.5% (4/38). Upstaging was noted more in women with radial scar greater than 1.0 cm, and with those with radiologic/pathologic discordance on the initial core biopsies.
RS was first described by Hamperl in 1975, and later by Eusebi in 1972[13, 19, 20]. The incidence rates of RS have been reported as 0.03% and 0.09% in screening populations.[3, 12] Lesions smaller than 1.0 cm has been referred to as radial scars, whereas those greater than 1.0 cm is being described as “complex sclerosing lesions”.[3] RS poses diagnostic challenges for the following reasons: first its mammographic similarity to breast cancer, and secondly its association with cancer on further excision. [2] In some cohort studies of benign breast disease, radial scar was not found to be independently associated with an increased BC risk, and attributed the mild elevation in cancer risk secondary to the frequent association of RS with other proliferative disease [6, 7, 21]; however others have found that RS is independently associated with an increased BC risk.[7] The presence of atypia and carcinoma are usually seen at the periphery of the lesion. [3, 4, 7]
About 7.0% of women with RS developed invasive BC. [6] Metaplastic carcinoma was noted to be very commonly associated with radial sclerosing lesions.[10, 22] This observation was also noted by our group in the current study as two of the women with follow-up invasive cancers have metaplastic carcinoma.
In a survey of breast surgeons (members of the American Society of breast surgeons) excision of radial scar was much more variable with only 50% recommending routine excision. [23] In one study, using a larger-gauge needle (8 or 11) sampled radial scars adequately, that there were no upgrade lesions on follow-up surgical excision. [14, 15]. Therefore, some investigators recommend that no excisional biopsy of RS is required if the initial CNB was obtained using a large-gauge needle with vacuum–assisted biopsy specifically when the RS is not associated with atypia on prior needle biopsy and radiologic and histologic findings are concordant. [3, 15, 17, 18] Furthermore, if 12 or more cores are sampled, there is no upstaging to cancer. [18]
Some investigators have found several risk factors associated with cancer upstaging of radial scar including older postmenopausal women (greater than 50 years of age), large radiographic size of radial scar and presence of atypical hyperplasia within RS. [4, 7, 9] The average size of radial scars associated with cancer was reported as 1.4 cm [2, 10, 24]. Some groups did not find cancer when the RS was an incidental microscopic finding. [2, 24]. However, when radial scar was the targeted lesion, several investigators have found an association with atypical hyperplasia and carcinoma in situ. [2, 4, 7, 11]
Some investigators has found that RS is associated with high-risk lesions such atypical hyperplasia, in-situ and invasive carcinoma on follow-up excisional biopsy. [9, 15]. However, others did not find an increased risk of malignancy on follow-up excisional biopsy.[15] The high-risk lesion and cancer upstage rate in follow-up excisional biopsies ranged from 3% – 46% in several reports. [2, 5, 6, 8–10, 12, 13, 17, 18, 25]
Sloane and Mayers have found atypical hyperplasia and carcinoma in situ in 10.8% (8/74) and 9.5% (7/74) of RSs respectively.[4] Similarly, Jacobs et al. have identified atypical hyperplasia in 8.1% (8/99) of RSs. [7]. Moreover, King et al. have found atypical hyperplasia and carcinoma in situ in 31% (5/16) and 6.3% (1/16) of RSs respectively. [2] Patterson et al. have found atypical hyperplasia and carcinoma in situ in 18.9% (33/175) and 10.9% (19/175) respectively.[11] The latter group found invasive carcinoma in 9.1% (16/275) of women with follow-up excisional biopsy.[11]
Some investigators have found that women with RS do not need any additional follow-up beyond routine mammographic screening.[26]
In conclusion, our data support that women with a larger radial scars greater than 1.0 cm, and those with pathologic and radiologic discordance should undergo follow-up excisional biopsy to detect more significant lesions.
References
- 1.Eusebi V, Millis RR. Epitheliosis, infiltrating epitheliosis, and radial scar. Semin Diagn Pathol. 2010;27(1):5–12. doi: 10.1053/j.semdp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.King TA, Scharfenberg JC, Smetherman DH, Farkas EA, Bolton JS, Fuhrman GM. A better understanding of the term radial scar. Am J Surg. 2000;180(6):428–432. doi: 10.1016/s0002-9610(00)00506-7. discussion 432-423. [DOI] [PubMed] [Google Scholar]
- 3.Loane J. Benign sclerosing lesions of the breast. Diagnostic histopathology. 2009;15(8):395–401. [Google Scholar]
- 4.Sloane JP, Mayers MM. Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology. 1993;23(3):225–231. doi: 10.1111/j.1365-2559.1993.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 5.Osborn G, Wilton F, Stevens G, Vaughan-Williams E, Gower-Thomas K. A review of needle core biopsy diagnosed radial scars in the Welsh Breast Screening Programme. Ann R Coll Surg Engl. 2011;93(2):123–126. doi: 10.1308/003588411X12851639107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders ME, Page DL, Simpson JF, Schuyler PA, Dale Plummer W, Dupont WD. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer. 2006;106(7):1453–1461. doi: 10.1002/cncr.21730. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340(6):430–436. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 8.Linda A, Zuiani C, Furlan A, Londero V, Girometti R, Machin P, Bazzocchi M. Radial scars without atypia diagnosed at imaging-guided needle biopsy: how often is associated malignancy found at subsequent surgical excision, and do mammography and sonography predict which lesions are malignant? AJR Am J Roentgenol. 2010;194(4):1146–1151. doi: 10.2214/AJR.09.2326. [DOI] [PubMed] [Google Scholar]
- 9.Andacoglu O, Kanbour-Shakir A, Teh YC, Bonaventura M, Ozbek U, Anello M, Ganott M, Kelley J, Dirican A, Soran A. Rationale of excisional biopsy after the diagnosis of benign radial scar on core biopsy: a single institutional outcome analysis. Am J Clin Oncol. 2013;36(1):7–11. doi: 10.1097/COC.0b013e3182354a3f. [DOI] [PubMed] [Google Scholar]
- 10.Morgan C, Shah ZA, Hamilton R, Wang J, Spigel J, Deleon W, Deleon P, Leete T, Fulmer JM. The radial scar of the breast diagnosed at core needle biopsy. Proc (Bayl Univ Med Cent) 2012;25(1):3–5. doi: 10.1080/08998280.2012.11928768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson JA, Scott M, Anderson N, Kirk SJ. Radial scar, complex sclerosing lesion and risk of breast cancer. Analysis of 175 cases in Northern Ireland. Eur J Surg Oncol. 2004;30(10):1065–1068. doi: 10.1016/j.ejso.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Fasih T, Jain M, Shrimankar J, Staunton M, Hubbard J, Griffith CD. All radial scars/complex sclerosing lesions seen on breast screening mammograms should be excised. Eur J Surg Oncol. 2005;31(10):1125–1128. doi: 10.1016/j.ejso.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Toth D, Sebo E, Sarkadi L, Kovacs I, Kiss C, Damjanovich L. Role of core needle biopsy in the treatment of radial scar. Breast. 2012;21(6):761–763. doi: 10.1016/j.breast.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Sohn VY, Causey MW, Steele SR, Keylock JB, Brown TA. The treatment of radial scars in the modern era--surgical excision is not required. Am Surg. 2010;76(5):522–525. [PubMed] [Google Scholar]
- 15.Rakha EA, Ho BC, Naik V, Sen S, Hamilton LJ, Hodi Z, Ellis IO, Lee AH. Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology. 2011;58(4):626–632. doi: 10.1111/j.1365-2559.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 16.Crystal P, Sadaf A, Bukhanov K, McCready D, O'Malley F, Helbich TH. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: can underestimation be predicted? Eur Radiol. 2011;21(3):582–589. doi: 10.1007/s00330-010-1949-6. [DOI] [PubMed] [Google Scholar]
- 17.Linda A, Zuiani C, Furlan A, Lorenzon M, Londero V, Girometti R, Bazzocchi M. Nonsurgical management of high-risk lesions diagnosed at core needle biopsy: can malignancy be ruled out safely with breast MRI? AJR Am J Roentgenol. 2012;198(2):272–280. doi: 10.2214/AJR.11.7040. [DOI] [PubMed] [Google Scholar]
- 18.Brenner RJ, Jackman RJ, Parker SH, Evans WP, 3rd, Philpotts L, Deutch BM, Lechner MC, Lehrer D, Sylvan P, Hunt R, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol. 2002;179(5):1179–1184. doi: 10.2214/ajr.179.5.1791179. [DOI] [PubMed] [Google Scholar]
- 19.Hamperl H. Strahlige narben und obliterierende mastopathie. Virchows Arch A (Pathol Anat) 1975;369:55–68. doi: 10.1007/BF00432461. [DOI] [PubMed] [Google Scholar]
- 20.Eusebi V, Grassigli A, Gross F. Lesioni focali sclero-elastotiche mammarie simulanti il carcinoma infiltrante. Pathologica. 1976;68:507–518. [PubMed] [Google Scholar]
- 21.Kabat GC, Jones JG, Olson N, Negassa A, Duggan C, Ginsberg M, Kandel RA, Glass AG, Rohan TE. A multi-center prospective cohort study of benign breast disease and risk of subsequent breast cancer. Cancer Causes Control. 2010;21(6):821–828. doi: 10.1007/s10552-010-9508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denley H, Pinder SE, Tan PH, Sim CS, Brown R, Barker T, Gearty J, Elston CW, Ellis IO. Metaplastic carcinoma of the breast arising within complex sclerosing lesion: a report of five cases. Histopathology. 2000;36(3):203–209. doi: 10.1046/j.1365-2559.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 23.Nizri E, Schneebaum S, Klausner JM, Menes TS. Current management practice of breast borderline lesions--need for further research and guidelines. Am J Surg. 2012;203(6):721–725. doi: 10.1016/j.amjsurg.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 24.Lee KA, Zuley ML, Chivukula M, Choksi ND, Ganott MA, Sumkin JH. Risk of malignancy when microscopic radial scars and microscopic papillomas are found at percutaneous biopsy. AJR Am J Roentgenol. 2012;198(2):W141–W145. doi: 10.2214/AJR.11.7712. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi S, Giannotti E, Vanzi E, Marziali M, Abdulcadir D, Boeri C, Livi L, Orzalesi L, Sanchez LJ, Susini T, et al. Radial scar without associated atypical epithelial proliferation on image-guided 14-gauge needle core biopsy: analysis of 49 cases from a single-centre and review of the literature. Breast. 2012;21(2):159–164. doi: 10.1016/j.breast.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Bunting DM, Steel JR, Holgate CS, Watkins RM. Long term follow-up and risk of breast cancer after a radial scar or complex sclerosing lesion has been identified in a benign open breast biopsy. Eur J Surg Oncol. 2011;37(8):709–713. doi: 10.1016/j.ejso.2011.04.011. [DOI] [PubMed] [Google Scholar]


